Abstract
Since the discovery of the chloroquine (CQ) resistance reversal properties of several different, structurally unrelated classes of compounds, including antidepressants, the way is again open to employ the aminoquinoline drugs to combat malaria effectively. In this study, CQ sensitivity was restored to varying extents in vitro in the CQ-resistant Plasmodium falciparum strain RSA11 by using the antidepressants amitriptyline, citalopram, oxaprotiline, and nomifensine. The 50% inhibitory concentrations (IC50) of CQ were reduced from 360 to as low as 11 nM when antidepressants were present. These particular antidepressants are highly specific for blocking the ATP-binding cassette transport protein-mediated reuptake of different neurotransmitters at the synaptic level. This study was aimed at determining the extent to which the neurotransmitter reuptake-blocking properties of these antidepressants play a role in the reversal process. None of the compounds or CQ-antidepressant combinations tested had innate antimalarial activity. No chemosensitizer or combination showed an increased CQ accumulation or significant shift in the IC50 in the CQ-sensitive clone D10. Of the compounds tested, citalopram, a highly specific serotonin reuptake blocker, produced the largest shift observed in the IC50 for the resistant isolate RSA11. No particular class of antidepressant was found to be better than any other at restoring CQ sensitivity. We conclude that the resistance-reversing properties of these compounds do not correlate with their activities as reuptake blockers.
During a single year, 300 million people become infected with malaria, which is responsible for approximately 2 million deaths annually. Parasite resistance to chloroquine (CQ)—the most commonly used antimalarial—has been reported in most regions in which malaria is endemic worldwide. The exact mechanisms of this resistance are still unclear (5).
The ability of certain drugs to reverse resistance was first discovered in 1982 in multidrug-resistant tumor cell lines (17). In 1987, CQ resistance was successfully reversed in Plasmodium falciparum using verapamil (VPL), a calcium channel blocker (12). CQ resistance is typically characterized by decreased CQ accumulation and an increase in the 50% inhibitory concentration (IC50) of CQ. Reversing resistance involves lowering the CQ IC50 by using a CQ-chemosensitizer combination. Since 1987, several structurally unrelated compounds have also demonstrated an ability to reverse CQ resistance in vitro (2, 8, 15). Examples include other calcium channel blockers (nicardipine), antidepressants (8), antihistamines (16), and antipsychotics (1). While these compounds bear little structural similarity to one another or to verapamil, some of them have since been shown to increase the uptake of CQ into resistant parasites (3).
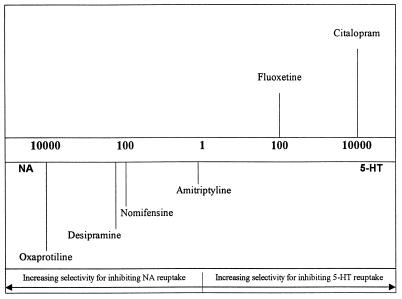
In addition to the various antihistamines, calcium channel blockers, and antipsychotics, antidepressants have featured prominently in resistance reversal. Desipramine, a tricyclic antidepressant, has been used in vitro in P. falciparum and in vivo for P. falciparum infection in Panamanian Aotus monkeys (3). However, trials with desipramine in humans have not been successful (21). It is believed that because the drug is highly plasma bound, a standard therapeutic dose does not reach the required level in the blood to allow for reversal to occur in humans. Another antidepressant, fluoxetine, has also been utilized in vitro. Antidepressants are believed to relieve depression through their ability to increase concentrations of several different neurotransmitters in the synaptic cleft (10). This is believed to occur by preventing the reuptake of the neurotransmitters, a process which is mediated via ATP-binding cassette (ABC) transport proteins on the synaptic membrane (6). The P. falciparum P-glycoprotein homologue (Pgh1) is also an ABC protein (22). Fluoxetine shows high specificity for blocking the reuptake of the neurotransmitter serotonin (5-HT), while desipramine shows preference for noradrenaline (NA) reuptake systems (Fig. 1). A previously published study indicated that carbamazepine, a compound that, like desipramine, contains a tricyclic core, was unable to reverse CQ resistance in vitro. Unlike desipramine and fluoxetine, however, carbamazepine has only a very weak ability to block neurotransmitter reuptake (6).
FIG. 1.
Relative specificities of antidepressants for blocking different neurotransmitter reuptake systems. The scale represents the fold increase in specificity of each antidepressant for inhibiting either 5-HT or NA reuptake. Reprinted from B. Leonard, Fundamentals of Psychopharmacology (10), with permission of the publisher.
Our study was devised in order to examine the differential effects of specific reuptake-blocking properties on the CQ resistance reversal phenomenon. The antidepressants we tested were highly specific for blocking different neurotransmitter reuptake systems (Fig. 1). Amitriptyline (AMT) blocks reuptake of both 5-HT and NA to approximately equal extents (10). Citalopram (CTL), like fluoxetine, is a specific 5-HT reuptake inhibitor, but it is some 100-fold more selective than fluoxetine in blocking the 5-HT reuptake system relative to the NA reuptake system. Similarly, oxaprotiline (OXP), the NA system blocker we used, is 100-fold more specific than desipramine for NA reuptake systems. Our dopamine (DA) reuptake blocker, nomifensine (NOM), is a highly specific DA reuptake blocker with moderate NA reuptake-blocking activity. The aim of this study was to determine whether the specificity of each antidepressant in terms of its particular reuptake-blocking effect is related to its ability to increase CQ accumulation, as well to increase CQ sensitivity when resistant parasites are incubated in the presence of antidepressants.
MATERIALS AND METHODS
Parasite culture.
Two culture-adapted isolates of P. falciparum were used in this study. Clone D10 (CQ sensitive; IC50, ca. 27 nM) was kindly donated by Alan Cowman of the Walter and Eliza Hall Institute, Melbourne, Australia. The South African strain RSA11 (CQ resistant; IC50, ca. 360 nM) was purchased from the Medical Research Council, Durban, South Africa. Cultures were maintained according to the methods of Trager and Jensen (19) in A+ human serum and O+ human erythrocytes. Growth medium was supplemented with gentamicin (1 mg/ml). Parasites were synchronized at the trophozoite stage using the protocol of Lambros and Vanderberg (9) with 5% (wt/vol) d-sorbitol (Sigma).
Chemicals.
Stock solutions of CQ diphosphate (Sigma), nomifensine hydrochloride (Sigma), amitriptyline hydrochloride (Sigma), verapamil hydrochloride (Sigma), and citalopram hydrobromide (donated by John Hyttel of H. Lundbeck and Sons, Valby, Denmark) were made in Milli-Q water. Oxaprotiline hydrochloride (a gift from A. P. Aucamp of Novartis S.A.) was dissolved in 100% dimethyl sulfoxide (DMSO) to 30 mM and diluted to the desired concentrations in Milli-Q water. Constituents for growth medium, as well as d-sorbitol, were obtained from Sigma. Phosphate-buffered saline tablets were purchased from Oxoid.
In vitro drug activity measurement and CQ resistance reversal.
The parasite lactate dehydrogenase (pLDH) assay was used to measure drug activity in vitro (11). After the intrinsic antimalarial activity of each drug had been determined, dose-response experiments were performed to test for CQ resistance reversal. CQ was serially diluted twofold in a microtiter plate to yield a concentration range from 1,000 to about 1 ng/ml. A fixed dose of the test drug was added to the CQ in each well. After a 48-h incubation, plates were developed as described by Makler et al. (11). The shift in the CQ IC50 was determined via regression analysis using Prism, version 2.01 (GraphPad Software, Inc., San Diego, Calif.).
Tritiated CQ accumulation.
Tritiated CQ uptake was carried out using parasite suspensions of 5% parasitemia and 1% hematocrit. Parasites were incubated at 37°C with no chemosensitizer (neutral control), 5 μM verapamil (positive control), or the desired concentration of the test drug for 15 min. Unparasitized erythrocytes (1% hematocrit; erythrocyte control) were also incubated at 37°C for 15 min. Both unparasitized erythrocytes and parasitized erythrocytes were then incubated with 1 nM [3H]CQ (18.8 Ci/mmol; Amersham) at 37°C for 1 h. Cultures were shaken regularly to maintain an even suspension. After incubation, cells were pelleted via centrifugation and washed twice in phosphate-buffered saline. The tip containing the pellet was removed, and accumulation was measured by liquid scintillation counting (Packard Canberra) using Beckman Quiksafe A scintillation fluid. Disintegrations per minute obtained were corrected for erythrocyte uptake. Mean values were calculated, and statistical analysis (Student's two-tailed t test) was carried out using Prism, version 2.01.
RESULTS
Antimalarial activity.
Table 1 shows the inherent antimalarial activities of the drugs tested. With the exception of amitriptyline, no significant differences were observed between the two strains. All the test IC50s were far greater than that of CQ. Each IC50 was above 2 μM, and thus each of the compounds was less potent than CQ against Plasmodium.
TABLE 1.
Comparison of IC50s for D10 and RSA11a
| Drug | IC50 (nM) for strain:
|
|
|---|---|---|
| D10 | RSA11 | |
| CQ | 14.01 ± 2.65 | 380.04 ± 4.85 |
| Amitriptyline | 5,578 ± 1,964 | 12,070 ± 1,238 |
| Citalopram | 2,222 ± 1,380 | 3,662 ± 1,428 |
| Nomifensine | 4,701 ± 1,422 | 5,136 ± 1,560 |
| Oxaprotiline | 2,448 ± 1,347 | 3,392 ± 1,046 |
n = 3.
CQ uptake.
Without the addition of neurotransmitter reuptake blockers, CQ accumulated to levels of about 245 ± 7 fmol/106 parasites in P. falciparum RSA11 and 1,573 ± 147 fmol/106 parasites in P. falciparum D10 (data not shown). This shows a sixfold difference between the sensitive and resistant isolates and is consistent with previous findings. Figure 2 shows the effect of each drug on the accumulation of tritiated CQ. Each drug was able to increase CQ uptake in RSA11 across a broad concentration range. As expected, no significant alteration in CQ uptake was observed in the CQ-sensitive clone D10 (data not shown).
FIG. 2.
Effects of antidepressants on the uptake of [3H]CQ in the resistant P. falciparum strain RSA11.
For the different antidepressants, the maximum increase in uptake observed in RSA11 was approximately 4.2- to 4.5-fold (1,060 to 1,175 fmol/106 parasites) over the parasite control. The individual antidepressant maxima were not significantly different from one another or 5 μM verapamil. From these data we determined the two concentrations that were used for the reversal experiments. The drug concentrations chosen had to be nontoxic to Plasmodium. For RSA11, parasites incubated with both tritiated CQ and any one of the antidepressants at either concentration had to exhibit a one- to twofold increase in CQ uptake over that for parasites incubated with CQ alone. These concentrations are shown in Table 2, footnote a. Without exception, the lower drug concentration was exactly half of the higher. Both concentrations are below the IC50 for each drug.
TABLE 2.
Reversal of resistance using CQ combined with a single antidepressant or with 1 μM verapamil in both CQ-sensitive (D10) and CQ-resistant (RSA11) parasites
| Druga | D10
|
RSA11
|
||
|---|---|---|---|---|
| IC50 (nM)b | RMIc | IC50 (nM)b | RMIc | |
| CQ only | 17.89 ± 1.35 | 360.7 ± 2.45 | ||
| Verapamil (1 μM) | 18.41 ± 1.02 | 1.02 | 18.01 ± 2.11 | 0.050 |
| Amitriptyline[high] | 9.28 ± 2.13 | 0.51 | 16.22 ± 7.36 | 0.045 |
| Amitriptyline[low] | 31.40 ± 2.11 | 1.76 | 43.65 ± 10.09 | 0.121 |
| Citalopram[high] | 11.23 ± 1.48 | 0.63 | 11.29 ± 2.54 | 0.031 |
| Citalopram[low] | 17.52 ± 1.46 | 0.98 | 17.3 ± 5.20 | 0.048 |
| Nomifensine[high] | 21.08 ± 1.34 | 1.19 | 21.56 ± 8.08 | 0.060 |
| Nomifensine[low] | 31.57 ± 1.34 | 1.76 | 87.52 ± 29.56 | 0.243 |
| Oxaprotiline[high] | 28.57 ± 1.43 | 1.60 | 13.00 ± 1.45 | 0.036 |
| Oxaprotiline[low] | 31.44 ± 1.19 | 1.76 | 38.66 ± 2.07 | 0.107 |
High and low doses, respectively, were as follows: for amitriptyline, 509.7 and 254.9 nM; for citalopram, 1,234 and 616.8 nM; for nomifensine, 1,100 and 550 nM; and for oxaprotiline, 728 and 364 nM.
Data are means from three separate experiments performed in duplicate ± standard errors of the means.
Calculated as [IC50 of CQ]/[IC50 of (CQ + antidepressant)].
CQ potentiation.
Table 2 shows the effect of each drug administered at each of the chosen concentrations with CQ. Results are expressed in terms of the shift of the IC50 and the response modification index (RMI) as described by Gerena et al. (8). The RMI represents the ratio of the IC50 of the combination to the IC50 of CQ alone. Each drug was able to reverse CQ resistance in RSA11; as expected, no reversal effect was observed in D10.
In RSA11, the shift in the IC50 appears to be most pronounced in the case of citalopram. The RMI values for the different concentrations of citalopram were not found to be significantly different from each other (P < 0.05) or from that for 1 μM verapamil; nor were those for the concentrations of amitriptyline or oxaprotiline. The RMI of CQ combined with the lower nomifensine dose (Nomifensine[low]), however, was significantly different from that of CQ with the higher nomifensine dose (Nomifensine[high]), as well as from that of CQ with 5 μM verapamil.
DISCUSSION
Each of the antidepressants tested has the ability to increase the accumulation of tritiated CQ into parasitized erythrocytes across a 100-fold concentration range (Fig. 2). These concentrations are nonlethal to Plasmodium. Other chemosensitizers such as verapamil have demonstrated similar increases (12). The maximum increase achieved with each antidepressant drug was not significantly different from that achieved by any other antidepressant or 5 μM verapamil itself (P > 0.05).
It has been shown that sensitive isolates of P. falciparum accumulate more CQ than their resistant counterparts (4). No increase in uptake is observed at comparable chemosensitizer concentrations in D10. Our data are consistent with these previous findings. We observed a sixfold difference in CQ uptake between RSA11 and D10 parasites incubated without antidepressants (data not shown). The chosen antidepressant concentrations were able to increase uptake one- to twofold over that for the parasite control in the resistant parasites. Although this represents a significant increase (maximum increases achieved with each antidepressant were between 4.2- and 4.5-fold), the increased accumulation is still significantly lower than the accumulation of CQ in the sensitive isolate. It is clear that this sensitivity modulation is related to increased CQ uptake; however, full restoration of CQ uptake is not necessary for complete CQ sensitization. It does suggest that the mechanism(s) for both CQ resistance and resistance reversal may be related in part to the transport of CQ into the parasite system.
While most chemosensitizers bear little structural similarity to one another, several essential components are believed to play a role in resistance reversal. These include the presence of planar (benzene) groups, a secondary or tertiary nitrogen, and a cationic charge (8). Lipophilicity is also considered important. Each of the four drugs tested meets the structural requirements necessary for resistance modulation (Fig. 3), and amitriptyline has also been shown to accumulate in acidic compartments (7); the food vacuole is one such compartment. Neurotransmitter reuptake at the synaptic level is governed by proteins belonging to the ABC superfamily of transport proteins, as do both Pgh1 in Plasmodium and the P glycoprotein responsible for multidrug resistance observed in tumor cells (6, 22). Verapamil has shown in vitro resistance-modifying capabilities both in multidrug-resistant tumor cells and in Plasmodium (12). Although its resistance-reversing mechanism in Plasmodium remains undefined, it is believed to exert its effect through the P glycoprotein (Pgp) in tumor cells (16). Desipramine, itself a tricyclic antidepressant and an NA reuptake inhibitor (10), has also shown resistance-modifying properties in Plasmodium (3), and amitriptyline has reversed multidrug resistance in tumor cells (20). These factors probably account for the increased CQ accumulation that we observed in RSA11.
FIG. 3.
Structures of the antidepressants tested.
From Table 2, it can be seen that each of the neurotransmitter reuptake blockers is able to alter CQ resistance in P. falciparum RSA11. This reversal resulted in a level of sensitivity (14 to 20 nM) to CQ similar to that seen in D10, and the resistance reversal achieved is comparable to that achieved using 1 μM verapamil (P < 0.05). The maximum reversal achieved with either concentration of any drug used was not significantly different from that with any other drug; hence, the data do not implicate any specific neurotransmitter reuptake-blocking activity in antidepressant-mediated CQ resistance modification. Although modest changes in the RMI are observed with D10, all the modified IC50s are between 9 and 32 nM, and thus D10 is clearly still sensitive to CQ. This indicates that CQ resistance reversal is occurring in RSA11 as opposed to a synergistic effect between the antidepressant and CQ.
It is worth noting that both doses of amitriptyline which reversed resistance are comparable to steady-state serum levels reported in patients undergoing treatment with this antidepressant (13). These data certainly do not support immediate clinical implementation of CQ with amitriptyline as a combination therapy. In vivo studies should be considered to determine whether this would be a viable option for future use, because most other chemosensitizers would need to be administered at levels which would be toxic to patients. Desipramine showed similar promise at low levels in vitro but failed in vivo (21), and both promethazine and chlorpheniramine, which have demonstrated resistance reversal ability in vitro, are undergoing testing in volunteers (14, 18).
ACKNOWLEDGMENTS
We extend grateful thanks to the University of Cape Town Research Committee and the Medical Research Council of South Africa for financial assistance received for this study.
We also thank John Hyttel of H. H. Lundbeck for the donation of citalopram hydrobromide and A. P. Aucamp of Novartis S.A. for the oxaprotiline hydrochloride. We especially thank Janet Rawlings for technical assistance.
REFERENCES
- 1.Basco L, Le Bras J. In vitro activities of chloroquine in combination with chlorpromazine or prochlorperazine against isolates of Plasmodium falciparum. Antimicrob Agents Chemother. 1992;36:209–213. doi: 10.1128/aac.36.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basco L K, Le Bras J. In vitro reversal of chloroquine resistance with chlorpheniramine against African isolates of Plasmodium falciparum. Jpn J Med Sci Biol. 1994;47:59–63. doi: 10.7883/yoken1952.47.59. [DOI] [PubMed] [Google Scholar]
- 3.Bitonti A J, Sjoerdsma A, McCann P P, Kyle D E, Oduola A M, Rossan R N, Milhous W K, Davidson D E J. Reversal of chloroquine resistance in malaria parasite Plasmodium falciparum by desipramine. Science. 1988;242:1301–1303. doi: 10.1126/science.3057629. [DOI] [PubMed] [Google Scholar]
- 4.Bray P G, Howells R E, Ritchie G Y, Ward S A. Rapid chloroquine efflux phenotype in both chloroquine-sensitive and chloroquine-resistant Plasmodium falciparum: a correlation of chloroquine sensitivity with energy-dependent drug accumulation. Biochem Pharmacol. 1992;44:1317–1324. doi: 10.1016/0006-2952(92)90532-n. [DOI] [PubMed] [Google Scholar]
- 5.Bray P G, Janneh O, Raynes K J, Mungthin M, Ginsburg H, Ward S A. Cellular uptake of chloroquine is dependent on binding to ferriprotoporphyrin IX and is independent of NHE activity in Plasmodium falciparum. J Cell Biol. 1999;145:363–376. doi: 10.1083/jcb.145.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coutaux A F, Mooney J J, Wirth D F. Neuronal monoamine reuptake inhibitors enhance in vitro susceptibility to chloroquine in resistant Plasmodium falciparum. Antimicrob Agents Chemother. 1994;38:1419–1421. doi: 10.1128/aac.38.6.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daniel W A, Wójcikowski J. Contribution of lysosomal trapping to the total tissue uptake of psychotropic drugs. Pharmacol Toxicol. 1997;80:62–68. doi: 10.1111/j.1600-0773.1997.tb00285.x. [DOI] [PubMed] [Google Scholar]
- 8.Gerena L, Bass G T S, Kyle D E, Oduola A M, Milhous W K, Martin R K. Fluoxetine hydrochloride enhances in vitro susceptibility to chloroquine in resistant Plasmodium falciparum. Antimicrob Agents Chemother. 1992;36:2761–2765. doi: 10.1128/aac.36.12.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lambros C, Vanderberg J P. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979;65:418–420. [PubMed] [Google Scholar]
- 10.Leonard B. Fundamentals of psychopharmacology. Chichester, Great Britain: John Wiley and Sons; 1992. pp. 66–68. [Google Scholar]
- 11.Makler M, Ries J M, Williams J A, Bancroft J E, Piper R C, Gibbins B L, Hinrichs D. Measurement of the lactate dehydrogenase activity of Plasmodium falciparum as an assessment of parasitemia. Am J Trop Med Hyg. 1993;48:205–210. doi: 10.4269/ajtmh.1993.48.205. [DOI] [PubMed] [Google Scholar]
- 12.Martin S K, Oduola A M J, Milhous W K. Reversal of chloroquine resistance in Plasmodium falciparum by verapamil. Science. 1987;235:899–901. doi: 10.1126/science.3544220. [DOI] [PubMed] [Google Scholar]
- 13.Moffat A C, editor. Clarke's identification and isolation of drugs. London, Great Britain: The Pharmaceutical Press; 1986. [Google Scholar]
- 14.Oduola A M J, Sowunmi A, Milhous W K, Brewer T G, Kyle D E, Gerena L, Rossan R N, Salako L A, Schuster B G. In vitro and in vivo reversal of chloroquine resistance in Plasmodium falciparum with promethazine. Am J Trop Med Hyg. 1998;58:625–629. doi: 10.4269/ajtmh.1998.58.625. [DOI] [PubMed] [Google Scholar]
- 15.Peters W, Ekong R, Robinson B L, Warhurst D C, Pan X Q. The chemotherapy of rodent malaria. XLV. Reversal of chloroquine resistance in rodent and human Plasmodium by antihistaminic agents. Ann Trop Med Parasitol. 1990;84:541–551. doi: 10.1080/00034983.1990.11812509. [DOI] [PubMed] [Google Scholar]
- 16.Safa A R. Photoaffinity labeling of the multidrug-resistance-related P-glycoprotein with photoactive analogs of verapamil. Proc Natl Acad Sci USA. 1988;85:7187–7191. doi: 10.1073/pnas.85.19.7187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Slater L M, Murray S L, Wetzel M W, Wisdom R M, DuVall E M. Verapamil restoration of daunorubicin responsiveness in daunorubicin-resistant Ehrlich ascites carcinoma. J Clin Investig. 1982;70:1131–1134. doi: 10.1172/JCI110702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sowunmi A, Fehintola F A, Ogundahunsi O A, Oduola A M. Comparative efficacy of chloroquine plus chlorpheniramine and halofantrine in acute uncomplicated falciparum malaria in Nigerian children. Trans R Soc Trop Med Hyg. 1998;92:441–445. doi: 10.1016/s0035-9203(98)91084-7. [DOI] [PubMed] [Google Scholar]
- 19.Trager W, Jensen J B. Human malaria parasites in continuous culture. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 20.Varga A, Nugel H, Baehr R, Marx U, Hevér A, Nacsa J, Ocsovszky I, Molnar J. Reversal of multidrug resistance by amitriptyline in vitro. Anticancer Res. 1996;16:209–211. [PubMed] [Google Scholar]
- 21.Warsame M, Wernsdorfer W H, Björkman A. Lack of effect of desipramine on the response to chloroquine of patients with chloroquine-resistant falciparum malaria. Trans R Soc Trop Med Hyg. 1992;86:235–236. doi: 10.1016/0035-9203(92)90288-n. [DOI] [PubMed] [Google Scholar]
- 22.Wilson C, Serrano A, Wasley A, Bogenschutz M, Shankar A, Wirth D. Amplification of a gene related to mammalian mdr genes in drug-resistant Plasmodium falciparum. Science. 1989;244:1184–1186. doi: 10.1126/science.2658061. [DOI] [PubMed] [Google Scholar]