Dear Editor,
Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been found to have many neuro-ophthalmologic manifestations, including optic neuritis and ischemic optic neuropathy [1], while vaccines against SARS-CoV-2 can also result in the rare development of thrombotic events [2]. Nonarteritic ischemic optic neuropathy (NAION) attributed to lack of perfusion to the optic nerve classically affects older patients having a small, crowded optic disc and with vasculopathic risk factors, or sleep apnea [3]. We report a novel case of NAION developed after vaccination with the recombinant adenoviral vector encoding the spike protein antigen of SARS-CoV-2 (AstraZeneca, Cambridge, UK) in a patient without well-known risk factors for NAION except old age.
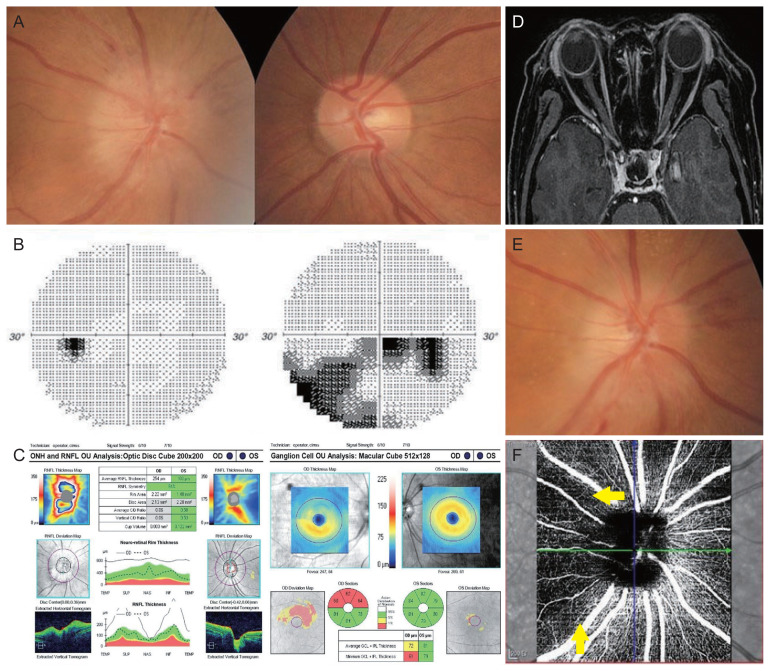
A previously healthy 65-year-old female patient presented with sudden painless diminution of inferior visual field in the right eye 15 days after receiving her second dose of the COVID-19 vaccine. She was referred to our neurology department for persistent vision loss despite a 3-day intravenous course of methylprednisolone (125 mg/day) at the neurology clinics outside. She denied a history of trauma, snoring or apneic episodes while sleeping, jaw claudication, fever, headache, scalp tenderness, or eye pain, while she had experienced mild fatigue and myalgia particularly in upper limbs for a few days following vaccination. Her social and medical history was unremarkable without any medication. On examination, her best-corrected visual acuities were counting fingers in the right eye and 20 / 20 in the left eye with normal color vision in each eye. Pupils responded to light properly, while there was a relative afferent pupillary defect in the right eye. Fundus examination demonstrated a swollen disc with several splinter hemorrhages in the right eye and a normal disc with cupping (not a disc-at-risk) in the fellow eye (Fig. 1A). Automatic visual field of the right eye revealed inferior arcuate and cecocentral visual field defects respecting the horizontal meridian, which was consistent with the findings of optical coherence tomography (OCT) (Fig. 1B, 1C). A magnetic resonance imaging of the brain and orbits revealed normal optic nerves without any increased signal intensity or abnormal enhancement, and no abnormalities of the brain (Fig. 1D). The remainder of her ocular finding was unremarkable. She had a body mass index of 25.1 kg/m2 and was normotensive. A majority of laboratory workup was noncontributory which included negative serologies for erythrocyte sedimentation rate, C-reactive protein, hemoglobin A1c, complete metabolic panel, prothrombin, partial thromboplastin time, antithrombin III, D-dimer, homocysteine, protein C, protein S, lupus anticoagulant, anti-aquaporin-4 antibody, and anti-myelin oligodendrocyte glycoprotein antibody. A polymerase chain reaction assay for SARS-CoV-2 was negative. Despite no signs of other cranial nerve or meningeal involvement, she was started on a 3-day additional course of intravenous methylprednisolone (1 g/day) given the concerns for vaccine-related immune reaction (mildly increased protein on the cerebrospinal fluid study, 54.1 mg/dL; normal, <40 mg/dL) and the previous insufficient steroid doses. Her visual acuity mildly improved to 20 / 200 in the right eye with the supero-temporal pallor of the optic disc and no improvement in the visual field defect when she completed a 3-week course of oral steroid tapering (Fig. 1E). Four months after vaccination, her visual acuity and field defect remained unchanged and there was a reduced peripapillary capillary density on OCT angiography corresponding to the previous optic disc lesion (Fig. 1F).
Fig. 1.
Nonarteritic ischemic optic neuropathy in the right eye after vaccination against Coronavirus disease 2019. (A) Disc photographs at presentation showing a swollen disc with several splinter peripapillary hemorrhages in the right eye and a normal disc with cupping (not a disc-at-risk) in the fellow eye. (B) Inferior arcuate and cecocentral visual field defects respecting the horizontal meridian in the right eye. (C) Optical coherence tomography showing thickened peripapillary retinal nerve fiber layer (RNFL) and thinning of macular retinal nerve fiber layer, corresponding to the visual field defect. (D) Gadolinium-enhancing T1-weighted magnetic resonance imaging of the brain and orbits showing normal optic nerves in both eyes. (E) Disc photograph after high-dose steroid treatment showing a progression of atrophy in the supero-temporal sector of the right optic disc. (F) Optical coherence tomography angiography 4 months after vaccination, indicating a decreased peripapillary capillary density adjacent to the superior and temporal sectors of optic disc (indicated by yellow arrows). The patient provided written informed consent for publication of the research details and clinical images. ONH = optic nerve head; OU = both eyes; OD = right eye; OS = left eye; C/D = cup/disc; TEMP = temporal; SUP = superior; NAS = nasal; INF = inferior; GCL = ganglion cell layer; IPL = internal plexiform layer.
Despite the report that vaccination with AstraZeneca can cause an immune thrombotic thrombocytopenia mediated by platelet-activating antibodies [2], it is uncertain whether the association of NAION and COVID-19 vaccination was causal or coincidental. However, there were several reports of ischemic optic neuropathy after influenza and varicella-zoster virus vaccinations, which could be explained by the interaction between vaccine antigens and specific HLA (human leukocyte antigen) molecules on the antigen presenting cells, resulting in an autoimmune reaction and vaccine adjuvants may also play a role in the induction of post-vaccine adverse events like vasculitis [4]. Although our patient with minimal cardiovascular risk factors developed the classic NAION based on the results of OCT angiography [5], she did not have abnormal serologic results for immune reaction, which was performed after steroid treatment. Nevertheless, NAION may occur in close temporal relationship to the COVID-19 vaccine. The association in this condition remains controversial, and further investigation is indicated to elucidate the pathophysiology of post-COVID-19 vaccination NAION.
Acknowledgements
None.
Footnotes
Conflicts of Interest
None.
Funding
None.
References
- 1.Tisdale AK, Dinkin M, Chwalisz BK. Afferent and efferent neuro-ophthalmic complications of coronavirus disease 19. J Neuroophthalmol. 2021;41:154–65. doi: 10.1097/WNO.0000000000001276. [DOI] [PubMed] [Google Scholar]
- 2.Greinacher A, Thiele T, Warkentin TE, et al. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384:2092–101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller NR, Arnold AC. Current concepts in the diagnosis, pathogenesis and management of nonarteritic anterior ischaemic optic neuropathy. Eye (Lond) 2015;29:65–79. doi: 10.1038/eye.2014.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nichani P, Micieli JA. Granuloma annulare, scalp necrosis, and ischemic optic neuropathy from giant cell arteritis after Varicella-Zoster virus vaccination. J Neuroophthalmol. 2021;41:e145–8. doi: 10.1097/WNO.0000000000000947. [DOI] [PubMed] [Google Scholar]
- 5.Augstburger E, Zeboulon P, Keilani C, et al. Retinal and choroidal microvasculature in nonarteritic anterior ischemic optic neuropathy: an optical coherence tomography angiography study. Invest Ophthalmol Vis Sci. 2018;59:870–7. doi: 10.1167/iovs.17-22996. [DOI] [PubMed] [Google Scholar]