Abstract
Diabetic foot ulcers (DFUs) are at risk for detrimental complications even with current, standard of care (SOC) treatments. The primary objective of this randomised controlled trial was to compare a unique resorbable glass microfiber matrix (Mirragen; Advanced Wound Matrix [BBGFM]; ETS Wound Care, Rolla, Missouri) compared with a standard of care group (SOC, collagen alginate dressing) at 12 weeks. Both groups received standard diabetic foot care including glucose monitoring, weekly debridements when needed and an offloading device. The primary endpoint was proportion of full‐thickness, non‐infected, non‐ischaemic wounds healed at 12 weeks, with secondary endpoints including percent area reduction (PAR) and changes in Semmes‐Weinstein monofilament testing. The result illustrated in the intent‐to‐treat analysis at 12 weeks showed that 70% (14/20) of the BBGFM‐treated DFUs healed compared with 25% (5/20) treated with SOC alone (adjusted P = .006). Mean PAR at 12 weeks was 79% in the BBGFM group compared with 37% in the SOC group (adjusted P = .027). Mean change in neuropathic score between baseline and up to 12 weeks of treatment was 2.0 in the BBGFM group compared with −0.6 in the SOC group where positive improvement in scores are better (adjusted P = .008). The mean number of BBGFM applications was 6.0. In conclusion, adding BBGFM to SOC significantly improved wound healing with no adverse events related to treatment compared with SOC alone.
Keywords: complete wound healing, diabetic foot ulcer, glass fibre matrix, randomised controlled trial, resorption
1. INTRODUCTION
Diabetic foot ulcers (DFUs) represent one of the many complications of long‐standing diabetes. 1 Not only are these wounds expensive to treat, with a recent systematic review showing that the mean cost was over $31 000 in 2015, 2 , 3 but complications, especially infection, can require prolonged antibiotic administration, deep and extensive debridement, and lower extremity amputations when these measures fail. Even relatively shallow (UT1A, Wagner 1) DFUs that do not respond to standard of care (SOC) are at risk for amputation of the affected area. 2 , 3 , 4 , 5 This risk increases for patients who have had prior DFUs or amputations. Any product, therefore, that can prevent infection or disrupt biofilm while promoting wound healing in a moist environment is worthy of further investigation.
Bioactive glass materials are biocompatible water‐soluble materials that release their constituent ions when immersed in body fluids. Although the main focus of these products has been the development of a scaffold material for bone tissue engineering, researchers have been fascinated with their potential to heal wounds through improving angiogenesis, 6 increasing metabolic activity and cell proliferation, 7 and serving as antimicrobial agents. 8 , 9 All these processes are essential components in the healing phases of chronic wounds.
While the first clearance by the United States Food and Drug Administration of a bioactive glass for a medical indication was in 1985, it was not until 2016 that a borate‐based bioactive glass (13‐93B3) was approved for use in acute and chronic soft tissue wounds and injuries. 6 Mirragen Advanced Wound Matrix (BBGFM) is a novel, borate‐based, bioactive glass nanofiber (ETS Wound Care; Rolla, Missouri) with a target composition of 53B2O3–6Na2O–12K2O–5MgO–20CaO–4P2O5 wt% (Figure 1). Borate‐based bioactive glasses have been formulated to degrade in the wound over a period of days or weeks. The rate of degradation depends in part on the wound exudate and thus be washed out or degraded as the wound heals. Bioactive glass matrix was recently tested in subjects with chronic wounds, three of which were DFUs. 9 These were wounds that had failed multiple previous advanced therapeutics and surgeries with a mean wound age of 10 months. After a mean of 3.5 treatments, all wound healed in 6–10 weeks.
FIGURE 1.
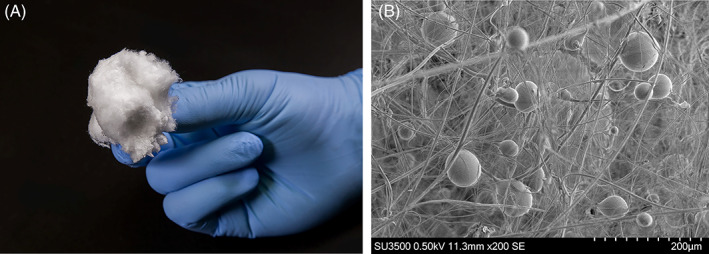
Gross and Microscopic Illustration of Mirragen Advanced Wound Matrix (BBGFM) (Mirragen) ETS Wound Care, Rolla, Missouri. A, Gross illustration of BBGFM. B, EM image of BBGFM at ×200 magnification demonstrating its intricate bioabsorbable glass fibre and sphere structure
Given that this case series was small, this unique material required further investigation with level one evidence to illustrate its effectiveness in hard‐to‐heal wounds. DFUs were selected due to their chronicity and high risk of amputation. The primary objective of our study was to further investigate the healing potential of BBGFM in subjects with chronic DFUs and comparing the healing rate to treatment with SOC alone. Forty patients were randomised to either SOC alone or BBGFM and SOC and treated for a period of 12 weeks. Secondary objectives included evaluating PAR, changes in neuropathy, the safety of the product, and to observe infection‐related complications.
2. METHODS
Following written consent prior to any study‐related procedure, subjects were eligible to be randomised 1:1 to BBGFM and SOC or SOC alone if they had a wound corresponding to University of Texas 1A/Wagner 1 in this parallel, two‐group, single‐blind randomised controlled trial (RCT). The Western Institutional Review Board approved both the study protocol and informed consent form on 1 December 2017 (No. 20172695). The study, registered at ClinicalTrials.gov (NCT02399826), was conducted under all applicable state and federal regulations in the United States at five outpatient wound care centres, adhered to Good Clinical Practice guidelines, and was in compliance with the Declaration of Helsinki. The first subject was enrolled on 16 September 2017 and the last subject exited the study on 31 October 2018.
2.1. Subject screening, eligibility, and standard of care
All subjects had to undergo a 2‐week screening period following the first day when a full physical examination was undertaken with documentation of medical history and selected demographics, and assessment of concomitant medications and therapies. A full evaluation of current wounds as well as history of chronic wounds was then conducted, as well as blood draws for serum creatinine and glycosylated haemoglobin (HbA1c) analysis (repeated at end‐of‐study visit), and pregnancy testing for females of childbearing age.
If multiple DFUs were eligible on a subject's feet, the largest wound was selected provided it met area requirements (the index wound). Peripheral neuropathy of the target foot was assessed with the standard 10‐point Semmes‐Weinstein monofilament exam using a 10 g target force 5.07 level monofilament pressed against the 10 predefined areas of the subject's foot. 10 After pain assessment, the index wound was evaluated for infection, 11 cleaned, and surgically debrided if necessary, using a 15 blade or curette to remove all necrotic tissue. Osteomyelitis screening was accomplished using the probe‐to‐bone test with further X‐ray and bone biopsy testing confirmation if suspected. Index wound assessment, including measurement and photographs, was performed. The lower extremity encompassing the index wound was also assessed using TCOM, ankle brachial index (ABI), or Doppler arterial waveform tests to exclude patients with any significant peripheral arterial disease.
At screening, all index wounds received a collagen alginate primary dressing (Fibracol; 3M corporation Minneapolis, Minnesota) topped with a padded three‐layer dressing (Dynaflex, 3M Corporation, Minneapolis, Minnesota) or equivalent. Offloading of the index wound was achieved with a removable diabetic cam‐walker (Royce Medical, Inc., Camarillo, California; or similar generic device) with the option of converting the removable walker into an instant total contact cast if patients had issues with maintaining the walker while ambulating. A complete total contact cast was also available if the patient could not be fit for a removable diabetic cam‐walker.
During the remainder of screening, further debridement was carried out as needed, and the index wound area was measured to calculate the percentage area reduction (PAR) over 2 weeks. Randomisation occurred 2 weeks after screening commenced provided all inclusion and exclusion criteria (Table 1) were still met.
TABLE 1.
Inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
|
|
2.2. Treatments
After randomisation, subjects were treated for 12 weeks unless the index wound closed and was confirmed closed 2 weeks later. The subject could be withdrawn from the trial due to an adverse event or medical monitor decision and had to be withdrawn if the PAR at 6 weeks was <50% so that other treatments could be pursued. Subjects were seen weekly and index wounds were cleansed with sterile normal saline solution, debrided if needed, photographed, and their surface area measured. If the wound was assessed as infected, anaerobic and aerobic cultures were obtained via wound swabs and appropriate systemic antibiotic treatment instituted until the infection was clinically resolved.
On the day of randomisation, the last step was application of BBGFM to the active treatment group that included shaping the advanced wound matrix to fit the size of the wound bed and pressing directly in contact with the wound, ensuring the entire wound area and wound margins were covered. A three‐layer padded dressing was then applied (Dynaflex) or equivalent. Given that the BBGFM dressing is completely bioabsorbable and is eventually absorbed at the wound site, only loose sections from prior applications were removed at subsequent visits during debridement. Re‐application was then performed at the weekly visits.
Index wounds in the SOC group received collagen alginate dressings (Fibracol) along with a padded three‐layer dressing or equivalent. The application of the collagen alginate was performed per indication and occurred on the weekly visits, and if the dressing was saturated, re‐application was performed at that time.
2.3. Healing validation
Initial healing by a site investigator was determined if there was complete (100%) epithelialisation without drainage or need for further dressing. Two weeks later, the wound was assessed to see if durable closure was still maintained. Final adjudication of healing was conducted by a panel composed of three plastic surgery wound experts blinded to the study assignment. This panel along with the medical monitor, also reviewed decisions made by site investigators regarding patient enrolment, healing, and study continuation.
All patients were fitted for and dispensed diabetic shoes and insoles provided by the sponsor at the study exit.
2.4. Study outcomes
The primary endpoint for the study was the proportion of completely healed wounds 12 weeks after randomisation. Secondary endpoints included PAR at 12 weeks, safety, and differences between scores for pain, Semmes‐Weinstein, and w‐QoL 12 (wound quality of life) determined at randomisation (first day of screening for w‐Qol) and 12 weeks. All endpoints were compared between treatment groups. If the subject's wound healed before 12 weeks or the subject was withdrawn from the trial, the last visit (end‐of‐study visit) was used as the point in time to measure these endpoints. PAR was calculated as ([AI – AXW]/AI)*100, where AI is the area of the index wound at randomisation and AXW the area at 12 weeks. The pain score was derived from a VAS scale of 0 to 10, and the Semmes‐Weinstein score had a theoretical range of 0 to 10 in which 0 meant that all 10 areas were insensate and 10 meaning that there was no neuropathy.
2.5. Randomisation and allocation concealment
A block size of 10 was used for randomisation achieved by using five sheets of paper with an SOC assignment and five with a BBGFM assignment, with each assignment placed in an opaque envelope, which was then sealed, and the envelopes shuffled by the study coordinator before being labelled 1 through 10. This process was repeated to obtain a sufficient number of assignments and was observed by the principal investigator and study staff, before envelopes were distributed to study sites. Site investigators had to open the envelopes in order and could only open the next envelope in order after a subject was ready to be randomised, thus accomplishing allocation concealment.
2.6. Sample size calculations and statistical analysis
Initial power calculations for the primary endpoint were based on a two‐sided Z test with pooled variance (independent proportions test): Group sample sizes of 20 in Group 1 and 20 in Group 2 achieve 82% power to detect a difference between the group proportions of 0.45. The proportion of wounds healed in Group 1 (the treatment group) was assumed to be 0.3 under the null hypothesis and 0.75 under the alternative hypothesis. The proportion in Group 2 (the control group) was 0.3. The significance level of the test was targeted at 0.05, while the significance level actually achieved by this design was 0.053.
The intent‐to‐treat (ITT) and safety populations comprised randomised patients who received at least one treatment. All analyses used the ITT approach. The last observation carried forward principle was used in regard to missing observations at any visit. Study variables were summarised as means and standard deviations (±SDs) for continuous variables, as well as medians for non‐normally distributed data. Categorical variables were presented as counts and percentages. For statistical testing of categorical variables between treatment groups, chi square or Fisher exact tests were performed; for continuous variables, independent t tests or Mann‐Whitney tests were used (depending on normality of variable values).
A logistic regression was carried out to analyse the proportions of wounds healed at 12 weeks, to account for imbalances between treatment groups of available covariates. Covariates were entered into one block with stepwise elimination of non‐significant covariates. Parsimonious model selection was confirmed by building model covariates one at a time and then in various combinations. The assumption of linearity in regard to the logit for continuous covariates, absence of multicollinearity between covariates, and lack of strongly influential outliers was examined.
To correct for the multiplicity of secondary endpoint statistical testing, P values were adjusted using the Holm step‐down procedure. Two‐sided P values < .05 were considered significant. PASW 25 (IBM, Chicago, Illinois) was used to perform all statistical testing.
3. RESULTS
Out of 44 subjects who were screened, 40 were eligible to be randomised to BBGFM and SOC or SOC alone resulting in 20 subjects per treatment group (Figure 2). In the BBGFM group, three subjects were lost to follow‐up: one to an adverse event and two subjects because the PAR of the index wounds was <50% at week 6. In the SOC group, five subjects experienced adverse events resulting in withdrawal, six index wounds failed to meet the 50% PAR rule at week 6 and were also withdrawn, and one index wound reopened at the confirmatory wound healing visit, causing this subject to be withdrawn from the study.
FIGURE 2.
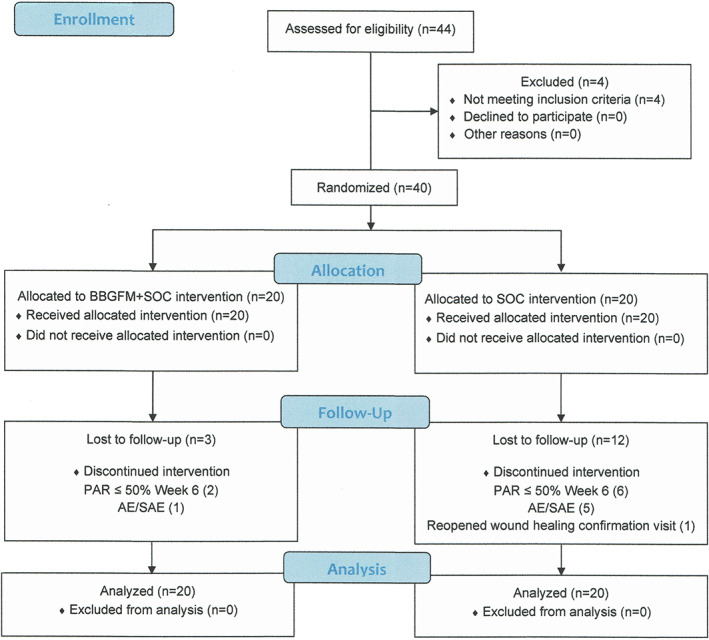
Flow chart of trial participants
Patient‐and wound‐related variables at baseline were generally well‐balanced between groups with only creatinine levels significantly lower in the BBGFM group compared with the SOC group (1.0 vs 1.4; P = .037) (Table 2).
TABLE 2.
Wound‐ and patient‐related variables between groups at randomisation compared with assess success of randomisation
| Variable | BBGFM | SOC | P |
|---|---|---|---|
| Patient age (years) | 61.0 (13.81) | 64.3 (9.32) | .38 |
| Race | |||
| Caucasian | 19 (95) | 17 (85) | .61 |
| African American | 1 (5) | 3 (15) | |
| Gender | |||
| Male | 10 (50) | 10 (50) | 1.0 |
| Female | 10 (50) | 10 (50) | |
| Hypertension | 14 (70) | 17 (85) | .45 |
| Neuropathy extends beyond dorsal surface | 15 (75) | 13 (65) | .49 |
| BMI | 34.1 (7.07) | 32.7 (5.18) | .46 |
| Smoker | 3 (15) | 4 (20) | 1.0 |
| HbA1c a | 7.85 (1.67) | 7.8 (1.76) | .99 |
| Creatinine | 1.0 (0.37) | 1.4 (0.58) | .037 |
| Wound area (cm2) | 3.5 (2.07) | 5.2 (4.67) | .42 |
| Wound age (weeks) | 23.0 (18.45) | 16.5 (16.73) | .16 |
| Wound plantar surface | 17 (85) | 17 (85) | 1.0 |
| Wound location | |||
| Toe | 1 (5) | 1 (5) | .9 |
| Forefoot | 5 (25) | 7 (35) | |
| Midfoot | 9 (45) | 7 (35) | |
| Heel/ankle/hindfoot | 5 (25) | 5 (25) | |
Note: Continuous variables are reported as means (standard deviations [SDs] in parentheses) and categorical variables as number (n) and percentage (%).
Average of HbA1c values (beginning and end of study).
Abbreviations: BBGFM, Mirragen Advanced Wound Matrix; BMI, body mass index; SOC, standard of care.
After 12 weeks of treatment 14 of 20 wounds (70%) healed in the BBGFM group compared with 5 of 20 (25%) in the SOC group (unadjusted P = .004). The corresponding number needed to treat was 2.0 (95% CI: 1.4‐5.8). The logistic regression model included treatment, BMI, and gender as main effects only. Bayesian information criterion for the model was 51.1 and dispersion was 1.01. Adjusted P value for treatment effect was .006 (Table 3). Being of male gender substantially lowered the odds of healing (odds ratio [OR]: 0.11), while both treatment (BBGFM) and body mass index (per unit increase) increased the odds of healing by 11.52 and 1.14, respectively. Weekly healing rates are shown in Figure 3.
TABLE 3.
Logistic regression of complete wound healing after 12 weeks of treatment
| Variable | B | P | OR | 95% CI | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Gender a | −2.21 | .01 | 0.11 | 0.02 | 0.59 |
| BMI | 0.13 | .034 | 1.14 | 1.01 | 1.29 |
| Treatment b | 2.45 | .006 | 11.52 | 2.02 | 65.70 |
Abbreviations: BMI, body mass index; OR, odds ratio.
Reference groups female.
Reference group: standard of care.
FIGURE 3.
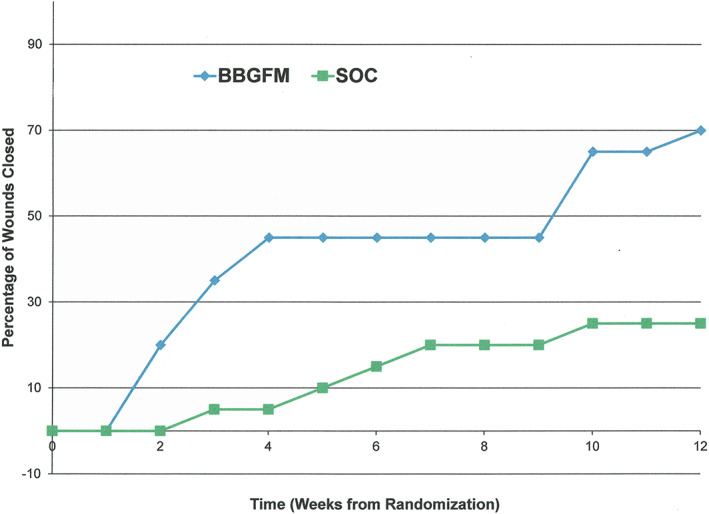
Weekly healing rates by treatment group
After 12 weeks of treatment, the PAR was 79% (SD: 48) in the BBGFM group compared with 37% (SD: 66) in the SOC group (unadjusted P = .009; adjusted P = .027). Weekly PAR values by treated group are shown in Figure 4. The mean change in neuropathic score (Semmes‐Weinstein) between baseline and up to 12 weeks of treatment was 2.0 (SD: 3.5) in the BBGFM group compared with −0.6 (SD: 1.60) in the SOC group (positive improvement in scores are better); unadjusted P = .002; adjusted P = .008). The mean weekly change in neuropathic score by treatment group is shown in Figure 5. The mean change in w‐QoL score between baseline and up to 12 weeks of treatment was 0.3 (SD: 2.1) in the BBGFM group compared with 0.33 (SD: 1.8) in the SOC group (positive reductions in scores are better); unadjusted P = .43; adjusted P = .86). Finally, the mean change in pain score between baseline and up to 12 weeks of treatment was 0.39 (SD: 0.63) in the BBGFM group compared with 0.24 (SD: 0.54) in the SOC group (positive reductions in scores are better); unadjusted P = .84; adjusted P = .86). The mean number of BBGFM applications was 6.0 (SD: 4.2); median: 6.
FIGURE 4.
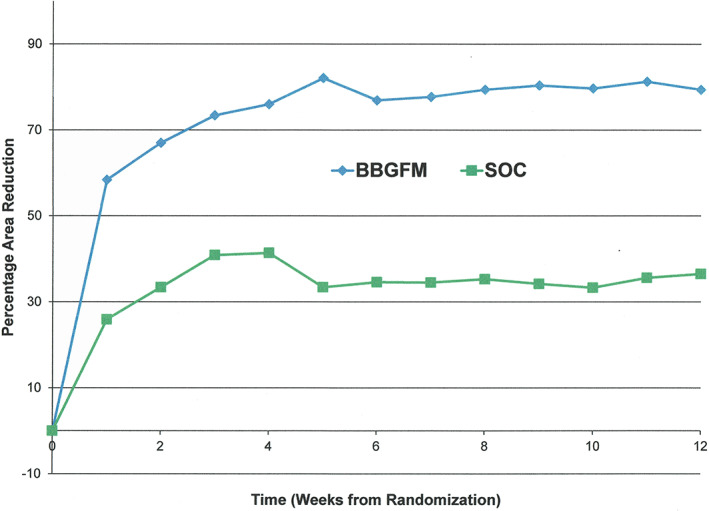
Weekly percentage wound area reduction by treatment group
FIGURE 5.
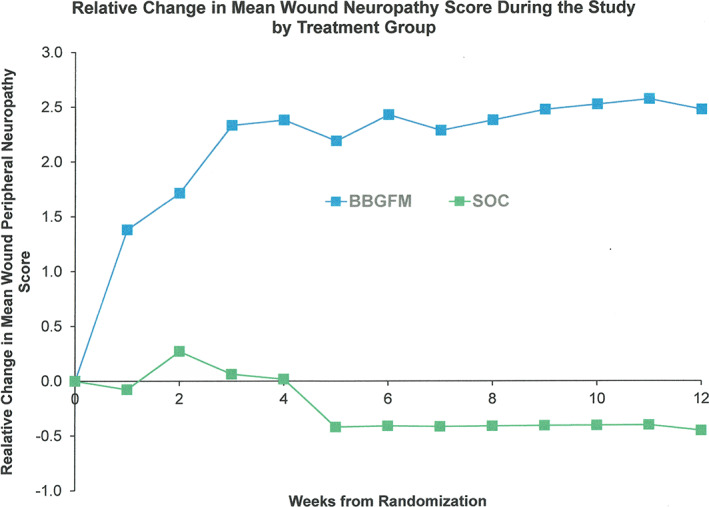
Weekly change in neuropathic score by treatment group
Nine adverse event (AEs) occurred in the SOC group, of which seven were SAEs, and five of the nine AEs were index‐wound‐related. The SAEs comprised: abscess, cellulitis, and osteomyelitis of the index wound that required hospitalisation, antibiotics, and surgical incision and drainage; an infection of the index wound that required hospitalisation, amputation of the second right toe and saucerization of the second metatarsal head; infection of the index ulcer that required antibiotics and hospitalisation; swelling of the left knee and cellulitis of left knee and ankle that required aspiration of the knee and antibiotics in hospital; infection of a non‐index ulcer that required antibiotics and culture; and two incidences of a fall that required hospitalisation. The remaining two AEs both involved the index ulcer, and included infection and cellulitis of the wound that also probed to bone but resolved with antibiotics, and infection that also was treated with antibiotics alone.
In the BBGFM group, there were four AEs that did not involve the index ulcer, of which three were SAEs: leg pain and cellulitis that required antibiotic treatment in hospital; diabetic ketoacidosis and a methicillin resistant S. aureus (MRSA) infection that required medication and treatment in hospital; and dyspnoea/exacerbation of an existing chronic heart failure condition that also required hospitalisation. The remaining AE was due to infection of the third metatarsal on the left foot and was successfully treated with antibiotics. There were no AEs related to study products.
4. DISCUSSION
Based on the primary endpoint of the trial, a significantly higher proportion of wounds healed after 12 weeks of BBGFM treatment and SOC compared with SOC alone (70% vs 25%; adjusted P = .006) with odds of healing over 11 times greater than that of SOC. The wound healing community has seen the proliferation of new cellular and/or tissue‐based products (CPTs) in the last 10 years with these types of products often seen as the ‘go to’ treatments for clinicians when chronic DFUs fail to respond to good SOC. Therefore, the finding that the difference between treatment groups in terms of percentage of wounds healed in the present study (45%) is comparable to or exceeds those from many other RCTs investigating CPTs in similar populations and DFU wound severity 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 is noteworthy, given the paucity of data regarding bioactive glasses.
Because the majority of these CTPs are created with synthetic, allogenic, or xenogeneic tissue constructs in which the goal is to replace the ‘missing’ numerous growth factors and cytokines locally at the site of the wound, they are expensive to manufacture, even if minimally manipulated in many instances. This means that a single treatment can cost several thousands of dollars. Indeed, Winters et al note that ‘Reaching a consensus on whether higher priced treatment options are cost effective has been challenging despite the many publications on the matter’. 21 The cost of healing BBGFM‐treated wounds was far less than half when compared with the majority of CTPs as the bioactive glass in the BBGFM is a relatively cost‐effective dressing option. Just as important, BBGFM achieved wound healing results comparable to or better than CTPs by what the authors suggest is a different mechanism of action when the bioactive glass wound dressing is applied to the non‐healing diabetic wound.
Angiogenesis occurs in the third phase of wound healing. Many factors such as vascular endothelial growth factor (VEGF) and basic fibroblast growth factor are necessary for new capillaries, especially tortuous microvessels, to develop, but prior transient inflammation and cytoskeletal rearrangements are also necessary for this process to proceed normally. 22 Many CTPs deliver angiogenic growth factors and extracellular matrices directly into the wound to replace deficits or normalise imbalances that exist in the chronic wound. In the case of BBGFM, while it mimics the microstructure of a fibrin clot, and such microstructure, porosity, and other environmental features are critical, bioactive ions are also released into the wound environment as a result of degradation, which occurs predictably over time. 23 Animal models also suggest that angiogenic effects may be promoted through the addition of doped copper ions in the bioactive glass fibres as a result of upregulation of VEGF. 6 , 24 Our own results in a clinical context suggest that angiogenesis is probably rapid because wound area reduction on average is high over the first week (Figure 4).
Another significant finding was the reduction in the Semmes‐Weinstein score, which was significant in the BBGFM group compared with the SOC group (P = .008). Only 40% of BBGFM subjects had a positive increase of ≥1 point, whereas no subjects in the SOC group had such an increase. The cause of diabetic peripheral neuropathy is not known but the prevalence does increase with diabetes duration. 25 We did not have data on diabetes duration for subjects so it is not possible to determine if the 40% who were clinical respondents were recently diagnosed. While the improvement in sensation around the wound is impressive in these cases, the mechanism involved and the likely duration of improvement remains unknown and further through investigation is needed with additional objective testing to either confirm or refute the finding in this trial.
Finally, it should be noted that in the study, the BBGFM group experienced no AEs related to infection of the index ulcer, while for the SOC group, there were five such infections. Faster healing times could explain some of the difference (less time for infection), but the antimicrobial properties of BBGFM could also have played a role to prevent infection. Bioactive glasses that contain boron, including BBGFM, release the ion in a controlled manner at the wound interface. Boron ions by themselves inhibit the growth of both gram‐positive and gram‐negative bacteria, including E. coli, S. sonnei, V. natriegens, S. epidermidis, Serratia marscesens, and MRSA, although it appears that for gram‐positive species the mechanism of action is disruption of the cell membrane integrity. 26 Boron‐based bioactive glasses are also able to disrupt biofilm, which is now understood to affect as much as two‐thirds of chronic wounds. 27 , 28 In one study of gram‐negative bacteria (P. aeruginosa and A. baumannii), gram‐positive bacteria (S. aureus), and fungi (C. albicans), boron‐containing bioactive glasses were found to have a strong effect on biofilm after 2 days, with the exception of S. aureus. Both boron and alkali release, resulting in pH increase were thought to be relevant mechanisms for biofilm disruption. 7 , 8
The strengths of the present study include a robust trial design, adequate statistical power for the primary endpoint, multiple investigation sites, a standardised approach to SOC, satisfactory allocation concealment, ITT analysis, appropriate adjustment for multiple statistical testing, and reporting according to CONSORT guidelines. However, there are some weaknesses in the study, which include lack of investigator blinding (it was not possible to create a sham product), as well as the need to withdraw subjects at 6 weeks if wounds were not sufficiently responding to treatment so that subjects could be offered an alternative and safer treatment pathway. 29 This resulted in censoring of outcomes. Future trials should consider a larger more robust number of patients and include more complex wounds including ulcers down to tendon, capsule, and bone to determine if BBGFM can be beneficial in helping these kinds of wounds heal.
In conclusion, this trial has demonstrated that the addition of a bioactive glass microfiber matrix containing boron to SOC results in significantly improved wound healing in Wagner 1 DFUs compared with SOC alone, with encouraging results regarding infection and neuropathy. Further studies will be needed to confirm these findings.
CONFLICT OF INTEREST
This study was funded through a research grant from ETS WoundCare provided to the Professional Education and Research Institute (PERI), for which Charles M Zelen, DPM is medical director. David Armstrong, DPM, MD, PhD received research funds from PERI to serve as Principal Investigator for this trial and to design and administrate the trial and also assist with the writing and review of the manuscript. Dennis Orgill, MD, PhD received research funds to serve as a validating/adjudicating plastic surgeon to review study photos and assist with the writing and review of the manuscript. Robert Galiano, MD received research funds to serve as a validating/adjudicating plastic surgeon to review study photos and assist with the writing and review of the manuscript. Paul Glat, MD received research funds to serve as a validating/adjudicating plastic surgeon to review study photos and assist with the writing and review of the manuscript. Lawrence Didomenico, DPM received research funds and served as a site investigator for this trial and assisted with the writing and review of the manuscript. Marissa Carter, PhD received research funds to provide the statistical analysis plan, and provide the statistical analysis for this trial and assist with the writing of the result section of the manuscript. Charles M Zelen, DPM is the medical director of the PERI and his company received research funds to administrate the clinical trial and write the paper for publication. There are no other conflict of interests with any of the authors in relationship to this study, or with regard to ETS WoundCare. IRB conflict of interest statements are on file with PERI.
ACKNOWLEDGEMENTS
The authors would like to acknowledge Ted Day, former CEO of ETS WoundCare who recently passed away for his discovery of BBGFM and his desire to advance science in wound healing. This study was funded with a grant from ETS WoundCare LLC, North Rolla, Missouri.
Armstrong DG, Orgill DP, Galiano RD, et al. A multi‐centre, single‐blinded randomised controlled clinical trial evaluating the effect of resorbable glass fibre matrix in the treatment of diabetic foot ulcers. Int Wound J. 2022;19(4):791‐801. doi: 10.1111/iwj.13675
Funding information ETS WoundCare; Rolla Missouri, Grant/Award Number: 001
Contributor Information
David G. Armstrong, Email: armstrong@usa.net.
Dennis P. Orgill, Email: dorgill@partners.org.
Robert D. Galiano, Email: Rgaliano@nw.org.
Paul M. Glat, Email: pmg0804@msn.com.
Marissa J. Carter, Email: mcarter@strategic-solution-inc.com.
Charles M. Zelen, Email: cmzelen@periedu.com.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med. 2017;376(24):2367‐2375. [DOI] [PubMed] [Google Scholar]
- 2. Armstrong DG, Lavery LA, Harkless LB. Validation of a diabetic wound classification system. The contribution of depth, infection, and ischemia to risk of amputation. Diabetes Care. 1998;21(5):855‐859. [DOI] [PubMed] [Google Scholar]
- 3. Chan B, Cadarette S, Wodchis W, Wong J, Mittmann N, Krahn M. Cost‐of‐illness studies in chronic ulcers: a systematic review. J Wound Care. 2017;26(sup4):S4‐S14. [DOI] [PubMed] [Google Scholar]
- 4. Jeon BJ, Choi HJ, Kang JS, Tak MS, Park ES. Comparison of five systems of classification of diabetic foot ulcers and predictive factors for amputation. Int Wound J. 2017;14(3):537‐545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yesil S, Akinci B, Yener S, et al. Predictors of amputation in diabetics with foot ulcer: single center experience in a large Turkish cohort. Hormones (Athens). 2009;8(4):286‐295. [DOI] [PubMed] [Google Scholar]
- 6. Rahaman MN, Day DE, Bal BS, et al. Bioactive glass in tissue engineering. Acta Biomater. 2011;7(6):2355‐2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schreiber R. Ca2+ signaling, intracellular pH and cell volume in cell proliferation. J Membr Biol. 2005;205(3):129‐137. [DOI] [PubMed] [Google Scholar]
- 8. Jung S, Day T, Boone T, Buziak B, Omar A. Antibiofilm activity of two novel, borate‐based, bioactive glass wound dressings. Biomed Glasses. 2019;5:57‐75. [Google Scholar]
- 9. Kaya S, Cresswell M, Boccaccini AR. Mesoporous silica‐based bioactive glasses for antibiotic‐free antibacterial applications. Korean J Couns Psychother. 2018;83:99‐107. [DOI] [PubMed] [Google Scholar]
- 10. Armstrong DG, Lavery LA, Vela SA, Quebedeaux TL, Fleischli JG. Choosing a practical screening instrument to identify patients at risk for diabetic foot ulceration. Arch Intern Med. 1998;158(3):289‐292. [DOI] [PubMed] [Google Scholar]
- 11. Woo KY, Sibbald RG. A cross‐sectional validation study of using NERDS and STONEES to assess bacterial burden. Ostomy Wound Manage. 2009;55(8):40‐48. [PubMed] [Google Scholar]
- 12. Sommer R, von Stülpnagel CC, Fife CE, et al. Development and psychometric evaluation of the U.S. English wound‐QoL questionnaire to assess health‐related quality of life in people with chronic wounds. Wound Repair Regen. 2020;28(5):609‐616. [DOI] [PubMed] [Google Scholar]
- 13. Serena TE, Yaakov R, Moore S, et al. A randomized controlled clinical trial of a hypothermically stored amniotic membrane for use in diabetic foot ulcers. J Comp Eff Res. 2020;9(1):23‐34. [DOI] [PubMed] [Google Scholar]
- 14. Tettelbach W, Cazzell S, Reyzelman AM, Sigal F, Caporusso JM, Agnew PS. A confirmatory study on the efficacy of dehydrated human amnion/chorion membrane dHACM allograft in the management of diabetic foot ulcers: a prospective, multicentre, randomised, controlled study of 110 patients from 14 wound clinics. Int Wound J. 2019;16(1):19‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zelen CM, Orgill DP, Serena T, et al. A prospective, randomised, controlled, multicentre clinical trial examining healing rates, safety and cost to closure of an acellular reticular allogenic human dermis versus standard of care in the treatment of chronic diabetic foot ulcers. Int Wound J. 2017;14(2):307‐315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tettelbach W, Cazzell S, Sigal F, et al. A multicentre prospective randomised controlled comparative parallel study of dehydrated human umbilical cord (EpiCord) allograft for the treatment of diabetic foot ulcers. Int Wound J. 2019;16(1):122‐130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zelen CM, Serena TE, Gould L, et al. Treatment of chronic diabetic lower extremity ulcers with advanced therapies: a prospective, randomised, controlled, multi‐Centre comparative study examining clinical efficacy and cost. Int Wound J. 2016;13(2):272‐282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cazzell SM, Lange DL, Dickerson JE Jr, Slade HB. The management of diabetic foot ulcers with porcine small intestine submucosa tri‐layer matrix: a randomized controlled trial. Adv Wound Care. 2015;4(12):711‐718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Driver VR, Lavery LA, Reyzelman AM, et al. A clinical trial of Integra template for diabetic foot ulcer treatment. Wound Repair Regen. 2015;23(6):891‐900. [DOI] [PubMed] [Google Scholar]
- 20. Lavery LA, Fulmer J, Shebetka KA, et al. The efficacy and safety of Grafix(®) for the treatment of chronic diabetic foot ulcers: results of a multi‐centre, controlled, randomised, blinded, clinical trial. Int Wound J. 2014;11(5):554‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Winters C, Kirsner RS, Margolis DJ, Lantis JC. Cost effectiveness of fish skin grafts versus standard of care on wound healing of chronic diabetic foot ulcers: a retrospective comparative cohort study. Wounds. 2020;32(10):283‐290. [PubMed] [Google Scholar]
- 22. Chong DC, Yu Z, Brighton HE, Bear JE, Bautch VL. Tortuous microvessels contribute to wound healing via sprouting angiogenesis. Arterioscler Thromb Vasc Biol. 2017;37(10):1903‐1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Miguez‐Pacheco V, Hench LL, Boccaccini AR. Bioactive glasses beyond bone and teeth: emerging applications in contact with soft tissues. Acta Biomater. 2015;13:1‐15. [DOI] [PubMed] [Google Scholar]
- 24. Lin Y, Brown RF, Jung SB, Day DE. Angiogenic effects of borate glass microfibers in a rodent model. J Biomed Mater Res A. 2014;102(12):4491‐4499. [DOI] [PubMed] [Google Scholar]
- 25. Dyck PJ, Kratz KM, Karnes JL, et al. The prevalence by staged severity of various types of diabetic neuropathy, retinopathy, and nephropathy in a population‐based cohort: the Rochester diabetic neuropathy study. Neurology. 1993;43(4):817‐824. [DOI] [PubMed] [Google Scholar]
- 26. Ottomeyer M, Mohammadkah A, Day D, Westenberg D. Broad‐spectrum antibacterial characteristics of four novel borate‐based bioactive glasses. Adv Microbiol. 2016;6:776‐87.2. [Google Scholar]
- 27. James GA, Swogger E, Wolcott R, et al. Biofilms in chronic wounds. Wound Repair Regen. 2008;16(1):37‐44. [DOI] [PubMed] [Google Scholar]
- 28. Malone M, Bjarnsholt T, McBain AJ, et al. The prevalence of biofilms in chronic wounds: a systematic review and meta‐analysis of published data. J Wound Care. 2017;26(1):20‐25. [DOI] [PubMed] [Google Scholar]
- 29. DiDomenico LA, Orgill DP, Galiano RD, et al. Aseptically processed placental membrane improves healing of diabetic foot ulcerations: prospective, randomized clinical trial. Plast Reconstr Surg Glob Open. 2016;4:e1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


