Abstract
Keywords: axillary, hidradenitis suppurativa, negative‐pressure wound therapy, wide surgical excision
1. INTRODUCTION
Hidradenitis suppurativa (HS), or acne inversa, is a chronic inflammatory dermatosis, which affects the apocrine glands and pilosebaceous unit, is a characteristic of the intertriginous regions and, more frequently, is found in the axillary and inguinal areas. It profoundly alters the quality of life of patients. The prevalence of HS is 0.3% to 4% in industrialised countries and primarily affects younger individuals (mean age of onset 23 years), with a female to male ratio of 4:1. 1 Several studies have shown that axillary involvement occurs in around 72% of cases, followed by perianal (32%), groin (24%) and mammary involvement (8%). 2 , 3
A genetic factor with an autosomal dominant heritance has been identified in one‐third of patients with HS. 2 Smoking and obesity are both well‐known factors related to the development and severity of HS. 4 , 5
The diagnosis of HS is made clinically, and biopsies are seldom required. Various staging systems have been created to objectify and clarify the severity of the disease. The Hurley staging system is most frequently used for HS. Stage I consists of a solitary or multiple isolated abscesses, without sinus tracts or scarring. Stage II is characterised by recurrent abscesses, single or multiple widely separated lesions, with sinus tract formation, and stage III relates to diffuse or broad involvement across a regional area, with multiple interconnected sinus tracts and abscesses. 6 Clinical management with topical and systemic antibiotics has been described, as well as more recently, the use of immunosuppressants, such as inhibitors of tumour necrosis factor with encouraging results. 7 , 8 , 9 , 10 In severe cases of HS, medical therapy alone is inadequate, and wide local excision of all skin in the affected region offers the best hope for local disease control. 2 The most common surgical procedures are incision with drainage, deroofing and limited or wide excision. Incision with drainage is usually performed in acute stages to drain pus from abscesses and to relieve pain, but recurrence rates of up to 100% have been reported. 2 , 11 , 12 Deroofing is a surgical technique that consists of surgical removal of the skin covering a tunnel, laying open the partially tunnel floor for healing by secondary intention. It has been proposed as an efficient means for recurrent lesions in a mild phase of the disease. 13 If the disease is localised, limited excision can be performed. Simple local excision includes the removal of the entire sinus tract and can be closed directly. This technique has a short duration of care but a very high recurrence rate. Mehdizadeh et al 12 in a systematic review and meta‐analysis published in 2015 showed a recurrence rate of 13% for wide excisions and 22% for limited excisions. Wide excision is the standard treatment for advanced cases. However, it creates a large defect. Hence, the problem with wide local excision is how to close the defect. Several studies have examined different methods of closure after excisions of HS, including skin grafts, locoregional and free flaps and healing by secondary intention. 2 , 3
Wide local excision in the axillary areas often leads to large defects that are difficult to close primarily as function and range of motion are prioritised. 14 This is probably the reason why surgical techniques used to reconstruct axillary defects include the most numerous options. 15 Wound healing by secondary intention provides good results, and recent studies have confirmed the success of earlier reports. 15 This procedure has essentially one disadvantage: a long recovery time (several weeks), with heavy dressings. To try to reduce the axillary healing time, several other techniques have been developed. Split‐thickness skin grafts (STSG) or flaps have shorter healing time and give satisfactory results but need immobilisation of the arm, leave sequelae in the donor sites and do not always prevent retractile scarring. Furthermore, the aesthetic result is pretty poor 16 and concerns persist about the recurrence rate as compared with healing by secondary intention. British guidelines 10 consider healing by secondary intention or thoracodorsal artery perforator (TDAP) flap closure for axillary wounds in people with HS following extensive excision. Therefore, to date, no randomised study has demonstrated an optimal approach to the surgical treatment of axillary HS, and none of the surgical procedures are superior to the others. 17
The current study aimed to describe and evaluate the results of the use of negative‐pressure wound therapy (NPWT) as a method of healing axillary defects after wide surgical excision for HS.
2. METHODS
We designed the study as a retrospective analysis in surgery department in Begin Hospital, St Mandé, France. This study is based on the review of all consecutives patients undergoing surgical treatment for axillary HS between September 2017 and December 2019 and treated with combined wide surgical resection and NPWT. Surgery was indicated essentially for Hurley stages II and III, after antibiotic failure.
2.1. Surgical technique
For each patient, we performed a radical wide excision in the operating room under general anaesthesia. Complete excision of all involved skin and subcutaneous tissue was performed. The width and depth of dissection were guided by extent of disease, with a disease‐free surgical margin of 1 cm. The surgical specimen was sent to the pathologist. The final dressing for all patients was an NPWT system set at 100 mmHg. This was changed every 2 to 3 days and establishes for a maximum period of 15 days after surgery. After stopping TPN, patients had daily care with alginate wicking.
2.2. Postoperative wound care
The goal of postoperative wound care was to maintain a moist and clean wound, being achieved with wound dressing changes. Patients were followed up 2 weeks postoperatively and subsequently every month until complete wound healing was achieved.
Demographic data were collected on age, sex, comorbidity, smoking, body mass index, American Society of Anesthesiologists (ASA) score duration of the disease and HS treatment. The severity of HS was classified using the Hurley classification. 6 Operative variables that were measured included operating time, size of excised area (according to the pathology reports), hospital stay, duration of NPWT, complications, time to complete healing and recurrence of HS.
We assessed patients' satisfaction by using a standardised form. The form consisted of questions:
Are you satisfied concerning shoulder mobility?
Are you satisfied concerning aesthetic results?
Are you satisfied concerning NPWT?
Would you do this surgery with NPWT again?
Would you recommend the surgery to another HS patient?
For each question, one answer was possible: completely satisfied, partially satisfied, moderately satisfied, not completely satisfied and not at all satisfied. The patients were interviewed by the same person either in consultation, by telephone or by e‐mail.
The judgement criteria were length of hospitalisation, time to complete healing, complications, patient satisfaction scores about the surgery and the presence or absence of local recurrence of the disease.
2.3. Statistical analysis
Continuous variables are presented as means (standard deviations [SDs]) or medians (ranges), and categorial variables are presented with numbers (%).We used an Microsoft Excel spreadsheet, in its version of Microsoft Office 2019, to tabulate the data, and XLStat software to perform the statistical analysis.
3. RESULTS
3.1. Population characteristics (Table 1)
TABLE 1.
Patients demographics
| Number of patients (n, %) | Mean ± SD | Ranges | ||
|---|---|---|---|---|
| Patients | 20 | |||
| Men | 8 (40%) | |||
| Women | 12 (60%) | |||
| Age (years) | 27.1 ± 7.7 | 15 to 40 | ||
| BMI (kg/m2) | 28.2 ± 5 | 20.3 to 42.5 | ||
| Tabaco (yes) | 11 (55%) | |||
| ASA score | ||||
| ASA 1 | 9 (45%) | |||
| ASA 2 | 11 (55%) | |||
| Medical treatments | ||||
| Systemic antibiotics | 15 (75%) | |||
| Anti‐TNF | 2 (5%) | |||
| Disease duration years | 7.1 ± 6.7 | 1 to 20 | ||
| Age of illness onset | 19.9 ± 5.7 | 11 to 37 | ||
| Patients with unilateral excision | 4 (20%) | |||
| Patient with bilateral excision | 16 (80%) | |||
| Size of the operated axilla | 35 | |||
| Left | 17 (48.6%) | |||
| Right | 18 (51.4%) | |||
| Hurley's stage | ||||
| Stage 2 | 10 (50%) | |||
| Stage 3 | 10 (50%) | |||
Abbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index; TNF, tumour necrosis factor.
In this retrospective study, we analysed data from 36 surgical procedures consecutively performed for 20 patients with HS between 2017 and 2019 (16 patients underwent bilateral procedures and 4 underwent unilateral procedure). The mean age of patients was 27.1 ± 7.7 years, 60% were female, the average BMI was 28.2 ± 5 kg/m2 and 55% were smokers. The Hurley stage at surgery was III for 10 (50%) patients and II for the remainder of the patients. The mean duration of disease before surgery was 7.1 ± 6.7 years. Fifteen patients (75%) had received treatment with antibiotics, and five patients (15%) had received treatment with .
3.2. Surgical characteristics (Table 2 and Figure 1)
TABLE 2.
Results
| Number of patients (n, %) | Mean ± SD | Extremes | ||
|---|---|---|---|---|
| Excised area (cm2) | 46.6 ± 43 | 10 to 195 | ||
| Operative time (minutes) | 39.4 ± 13 | 20 to 64 | ||
| Length of hospitalisation (days) | 3 ± 0.2 | 3 to 4 | ||
| Length of NPWT healing (days) | 18.5 ± 9 | 5 to 39 | ||
| Length of complete healing | 115 ± 85 | 21 to 407 | ||
| Complication | 5 (25%) | |||
| Infection | 2 (5.7%) | |||
| Bleeding | 0 | |||
| Pain | 4 (11.4%) | |||
| Loss of mobility | 1 (2.8%) | |||
| Length of follow‐up (days) | 375.5 ± 226.3 | 37 to 764 | ||
| Recurrences of HS | 1 (5%) | |||
Abbreviations: HS, hidradenitis suppurativa; NPWT, negative‐pressure wound therapy.
FIGURE 1.
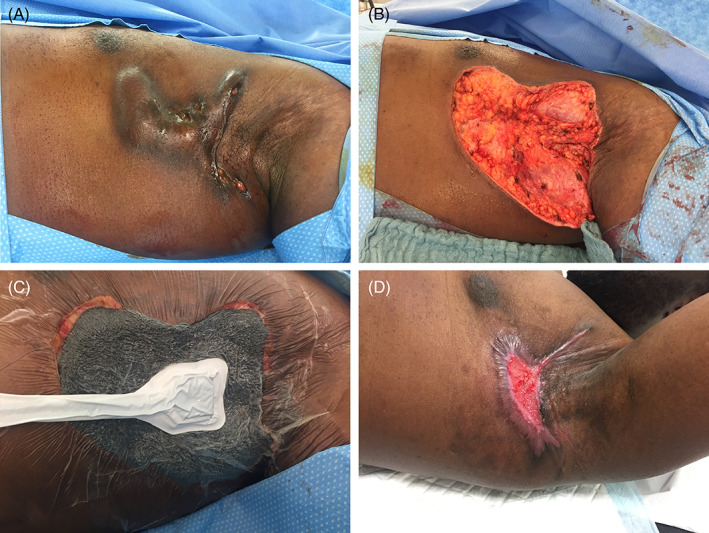
A, Before wide excision. B, After wide excision. C, negative‐pressure wound therapy (NPWT). D, 2 months after wide excision
Each patient was free to choose a unilateral or bilateral procedure (in cases of bilateral pathology). Among the 20 patients, 16 (80%) patients underwent bilateral procedures. The average excised area was 46.6 ± 43.0 m2, and the mean operative time was 39.4 ± 13 minutes. The average length of hospitalisation was 3 days. The average duration of NPWT was 18.5 ± 9 days, and the mean complete healing time was 115 ± 85 days. Among the 36 procedures, there were five (13.8%) complications. The most frequent were pain (11.4%), infection (5.7%) and loss of mobility (2.8%). None of the complications required a new hospitalisation.
3.3. Follow‐up and recurrence
The mean follow‐up was 412.5 ± 195.2 days. We recorded only one recurrence among the 19 patients who were followed. One patient was lost to follow‐up 3 months after surgery.
3.4. Satisfaction study (Table 3)
TABLE 3.
Satisfaction study
| Not at all (n, %) | Not totally (n, %) | Moderately satisfied (n, %) | Partially satisfied (n, %) | Completely satisfied (n, %) | |
|---|---|---|---|---|---|
| 1‐Are you satisfied concerning shoulder mobility? | 0 | 0 | 3 (15.8%) | 7 (36.8) | 9 (47.4) |
| 2‐Are you satisfied concerning aesthetic results? | 1 (5.3%) | 1 (5.3%) | 3 (15.8%) | 8 (42.1%) | 6 (31.5%) |
| 3‐Are you satisfied concerning NPWT? | 1 (5.3)% | 0 | 2 (10.5%) | 2 (10.5%) | 14 (73.7%) |
| 4‐Would you do this surgery with NPWT again? | 4 (21%) | 1 (5.3%) | 2 (10.5%) | 4 (21%) | 8 (42.1%) |
| 5‐Would you recommend the surgery to another HS patient? | 2 (10.5%) | 0 | 2 (10.5%) | 3 (15.8%) | 12 (63.2%) |
Abbreviations: HS, hidradenitis suppurativa; NPWT, negative‐pressure wound therapy.
Among the 20 patients, 19 patients returned the satisfaction form. For shoulder mobility results, a total of 9 (47.4%) patients were completely satisfied, 36.8% were partially satisfied, and 15.8% were poorly satisfied. For the aesthetic results, only 6 (31.6%) patients were completely satisfied. Concerning NWPT, 14 (73.7%) patients were completely satisfied, with only 8 (42.1%) patients accepting another surgery with the same procedure. In all, 12 (63.1%) patients would recommend this surgery to another patient (Table 3). The average score on the satisfaction questionnaire was 20.5 ± 4.2 points (min 11–max 25).
4. DISCUSSION
Multiple reconstructive modalities after wide axillary excisions have been described, including skin grafts, flaps and healing by secondary intention. Much discussion has been dedicated to determining the ideal postexcisional reconstructive modality, with pertinent outcomes of local disease recurrence rate, time to complete healing, donor site morbidity, function and aesthetics. Healing by secondary intention after excision of HS has been described, with successful wound healing results, 18 and seems to have less pain than grafting, 19 cosmetically acceptable scars, lack of donor sites, no flap or graft loss and an acceptable range of motion with a low incidence of contractures. 18 Disadvantages include relatively long healing times, painful dressing changes, need for meticulous wound care and risk of wound contracture, particularly with large excisions. The TDAP and STSG in the management of chronic axillary HS improved the time to healing. However, these methods have significant morbidities and donor‐site limitations. Moreover, STSGs and flaps increased both operative time and length of hospitalisation, as well as may lead to an increased number of surgical procedures. 20 Chen et al demonstrated in 2011 21 that only the extremely huge defects will require a skin graft or flap, 21 but in some cases, the excision creates very large skin defects such that local or perforator flaps may not be adequate for the reconstruction. 12 , 22 , 23
According to the patient's choice, wound size and all the disadvantages already mentioned for skin grafts or flaps, our centre prefers wound healing by secondary intention. We argue that wound healing by secondary intention has advantages, including cosmetically acceptable scars, lack of donor sites, no flap or graft loss and functional outcomes with a low incidence of contractures. 18 Moreover, in a small study (n = 10), Morgan et al 19 implied that patients possibly prefer healing by secondary intention over skin grafting. Bieniek et al 24 demonstrated favourable cosmetic results with healing by secondary intention after surgery for HS. In order to reduce the healing time, we proposed a two‐step healing strategy. The use of NPWT for 2 weeks on an open wound created after wide excision of HS lesions to remove residual infections provided drainage and improved perfusion before secondary healing with mesh. Our time to complete healing was higher than that in previous studies and this whatever the mode of wound care: grafts flaps or secondary intention (115 days). The average excised area was 46.6 ± 43 cm2. This size seems to be lower than that in other studies. 16 , 25 , 26 , 27 , 28 , 29 , 30 , 31 In this analysis of 35 wide excisions with wound healing with NPWT, the operative time was 39.4 minutes, and length of hospitalisation was 3 days. A systematic review of the literature on axillary surgery for HS (Table 4) 16 , 17 , 25 , 26 , 27 , 28 , 29 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 from 2010 to 2020 showed a mean operative time of 125.9 minutes, mean length of hospitalisation of 4.9 days and time to complete healing of 38.2 days. However, in several studies, these points are not reported, which makes comparisons to our study difficult. The studies carried out essentially sought to evaluate the rate of recurrence, mobility of the shoulder and aesthetic results, as well as time to healing after excision of HS. The scant data available described a time to healing between 20 and 150 days. 14 , 39 , 40 , 41
TABLE 4.
Summary of articles on surgical interventions in patients with axillary hydratenitis suppuritiva published since 2010
| Study, year | No. of patients | Closure type | Length of surgery (min) | Excised area (cm2) | Length of hospitalisation (day) | Length of healing (days) | Complication (n; %) | Recurrence rate (%) | Length follow up (month) |
|---|---|---|---|---|---|---|---|---|---|
| Busnardo et al (2011) 32 | 12 | Flap | ND | ND | ND | ND | ND | 38.8% | 6 months |
| Afsharfard et al (2020) 17 | 13 | STSG and flap | ND | ND | ND | ND | ND | 0 | 1 year |
| Wormald et al (2014) 33 | 27 | Graft and flap | 146.5 ± 69.8 | ND | 5.6 ± 2.9 | 70.7 ± 89.6 | ND | 3.7% | 1 year |
| Ortiz et al (2010) 34 | 16 | Thoracodorsal artery perforator flap | ND | ND | ND | ND | ND | 0 | ND |
| Marchesi et al (2018) 25 | 17 | Thoracodorsal artery perforator flap | 151 ± 31 | 94.5 ± 38 | 1 to 5 | 20 ± 9 | N = 6; 35% | ND | ND |
| Pearce et al (2017) 26 | 7 | NPWT + STSG | ND | 335 | 8.8 ± 4 | 38.7 ± 15,3 | ND | ND | 1 months |
| Elgohary et al (2018) 30 | 20 | Thoracodorsal artery perforator flap | 210 ± 25 | Range 96 to 204 | 6 ± 3 | ND | N = 7; 35% | ND | 30 months ± 5.2 |
| Elboraey et al (2019) 31 | 6 | Propeller flap | ND | Range 66 to 252 | 4 | ND | N = 2; 33% | 0 | 10 months mean |
| Ching et al (2017) 35 | 4 | Transposition flap | ND | ND | ND | ND | N = 1; 25% | 0 | 18 months |
| Wu et al (2020) 28 | 34 | Rotation flap | 4 ± 16 | 84 | 0 | ND | N = 13; 25% | 10% | 32 months |
| Gonzaga et al (2013) 27 | 4 | Graft with bilayer dermal regeneration | ND | Range 200 to 300 | ND | ND | N = 1; 25% | 0 | 23 months |
| Hallock et al (2013) 36 | 2 | Thoracodorsal artery perforator based V‐Y advancement | ND | 90 | ND | 45,5 | 1 (minor) | 0 | 9.5 months |
| Varkarakis et al (2010) 37 | 15 | Rotation flap | ND | 212 | ND | ND | N = 4; 26.6% | 0 | 12 months |
| Nail‐Barthelemy et al (2019) 16 | 13 | Perforator flap | 76.2 ± 18.8 | 58 ± 31.5 | 5.1 ± 3.1 | 20.5 ± 13.5 | N = 6; 46% | 0 | 9 months |
| Calibre et al (2013) 38 | 5 | Skin grafting + NPWT | ND | ND | ND | 34 (20–43) | 0 | ND | ND |
| Jandali et al (2013) 29 | 9 | Thoracodorsal artery perforator | ND | 74 | ND | ND | N = 2; 22;% | 11.1% | 20 months |
| Mean | 12.7 | 125.94 | 4.9 | 38.2 | 26.4% | 1.4% | ±15 |
Abbreviations: NPWT, negative‐pressure wound therapy; STSG, split‐thickness skin graft.
In this study, we measured the local recurrence rate of HS at 5% (one patient) with a mean follow‐up of 1 year. The recurrence risk after wide excision was estimated at 6% to 38%. 18 , 42 , 43 , 44 Recurrence rates were reported for primary closure (54%–70%), 45 STSGs (0%–33%) 18 , 46 , 47 and flaps (0%–6.6%). 18 , 33 , 37 Our recurrence rate seems very low and must be confirmed with a longer follow‐up study. Mehdizadeh et al, 12 in a systematic review and meta‐analysis published in 2015, showed a recurrence rate of 13% (95% CI 5.0–22.0) for wide excisions and 22% (95% CI 10.0–37.0) for limited excisions. Fertitta et al 43 updated this analysis from 2015 to 2019 and found a recurrence rate between 12.4% and 54.2%.
For severe cases, NPWT has been developed and is commonly used in the management of open and contaminated wounds nowadays. 48 NPWT in HS has been used more frequently in severe perineal cases. 21 , 39 , 40 , 41 It has been suggested that this system improves wound healing by increasing blood flow and formation of granulation tissue, reducing the bacterial load and thereby reducing the size and complexity of the wound. 39 , 48 , 49 It should be noted that in this series, the patients benefited from an average of 18.5 ± 9 days of healing by NPWT. In two cases, the TPN healing method was stopped earlier than expected (before the 15th day) because of pain during dressings. This is quite frequent with this device in spite of adapted analgesics.
In our study, the satisfaction score seems high, with an average score of 20.5 points (out of 25 points). We deliberately chose to create a satisfaction questionnaire for our patients, because we did not find an appropriate questionnaire in the literature. The ‘DLQI’ is a tool often used to assess the quality of life of many dermatologic diseases, but it was not routinely given pre‐operatively to our patients. We admit that our questionnaire, which is very simple and non‐specific, has its own limitations and is not as standardised as the DLQI. However, it allows us to see that the NPWT was well‐perceived by our patients. The satisfaction study suggests that patients were completely or partially satisfied with the surgery because 75% them recommend the surgery to other patients with HS.
4.1. Limitations
The limitations of our study are its lack of power (because it is a single‐centre retrospective study), small sample size and lack of an arm to compare the results to the use of healing by secondary intention without NPWT. Therefore, this study aims to be a preliminary work to further studies.
5. CONCLUSION
Axillary HS is a difficult disease to manage. Several reconstructive modalities after wide axillary excision have been described, but none have shown their superiority. Our study allows to show despite the small number of patient that the use of NPWT for axillary wound management after wide excision in HS is not only safe and simple but also has a shorter operative time and hospital stay, with an acceptable complication rate and lower recurrence rate.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
INFORMED CONSENT
The patients in this manuscript have given written informed consent to publication of their case details.
Supporting information
Table S4. Summary of articles on surgical interventions in patients with axillary hydratenitis suppuritiva published since 2010.
Ezanno A‐C, Fougerousse A‐C, Guillem P, GEM Resoverneuil . The role of negative‐pressure wound therapy in the management of axillary hidradenitis suppurativa. Int Wound J. 2022;19(4):802‐810. doi: 10.1111/iwj.13678
GEM Resoverneuil: French Multicentric Study Group.
DATA AVAILABILITY STATEMENT
Data available on request due to privacy/ethical restrictions.
REFERENCES
- 1. Revuz JE, Canoui‐Poitrine F, Wolkenstein P, et al. Prevalence and factors associated with hidradenitis suppurativa: results from two case‐control studies. J Am Acad Dermatol. 2008;59(4):596‐601. [DOI] [PubMed] [Google Scholar]
- 2. Jemec GBE. Clinical practice. Hidradenitis suppurativa. N Engl J Med. 2012;366(2):158‐164. [DOI] [PubMed] [Google Scholar]
- 3. Benhadou F, Villani AP, Guillem P. Which factors determine affected sites in hidradenitis suppurativa? Dermatology. 2020;236(1):15‐20. [DOI] [PubMed] [Google Scholar]
- 4. Sartorius K, Emtestam L, Jemec GBE, Lapins J. Objective scoring of hidradenitis suppurativa reflecting the role of tobacco smoking and obesity. Br J Dermatol. 2009;161(4):831‐839. [DOI] [PubMed] [Google Scholar]
- 5. König A, Lehmann C, Rompel R, Happle R. Cigarette smoking as a triggering factor of hidradenitis suppurativa. Dermatology. 1999;198(3):261‐264. [DOI] [PubMed] [Google Scholar]
- 6. Hurley H. Dermatological Surgery: Principles and Practice. New York, NY: Marcel Dekker; 1989. [Google Scholar]
- 7. Jemec GBE, Okun MM, Forman SB, et al. Adalimumab medium‐term dosing strategy in moderate‐to‐severe hidradenitis suppurativa: integrated results from the phase 3, randomized, placebo‐controlled PIONEER trials. Br J Dermatol. 2019;181(5):967‐975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zouboulis CC, Desai N, Emtestam L, et al. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatol Venereol. 2015;29(4):619‐644. [DOI] [PubMed] [Google Scholar]
- 9. Gulliver W, Zouboulis CC, Prens E, Jemec GBE, Tzellos T. Evidence‐based approach to the treatment of hidradenitis suppurativa/acne inversa, based on the European guidelines for hidradenitis suppurativa. Rev Endocr Metab Disord. 2016;17(3):343‐351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ingram JR, Collier F, Brown D, et al. British Association of Dermatologists guidelines for the management of hidradenitis suppurativa (acne inversa) 2018. Br J Dermatol. 2019;180(5):1009‐1017. [DOI] [PubMed] [Google Scholar]
- 11. Danby FW, Hazen PG, Boer J. New and traditional surgical approaches to hidradenitis suppurativa. J Am Acad Dermatol. 2015;73(5 Suppl 1):S62–S65. [DOI] [PubMed] [Google Scholar]
- 12. Mehdizadeh A, Hazen PG, Bechara FG, et al. Recurrence of hidradenitis suppurativa after surgical management: a systematic review and meta‐analysis. J Am Acad Dermatol. 2015;73(5 Suppl 1):S70–S77. [DOI] [PubMed] [Google Scholar]
- 13. van Hattem S, Spoo JR, Horváth B, Jonkman MF, Leeman FWJ. Surgical treatment of sinuses by deroofing in hidradenitis suppurativa. Dermatol Surg. 2012;38(3):494‐497. [DOI] [PubMed] [Google Scholar]
- 14. Parrado R, Cadena M, Vergara A, Cadena D, Chalela JG. The role of negative pressure wound therapy in the management of hidradenitis suppurativa: a case report and literature review. Int Wound J. 2017;14(1):35‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chuang CJ, Lee CH, Chen TM, Wang HJ, Chen SG. Use of a versatile transpositional flap in the surgical treatment of axillary hidradenitis suppurativa. J Formos Med Assoc. 2004;103(8):644‐647. [PubMed] [Google Scholar]
- 16. Nail‐Barthelemy R, Stroumza N, Qassemyar Q, Delage M, Nassif A, Atlan M. Evaluation of the mobility of the shoulder and quality of life after perforator flaps for recalcitrant axillary hidradenitis. Ann Chir Plast Esthet. 2019;64(1):68‐77. [DOI] [PubMed] [Google Scholar]
- 17. Afsharfard A, Khodaparast MB, Zarrintan S, Yavari N. Comparison of split thickness skin grafts and flaps in bilateral chronic axillary Hidradenitis Suppurativa. World J Plast Surg. 2020;9(1):55‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Humphries LS, Kueberuwa E, Beederman M, Gottlieb LJ. Wide excision and healing by secondary intent for the surgical treatment of hidradenitis suppurativa: a single‐center experience. J Plast Reconstr Aesthetic Surg. 2016;69(4):554‐566. [DOI] [PubMed] [Google Scholar]
- 19. Morgan WP, Harding KG, Hughes LE. A comparison of skin grafting and healing by granulation, following axillary excision for hidradenitis suppurativa. Ann R Coll Surg Engl. 1983;65(4):235‐236. [PMC free article] [PubMed] [Google Scholar]
- 20. Ruan QZ, Chen AD, Singhal D, Lee BT, Fukudome EY. Surgical management of hidradenitis suppurativa: procedural trends and risk factors. J Surg Res. 2018;229:200‐207. [DOI] [PubMed] [Google Scholar]
- 21. Chen E, Friedman HI. Management of regional hidradenitis suppurativa with vacuum‐assisted closure and split thickness skin grafts. Ann Plast Surg. 2011;67(4):397‐401. [DOI] [PubMed] [Google Scholar]
- 22. Ge S, Orbay H, Silverman RP, Rasko YM. Negative pressure wound therapy with instillation and dwell time in the surgical management of severe hidradenitis suppurativa. Cureus. 2018;10(9):e3319. doi: 10.7759/cureus.3319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Alharbi M, Perignon D, Assaf N, Qassemyar Q, Elsamad Y, Sinna R. Application of the inner arm perforator flap in the management of axillary hidradenitis suppurativa. Ann Chir Plast Esthet. 2014;59(1):29‐34. [DOI] [PubMed] [Google Scholar]
- 24. Bieniek A, Matusiak Ł, Chlebicka I, Szepietowski JC. Secondary intention healing in skin surgery: our own experience and expanded indications in hidradenitis suppurativa, rhinophyma and non‐melanoma skin cancers. J Eur Acad Dermatol Venereol. 2013;27(8):1015‐1021. [DOI] [PubMed] [Google Scholar]
- 25. Marchesi A, Marcelli S, Zingaretti N, Parodi PC, Vaienti L. Pedicled thoracodorsal artery perforator and muscle‐sparing latissimus dorsi flaps in the axillary reconstruction after hidradenitis suppurativa excision: functional and aesthetic issues. Ann Plast Surg. 2018;81(6):694‐701. [DOI] [PubMed] [Google Scholar]
- 26. Pearce FB, Richardson KA. Negative pressure wound therapy, staged excision and definitive closure with split‐thickness skin graft for axillary hidradenitis suppurativa: a retrospective study. J Wound Care. 2017;26(Supp 1):S36–S42. [DOI] [PubMed] [Google Scholar]
- 27. Gonzaga TA, Endorf FW, Mohr WJ, Ahrenholz DH. Novel surgical approach for axillary hidradenitis suppurativa using a bilayer dermal regeneration template: a retrospective case study. J Burn Care Res. 2013;34(1):51‐57. doi: 10.1097/BCR.0b013e31826a7be7 [DOI] [PubMed] [Google Scholar]
- 28. Wu Y, Ngaage LM, Ge S, Rada EM, Silverman RP, Rasko YM. Reconstruction for axillary hidradenitis suppurativa using one‐stage local tissue rearrangement: a retrospective analysis of 53 cases. Int Wound J. 2020;17(3):701‐707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jandali S, Mirzabeigi MN, Fosnot J, Low DW. Thoracodorsal artery perforator flaps and muscle‐sparing latissimus dorsi myocutaneous flaps for the treatment of axillary hidradenitis. Ann Plast Surg. 2012;69(4):371‐375. [DOI] [PubMed] [Google Scholar]
- 30. Elgohary H, Nawar AM, Zidan A, Shoulah AA, Younes MT, Hamed AM. Outcome of pedicled thoracodorsal artery perforator flap in the surgical treatment of stage II and III hidradenitis suppurativa of axilla. Ann Plast Surg. 2018;81(6):688‐693. [DOI] [PubMed] [Google Scholar]
- 31. Elboraey MA, Alali AB, Alkandari QA. Immediate and delayed reconstruction after excision of axillary hidradenitis suppurativa using a propeller flap. Plast Reconstr Surg. 2019;7(8):e2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Busnardo FF, Coltro PS, Olivan MV, Busnardo APV, Ferreira MC. The thoracodorsal artery perforator flap in the treatment of axillary hidradenitis suppurativa: effect on preservation of arm abduction. Plast Reconstr Surg. 2011;128(4):949‐953. [DOI] [PubMed] [Google Scholar]
- 33. Wormald JCR, Balzano A, Clibbon JJ, Figus A. Surgical treatment of severe hidradenitis suppurativa of the axilla: thoracodorsal artery perforator (TDAP) flap versus split skin graft. J Plast Reconstr Aesthetic Surg. 2014;67(8):1118‐1124. [DOI] [PubMed] [Google Scholar]
- 34. Ortiz CL, Castillo VL, Pilarte FS, Barraguer EL. Experience using the thoracodorsal artery perforator flap in axillary hidradentitis suppurativa cases. Aesthetic Plast Surg. 2010;34(6):785‐792. [DOI] [PubMed] [Google Scholar]
- 35. Ching DL, Mughal M, Papas A, Soldin M. Axillary reconstruction for hidradenitis suppurativa with an inner‐arm transposition flap creating a brachioplasty effect. Arch Plast Surg. 2017;44(3):228‐233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hallock GG. Island thoracodorsal artery perforator‐based V‐Y advancement flap after radical excision of axillary hidradenitis. Ann Plast Surg. 2013;71(4):390‐393. [DOI] [PubMed] [Google Scholar]
- 37. Varkarakis G, Daniels J, Coker K, Oswald T, Akdemir O, Lineaweaver WC. Treatment of axillary hidradenitis with transposition flaps: a 6‐year experience. Ann Plast Surg. 2010;64(5):592‐594. [DOI] [PubMed] [Google Scholar]
- 38. Calibre C, Bouhanna A, Salmin J‐P, Bodin F, Benaïssa‐Beck M, Bruant‐Rodier C. Axillary hidradenitis suppurativa: a single‐stage surgical treatment. Ann Chir Plast Esthet. 2013;58(6):670‐675. [DOI] [PubMed] [Google Scholar]
- 39. Chen YE, Gerstle T, Verma K, Treiser MD, Kimball AB, Orgill DP. Management of hidradenitis suppurativa wounds with an internal vacuum‐assisted closure device. Plast Reconstr Surg. 2014;133(3):370e–7e. [DOI] [PubMed] [Google Scholar]
- 40. Hynes PJ, Earley MJ, Lawlor D. Split‐thickness skin grafts and negative‐pressure dressings in the treatment of axillary hidradenitis suppurativa. Br J Plast Surg. 2002;55(6):507‐509. [DOI] [PubMed] [Google Scholar]
- 41. Elwood ET, Bolitho DG. Negative‐pressure dressings in the treatment of hidradenitis suppurativa. Ann Plast Surg. 2001;46(1):49‐51. [DOI] [PubMed] [Google Scholar]
- 42. Deckers IE, Dahi Y, van der Zee HH, Prens EP. Hidradenitis suppurativa treated with wide excision and second intention healing: a meaningful local cure rate after 253 procedures. J Eur Acad Dermatol Venereol. 2018;32(3):459‐462. [DOI] [PubMed] [Google Scholar]
- 43. Fertitta L, Hotz C, Wolkenstein P, et al. Efficacy and satisfaction of surgical treatment for hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2020;34(4):839‐845. [DOI] [PubMed] [Google Scholar]
- 44. Yamashita Y, Hashimoto I, Matsuo S, Abe Y, Ishida S, Nakanishi H. Two‐stage surgery for hidradenitis suppurativa: staged artificial dermis and skin grafting. Dermatol Surg. 2014;40(2):110‐115. [DOI] [PubMed] [Google Scholar]
- 45. Büyükaşik O, Hasdemir AO, Kahramansoy N, Çöl C, Erkol H. Surgical approach to extensive hidradenitis suppurativa. Dermatol Surg. 2011;37(6):835‐842. [DOI] [PubMed] [Google Scholar]
- 46. Mandal A, Watson J. Experience with different treatment modules in hidradenitis suppuritiva: a study of 106 cases. Surg J R Coll Surg. 2005;3(1):23‐26. [DOI] [PubMed] [Google Scholar]
- 47. Jemec GB. Effect of localized surgical excisions in hidradenitis suppurativa. J Am Acad Dermatol. 1988;18(5 Pt 1):1103‐1107. [DOI] [PubMed] [Google Scholar]
- 48. Argenta LC, Morykwas MJ. Vacuum‐assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg. 1997;38(6):563‐576. discussion 577. [PubMed] [Google Scholar]
- 49. Mouës CM, Heule F, Hovius SER. A review of topical negative pressure therapy in wound healing: sufficient evidence? Am J Surg. 2011;201(4):544‐556. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S4. Summary of articles on surgical interventions in patients with axillary hydratenitis suppuritiva published since 2010.
Data Availability Statement
Data available on request due to privacy/ethical restrictions.


