Abstract
Among the available dressings for partial‐thickness burn wound treatment, SUPRATHEL has shown good usability and effectiveness for wound healing and patient comfort and has been used in many burn centres in the last decade. Recently, bacterial nanocellulose (BNC) has become popular for the treatment of wounds, and many studies have demonstrated its efficacy. epicitehydro, consisting of BNC and 95% water, is a promising product and has recently been introduced in numerous burn centres. To date, no studies including direct comparisons to existing products like SUPRATHEL have been conducted. Therefore, we aimed to compare epicitehydro to SUPRATHEL in the treatment of partial‐thickness burns. Twenty patients with partial‐thickness burns affecting more than 0.5% of their total body surface area (TBSA) were enrolled in this prospective, unicentric, open, comparative, intra‐individual clinical study. After debridement, the wounds were divided into two areas: one was treated with SUPRATHEL and the other with epicitehydro. Wound healing, infection, bleeding, exudation, dressing changes, and pain were documented. The quality of the scar tissue was assessed subjectively using the Patient and Observer Scar Scale. Wound healing in patients with a mean TBSA of 9.2% took 15 to 16 days for both treatments without dressing changes. All wounds showed minimal exudation, and patients reported decreased pain with the only significant difference between the two dressings on day 1. No infection or bleeding occurred in any of the wounds. Regarding scar evaluation, SUPRATHEL and epicitehydro did not differ significantly. Both wound dressings were easy to use, were highly flexible, created a safe healing environment, had similar effects on pain reduction, and showed good cosmetic and functional results without necessary dressing changes. Therefore, epicitehydro can be used as an alternative to SUPRATHEL for the treatment of partial‐thickness burn wounds.
Keywords: biological dressing, burns, reepithelialisation, wound healing
1. INTRODUCTION
Burn injuries are the fourth most common type of injury among all injuries worldwide. 1 While deep burn wounds require surgical treatment, various wound dressings have been developed for the treatment of superficial burns in the last decade. 2 , 3 , 4 The optimal wound dressing protects the wound against infection, has good biocompatibility, maintains a moist wound environment, and accelerates wound healing. 5 , 6 , 7 , 8 Furthermore, pain reduction during wound healing and decreased scar formation are important criteria for selecting an ideal wound dressing. 8 , 9 , 10 , 11 Nowadays, cost‐effectiveness also plays a central role in the selection of suitable wound treatment products. 5 , 6
Among the wide variety of available dressings, the synthetic dressing SUPRATHEL (PolyMedics Innovations GmbH, Denkendorf, Germany) has shown good usability and effectiveness in the treatment of partial‐thickness burn injuries 6 , 11 , 12 , 13 , 14 and has been compared with a number of different wound dressings in the past. Schwarze et al compared SUPRATHEL with Omniderm (Omikron Scientific Ltd., Rehovot, Israel), a transparent, hydrophilic, polyurethane membrane, and found no significant difference in healing time but observed a significant reduction in pain scores and increased patient comfort in burn wounds treated with SUPRATHEL. 12 In another study, Hundeshagen et al compared SUPRATHEL with Mepilex Ag (Mölnlycke, Göteborg, Sweden), a silver‐coated foam dressing, which was seven times cheaper and demonstrated significantly lower pain ratings in the SUPRATHEL group, as well as better elasticity 1 month after the burn injury. 6
Owing to the high price of SUPRATHEL, the search for cost‐effective alternatives with comparable properties continues. 6 , 12 One such alternative dressing is epicitehydro (QRSKIN GmbH, Würzburg, Germany), which consists of biotechnologically generated bacterial nanocellulose (BNC) synthesised by Komagataeibacter xylinus and 95% water. 15 , 16 The high amount of water incorporated in the cellulose reduces the intradermal temperature, wound progression, and pain through an evaporative cooling effect 17 and is loadable with antiseptic substances. 7 , 16 , 18 , 19 , 20 , 21
The objective of this study was to directly compare epicitehydro with its significantly more expensive competitor SUPRATHEL for partial‐thickness burn wounds in terms of patient comfort, wound healing, and scarring. epicitehydro and SUPRATHEL are both currently approved on the market as wound dressings and are therefore used within their intended range.
2. METHODS
Prior to enrolling patients in the study, approval was obtained from the appropriate institutional review board (Project No.: 5/2017), and all patients provided written informed consent. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki.
All patients aged 18 to 75 years who sustained partial‐thickness flame, scald, or contact burns with more than 0.5% of their total body surface area (TBSA) affected were enrolled after consenting to participate in this prospective, unicentric, open, comparative, intra‐individual clinical study.
Exclusion criteria were a lack of consent and compliance in the follow‐up examinations, an existence of inhalation trauma, burns caused by chemical substances or electricity, localisation of the burned area in the face, an ABSI score of 10, pregnancy or nursing, patients with an active infection or a suicide attempt within the last 12 months, or mentally unstable patients as well as patients who were treated with topical agents or pharmaceutical dressings prior to enrollment and admission. One patient who had an electrical burn was included in the study because the burn depth was only partial thickness and fasciotomy or escharotomy was not necessary.
A total of 20 patients meeting the eligibility criteria were enrolled between October 2018 and February 2020. Demographic data and data on medications administered by the treating emergency physicians were collected and documented.
On the day of admission, all wounds were cleaned mechanically with cotton gauze using Prontosan (B. Braun Melsungen AG, Melsungen, Germany) wound irrigation solution. The TBSA and burn depth were then estimated by a burn surgeon. If the attending physician assessed the depth as a partial‐thickness burn and the patients agreed to take part in the study, the wound was divided into two areas: one was treated with SUPRATHEL and the other with epicitehydro, simultaneously. After application, the wounds were covered with Jelonet (Smith & Nephew, Watford, England), Prontosan impregnated cotton gauze, and an external dressing.
The wound regime was analogous for both dressings in accordance with our standard of care. External dressing changes were performed regularly as long as exudation occurred. The two types of dressings both adhered to the wound over the course of wound healing and detached themselves independently after complete reepithelialisation.
The primary outcome measures investigated in this trial were infection, bleeding, exudation, and pain. Necessary dressing changes were also documented.
Exudation was analysed on days 1, 2, 4, 8, and 16 by visual inspection of an observer using the verbal rating scale (VRS) (0 = no exudate to 10 = maximal exudation). Pain was also analysed on days 1, 2, 4, 8, and 16 using a numerical rating scale (VRS) for pain (0 = no pain to 10 = the most extreme pain) as reported by the patient.
The secondary outcomes investigated in this study were time from wound treatment until wound healing (defined as <5% residual defect) and assessment of scar quality 3 and 6 months after injury. During these follow‐up examinations, scar quality was assessed using the Patient and Observer Scar Scale (POSAS). 22 , 23 , 24 , 25 , 26
2.1. Statistical methods
Comparisons of a priori hypotheses using t‐tests and descriptive statistics were performed using Prism 9 software Version 9.0.0 (GraphPad Software, LLC., San Diego, California).
Statistical significance was accepted at P ≥ .05.
3. RESULTS
Among the 20 patients who completed the trial, the partial‐thickness burns were mainly caused by scalds (55%), followed by flames (30%), and the injured body regions were mostly arms (55%), followed by legs (35%). The average TBSA was 9.2%, with a minimum of 1% and a maximum of 23%. The proportions of males and females were 65% and 35%, respectively, with a mean age of 36.6 years (Table 1).
TABLE 1.
Patient aetiology
| Pat. ID | Gender | Age (y) | TBSA | Burn cause | Study area |
|---|---|---|---|---|---|
| 1 | Male | 46 | 11 | Flame | Left and right knee |
| 2 | Female | 36 | 7.5 | Scald | Right arm |
| 3 | Male | 41 | 12 | Electricity | Right elbow/trunk |
| 4 | Male | 46 | 9.5 | Flame | Left and right forearm |
| 5 | Male | 25 | 14 | Explosion | Left and right forearm |
| 6 | Female | 29 | 7 | Scald | Left and right leg |
| 7 | Male | 26 | 5 | Flame | Left and right forearm |
| 8 | Female | 44 | 5 | Flame | Right hand |
| 9 | Male | 61 | 23 | Scald | Left shoulder and trunk |
| 10 | Male | 18 | 10.5 | Scald | Right forearm and leg |
| 11 | Male | 55 | 19 | Flame | Right arm |
| 12 | Male | 20 | 13.25 | Scald | Left leg |
| 13 | Female | 23 | 13 | Scald | Right arm |
| 14 | Male | 27 | 12 | Scald | Left leg |
| 15 | Male | 33 | 1 | Scald | Left and right hand |
| 16 | Female | 36 | 2 | Scald | Right forearm |
| 17 | Female | 29 | 5 | Flame | Left forearm and hand |
| 18 | Male | 51 | 8 | Scald | Right arm |
| 19 | Male | 45 | 1 | Explosion | Right hand |
| 20 | Female | 40 | 5 | Scald | Left and right leg |
| Mean | 36.6 | 9.2 | |||
| SD | 11.7 | 5.6 |
Abbreviations: TBSA, total body surface area.
3.1. Wound healing
Both dressings were placed on the wounds and gradually cut back as reepithelialisation progressed until the dressings were completely detached. They were highly flexible, adapted to the skin surface easily during the initial application, and became stiff as they slowly dried out during the wound healing process. The primary dressings did not need to be changed during the study period, and no infections or bleeding after wound debridement were observed. A difference in application time was not observed. Dressing changes did not occur for both epicitehydro and SUPRATHEL.
3.2. Exudation
Wounds in general showed low exudation rates (mean of 2.7 VRS on day 1; P = 1.0) that decreased during the healing process (mean of 0.1 VRS on day 16; P = 1.0) without a significant difference between the two dressings (Figure 1).
FIGURE 1.
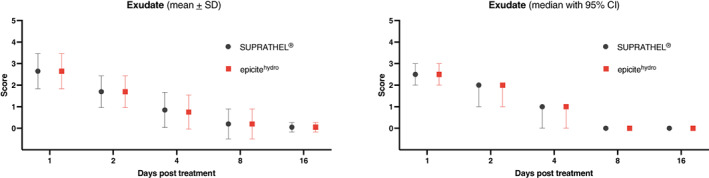
Exudation during wound healing (verbal rating scale 0‐10). CI, confidence interval
3.3. Pain
Patients rated their pain level using the VRS (0 = no pain at all to 10 = extreme pain). Wound‐related pain scores were low (mean of 2.6 for epicitehydro and 2.8 for SUPRATHEL on day 1; P = .041) and decreased during the healing process (Figure 2), solely with a significant difference between the two dressings on day 1 (P = .083 on day 2; P = .163 on day 4; P = 1.0 on day 8 and 16).
FIGURE 2.
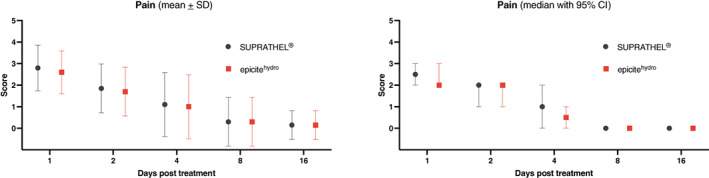
Pain during wound healing (verbal rating scale 0‐10). CI, confidence interval
3.4. Time for wound closure
Wound closure was documented for 18 of the 20 participating patients with a mean of 15.4 ± 4.9 days for wounds treated with SUPRATHEL and 16.1 ± 4.8 days (P = .111) for wounds treated with epicitehydro (Figures 3 and 4).
FIGURE 3.
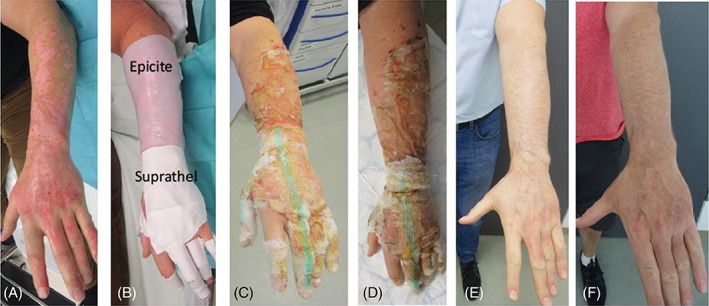
Partial‐thickness burns of the arm and hand, A, after debridement, B, after application of the dressings, C, day 5, D, day 8, E, after 3 months, F, after 6 months
FIGURE 4.
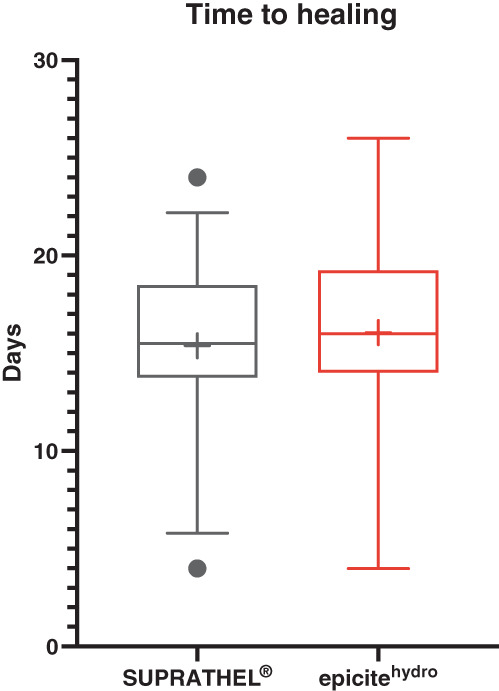
Graphical Analysis of data for the time up to conclusion of wound healing (Box and whiskers, 10%‐90% percentile, + = mean)
3.5. Scar evaluation
3.5.1. POSAS by patient
Scores for pain, itching, stiffness, thickness, and irregularity after 3 and 6 months were generally low for both dressings. Overall scores including those for wound colour were in the midrange and decreased from 3 to 6 months post‐injury. The distribution of scores showed a very similar pattern for both treatments, as shown in Table 2.
TABLE 2.
POSAS Scores surveyed by patients or observers after 3 and 6 months
| SUPRATHEL | epicitehydro | ||||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Median | Mean | SD | Median | P‐value | |
| POSAS patient | |||||||
| Pain 3 mo | 1.20 | 0.52 | 1 | 1.20 | 0.89 | 1 | 1.000 |
| Pain 6 mo | 1.45 | 1.60 | 1 | 1.55 | 1.32 | 1 | .541 |
| Itching 3 mo | 2.20 | 1.82 | 1 | 2.05 | 1.82 | 1 | .762 |
| Itching 6 mo | 2.40 | 2.80 | 1 | 2.30 | 2.48 | 1 | .541 |
| Colour 3 mo | 5.35 | 2.62 | 5 | 5.30 | 2.90 | 5 | .938 |
| Colour 6 mo | 4.20 | 2.57 | 4 | 4.05 | 1.93 | 4 | .769 |
| Stiffness 3 mo | 1.60 | 1.50 | 1 | 1.65 | 1.72 | 1 | .909 |
| Stiffness 6 mo | 2.05 | 2.26 | 1 | 1.35 | 0.93 | 1 | .209 |
| Thickness 3 mo | 2.10 | 1.83 | 1 | 2.25 | 2.07 | 1 | .774 |
| Thickness 6 mo | 1.65 | 1.39 | 1 | 1.60 | 1.05 | 1 | .895 |
| Irregularity 3 mo | 2.75 | 2.47 | 1 | 2.65 | 2.48 | 1 | .837 |
| Irregularity 6 mo | 2.00 | 1.59 | 1 | 1.65 | 1.18 | 1 | .330 |
| Overall 3 mo | 4.50 | 2.44 | 5 | 4.50 | 2.80 | 4 | 1.000 |
| Overall 6 mo | 3.70 | 2.08 | 3 | 3.55 | 1.99 | 3 | .643 |
| POSAS by observer | |||||||
| Pain 3 mo | 4.05 | 1.50 | 4 | 3.55 | 1.64 | 3 | .163 |
| Pain 6 mo | 2.50 | 1.54 | 2 | 2.25 | 1.41 | 2 | .449 |
| Itching 3 mo | 3.55 | 1.10 | 4 | 3.45 | 1.94 | 3 | .807 |
| Itching 6 mo | 2.70 | 1.63 | 3 | 2.75 | 1.41 | 3 | .874 |
| Colour 3 mo | 1.55 | 1.67 | 1 | 1.25 | 0.72 | 1 | .467 |
| Colour 6 mo | 1.45 | 1.15 | 1 | 1.20 | 0.52 | 1 | .349 |
| Stiffness 3 mo | 1.60 | 1.70 | 1 | 1.10 | 0.31 | 1 | .196 |
| Stiffness 6 mo | 1.40 | 1.00 | 1 | 1.35 | 0.67 | 1 | .825 |
| Thickness 3 mo | 1.00 | 0.00 | 1 | 1.10 | 0.31 | 1 | .163 |
| Thickness 6 mo | 1.40 | 1.27 | 1 | 1.15 | 0.67 | 1 | .171 |
| Irregularity 3 mo | 1.30 | 0.92 | 1 | 1.05 | 0.22 | 1 | .262 |
| Irregularity 6 mo | 1.25 | 0.91 | 1 | 1.20 | 0.62 | 1 | .748 |
| Overall 3 mo | 3.70 | 1.63 | 4 | 3.10 | 1.45 | 3 | .163 |
| Overall 6 mo | 2.95 | 1.40 | 3 | 2.70 | 0.89 | 3 | .383 |
Abbreviation: POSAS, Patient and Observer Scar Scale.
3.5.2. POSAS by observer
Scoring performed by an observer was performed using a VRS (0 = normal skin to 10 = worst scar imaginable). Scores for thickness, relief, pliability, and surface area after 3 and 6 months were generally low. Overall, vascularity and pigmentation scores were in the midrange and decreased from 3 to 6 months (Table 2).
The majority of the collected data showed very low average values that did not show any significant difference or trend to a difference between treatments with the two dressings.
4. DISCUSSION
Scars, which are clearly visible to everyone, can be a burden for patients and may have negative effects on social life and self‐confidence, and can even lead to depression. 27 , 28 , 29 Therefore, the cosmetic appearance of scars is often very important to patients. In the past few years, biosynthetic dressings have been produced to create an ideal replacement for human skin. Ideally, these dressings should: fit closely to the wound bed, be highly flexible, allow for exchange of water vapour, be long‐lasting, create a barrier against bacteria and contamination, be transparent in order to recognise infections easily, be easy to handle with a simple application and removal process, accelerate wound healing, produce acceptable cosmetic results, and be low in cost.
epicitehydro and SUPRATHEL both seem to provide these aspects. For an in‐depth comparison, an intra‐individual, prospective, clinical study was conducted.
In this study, both dressings offered a safe healing environment without infection and high levels of patient comfort for the treatment of partial‐thickness burns, without the need for painful dressing changes.
Karlsson et al described their experiences in the use of biosynthetic cellulose dressings in burns and reported an infection rate of 39%. 7 They treated 18 patients with superficial burn injuries with a mean TBSA of 8.2%. The median healing time was 28 days (13‐80 days), which is appreciably higher than the time for final closure in the epicitehydro treated wounds in the current study (16.1 days). 7 Karlsson et al also reported patients who described that the cellulose got “stiff” over their joints. 7 In the current study, no patient complaints about stiff wound dressings were documented, but most dressings were not applied over joints. In addition, Maurer et al compared polyurethane foam dressings with BNC sheets and reported that the length of hospitalised care and procedures requiring anaesthesia were significantly reduced in the nanocellulose group. 8
Partial‐thickness burn injuries are accompanied by pain. Therefore, pain reduction is one of the most important properties of wound dressings. In the literature, SUPRATHEL showed better pain reduction than Mepilex Ag 6 and Omiderm. 12 There are even studies that report an analgesic effect on burn wounds 6 , 30 and donor sites 31 for SUPRATHEL. In this study, the reported pain scores for SUPRATHEL‐ and epicitehydro‐treated burn wounds were generally low and decreased during the healing process. Due to ethical reasons, no burn wound received none of the two dressings. Therefore, we do not know, if both dressings had an analgesic effect. Nevertheless, epicitehydro showed similar pain scores compared with SUPRATHEL, which is in accordance with results found in previous studies. 7 , 8 , 32
Superficial partial‐thickness burn wounds normally heal without scarring or with minimal scarring. With professional wound care and infection prevention techniques, wound healing is completed in 2 to 4 weeks. 33 epicitehydro and SUPRATHEL are modern biosynthetic wound dressings that accelerate wound healing and minimise scarring. 34 , 35 , 36 In this study, wound healing of partial‐thickness burn wounds was completed after 15.4 and 16.1 days with SUPRATHEL and epicitehydro, respectively, which was higher than the time of reepithelialisation reported in previous studies dealing with partial‐thickness burn wounds of 12 6 and 13 days 12 for adults as well as 10.2 days, 11 10 days, 37 and 12 days 8 for children, respectively. One explanation for this phenomenon is the higher mean of affected TBSA in this study compared with other studies (9.2% vs 5.5% and 4.0%, respectively) 6 , 11 as well as the inclusion of partial‐thickness burn wounds with deeper areas, which also prolongs wound healing.
Some cases reported in the literature showed a higher degree of scarring or intermittent scarring in the treated areas, or a skin reaction, such as dermatitis, after application of wound dressings. 38 These findings were not confirmed in this trial, solely a slightly higher pigmentation rate in the burned areas treated with SUPRATHEL was observed.
In contrast, other groups described improved scar properties for SUPRATHEL‐treated superficial burn wounds and donor sites. 14 , 39
In a previous study, it was described that SUPRATHEL costs 0.56$ (United States Dollar) per square centimetre. 6 For our hospital epicitehydro is 3.6 times cheaper than SUPRATHEL, usually depending on the individual price negotiation between the hospital and the manufacturer. Therefore, epicitehydro is more cost‐effective than SUPRATHEL.
4.1. Limitations
This study had several limitations. First, the study group was rather small and comprised only 20 patients. Multicentre studies with larger sample sizes are needed to validate our results. Furthermore, burn depth was only assessed clinically.
5. CONCLUSION
Both wound dressings used in this study were easily handled, did not need to be removed or exchanged, were highly flexible, created a barrier against bacteria, showed no infections, had similar effects in pain reduction, and showed good cosmetic and functional results. Additionally, comparable healing times were observed with epicitehydro, which is more cost‐effective than SUPRATHEL. Therefore, epicitehydro can be used as an alternative, cost‐effective, wound dressing to SUPRATHEL for the treatment of partial‐thickness burn injuries.
CONFLICT OF INTEREST
The authors disclose the following commercial associations that might create a conflict of interest in connection with the submitted manuscript: This research was supported by QRskin (Germany). Hereby QRskin had no influence in the planning and implementation of the study. Furthermore, QRskin had no influence in the data collection.
Schiefer JL, Aretz GF, Fuchs PC, et al. Comparison of wound healing and patient comfort in partial‐thickness burn wounds treated with SUPRATHEL and epictehydro wound dressings. Int Wound J. 2022;19(4):782‐790. 10.1111/iwj.13674
Alexandra Schulz and Marc Daniels share equal authorship.
Funding information QRskin
DATA AVAILABILITY STATEMENT
Data is available upon request.
REFERENCES
- 1. Peck MD. Epidemiology of burns throughout the world. Part I: distribution and risk factors. Burns. 2011;37(7):1087‐1100. 10.1016/j.burns.2011.06.005 [DOI] [PubMed] [Google Scholar]
- 2. Collier M. Understanding the principles of wound management. J Wound Care. 2006;15(1 Suppl):S7‐S10. [PubMed] [Google Scholar]
- 3. Beele H. Artificial skin: past, present and future. Int J Artif Organs. 2002;25(3):163‐173. 10.1177/039139880202500302 [DOI] [PubMed] [Google Scholar]
- 4. Przybilski M, Deb R, Erdmann D, Germann G. New developments in skin replacement materials. Chirurg. 2004;75(6):579‐587. 10.1007/s00104-004-0860-6 [DOI] [PubMed] [Google Scholar]
- 5. Schiefer JL, Daniels M, Grigutsch D, Fuchs PC, Schulz A. Feasibility of pure silk for the treatment of large superficial burn wounds covering over 10% of the total body surface. J Burn Care Res. 2020;41(1):131‐140. 10.1093/jbcr/irz131 [DOI] [PubMed] [Google Scholar]
- 6. Hundeshagen G, Collins VN, Wurzer P, et al. A prospective, randomized, controlled trial comparing the outpatient treatment of pediatric and adult partial‐thickness burns with suprathel or mepilex Ag. J Burn Care Res. 2018;39(2):261‐267. 10.1097/BCR.0000000000000584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Karlsson M, Olofsson P, Steinvall I, Sjoberg F, Thorfinn J, Elmasry M. Three years' experience of a novel biosynthetic cellulose dressing in burns. Adv Wound Care (New Rochelle). 2019;8(2):71‐76. 10.1089/wound.2018.0790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Maurer K, Renkert M, Duis M, Weiss C, Wessel LM, Lange B. Application of bacterial nanocellulose‐based wound dressings in the management of thermal injuries: experience in 92 children. Burns. 2021. 10.1016/j.burns.2021.07.002. online ahead of print. [DOI] [PubMed] [Google Scholar]
- 9. Vloemans AF, Hermans MH, van der Wal MB, Liebregts J, Middelkoop E. Optimal treatment of partial thickness burns in children: a systematic review. Burns. 2014;40(2):177‐190. 10.1016/j.burns.2013.09.016 [DOI] [PubMed] [Google Scholar]
- 10. Kim LK, Martin HC, Holland AJ. Medical management of paediatric burn injuries: best practice. J Paediatr Child Health. 2012;48(4):290‐295. 10.1111/j.1440-1754.2011.02128.x [DOI] [PubMed] [Google Scholar]
- 11. Rashaan ZM, Krijnen P, Allema JH, Vloemans AF, Schipper IB, Breederveld RS. Usability and effectiveness of Suprathel((R)) in partial thickness burns in children. Eur J Trauma Emerg Surg. 2017;43(4):549‐556. 10.1007/s00068-016-0708-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schwarze H, Kuntscher M, Uhlig C, et al. Suprathel, a new skin substitute, in the management of partial‐thickness burn wounds: results of a clinical study. Ann Plast Surg. 2008;60(2):181‐185. 10.1097/SAP.0b013e318056bbf6 [DOI] [PubMed] [Google Scholar]
- 13. Highton L, Wallace C, Shah M. Use of Suprathel(R) for partial thickness burns in children. Burns. 2013;39(1):136‐141. 10.1016/j.burns.2012.05.005 [DOI] [PubMed] [Google Scholar]
- 14. Keck M, Selig HF, Lumenta DB, Kamolz LP, Mittlbock M, Frey M. The use of Suprathel((R)) in deep dermal burns: first results of a prospective study. Burns. 2012;38(3):388‐395. 10.1016/j.burns.2011.09.026 [DOI] [PubMed] [Google Scholar]
- 15. Klemm D, Petzold‐Welcke K, Kramer F, et al. Biotech nanocellulose: a review on progress in product design and today's state of technical and medical applications. Carbohydr Polym. 2021;254:117313. 10.1016/j.carbpol.2020.117313 [DOI] [PubMed] [Google Scholar]
- 16. Nischwitz SP, Bernardelli de Mattos I, Hofmann E, et al. Continuous pH monitoring in wounds using a composite indicator dressing ‐ a feasibility study. Burns. 2019;45(6):1336‐1341. 10.1016/j.burns.2019.02.021 [DOI] [PubMed] [Google Scholar]
- 17. Holzer JCJ, Tiffner K, Kainz S, et al. A novel human ex‐vivo burn model and the local cooling effect of a bacterial nanocellulose‐based wound dressing. Burns. 2020;46(8):1924‐1932. 10.1016/j.burns.2020.06.024 [DOI] [PubMed] [Google Scholar]
- 18. Fontana JD, de Souza AM, Fontana CK, et al. Acetobacter cellulose pellicle as a temporary skin substitute. Appl Biochem Biotechnol. 1990;24–25:253‐264. 10.1007/BF02920250 [DOI] [PubMed] [Google Scholar]
- 19. Picheth GF, Pirich CL, Sierakowski MR, et al. Bacterial cellulose in biomedical applications: a review. Int J Biol Macromol. 2017;104(Pt A):97‐106. 10.1016/j.ijbiomac.2017.05.171 [DOI] [PubMed] [Google Scholar]
- 20. Bernardelli de Mattos I, Nischwitz SP, Tuca AC, et al. Delivery of antiseptic solutions by a bacterial cellulose wound dressing: uptake, release and antibacterial efficacy of octenidine and povidone‐iodine. Burns. 2020;46(4):918‐927. 10.1016/j.burns.2019.10.006 [DOI] [PubMed] [Google Scholar]
- 21. de Mattos IB, Holzer JCJ, Tuca AC, et al. Uptake of PHMB in a bacterial nanocellulose‐based wound dressing: a feasible clinical procedure. Burns. 2019;45(4):898‐904. 10.1016/j.burns.2018.10.023 [DOI] [PubMed] [Google Scholar]
- 22. DeJong HM, Phillips M, Edgar DW, Wood FM. Response to letter to the editor: 'Patient opinion of scarring is multidimensional: an investigation of the POSAS with confirmatory factor analysis'. Burns. 2017;43(6):1361‐1362. 10.1016/j.burns.2016.11.010 [DOI] [PubMed] [Google Scholar]
- 23. Baryza MJ, Baryza GA. The Vancouver Scar Scale: an administration tool and its interrater reliability. J Burn Care Rehabil. 1995;16(5):535‐538. [DOI] [PubMed] [Google Scholar]
- 24. DeJong HM, Phillips M, Edgar DW, Wood FM. Patient opinion of scarring is multidimensional: an investigation of the POSAS with confirmatory factor analysis. Burns. 2017;43(1):58‐68. 10.1016/j.burns.2016.06.026 [DOI] [PubMed] [Google Scholar]
- 25. Finlay V, Burrows S, Kendell R, et al. Modified Vancouver Scar Scale score is linked with quality of life after burn. Burns. 2017;43(4):741‐746. 10.1016/j.burns.2016.11.007 [DOI] [PubMed] [Google Scholar]
- 26. Goei H, van der Vlies CH, Tuinebreijer WE, van Zuijlen PPM, Middelkoop E, van Baar ME. Predictive validity of short term scar quality on final burn scar outcome using the Patient and Observer Scar Assessment Scale in patients with minor to moderate burn severity. Burns. 2017;43(4):715‐723. 10.1016/j.burns.2016.10.012 [DOI] [PubMed] [Google Scholar]
- 27. Spronk I, Legemate CM, Dokter J, van Loey NEE, van Baar ME, Polinder S. Predictors of health‐related quality of life after burn injuries: a systematic review. Crit Care. 2018;22(1):160. 10.1186/s13054-018-2071-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lawrence JW, Fauerbach JA, Heinberg L, Doctor M. Visible vs hidden scars and their relation to body esteem. J Burn Care Rehabil. 2004;25(1):25‐32. 10.1097/01.BCR.0000105090.99736.48 [DOI] [PubMed] [Google Scholar]
- 29. Lawrence JW, Mason ST, Schomer K, Klein MB. Epidemiology and impact of scarring after burn injury: a systematic review of the literature. J Burn Care Res. 2012;33(1):136‐146. 10.1097/BCR.0b013e3182374452 [DOI] [PubMed] [Google Scholar]
- 30. Uhlig C, Rapp M, Hartmann B, Hierlemann H, Planck H, Dittel KK. Suprathel‐an innovative, resorbable skin substitute for the treatment of burn victims. Burns. 2007;33(2):221‐229. 10.1016/j.burns.2006.04.024 [DOI] [PubMed] [Google Scholar]
- 31. Schwarze H, Kuntscher M, Uhlig C, et al. Suprathel, a new skin substitute, in the management of donor sites of split‐thickness skin grafts: results of a clinical study. Burns. 2007;33(7):850‐854. 10.1016/j.burns.2006.10.393 [DOI] [PubMed] [Google Scholar]
- 32. Portela da Gama FM, Dourado F. Bacterial NanoCellulose: what future? Bioimpacts. 2018;8(1):1‐3. 10.15171/bi.2018.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Brunelli C, Zecca E, Martini C, et al. Comparison of numerical and verbal rating scales to measure pain exacerbations in patients with chronic cancer pain. Health Qual Life Outcomes. 2010;8:42. 10.1186/1477-7525-8-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Barret JP, Dziewulski P, Ramzy PI, Wolf SE, Desai MH, Herndon DN. Biobrane versus 1% silver sulfadiazine in second‐degree pediatric burns. Plast Reconstr Surg. 2000;105(1):62‐65. 10.1097/00006534-200001000-00010 [DOI] [PubMed] [Google Scholar]
- 35. Balasubramani M, Kumar TR, Babu M. Skin substitutes: a review. Burns. 2001;27(5):534‐544. 10.1016/s0305-4179(01)00018-3 [DOI] [PubMed] [Google Scholar]
- 36. Cattelaens J, Turco L, Berclaz LM, et al. The impact of a nanocellulose‐based wound dressing in the management of thermal injuries in children: results of a retrospective evaluation. Life (Basel). 2020;10(9):212. 10.3390/life10090212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Resch A, Staud C, Radtke C. Nanocellulose‐based wound dressing for conservative wound management in children with second‐degree burns. Int Wound J. 2021;18(4):478‐486. 10.1111/iwj.13548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Whitaker IS, Worthington S, Jivan S, Phipps A. The use of biobrane by burn units in the United Kingdom: a national study. Burns. 2007;33(8):1015‐1020. 10.1016/j.burns.2006.11.017 [DOI] [PubMed] [Google Scholar]
- 39. Kaartinen IS, Kuokkanen HO. Suprathel((R)) causes less bleeding and scarring than Mepilex((R)) transfer in the treatment of donor sites of split‐thickness skin grafts. J Plast Surg Hand Surg. 2011;45(4–5):200‐203. 10.3109/2000656X.2011.583515 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is available upon request.


