Abstract
Using a novel, automated robotic phantom system containing multiple wound simulants, we determined the fluid handling performance of the curea P1 multipurpose dressing vs market‐leading comparator superabsorbent and foam‐based dressings (FBDs). Specifically, we measured the retained, residual, evaporated, and (potentially occurring) spillover fluid shares for high‐ vs low‐viscosity exudate‐simulant test fluids, at 12, 24, and 30 hours postapplication of the dressings. These experiments were conducted for off‐loaded (‘prone’), non‐off‐loaded (‘supine’), and vertical (‘side‐lying’) simulated body positions. We found that the multipurpose dressing exhibited the best and most robust fluid handling performance across all the test configurations, for both the low‐ and high‐viscosity fluids. The FBD consistently showed the poorest performance compared to the other dressings, rendering it unlikely to be able to manage viscous exudates in ambulant patients (such as when applied to venous leg ulcers) as effectively as the other dressings. The superabsorbent dressing performed better than the foam dressing, but its fluid handling metrics were inferior to those of the multipurpose dressing. The current comparative quantification of the shares of retained, residual, evaporated, and spillover fluid, acquired through standardised laboratory tests, should help decision‐makers to select dressings that best meet their patient needs.
Keywords: exudate management, laboratory testing, robotic wound simulator, tissue phantom, wound care
Abbreviations
- ANOVA
Analyses of variance
- ASL
Adipose simulant layer
- FBD
Foam‐based dressing
- MPD
Multipurpose dressing
- MSL
Muscle simulant layer
- SAD
Superabsorbent dressing
- SAP
Superabsorbent polymer
- SSL
Skin simulant layer
- VLU
Venous leg ulcer
1. INTRODUCTION
Pressure ulcers/injuries, diabetic foot ulcers, and venous leg ulcers (VLUs), altogether known as chronic wounds, are one of the most common, difficult, complex, and costly medical problems in both developed and developing countries. 1 , 2 The burden of chronic wounds is constantly escalating, with the ageing of the population and the spread of obesity and diabetes fuelling the growth, and more recently, the Coronavirus disease 2019 (COVID‐19) pandemic compromising prevention efforts in the community. One of the fundamentals of modern wound care is exudate management, and inducing a moist (but not wet) wound environment is a well‐establish evidence‐based practice to enhance the healing process. 3 In a wound that is not healing as expected, exudate production may continue and be excessive due to an ongoing inflammatory process or the presence of infection. 4 , 5 Excess exudate amounts should, therefore, be absorbed and retained in effective wound dressings to facilitate the repair of tissues.
Wound dressings alter the wound‐bed environment and interact with the wound surfaces to support and progress the healing. 3 Poor absorbency and low retention of exudate by the applied dressing may delay the healing, or even exacerbate the wound, mainly due to over‐hydration of the wound‐bed and peri‐wound skin, leading to maceration. 1 Clinically effective wound dressings are, therefore, required to manage exudates across all the possible scenarios, from a normal course of healing and closure to wound chronicity, through the potential range of exudate volumes and viscosities in a particular wound over time, as well as for the variety of the wound aetiologies for which the dressing is indicated. While multiple dressing technologies and material types are available in the arsenal of contemporary wound care, including hydrocolloids, hydrofibres, hydrogels, alginates, superabsorbents, and the traditional foams (to mention a few), not all dressing types would meet the above criteria of handling a wide range of exudate volumes and viscosities. 6 , 7 Among the advanced dressings that are indicated for moderately to highly exuding wounds are those that contain superabsorbent particles. 8 , 9 Though the majority of manufacturers of these dressings claim that they prevent leakage and maceration, there are fundamental differences in the engineering design and structure of commercially available superabsorbent dressings (SADs), leading to a large diversity of performance in their absorbency and retention metrics, particularly when the fluid handling performance of these dressings is challenged by high‐viscosity test fluids that represent thick (and sometimes sticky) exudates. 10 , 11
Recently, a new category of advanced wound dressings was proposed, to address the aforementioned variety of expected wound and patient conditions, namely a multipurpose dressing (MPD). The curea P1 (manufactured by curea medical GmbH, Steinfurt, Germany) is such an MPD, which has a non‐sagging air‐laid core of natural fibres in which superabsorbent (sodium polyacrylate) particles are embedded, to form curea's proprietary SuperCore technology. 10 In addition, this MPD has a non‐woven interface layer for soft debridement, laminated edges for prevention of leakage and high durability, and a breathable backsheet for effective moisture‐vapour transmission and gas exchange, as well as for a bacterial barrier. 10 Unlike standard SADs, this MPD technology was demonstrated to be able to absorb and retain considerable quantities of whole blood (representing thick exudates), and overall, its multipurpose design facilitates management of a particularly wide range of exudate volumes and viscosities. 10
This study was aimed to experimentally investigate and compare the absorbency and retention performance of this MPD to those of alternative advanced dressings, particularly dressings that are based upon a superabsorbent technology or a foam technology, the latter is traditionally used due to its relatively low cost and availability, despite that it may not be suitable to handle highly exuding wounds or viscous exudates, such as for some of the VLUs. The current laboratory investigations utilised a novel, robotic wound phantom system, in which a computerised unit controls multiple identical exuding wound simulants where the rate of exudation, the viscosity of the exudate‐like fluid, and the orientation of the wound and tested dressing with respect to the gravity vector can all be controlled and modified. The latter, wound orientation factor, is particularly important, in the context of treating the variety of the potential clinical scenarios that a wound‐dressing must be able to handle, such as non‐off‐loaded vs off‐loaded wounds, and exudates that flow along vs opposed to the direction of the gravity vector. These considerations were, therefore, integrated into the tests reported here. This work is also relevant and important in the context of improving the industry standards for testing the performance of wound dressings, as existing test standards commonly used by industry do not consider many of the above factors, as discussed in Reference 10. Our current experimental system and protocol were, therefore, designed with emphasis on the clinical relevance of the bioengineering laboratory testing reported herein, to reproduce as much as possible of the practical and the real‐world scenarios that may be associated with failure of wound dressings in clinical practice.
2. METHODS
2.1. Robotic phantom system of multiple cavity wounds for automated dressing tests
We developed, built, and utilised a versatile robotic phantom system of six open cavity wound replicates, each simulating an exuding, frustum conically shaped wound (ie, a crater‐like wound). The dimensions of each wound unit were as follows: radius of the wound opening at the surface (‘skin’ level) R = 2.25 cm, radius of the bottom of the simulated wound‐bed r = 1.1 cm, and maximum depth of the wound d = 2 cm (Figure 1). At the bottom of the wound cavity, a wound‐bed tissue simulant made of soft, open‐celled, non‐reticulated polyurethane foam with low apparent density of 0.02 g/cm3 was placed (for additional realism, to simulate a partially or fully necrotic wound‐bed), so that the depth of the cavity space was reduced to 7 mm (Figures 1 and 2).
FIGURE 1.
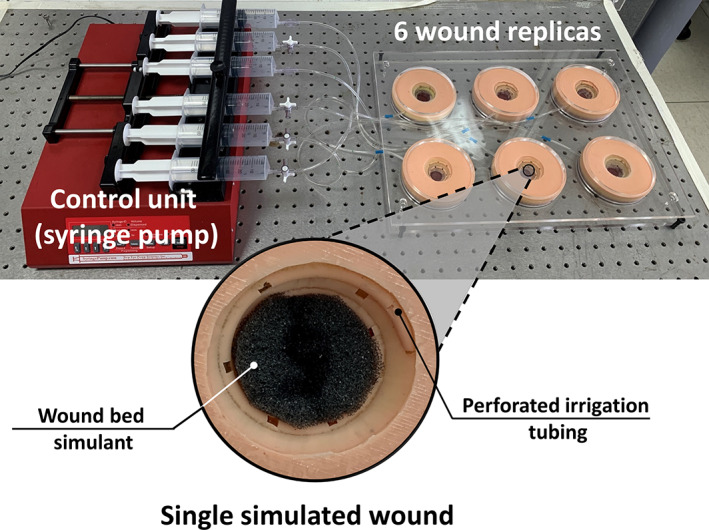
The robotic phantom system including the six wound replicas (with a close‐up view of a single simulated wound) and the control unit
FIGURE 2.
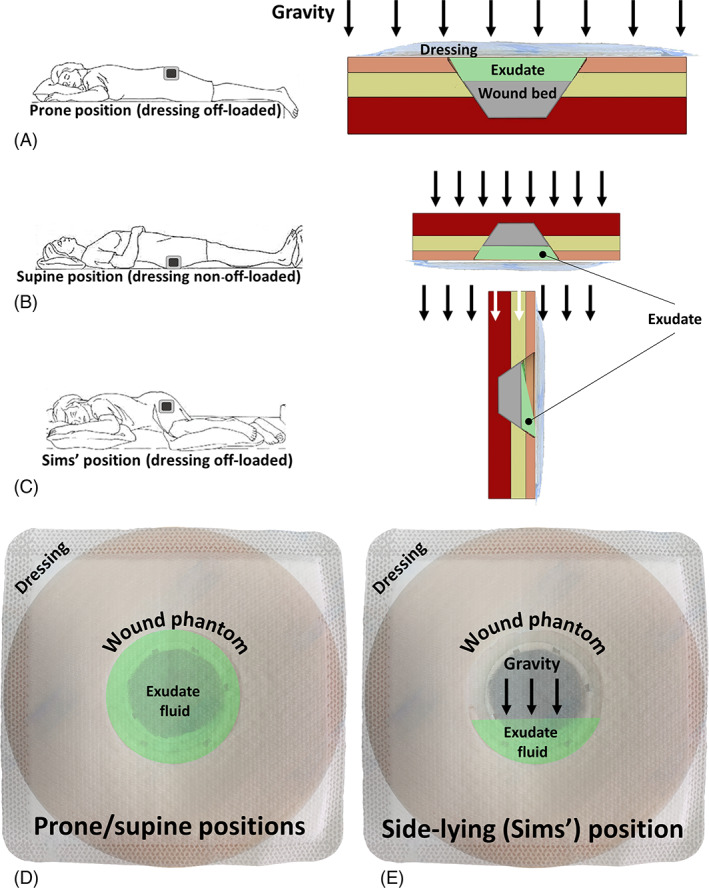
The wound configurations that were studied in this work (with respect to the direction of the gravity vector): (A) prone, (B) supine, and (C) Sims' positions where the wound is facing upward, downward, and sideway, respectively. Although the exudate needs to be transported into the dressing through capillary motion in the prone configuration (A), as opposed to being pushed by gravity for the supine position (B), in both of these cases, the effective area of the dressing for potential contact with the exudate is the entire wound pad. Contrarily, for a side‐lying (Sims') position, gravity transfers the exudate towards the lower portion of the pad, and hence, not all the wound pad area can be used for absorption of the wound fluids (C). Accordingly, whether the transport mechanism is capillary action (A) or the self‐weight of the exudate (B) may affect the fluid flow rate into the dressing, but not the effective area of the wound pad for fluid handling, which is identical for the prone or supine test configurations (D). For the side‐lying position, however, only a portion of the wound pad is effectively in contact with the exudate due to gravity concentrating the fluid at the lower region of the wound (E). We, therefore, consider the latter test configuration to present the greatest challenge to the dressings under investigation
All the six (identical) wound units included three layers of synthetic soft tissue simulants (Figure 1). The top layer representing the peri‐wound skin, which is termed here as the skin simulant layer (SSL), consisted of a 3 mm‐thick commercial silicone that is commonly accepted for representing skin in medical and cosmetic applications (Dragon Skin 20, Smooth‐On, Inc., Macungie, PA, USA). The adipose simulant layer (ASL) was similarly made of an 8 mm‐thick layer of a Dragon Skin 20 mixed with Slacker (Smooth‐On, Inc., Macungie, PA, USA), which lowers the shore hardness of the silicone to approximate that of native adipose tissue, as recommended by the manufacturer. The underlying layer representing skeletal muscle, that is, the muscle simulant layer (MSL), was made of the same silicone type of the SSL, but at a thickness of 12 mm. According to the manufacturer's technical datasheets, the tensile strength and tangent modulus at 100% strain of the Dragon Skin 20 silicone material (following the relevant testing standard ASTM D412‐06 12 ) are 3.8 MPa and 338 kPa, respectively, which are characteristic to both human skin and muscle tissues under large tensile deformations. 13 , 14 , 15 The addition of the Slacker component to the silicone, to represent the subcutaneous fat tissue as indicated above, reduced the strength and stiffness of the treated silicone by approximately an order of magnitude, to the levels of a silicone gel (or paraffin gel), which is suitable for representing adipose tissue. 16 , 17 , 18 The complete structure of each wound unit was formed by casting the ASL on top of the MSL, and then layering the SSL onto the ASL, which altogether resulted in a realistic soft tissue simulant with the ‘look and feel’ of human tissues.
To simulate the continuous secretion of exudate from the robotic wounds, a spiral perforated irrigation tube was incorporated in each wound unit and was helically tunnelled through the walls of the wound to connect to a multi‐channel electromechanical syringe pump system (NE‐1600, New Era Pump Systems Inc., Farmingdale, NY, USA) (Figure 1). This syringe pump system provided precision control over the flow volumes and release rates of an exudate‐substitute fluid, which was delivered into the simulated wounds. The effective wet surface of each wound unit, that is, the size of the wall area from which the exudate‐like fluid was released, was 24 cm2 and the irrigation depth was 1.8 cm. Two safe and reproducible exudate‐substitute fluids were prepared for use with the robotic wound phantom system, based on a Xanthan gum thickener, consistent with our published research. 19 , 20 , 21 These exudate‐like fluids were produced to have high vs low‐viscosity values, of 0.71 and 0.23 Pa×s, respectively; the density was 1.03 g/cc for both fluid types.
2.2. Simulated treatments of the robotic wounds by means of dressings
Three types of wound dressings, with sizes of 10 × 10 cm, produced by different manufacturers were applied to the robotic wounds for systematically testing and comparing their fluid management and handling performance. One product was the curea P1 MPD, 10 which was compared against another commercially available and market‐dominant SAD described in Reference 22, as well as to an additional market‐leading foam‐based dressing (FBD) as detailed in Reference 23. All these dressing products were weighed prior to applying them onto the wound units, and application of the products to ‘treat’ the simulated wounds was performed according to the manufacturer's instructions for use per each product type.
The wound units were placed in a custom‐made Perspex frame, which facilitated convenient and accurate positioning of the entire set of the simulated wounds at different orientations with respect to the direction of the gravity vector, to form different exudate flow regimes associated with a simulated body position. Accordingly, the simulated wounds were studied in prone, supine, and Sims' (side‐lying) positions, that is, where the wound units were facing upwards (Figure 1), downwards, and sideway, respectively. The tested wound dressings were correspondingly required to absorb and retain the exudate substitutes through capillary motion in the ‘prone’ configuration (Figure 2A); where the fluid was pulled down into the wound pad by gravity in the ‘supine’ position (Figure 2B); and where the exudate‐substitute tended to flow to the lower portion of the wound pad in each tested dressing and concentrate there for the ‘Sims’ position (Figure 2C). The latter, side‐lying test condition, which also resembles the clinical situation of treating a VLU in a standing person, was particularly challenging for the dressing products under investigation, as gravity drives the fluid to concentrate at the lower part of the wound pad, thereby limiting the effective wound pad area for absorption (Figure 2C). For the ‘supine’ condition, compressive forces were further applied to the superior surface of the frame; at the top of each wound unit, for example, to represent the bodyweight forces that occur in the sacral region for a non‐off‐loaded pressure ulcer, and which deform the wound and surrounding soft tissues, as well as the dressing. These forces, which may affect the fluid management and handling performance of dressings applied to non‐off‐loaded wounds, were calculated based on human interface pressure mapping data for the sacral region in a supine position. 24 These interface pressure data indicated that the compressive force expected at the sacral region is approximately 100 N (or 10 kg). To determine how the fluid management and handling performance of each tested dressing type changes over time, the products were evaluated during multiple durations of simulated use: 12, 24, and 30 hours, under a constant flow rate of 2 mL/h (separately for each exudate fluid viscosity level). All the reported tests were conducted under climate control, at ambient temperature, and humidity of approximately 25°C and 50%, respectively.
2.3. Absorbency and retention studies
After each simulated use session, the dressings were weighed again, and the net fluid mass gained in each dressing due to absorption and retention was calculated. Any exudate‐simulant fluids, which remained in the wound cavities (ie, the residual fluid), and, likewise, any fluid spillovers onto the simulated peri‐wound environment, were carefully collected and weighed as well. Next, the measured fluid masses were converted to volumes, by dividing the absorbed, residual, and spillover masses by the fluid density. The total amount of the exudate‐like fluid volume delivered to each wound unit over the time course of a certain test was then calculated, as the sum of the retained fluid volume in the dressing plus the residual and spillover fluid volumes (if any). Furthermore, the percentage retention of fluid in each dressing specimen was calculated, based on the ratio of the fluid volume that was retained in the dressing over the total exudate‐like fluid volume that was delivered to the wound unit by the syringe pump system throughout the duration of the test (separately for each wound unit and test condition). Similarly, the percentages of the residual and spillover fluid volumes were calculated, by normalising these fluid volumes with respect to the total fluid volume delivered to the wound unit throughout the test time. In addition, the percentage of the evaporated fluid was calculated, as 100% minus the sum of the fluid percentage retained in the dressing, and the residual and the spillover (if occurred) fluid shares.
2.4. Data and statistical analyses of the fluid management and handling outcome measures
All the experiments were conducted in replicates of three and descriptive statistics of means and standard deviations were calculated for the retained, residual, spillover, and evaporated fluid volume shares (percentages) per each simulated use duration (12, 24, and 30 hours). From the aforementioned measured fluid volumes, we also calculated an additional dimensionless parameter, α = ratio of the cavity plus the spillover fluid volumes over the volume of the fluid retained in the dressing. This α parameter is an important performance outcome measure indicating both safety and efficacy, as it formulates and quantifies the likelihood of a dressing failure event related to fluid management and handling in clinical scenarios and settings. That is, a zero α value is ideal and indicates no pooling of exudate in the wound cavity, and no occurrence of spillovers. For realistic (non‐zero) α values, the greater the α is, the higher the risk that these adverse dressing failure events will occur in real‐world scenarios, and thereby, potentially lead to degradation of the wound, maceration of the peri‐wound skin, spread of infection out of the wound site, or any combination of these.
To identify potential statistically significant differences across dressing performance outcome measures for each tested product, analyses of variance (ANOVA) followed by post‐hoc Tukey‐Kramer multiple pairwise comparisons were conducted. Specifically, for each robotic wound system orientation (ie, prone, supine, or Sims'), two‐way ANOVAs followed by Tukey‐Kramer comparisons were ran for the factors of the usage time and the dressing type, to determine potential significant differences in fluid distribution (ie, shares of retained, residual, spillover, and evaporated fluid), separately for the low and high‐viscosity test fluid conditions. In addition, 2‐way ANOVAs were ran for the α outcome measure, likewise for the factor of the usage time and dressing type, per each fluid viscosity level. Finally, for each fluid viscosity case, a one‐way ANOVA for the factor of the dressing type was performed specifically for the Sims' configuration at the 30 hours time point (which is considered here as the test condition and duration imposing the greatest challenge to the fluid management and handling performance of the dressing products under investigation, as explained above). A P‐value lower than .05 was considered statistically significant.
3. RESULTS
For the ‘prone’ position, where the dressings were completely off‐loaded and were placed above the simulated wounds (requiring that fluid transfer into the tested dressings will be driven purely by capillary action), the MPD demonstrated an increasing trend of the share of the retained fluid volume over time, as opposed to the SAD under investigation, for which the retained fluid volume share decreased (Figure 3A,B). This observation was consistent for the two test fluid viscosity levels (Figure 3A,B). The decrease in the share of the retained fluid in the SAD was on the account of approximately 1.4‐times greater evaporation to the environment (following 24 hours), and also, greater residual fluid remaining in the wound cavities for that SAD with respect to the MPD, particularly for the longer (24 and 30 hours) test durations. For example, at the 30‐hour time point, the mean residual fluid share for the MPD was approximately half the corresponding quantity for the SAD (Figure 3A,B). The FBD exhibited an increase in the volume share of the retained fluid over time (similarly to the MPD), but that was not as consistent across the test fluid viscosity conditions, with a weaker trend when the more viscous test fluid was applied to the FBD (Figure 3A,B). Moreover, the FBD showed limited and statistically significantly lower retention than the MPD following 24 hours (P < .05; Figure 3A,B), resulting in approximately twice to triple the amounts of residual fluid remaining in the wound cavities compared to the cavity fluid left when the MPD product was used. The FBD also clearly failed to handle the viscous test fluid, leading to a mean spillover share of 14% of the total fluid delivered to the wound units at the 30 hours time point (Figure 3B). Even prior to that adverse event, and likely leading to it, the FBD demonstrated a remarkably inferior capacity to absorb the viscous fluid with respect to the other dressings, which was manifested as mean residual (cavity) shares of the fluid that even exceeded the shares of the retained fluid 2.3‐fold and 2.2‐fold at the 12 and 24 hour time points, respectively (Figure 3B), thereby indicating flow insufficiency into this FBD. Absorbing and retaining less fluid than the amount of fluid, which pools in the wound cavity, as evident for the currently tested FBD dressing, indicates insufficient efficacy in the management and handling of viscous fluids (Figure 3B).
FIGURE 3.
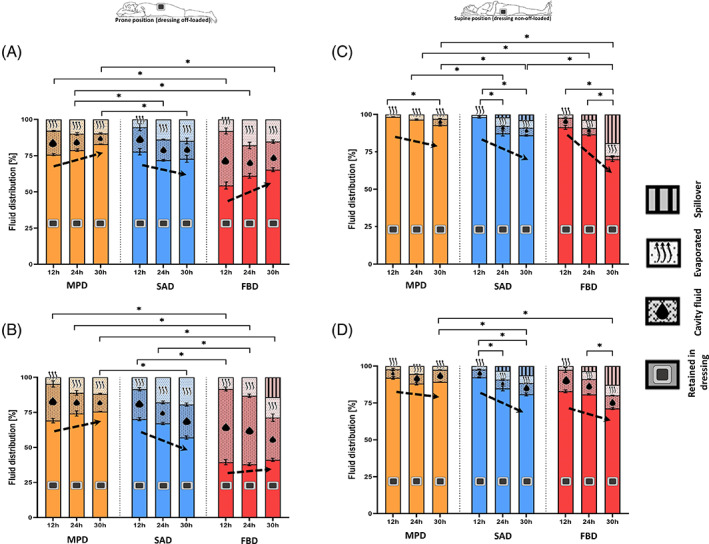
Retention performances of the tested dressings for the prone (off‐loaded; A,B) and supine (non‐off‐loaded; C,D) test configurations, and for the low (A,C) vs high (B,D) exudate‐simulant viscosities, after 12, 24, and 30 hours of simulated use. The error bars are the standard errors from the mean values of three test repetitions per test configuration and an asterisk indicates a statistically significant difference in the relevant outcome measure (P < .01). FBD, foam‐based dressing; MPD, multipurpose dressings; SAD, superabsorbent dressing
For the ‘supine’ position of the wound units, which involved external forces simulating the bodyweight acting on the wounds (that potentially affected the retention capacities of the dressings), but where gravity was the driving force for pulling the fluid down to the wound pads, all the tested dressings demonstrated greater shares of retained fluid with respect to the ‘prone’ test condition (Figure 3C,D). The fluid management and handling performance of the MPD were again mildly affected by the viscosity of the test fluid, and after 30 hours of simulated use, this dressing consistently and statistically significantly retained the greatest mean shares of fluid (93% and 89% for the low‐ and high‐viscosity test fluids, respectively) compared to the other tested dressings (P < .05; Figure 3C,D). Moreover, no spillovers occurred for the MPD despite the push of fluid by gravity into this dressing, and the external compression that has been applied to challenge the dressing reservoir in this regard. Contrarily, the SAD and the FBD failed to prevent spillovers after 24 hours and later; these spillovers occurred for both test fluid viscosities, and to a greater extent for the FBD (Figure 3C,D).
The likelihood of a functional dressing failure presenting itself as pooling of exudate in the wound cavity, or as spillover of exudate onto the peri‐wound skin, or potentially both, is elegantly depicted through comparisons of the dimensionless α parameter values for the different dressing types that were investigated here. The FBD always had the greatest α values, for either the ‘prone’ (Figure 4A,B) or ‘supine’ (Figure 4C,D) positions, as well as for each test time point and for both test fluid viscosities (with statistically significant differences against the other dressings for the viscous test fluid at both postures; P < .05; Figure 4). This identifies the FBD as being considerably more susceptible to failure via one or both of the above routes with respect to the other two dressings, particularly when managing viscous exudates. When compared against each other, the MPD and SAD also exhibited significantly different performance outcomes, with the α of the MPD consistently remaining below half the corresponding values of the SAD at the 30 hours time point, irrespective of the position or of the exudate viscosity level (a difference, which was statistically significant for the viscous test fluid regardless of the posture, and for both the low‐ and high‐viscosity fluids in the supine position; P < .05, Figure 4B‐D). While no dressing is theoretically ideal (ie, has α = 0), the MPD emerged as the safest and most effective dressing in fluid management and handling among all the tested dressing types (Figures 3 and 4).
FIGURE 4.
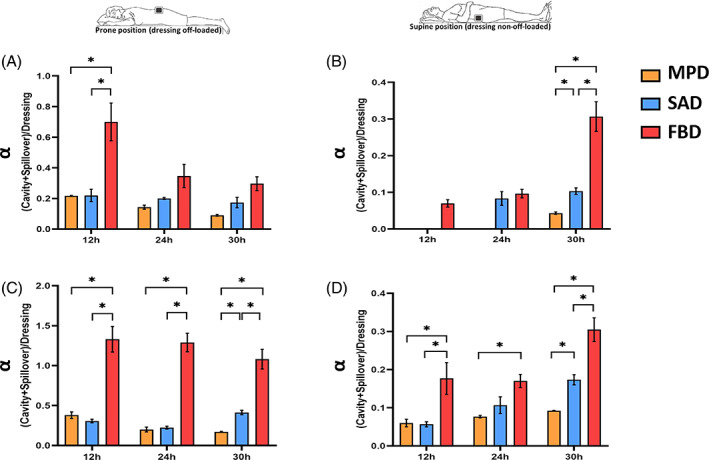
Ratios of exudate‐simulant fluid, which remained in the wound cavity plus any fluid spillovers onto the peri‐wound environment (if applicable, as per the results in Figure 3), over the corresponding volume of the fluid retained in the dressing. These ratios are plotted for the prone (off‐loaded; A,B) and supine (non‐off‐loaded; C,D) test configurations, and for the low (A,C) vs the high (B,D) exudate‐simulant viscosities, after 12, 24, and 30 hours of simulated use. We note that either pooling of fluid in the wound cavity or spillovers may be harmful, and, therefore, the smaller the value of this parameter, the better. The error bars are the standard errors from the mean values of three test repetitions per test configuration and an asterisk indicates a statistically significant difference in the relevant outcome measure (P < .01). FBD, foam‐based dressing; MPD, multipurpose dressings; SAD, superabsorbent dressing
As indicated in the methodology section, the ‘Sim's’ (side‐lying) position test condition generally introduces the greatest challenge to the fluid management and handling performance of wound dressings, as fluid is pulled down by gravity to concentrate at the lower portion of the wound pad (Figure 2C,E). This dictates a particular flow regime that does not allow a dressing to utilise its entire wound pad area as effectively as if it is placed horizontally on a wound (either below or above the wound), and typically, just the lower half of the wound pad (or sometimes less) is utilised for the wound‐dressing fluid transfer (Figure 2C,E). This situation is typical to multiple clinical scenarios, importantly including (in addition to the side‐lying position as indicated above) the treatment of VLUs in standing and ambulatory patients.
Since the results of the ‘prone’ and ‘supine’ studies reported above revealed that leakage only occurred at the later (24 and 30 hours) time points, and given that preliminary testing confirmed that absorbency and retention for the ‘Sim's’ position were indeed lower than for the ‘prone’ and ‘supine’ positions of the wound units (which resulted from the reduced effective wound pad area for fluid transfer for the ‘Sim's’ test condition; Figure 2C,E), we focused on dressing performance at the 30‐hour time point for the ‘Sim's’ trials. Consistent with the previous results, the FBD demonstrated the poorest performance, with the lowest retained fluid shares and the highest spillover shares for both test fluid viscosities. In particular, for the high‐viscosity test fluid, the mean spillover share of the FBD (64%) was 4‐fold greater than its retention (16%), which indicates total failure of the FBD in handling the high‐viscosity fluid under these vertical flow test conditions (Figure 5). The MPD and the SAD performed similarly for the low‐viscosity fluid, though a slight spillover was observed for the latter dressing (Figure 5A), however, differences in performance between these two dressings became remarkable when both were subjected to the viscous test fluid (Figure 5B). When tested with the high‐viscosity fluid, the MPD still retained a mean of 78% of the total fluid share whereas, for the SAD, the mean retention was only 36%. Importantly, for a viscous exudate‐like fluid, the spillover from the SAD was 1.2‐times greater than the share of the retained fluid, with respect to a negligible (less than 3%) spillover from the MPD under the same extreme vertical flow conditions (Figure 5B). At the 30‐hour time point, the retention share for the MPD was notably the most consistent in handling the high‐viscosity exudate across the different positions, that is, was 75%, 89%, and 78% for the ‘prone’, ‘supine’, and ‘Sim's’ test configurations, respectively, whereas for the SAD the corresponding values were 57%, 81%, and 36%, respectively, pointing to a substantially larger performance variation depending on the dressing orientation with respect to the gravity vector. All the above results associated with the ‘Sim's’ position are well reflected in the α analyses for this test configuration (Figure 6). The FBD had the greatest α for both fluid viscosity levels, whereas the MPD had the lowest α values (near‐zero for the low‐viscosity test fluid). The SAD exhibited intermediate α values, but those were still 6‐times and 7.6‐times greater than the corresponding α data of the MPD for the low‐ and high‐viscosity levels of the test fluid, respectively. To summarise, taken together, the above experimental results indicate that the FBD technology is unlikely to be able to handle a viscous exudate in a vertical wound and dressing configuration (such as when applied to a VLU) as effectively as the MPD and SAD would perform in such scenarios. Overall, dressings from different manufacturers also exhibit remarkably different performance metrics in fluid handling, particularly when tested in interaction with viscous fluids representing the thicker domain of wound exudates.
FIGURE 5.
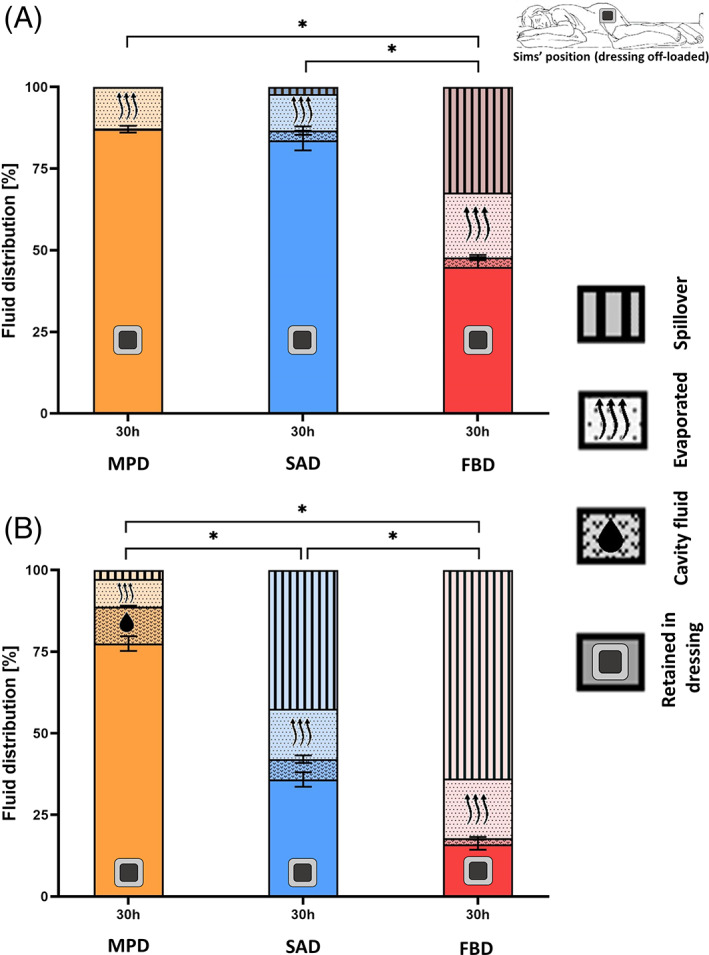
Retention performances of the tested dressings for the side‐lying (Sims') test configuration and for the low (A) vs the high (B) exudate‐simulant viscosities after 30 hours of simulated use. The side‐lying test configuration is a particularly demanding challenge for the dressings investigated here, as the dressings are unable to use the entire wound pad surface for absorption, as demonstrated in Figure 2. The error bars are the standard errors from the mean values of three test repetitions per test configuration and an asterisk indicates a statistically significant difference in the relevant outcome measure (P < .01). FBD, foam‐based dressing; MPD, multipurpose dressings; SAD, superabsorbent dressing
FIGURE 6.
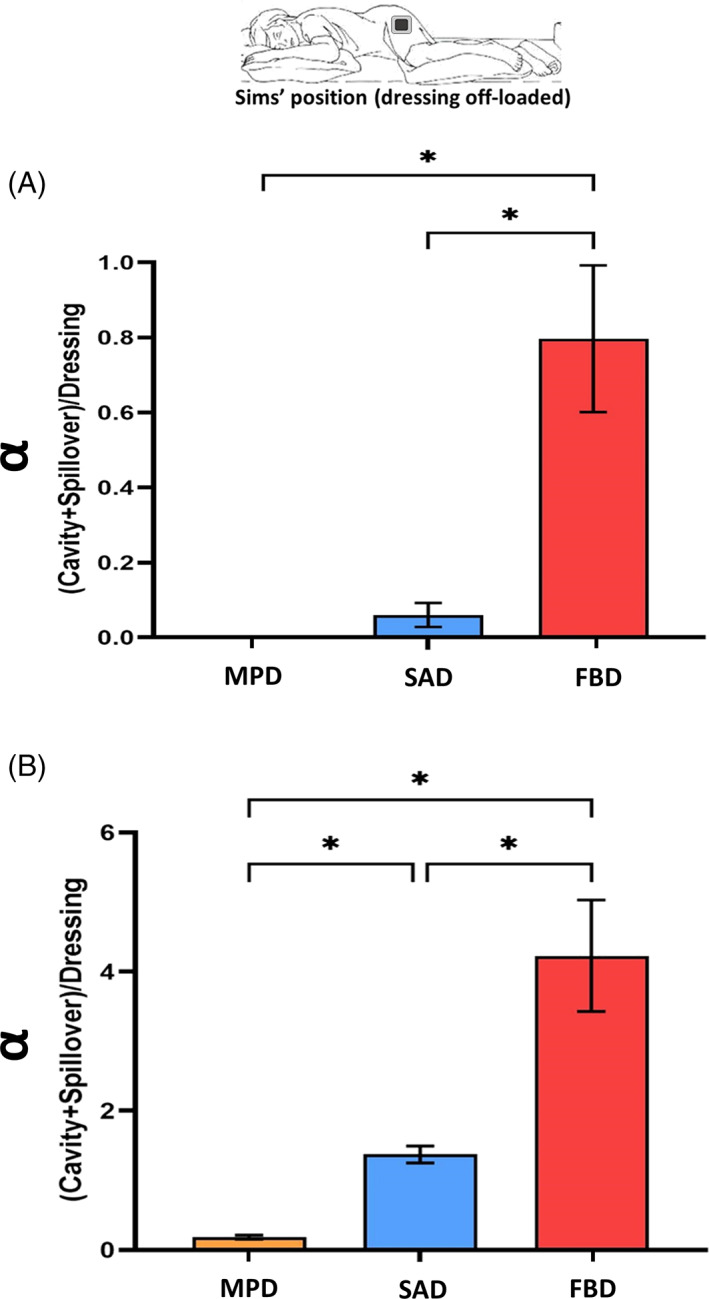
Ratios of exudate‐simulant fluid, which remained in the wound cavity plus any fluid spillovers onto the peri‐wound environment (if existed; Figure 5), over the corresponding volume of the fluid retained in the dressing for the side‐lying (Sims') test configuration. These ratios are shown for the low (A) vs the high (B) exudate‐simulant viscosities, after 30 hours of simulated use. The error bars are the standard errors from the mean values of three test repetitions per test configuration and an asterisk indicates a statistically significant difference in the relevant outcome measure (P < .01). FBD, foam‐based dressing; MPD, multipurpose dressings; SAD, superabsorbent dressing
4. DISCUSSION
The wound exudate plays an essential role in the healing process, as it forms an aqueous biological environment for cell proliferation, migration, and differentiation, and a medium for cell signalling (which is altogether known as moist wound healing), however, excessive exudate amounts may cause wound deterioration and peri‐wound maceration. 25 , 26 , 27 , 28 Managing excess exudate requires effective wound dressings that continuously absorb and retain the exudate while maintaining the wound‐bed moist, and that are safe to use throughout the intended treatment period, during which no backflow, pooling, or spillover of exudate should occur. 19 , 20 , 21 , 29 , 30 In this study, we have used a novel robotic phantom system representing open exuding wounds to evaluate the fluid absorbency and retention performances of three distinct dressing types, MPD, SAD, and FBD, which were tested at different simulated postures and subjected to off‐loaded vs non‐off‐loaded conditions, and to low‐ and high‐viscosity exudate‐like fluids.
Among the dressings under investigation, the MPD demonstrated the most effective fluid handling performance across all the test conditions. Specifically, at all the simulated body positions, and regardless of whether the dressings were off‐loaded or non‐off‐loaded, or if the exudate‐substitute was of low‐ or high‐viscosity, the retained fluid volumes were always the greatest for the MPD at the 30‐hours trial endpoint (Figures 3 and 5). Moreover, no spillovers occurred for the MPD, excluding a negligible amount of the high‐viscosity fluid at the simulated Sim's position (Figure 5C). In contrast, spillovers from the FBD occurred in all the tested cases except the proning with low‐viscosity fluid (which was the least challenging for the absorbency capacity of the dressings under investigation, as all fluid transfer occurred through capillary [upward] motion). The earliest spillover events occurred at the 24‐hour time point, for both the SAD and FBD, and the highest spillover amounts were from the FBD at the 30‐hour endpoint, for all the studied simulated positions.
The α parameter was introduced to quantify the relative likelihood of a functional dressing failure, through either fluid pooling in the cavity or spillovers. Consistent with the results reported above, these analyses revealed that the FBD always had the greatest α values, across all the studied positions, at all time points and for both test fluid viscosities, demonstrating that the FBD has inferior retention capacity with respect to the SAD and MPD (Figures 4 and 6). The SAD performed similarly to the MPD in the ‘prone’ and ‘supine’ tests for the first 24 hours (Figure 4), but exhibited substantially poorer performance at the 30‐hours endpoint, particularly for the high‐viscosity exudate‐substitute tests where the α of the SAD were always statistically significantly greater than those of the MPD, for all the tested postures (P < .05; Figures 4 and 6). This difference in functional (fluid handling) performance must relate to the fundamental structural differences between the studied SAD and MPD dressings. Gefen recently reviewed the structure–function relations of SADs and classified them into two main families, namely, dressings with a sandwich core structure of superabsorbent particles contained between two covers, which is also called a superabsorbent polymer (SAP)‐sheet core, vs dressings with a core of embedded SAP grains within a cellulose fluff. 10 The currently investigated SAD belongs to the former, SAP‐sheet core group, whereas the MPD is a member of the latter family. The bioengineering theory formulated in the Gefen article, based on the Darcy equations of fluid motion in porous media, established that wound dressings with an SAP‐sheet core are likely to perform inferiorly with respect to dressings having cores of embedded SAP grains, as the wound‐facing cover of the SAP‐sheet core may become a flow‐limiting element, particularly when the dressing is required to handle large amounts of viscous fluids (the wound‐facing aspect of the FBD may also be a flow‐limiting element in this regard). This subsequently limits the absorbency and retention performance of SADs with an SAP‐sheet core when handling viscous exudates. The currently reported findings (Figures 4 and 6) provide empirical confirmation to the Gefen theory concerning the fluid handling performance of SADs, and experimentally confirm the superiority of the MPD (embedded SAP grains) design over the SAP‐sheet core design of the SAD in managing viscous exudates.
As with any experimental work, there are inherent limitations that should be recognised in the ability to replicate the complex in vivo processes of wound management and the seemingly endless number of potential clinical scenarios in a laboratory setting. One particularly interesting scenario, which has not been considered in the current work but should be investigated in future research is the treatment of VLUs by means of compression therapy. This article explained the challenging nature of treating wounds where the dressing is applied vertically to the ground and parallel to the direction of the gravity vector (Figure 1C,E). However, we did not consider a non‐off‐loaded wound in such configuration, as would be the case for a dressing applied under compression bandaging, which should create an even greater challenge to the dressing design, due to the additional distortion and reduction of the dressing reservoir for fluid handling by the external compressive forces. The robotic wound simulants can also be further improved to facilitate, for example, continuous pH monitoring or the growth of microbiomes representing different infected wound aetiologies. It should also be noted that the experimental protocol in the current work is conservative with respect to the clinical demands from wound dressings in the real‐world, as we had tested the dressing performances for periods of up to 30 hours, whereas dressings applied to (non‐infected) open wounds are typically replaced every 3‐5 days. 31
In closure, we found that the MPD exhibited the best and most robust fluid handling performance across all the test configurations, and for both the low‐ and high‐viscosity test fluids. The FBD consistently showed the poorest performance, and, in particular, is unlikely to be able to handle a viscous exudate in a vertical wound and dressing configuration (such as in the current ‘side‐lying’ position or when applied to a VLU of ambulatory patients) as effectively as the other dressing technologies. The SAD performed better than the FBD, but its fluid handling metrics were overall inferior with respect to those of the MPD. Finally, the fluid management and handling performance metrics of wound dressings are technology‐specific and product‐specific, and depend on the material composition, structure, and manufacturing method of the brand. The comparative quantification of the shares of retained, residual, evaporated and spillover fluid, acquired through standardised laboratory testing, as reported here, should help clinical and non‐clinical decision‐makers to select wound dressings that best meet their patient needs.
ACKNOWLEDGEMENTS
This project has received funding from the European Union's Horizon 2020 research and innovation programme under the Marie Skłodowska‐Curie Grant Agreement No. 811965; project STINTS (Skin Tissue Integrity under Shear). This work was also partially supported by the Israeli Ministry of Science & Technology (Medical Devices Program Grant no. 3‐17421, awarded to Professor Amit Gefen in 2020) and by Curea Medical GmbH (Steinfurt, Germany).
Orlov A, Gefen A. The fluid handling performance of the curea P1 multipurpose dressing against superabsorbent and foam dressing technologies. Int Wound J. 2022;19(4):945‐956. doi: 10.1111/iwj.13774
Funding information Curea Medical GmbH (Steinfurt, Germany); European Union's Horizon 2020 research and innovation programme under the Marie Skłodowska‐Curie Grant, project STINTS (Skin Tissue Integrity under Shear), Grant/Award Number: 811965; Israeli Ministry of Science & Technology (Medical Devices Program), Grant/Award Number: 3‐17421
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Gefen A, Ousey K. Safe and effective wound care during the COVID‐19 pandemic. J Wound Care. 2020;29:622‐623. doi: 10.12968/jowc.2020.29.11.622 [DOI] [PubMed] [Google Scholar]
- 2. Guest JF, Fuller GW, Vowden P. Cohort study evaluating the burden of wounds to the UK's National Health Service in 2017/2018: update from 2012/2013. BMJ Open. 2020;10:e045253. doi: 10.1136/bmjopen-2020-045253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Han G, Ceilley R. Chronic wound healing: a review of current management and treatments. Adv Ther. 2017;34:599‐610. doi: 10.1007/S12325-017-0478-Y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Folestad A, Gilchrist B, Harding K, et al. Wound exudate and the role of dressings. A consensus document. Int Wound J. 2008;5(Suppl 1):iii‐12. doi: 10.1111/j.1742-481X.2008.00439.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. World Union of Wound Healing Societies . World Union of Wound Healing Societies (WUWHS) Consensus Document. Wound exudate: effective assessment and management. Wounds Int. 2019;1–33. https://www.woundsinternational.com/resources/details/wuwhs-consensus-document-wound-exudate-effective-assessment-and-management [Google Scholar]
- 6. Jones V, Grey JE, Harding KG. ABC of wound healing: wound dressings. BMJ. 2006;332:777‐780. doi: 10.1136/BMJ.332.7544.777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wiegand C, Hipler UC. A superabsorbent polymer‐containing wound dressing efficiently sequesters MMPs and inhibits collagenase activity in vitro. J Mater Sci Mater Med. 2013;24:2473‐2478. doi: 10.1007/S10856-013-4990-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ousey K, Atkin L, White R. Superabsorbent wound dressings: a literature review. Wounds UK. 2013;9:52‐60. https://www.wounds-uk.com/journals/issue/35/article-details/superabsorbent-wound-dressings-a-literature-review [Google Scholar]
- 9. Wiegand C, Tittelbach J, Hipler U‐C, Elsner P. Clinical efficacy of dressings for treatment of heavily exuding chronic wounds. Chronic Wound Care Manag Res. 2015;2:101‐111. doi: 10.2147/CWCMR.S60315 [DOI] [Google Scholar]
- 10. Gefen A. Not all superabsorbent wound dressings are born equal: theory and experiments. J Wound Care. 2021;30:738‐750. doi: 10.12968/JOWC.2021.30.9.738 [DOI] [PubMed] [Google Scholar]
- 11. Wiegand C, White RJ. Binding and inhibition of protease enzymes, including MMPs, by a superabsorbent dressing in vitro. J Wound Care. 2013;22:221‐227. doi: 10.12968/jowc.2013.22.5.221 [DOI] [PubMed] [Google Scholar]
- 12.ASTM D412‐06, Standard Test Methods for Vulcanized Rubber and Thermoplastic Elastomers‐Tension. West Conshohocken, PA: ASTM International; 2006, www.astm.org; n.d. 10.1520/D0412-06 [DOI]
- 13. Xu F, Lu TJ. Skin biothermomechanics: modeling and experimental characterization. Advances in Applied Mechanics. Vol 43. Amsterdam, Netherlands: Elsevier Inc.; 2009:147‐248:chap 3. doi: 10.1016/S0065-2156(09)43003-5 [DOI] [Google Scholar]
- 14. Kuthe CD, Uddanwadiker RV. Investigation of effect of fiber orientation on mechanical behavior of skeletal muscle. J Appl Biomater Funct Mater. 2016;14:e154‐e162. doi: 10.5301/jabfm.5000275 [DOI] [PubMed] [Google Scholar]
- 15. Graham HK, McConnell JC, Limbert G, Sherratt MJ. How stiff is skin? Exp Dermatol. 2019;28:4‐9. doi: 10.1111/exd.13826 [DOI] [PubMed] [Google Scholar]
- 16. Gefen A, Haberman E. Viscoelastic properties of ovine adipose tissue covering the gluteus muscles. J Biomech Eng. 2007;129:924‐930. doi: 10.1115/1.2800830 [DOI] [PubMed] [Google Scholar]
- 17. Vieira SL, Pavan TZ, Junior JE, Carneiro AAO. Paraffin‐gel tissue‐mimicking material for ultrasound‐guided needle biopsy phantom. Ultrasound Med Biol. 2013;39:2477‐2484. doi: 10.1016/J.ULTRASMEDBIO.2013.06.008 [DOI] [PubMed] [Google Scholar]
- 18. Orlov A, Lustig A, Grigatt A, Gefen A. Fluid handling dynamics and durability of silver‐containing gelling fiber dressings tested in a robotic wound system. Adv Skin Wound Care. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lustig A, Gefen A. Fluid management and strength post‐simulated use of primary and secondary dressings for treating diabetic foot ulcers: robotic phantom studies. Int Wound J. 2021;19:305‐315. doi: 10.1111/iwj.13631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lustig A, Alves P, Call E, Santamaria N, Gefen A. The sorptivity and durability of gelling fibre dressings tested in a simulated sacral pressure ulcer system. Int Wound J. 2020;18:194‐208. doi: 10.1111/iwj.13515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lustig A, Gefen A. Three‐dimensional shape‐conformation performances of wound dressings tested in a robotic sacral pressure ulcer phantom. Int Wound J. 2021;18:670‐680. doi: 10.1111/iwj.13569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jones J, Barraud J. An evaluation of KerraMax Care in the management of moderate to heavily exuding wounds. Br. J. Community Nurs. 2014;19:S48‐S53. doi: 10.12968/bjcn.2014.19.sup3.s48 [DOI] [PubMed] [Google Scholar]
- 23. Atkin L, Nierenberg N, Wild T. Case series: ALLEVYN LIFE™ non‐bordered foam dressing for managing moderate to heavily exuding wounds. Wounds Int. 2018;9:38‐42. [Google Scholar]
- 24. Bush T, Leitkam S, Aurino M, Cooper A, Basson M. A comparison of pressure mapping between two pressure‐reducing methods for the sacral region. J Wound Ostomy Continence Nurs: Off Publ Wound, Ostomy Cont Nurses Soc. 2015;42:338‐345. doi: 10.1097/WON.0000000000000144 [DOI] [PubMed] [Google Scholar]
- 25. Junker JPE, Kamel RA, Caterson EJ, Eriksson E. Clinical impact upon wound healing and inflammation in moist, wet, and dry environments. Adv Wound Care. 2013;2:348‐356. doi: 10.1089/WOUND.2012.0412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Okan D, Woo K, Ayello EA, Sibbald G. The role of moisture balance in wound healing. Adv Skin Wound Care. 2007;20:39‐53. doi: 10.1097/00129334-200701000-00013 [DOI] [PubMed] [Google Scholar]
- 27. Rodgers A, Watret L. Maceration and its effect on periwound margins. Wounds Int. 2003;6:2‐5. [Google Scholar]
- 28. Rippon MG, Ousey K, Rogers AA, Atkin L. Wound hydration versus maceration: understanding the differences. Wounds UK. 2016;12:62‐68. [Google Scholar]
- 29. Tickle J. Wound exudate assessment and management: a challenge for clinicans. Br J Nurs. 2015;24:S38‐S43. doi: 10.12968/BJON.2015.24.SUP20.S38 [DOI] [PubMed] [Google Scholar]
- 30. Nichols E. Wound assessment part 2: exudate. Wound Essent. 2016;10:36‐41. [Google Scholar]
- 31. Davies P, Stephenson J, Manners C. Understanding undisturbed wound healing in clinical practice — a global survey of healthcare professionals. Wounds UK. 2019;15:56‐65. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


