Abstract
Transjugular intrahepatic portosystemic shunt (TIPS) is an effective interventional procedure to relieve portal hypertension, which is a main mechanism for the development of complications of liver cirrhosis (LC), such as variceal hemorrhage, ascites, and hepatorenal syndrome. However, the high incidence of adverse events after TIPS implementation limits its application in clinical practice. Esophageal variceal hemorrhage is one of the major indications for TIPS. Recently, preemptively performed TIPS has been recommended, as several studies have shown that TIPS significantly reduced mortality as well as rebleeding or failure to control bleeding in patients who are at high risk of treatment failure for bleeding control with endoscopic variceal ligation and vasoactive drugs. Meanwhile, recurrent ascites is another indication for TIPS with a proven survival benefit. TIPS may also be considered as an effective treatment for other LC complications, usually as an alternative therapy. Although there are concerns about the development of hepatic encephalopathy and hepatic dysfunction after TIPS implementation, careful patient selection using prognostic scores can lead to excellent outcomes. Assessments of cardiac and renal function prior to TIPS may also be considered to improve patient prognosis.
Keywords: Transjugular intrahepatic portosystemic shunt; Liver cirrhosis; Hypertension, Portal; Portal pressure
INTRODUCTION
Complications of decompensated liver cirrhosis (LC) can seriously impair the quality of life and also increase the morbidity and mortality rates of patients. Although liver transplantation (LT) is the best way to reverse the clinical course of patients with decompensated LC, only a limited number of the patients may undergo LT due to a persistent shortage of viable organs. Portal hypertension is the main pathologic mechanism driving the occurrence of LC complications. First-line treatments for such complications, which include sodium restrictions and the use of diuretics for ascites or vasoactive drugs and endoscopic therapy for variceal bleeding, do not aim to alleviate portal hypertension itself but instead manage symptoms; therefore, patients may still suffer from recurrent episodes of these LC complications.
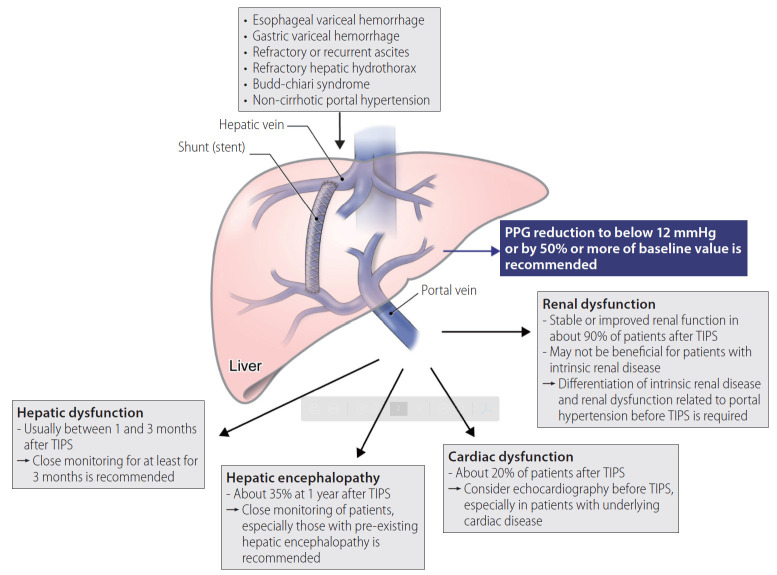
Transjugular intrahepatic portosystemic shunt (TIPS) robustly reduces portal hypertension by establishing a shunt within the liver. The procedure is performed by creating an intrahepatic portosystemic shunt connecting the right or main portal vein to the hepatic vein, aiming to reduce the porto-systemic pressure gradient (PPG) while maintaining adequate liver perfusion (Fig. 1). The most common indications of TIPS are uncontrolled bleeding from the esophageal varix and refractory or recurrent ascites. TIPS can also be considered in patients with gastric variceal hemorrhage, hepatic hydrothorax, Budd-Chiari syndrome (BCS), or noncirrhotic portal hypertension. In addition to the therapeutic purpose of LC complications, TIPS decompresses increased resistance of portal venous blood flow, leading to stabilization of the activated renin–angiotension and sympathetic systems. Moreover, enhanced translocation of the gut microbiome and systemic inflammation in patients with portal hypertension decrease after TIPS implementation [1]. Despite these advantages, however, there are non-negligible adverse events that may arise following TIPS, including hepatic encephalopathy (HE) and hepatic or cardiac dysfunction, which limit the use of TIPS in LC patients.
Figure 1.
Overview of TIPS. The main complications of TIPS and their countermeasures are presented. TIPS, transjugular intrahepatic portosystemic shunt; PPG, porto-systemic pressure gradient.
Until 2000, TIPS was performed using bare metal stents, thus carrying a significant drawback of shunt dysfunction due to stent stenosis occurring in more than 50% of patients within 1 year, mainly by the proliferation of the intima [2,3]. Among the remarkable advances in the TIPS procedure, the development of polytetrafluoroethylene (PTFE)-covered stents was the most outstanding achievement to date. PTFE can suppress the proliferation of intima, thus maintaining stent patency [4]. The rate of primary patency of TIPS was significantly improved to 86% and 80% at 1 and 2 years, respectively, compared to 47% and 19%, respectively, in the setting of TIPS with bare stent placement [4].
TIPS is usually indicated as a rescue therapy in LC patients who have developed complications with no response to first-line treatment, and sometimes as a bridging therapy for LT. However, practical clinicians often hesitate to decide whether to perform TIPS in LC patients due to the adverse effects of TIPS. In this review, we aimed to demonstrate the most suitable timing and candidates for performing TIPS in patients with LC complications. We also summarized the main complications of TIPS, as well as their countermeasures.
PROCEDURE
Since variceal bleeding or ascites usually occurs with a PPG of above 12 mmHg, TIPS is performed to reach a goal of reducing the PPG to below 12 mmHg or by 50% or more of the baseline value [2,5]. However, complications following TIPS, such as HE or hepatic dysfunction, usually occur when the PPG is adjusted to less than 5 to 10 mmHg; therefore, the targeted level is suggested to be higher than 10 mmHg with a narrow therapeutic window [3]. Technically, it is not easy to accurately relocate the target PPG to between 10 to 12 mmHg during the procedure. TIPS is generally performed by full or partial expansion of 8- or 10-mm stents [5,6]. Nowadays, a smaller 8-mm stent has been more commonly used, as it demonstrated similar efficacy to the 10-mm stent but a lower incidence of HE or hepatic dysfunction [7]. In addition, a recent prospective, multicenter study showed that TIPS with 8-mm stents led to significantly higher transplant-free survival rates compared to using 10-mm stents [6].
PATENCY
Although the incidence of shunt dysfunction significantly decreased after using PTFE-covered stents, guidelines suggest performing a doppler ultrasound to examine shunt patency at intervals of 6 or 12 months after the procedure [8,9]. Stenosis of the shunt is mainly caused by intimal proliferation; however, it can rarely result from thrombosis in patients with inadequate coverage of the stent, bile leakage into the stent, or a hypercoagulable condition, such as BCS [5,10,11]. A previous study showed that prophylactic use of antiplatelet or anticoagulation medications for preventing stent thrombosis after TIPS reduced the rate of shunt stenosis, and some centers have adopted the prophylactic use of anticoagulation as a strategy [12,13]. However, evidence is insufficient to encourage the prophylactic use of antiplatelet or anticoagulant drugs in patients with TIPS [14].
INDICATIONS
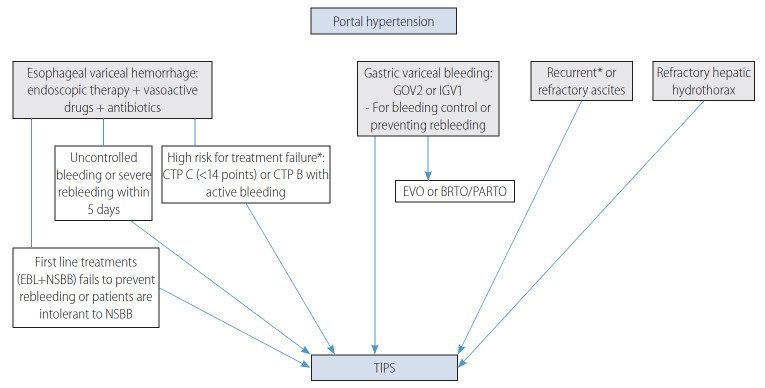
The optimal timing for TIPS in LC complications is schematically shown in Figure 2, and clinical indications for TIPS recommended in each guideline are summarized in Table 1.
Figure 2.
The main indications for TIPS are presented. CTP, Child-Turcotte-Pugh; EBL, endoscopic band ligation; NSBB, nonselective beta-blockers; GOV, gastroesophageal varices; IGV, isolated gastric varices; EVO, endoscopic variceal obliteration; BRTO, balloon occluded retrograde transvenous obliteration; PARTO, plug-assisted retrograde transvenous obliteration; TIPS, transjugular intrahepatic portosystemic shunt. *The survival benefit of TIPS was demonstrated in randomized controlled trials.
Table 1.
Summary of current international guidelines
| AASLD [9,14] | EASL [16] | Baveno VI [17] | KASL [20,88] | |
|---|---|---|---|---|
| Esophageal VH | ||||
| Rescue therapy | Recommended | Strong (1) | Recommended (B) | Weak (2) |
| Secondary prevention* | Recommended | Strong (1) | Recommended (B) | Strong (1) |
| Preemptive therapy† | Recommended | Weak (2) | Strongest (A) | Weak (2) |
| Debatable in patients with CTP B | ||||
| Gastric VH: GOV2 or IGV1 | ||||
| Control of bleeding | Recommended | Strong (1) | Preemptive TIPS for GOV2†, strongest (A) | TIPS or RTO, strong (1)‡ |
| Cf. BRTO; weak (2) | Cf. EVO for IGV (A) and GOV2 (D) | |||
| Secondary prevention | Recommended | Strong (1) | Weakest (D) | Weak (2)§ |
| Cf. BRTO is also TOC | ||||
| Refractory/recurrent ascites | Recommended | Strong (1) | No mention | Weak (2) |
| Refractory/recurrent hepatic hydrothorax | Recommended | Strong (1) | No mention | Weak (2) |
| Hepatorenal syndrome | Insufficient data | Insufficient data in HRS-AKI | No mention | Insufficient data |
| HRS-NAKI, weak (2) |
The recommendation level for each indication is shown in parentheses.
The grade of recommendations ranges from 1 (strong) to 2 (weak) in the guidelines of EASL and KASL, and from A (strongest) to D (weakest) in the BAVENO VI guideline. The AASLD guideline does not report levels of recommendation.
AASLD, American Association for the Study of Liver Disease; EASL, European Association for the Study of the Liver; KASL, Korean Association for the Study of the Liver; VH, variceal hemorrhage; CTP, Child-Turcotte-Pugh; GVO, gastroesophageal varices; BRTO, balloon-occluded retrograde transvenous obliteration; TIPS, transjugular intrahepatic portosystemic shunt; EVO, endoscopic variceal obliteration; IGV, isolated gastric varices; RTO retrograde transvenous obliteration; TOC, treatment of choice; HRS, hepatorenal syndrome; AKI, acute kidney injury; NAKI, non-acute kidney injury.
For secondary prevention of esophageal VH, guidelines suggest TIPS if the first-line treatment (endoscopic band ligation [EBL] + non-selective beta-blockers [NSBB]) fails or if patients are intolerant to NSBB.
Preemptive TIPS (placed within 72 hours after initial endoscopy) is recommended in patients at high risk of treatment failure with endoscopic therapy and vasoactive drugs for esophageal variceal hemorrhage. Patients with CTP class C (<14 points) or those with CTP class B and active bleeding at endoscopy are at high risk of treatment failure.
KASL recommends EVO as the first-line line treatment for gastric VH; strong (1). TIPS or RTO (BRTO or PARTO) can be performed instead of EVO.
KASL recommends EVO or BRTO as well as TIPS for secondary prevention of gastric VH (GOV2 or ICG1); weak (2).
Esophageal VH
Acute VH should be managed with endoscopic treatment within 12 hours of admission, if the patient is hemodynamically stable, together with the administration of antibiotics and vasoactive drugs, such as somatostatin, octreotide, or terlipressin. The implementation of TIPS can be considered for uncontrolled VH or for secondary prevention of esophageal VH. TIPS as a preemptive therapy for patients at high risk of treatment failure with standard treatment (e.g., endoscopic therapy, vasoactive drugs, and antibiotics) is nowadays highly recommended. TIPS is not indicated for primary prevention of esophageal VH.
Preemptive TIPS
Considering the high risk of liver decompensation or death after treatment failure for acute VH, the use of preemptive TIPS (pTIPS) was suggested in terms of early application of effective treatment [15]. The pTIPS refers to performing the procedure within 72 hours of initial endoscopy in patients with acute VH at high risk of treatment failure and poor outcome despite standard treatments consisting of vasoactive drugs, antibiotics, and endoscopic therapy [15]. Three randomized controlled trials (RCTs) and several observational studies have proven the efficacy of pTIPS in reducing rebleeding of the esophageal varix and mortality. Based on these results, recent guidelines recommend performing pTIPS in patients who are at high risk, such as those with Child-Turcotte-Pugh (CTP) C (<14 points) or CTP B with active bleeding after initial pharmacological and endoscopic therapy [9,16,17].
In the first RCT for pTIPS, LC patients who were admitted for acute VH and a hepatic venous pressure gradient (HVPG) greater than 20 mmHg were defined as a high-risk group for treatment failure and randomly allocated to undergo pTIPS (within 24 hours after admission) or not. Preemptive TIPS significantly reduced the treatment failure, in-hospital, and 1-year mortality rates compared to medical treatment. However, interpretation of the results should consider the limitations inherent with using medical treatment different from the current standard therapy and when using bare stents, recently exchanged for PTFE-covered stents [18]. In another European multicenter RCT, using PTFE-covered stents, LC patients with acute VH were enrolled to evaluate the efficacy of pTIPS. Patients with CTP C (10–13 points) or those with CTP B and active bleeding at initial endoscopy after admission were defined as a high-risk group for treatment failure and randomly assigned to pTIPS or standard treatment (vasoactive drugs + endoscopic treatment) groups. The incidence of remaining free from treatment failure within 1 year was significantly higher in the pTIPS group than that in the standard treatment group (97% and 50%, respectively; P<0.001). The 1-year survival rate was also significantly high at 86% in the pTIPS group versus 61% in the standard treatment group (P<0.001). There was no significant difference in the incidence of post-TIPS HE between the two groups [15]. Unlike other RCTs, the most recent RCT enrolled LC patients with CTP B regardless of active bleeding at endoscopy or CTP C (<14 points) and set survival benefit as the primary outcome. In this study, pTIPS independently reduced mortality (adjusted hazard ratio [aHR], 0.44; 95% confidence interval [CI], 0.22–0.88; P=0.02] and treatment failure (aHR, 0.25; 95% CI, 0.12–0.54; P<0.0001) compared to standard treatment. The incidence of post-TIPS HE was similar (35% vs. 36%; P=1.00) between the two groups [19]. Therefore, all three RCTs consistently demonstrated that pTIPS not only reduced variceal rebleeding but also improved survival. There was no difference in the incidence of post-TIPS HE between the pTIPS and standard treatment groups. However, this is not because pTIPS is relatively safe from this complication; the reason is that, if pTIPS is not performed, the increased risk of esophageal VH and combined other complications may precipitate the development of HE. Based on these results, the Baveno VI consensus states that pTIPS should be considered in patients at high risk for treatment failure (CTP C [<14 points] or CTP B with active bleeding) after initial pharmacological and endoscopic therapy [17].
However, some observational studies have demonstrated no survival benefit from pTIPS; therefore, guidelines of the American Association for the Study of Liver Diseases (AASLD), European Association for the Study of the Liver (EASL), and Korean Association for the Study of the Liver all recommend pTIPS, with the caveat that further studies are needed [9,16,20-22]. There are also concerns regarding the definition of high-risk patients, such as whether CTP B patients with active bleeding should be classified into this group [23]. A recent meta-analysis confirmed the efficacy of pTIPS in terms of reducing mortality to 56% compared to standard treatments in patients with CTP C (<14 points) or CTP B with active bleeding, but not in subgroup of patients with a CTP score of 7 points due to their good prognosis [24].
Regarding the timing of pTIPS, the procedure was usually performed within 3 days of index bleeding in previous studies, but the same effects have also been demonstrated when pTIPS was performed within 5 days of index bleeding [25]. However, there was no survival benefit when TIPS was performed for secondary prevention within 6–15 days after index bleeding, although clinical indications were somewhat different [26,27]. Considering that the effect of pTIPS is predominantly to reduce early rebleeding [19], it is recommended to perform pTIPS at the earliest time after VH.
Rescue therapy or secondary prevention
Although RCTs are still lacking, current guidelines recommend TIPS as a rescue therapy for patients who experience persistent bleeding or severe rebleeding despite standard treatment within the first 5 days after admission for acute VH, which occurs in 10 to 15% of the patients [9,16,17].
Rebleeding of esophageal VH is accompanied by a significant mortality rate of up to 33%; thus, nonselective beta-blockers (NSBB) in combination with endoscopic band ligation (EBL) are recommended for secondary prevention in patients who experienced a first episode of VH [9,17]. Performing TIPS in order to prevent rebleeding compared to standard treatments (EBL + NSBB) has been evaluated in previous studies. TIPS with an uncovered stent significantly reduced the rebleeding rate compared to medical treatments, but the incidence of HE was significantly higher in patients who underwent TIPS [28]. A recent RCT with PTFE-covered stents investigated the role of TIPS compared to elective EBLs with NSBB in patients who were hemodynamically stabilized after initial treatment for a first or second occurrence of esophageal or gastric VH. TIPS significantly reduced the rate of rebleeding but increased the incidence of post-TIPS HE by 35% within 1 year compared to standard treatment. However, during a long-term follow-up period, the difference in the incidence of post-TIPS HE disappeared (38% vs. 23%; P=0.121). Also, the survival rate was not significantly different between the two groups [26]. Another large randomized study, which enrolled patients with a mean CTP score of 7 points and model for end-stage liver disease (MELD) score of 10 points and who experienced VH more than 5 days ago, compared the effect of TIPS with an 8-mm covered stent to HVPG-guided medical treatment (NSBB therapy). Here, the TIPS group had significantly reduced rebleeding rate compared to the medical treatment group (P=0.002), but the survival rate was not different. In this study, patients in the TIPS group had a slightly greater 2-year incidence of HE (18% vs. 8%; P=0.05), but the quality of life was not different [27]. Collectively, TIPS for secondary prevention definitely reduced the risk of rebleeding with a slight increase in HE incidence compared to medical treatment. Nevertheless, there was no difference in the survival rate. Recent guidelines have recommended NSBB be combined with EBL as a firstline therapy for secondary prevention of esophageal VH; however, if the first-line treatment (NSBB + EBL) fails or patients are intolerant to NSBB, TIPS should be considered [9,16,17,20].
Gastric VH
Acute bleeding from gastroesophageal varices type 1 (GOV1) can be controlled by endoscopic therapy, either by EBL or cyanoacrylate glue injection. In the case of bleeding from cardiofundal varices (GOV2 or isolated gastric varices type 1), the guidelines of AASLD and EASL recommend TIPS as a treatment of choice for the control of acute bleeding and the prevention of rebleeding from cardiofundal varices [9,16]. A RCT evaluating the role of TIPS in the prevention of rebleeding from gastric varices showed that TIPS was more effective than endoscopic cyanoacrylate injection, with similar survival and complication rates [29]. Balloon occluded retrograde transvenous obliteration (BRTO) or technically modified methods, such as plug-assisted retrograde transvenous obliteration or coil-assisted retrograde transvenous obliteration, can also be used instead of TIPS with similar efficacy. A recent RCT demonstrated the high efficacy of BRTO in reducing the rebleeding rate of gastric varices compared to endoscopic cyanoacrylate injection, with similar complication rates or aggravation of esophageal varices [30]. In addition, a meta-analysis showed that the post-procedure rebleeding rate was significantly lower in patients treated with BRTO compared to those treated with TIPS (odds ratio [OR], 0.27; 95% CI, 0.09–0.81; P=0.02) [31]. BRTO has the advantage of being able to preserve blood flow to the liver; however, portal hypertension can be aggravated, leading to worsening of ascites in 9.2% of patients or the recurrence of esophageal varices in 33.3% of patients who received BRTO [32]. In summary, TIPS is recommended for the control of acute bleeding and the prevention of rebleeding from gastric varices. BRTO can also be used instead of TIPS with a similar or higher efficacy rate in reducing the rebleeding rate [31], although more data are needed for determining the best candidate.
Refractory or recurrent ascites
Although large-volume paracentesis (LVP) is the first-line treatment for uncontrolled ascites despite high-dose diuretics and salt restriction, TIPS can be considered an effective alternative in patients who require LVP at frequent intervals [16]. In previous RCTs comparing TIPS and LVP in patients with refractory or recurrent ascites, TIPS significantly improved ascites compared to LVP, but the incidence of post-TIPS HE increased and survival outcomes were inconsistent [33-37]. However, the role of TIPS for refractory or recurrent ascites needs to be reassessed, since these studies used bare stents for TIPS. In a meta-analysis of patients with variceal bleeding, mainly including patients who used PTFE-covered stents, TIPS improved the occurrence of ascites by 74% without increasing the risk of post-TIPS HE [24]. To date, the survival benefit of TIPS in patients with refractory ascites has not been identified. Instead, a recent RCT evaluating the effects of TIPS with PTFE-covered stents compared to LVP with albumin therapy in patients with recurrent ascites demonstrated that TIPS independently improved the 1-year transplant-free survival rate (HR, 2.1; 95% CI, 1.1–4.0). There was no difference in the incidence of post-TIPS HE, which was 35% in both groups [38].
In patients with refractory or recurrent ascites, TIPS can also improve sarcopenia due to increased absorption of intestinal nutrients by relieving portal hypertension and decreased protein loss by paracentesis [39]. Although the timing of TIPS for recurrent or refractory ascites has not yet been determined, a study which demonstrated 1-year survival benefit from TIPS in patients with recurrent ascites enrolled patients who required LVP at least two times within 3 months, while excluding patients who required more than six times during the same period [38]; this strategy of patient selection for early TIPS boasted a degree of cost-effectiveness compared to LVP with albumin therapy [40]. Another study showed that a high frequency of LVP (OR, 1.2; 95% CI, 1.3–2.4) and elevated creatinine level (OR, 2.6; 95% CI, 1.2–6.6) were significantly associated with TIPS failure for controlling ascites [41]. Therefore, performing early TIPS before patients suffer from highly frequent LVP that impairs their quality of life might improve clinical prognosis and also enhance the effectiveness of TIPS.
Hepatic hydrothorax
About 5–10% of patients with LC suffer from hepatic hydrothorax, and 20–25% of them have refractory hepatic hydrothorax that does not respond to salt restriction or high-dose diuretics [42,43]. In these patients, repeated thoracentesis is required, but procedure-related complications frequently accompany this procedure, such as pneumothorax, bleeding, or infection. For the same reasons, chronic drainage of pleural effusion is not recommended. Although pleurodesis can be considered for refractory hepatic hydrothorax, it is rarely used in practice due to its complication rate of about 82% [44]. Although there has been limited evidence to support the efficacy of TIPS for refractory hepatic hydrothorax, especially when using PTFE-covered stents, complete or partial resolution of hepatic hydrothorax after TIPS was reported to range from 59% to 81% in case series [45-50]. In addition, a recent study that compared TIPS using PTFE-covered stents with other modalities (i.e., medical treatment, thoracentesis, and catheter drainage) in patients with hepatic hydrothorax showed no difference in mortality among the various treatment groups [51]. Therefore, TIPS is the preferred alternative treatment in patients who require repeated thoracentesis due to refractory hepatic hydrothorax. Further investigation is required to disclose the efficacy of TIPS using PTFE-covered stents in refractory hepatic hydrothorax.
Others
Hepatocellular carcinoma (HCC)
Although HCC is a relative contraindication to TIPS, it can be considered if anatomically accessible and clinically necessary [14,52]. A few studies have evaluated the effect of TIPS implementation on HCC patients. A previous study including 209 HCC patients who underwent TIPS before or after an HCC diagnosis reported that TIPS successfully decreased HVPG, leading to an improvement in portal hypertension symptoms, such as esophageal VH or refractory ascites [53]. In a small number of HCC patients with refractory ascites, TIPS implementation improved CTP scores in 78% of the study population, mainly by controlling ascites. Improvements in CTP scores increase the chance of receiving treatments for HCC, which leads to a survival benefit [54]. TACE, a major treatment modality for HCC, can cause hepatic ischemia through arterial embolization; therefore, liver perfusion can be further reduced after TACE in patients with TIPS, which diverts the portal venous inflow. A previous study showed that TACE with drug-eluting beads in HCC patients with TIPS significantly reduced the incidence of hepatotoxicity after TACE and improved the overall survival rate compared to conventional TACE [55]. However, there are still concerns regarding the occurrence of hepatic dysfunction or HE after TIPS, which deprives patients of the opportunity to receive treatments for HCC or tumor dissemination during the procedure. TIPS implementation in HCC patients should be decided while considering each patient’s individual situation.
Hepatorenal syndrome (HRS)
There has been no RCT that compared the use of TIPS to medical treatment (i.e., vasoconstrictors and albumin) in patients with HRS. To date, the role of TIPS in HRS remains controversial. TIPS can alleviate circulatory and neurohormonal derangement by reducing portal hypertension; therefore, theoretically, TIPS can be beneficial for patients with HRS. In patients with type 1 or 2 HRS who received TIPS, limited data showed that renal function was improved [56,57]. In addition, a recent study using a large population cohort demonstrated a survival benefit from TIPS in patients with LC and HRS relative to non-TIPS (adjusted OR, 0.43; 95% CI, 0.30–0.62; P<0.001) [58]. However, TIPS is rarely indicated in patients with HRS, a complication of end-stage liver disease, as these individuals frequently have severe hepatic dysfunction and TIPS can further deteriorate their renal function due to reduced systemic arterial pressure.
BCS and noncirrhotic portal hypertension
Regarding BCS, current guidelines recommend TIPS to be performed in symptomatic patients if anticoagulation therapy or hepatic vein interventions, such as stenting or angioplasty, have failed [8,59]. Portal hypertensive complications resolved in more than 70% of BCS patients who received TIPS, and the 5-year survival rate was greater than 70% [8]. TIPS can also be used in patients with noncirrhotic portal hypertension with indications similar to those of LC patients [8]. The 1-year rebleeding rate range was 5–11% and the 5-year survival rate was nearly 90% in patients with noncirrhotic portal hypertension who underwent TIPS for recurrent VH [60,61].
CONTRAINDICATIONS
General contraindications for TIPS, as stated in guidelines, are as follows: congestive heart failure, pulmonary hypertension (absolutely contraindicated when pulmonary pressure is above a mean value of 45 mmHg), progressive renal failure, HE of grade 2 or higher according to the West Haven criteria, uncontrolled systemic infection, severe thrombocytopenia, or coagulopathy (Table 2) [14,16]. TIPS implementation increases blood flow to the central circulation, which can cause serious problems in patients with underlying cardiopulmonary diseases and worsen renal function in patients with underlying renal disease. TIPS implementation should be decided cautiously in patients aged over 65 years, as these patients are more prone to developing HE [8]. Existing guidelines suggest TIPS to be performed at experienced centers [16]. Medical centers that have performed TIPS at least 20 times per year had a significantly lower inpatient mortality rate compared to those that did not (adjusted OR, 2.5; P=0.04) [62].
Table 2.
Absolute and relative contraindications for TIPS
| Absolute | Relative |
|---|---|
| Primary prevention of variceal hemorrhage | Age >65 years |
| Hepatic encephalopathy (grade ≥2)*, recurrent or persistent | MELD score >15–18 |
| Uncontrolled systemic infection or sepsis | Total bilirubin >3–4 mg/dL |
| Severe pulmonary hypertension (>45 mmHg) | Severe thrombocytopenia or coagulopathy |
| Congestive heart failure | Progressive renal failure |
| Severe tricuspid regurgitation | Anatomical problems (such as central tumor, polycystic liver disease) |
| Unrelieved biliary obstruction |
TIPS, transjugular intrahepatic portosystemic shunt; MELD, model for end-stage liver disease.
The grade of hepatic encephalopathy was according to the West Haven criteria.
TIPS is relatively contraindicated in patients with severe hepatic dysfunction, such as a serum bilirubin level of greater than 4 mg/dL or MELD score of greater than 15 to 18 points [14]. However, it is unclear whether the effects of TIPS outweigh the risk of complications that can develop after TIPS in these patients. A few studies showed that pTIPS contributed to improving the prognosis in patients with severe hepatic dysfunction. A previous study of three French hospitals, which enrolled patients with a mean MELD score of 29 points and CTP score of 13, showed that 6-week and 1-year survival rates were significantly improved in patients treated with pTIPS compared to those treated with standard treatment: the 6-week survival rates were 76.5% and 35.3% in the pTIPS and control groups (P=0.023), respectively, and the 1-year survival rates were 76.5% and 23.5% (P=0.005), respectively [63]. In patients with acute on chronic liver failure (ACLF) due to acute VH, pTIPS was associated with a reduced 1-year mortality rate compared to standard treatments (22.7% vs. 56.5%; P=0.002). Preemptive TIPS also achieved survival benefit in patients with ACLF and a bilirubin level of greater than 5 mg/dL [64]. In a meta-analysis, the survival rate of the pTIPS group was significantly higher than that in the standard treatment group among patients with bilirubin levels of above 5 mg/dL or even those with levels of above 10 mg/dL [24]. However, these studies only included a small number of patients, and additional studies are needed to recommend TIPS in patients with severe hepatic dysfunction. Careful patient selection for TIPS is important to balance the benefits of TIPS over the occurrence of complications after TIPS.
PROGNOSIS
Hepatic dysfunction
Hepatic dysfunction can develop after TIPS, since the portal blood flow bypasses intrahepatic circulation and travels directly to the inferior vena cava through a portosystemic shunt. The liver can also be affected by hypotension during the procedure. A previous study reported a significant incidence of hepatic failure (death, LT, or MELD score >18 points) within 3 months after TIPS as 9.3% among patients with baseline MELD scores of 9.6 points (range, 6–12). In these patients, MELD scores of 11–12 points, low hemoglobin concentrations, and low platelet counts were independently associated with the development of early hepatic failure. However, the relatively high rate of hepatic failure might be due to the fact that this study enrolled patients who underwent TIPS between 1999 and 2012 at a single center [65].
The deterioration of hepatic function is usually a transient event after TIPS, and is manifested by the elevation of bilirubin level [5]. In a recent RCT for evaluating the role of pTIPS in acute VH, serum bilirubin level, international normalized ratio, and MELD score were slightly increased at 1 and 3 months and improved at 6 months after TIPS. In addition, the effect of pTIPS was evident in reducing liver-related mortality compared to standard treatments [19]. Therefore, although concerns about hepatic dysfunction after TIPS still exist, hepatic failure is not a common event if TIPS is performed according to indications. Close monitoring of hepatic function is warranted, at least for a short period of time, after TIPS.
HE
HE is the most concerning complication that may develop after TIPS; therefore, guidelines do not recommend TIPS implementation in patients on recurrent or persistent overt HE (grade ≥2 according to the West Haven criteria) [16]. For patients with covert HE, there are differences in suggestions according to the guidelines. A recent guideline does not recommend TIPS in patients with covert HE, while the AASLD guideline suggests avoiding TIPS only when the HE is uncontrollable with standard therapy [8,14]. Post-TIPS HE was more common in patients with minimal HE, and there was no case of recurrent overt HE after TIPS in patients with no history of overt HE and no current minimal HE diagnosed with the critical flicker frequency [66]. However, several prospective studies which evaluated the effect of TIPS in LC patients excluded only patients with overt HE and 1-year incidence of HE after TIPS was about 35%, similar to that in patients who received standard therapy [7,38,66]. Strict criteria in patients with HE might limit candidates for TIPS since the procedure is restricted to patients with decompensated LC. Therefore, although TIPS is not recommended for patients with overt HE, those with covert HE can receive TIPS, with close monitoring for the occurrence of post-TIPS HE.
A significant proportion of LC patients have sarcopenia and are at an increased risk of developing HE, as the role of muscle in the disposal of ammonia is enhanced in LC patients [67]. Previously, sarcopenia was identified as a predictor for the development of HE in patients who underwent TIPS [68]. However, in a recent study, although the risk of post-TIPS HE was significantly reduced in patients with a higher skeletal muscle index (SMI) used for evaluating sarcopenia in LC patients, sarcopenia itself was not associated with post-TIPS HE. Furthermore, SMI improved after TIPS in LC patients [39]. An average SMI increased as 17% after a mean 10 months of TIPS [69]. Therefore, although close monitoring of the development of HE after TIPS is important in patients with sarcopenia, performing TIPS in patients with sarcopenia should not necessarily be avoided.
A previous RCT showed that prophylactic use of rifaximin or lactitol was not effective in reducing the development of post-TIPS HE [70]. Therefore, guidelines do not recommend routine prophylactic treatment for HE in patients who underwent TIPS [71]. However, the most recent RCT evaluating the role of rifaximin as a prophylactic treatment for post-TIPS HE demonstrated that the incidence of overt HE after TIPS was significantly lower in patients who received rifaximin compared to that in patients who received placebo during a follow-up period of 6 months (34% vs. 53%, respectively; P=0.012) [72]. A possible reason for the relatively high incidence of HE in this study could be that PPG after TIPS was too low, at 6 mmHg. Further study of prophylactic treatments for the development of post-TIPS HE is required to gather long-term data.
Cardiac dysfunction
LC patients are in a vulnerable state for developing cardiac dysfunction. The prevalence of cirrhotic cardiomyopathy, which refers to cardiac dysfunction in LC patients without known cardiac disease, ranges from 26% to 81%, according to existing studies [73,74]. Although TIPS implementation can have the positive effects of increasing the cardiac preload and output as the portal flow directly enters the inferior vena cava through the shunt, sudden hemodynamic changes caused by TIPS can overwhelm the hearts of LC patients and lead to severe cardiac decompensation.
Several previous studies have focused on predicting cardiac complications after TIPS. In a retrospective study, 50.4% of LC patients had abnormal findings on echocardiography performed before TIPS, but the results of the exam did not predict mortality. However, this study was limited in that the predictive role of echocardiography for the development of cardiac complications after TIPS was not evaluated [75]. A recent prospective study showed that cardiac decompensation occurred in 20% of LC patients treated with TIPS. Brain natriuretic peptide (BNP), NT-proBNP level, and echocardiography before TIPS implementation discriminated patients at high risk for such. Cardiac decompensation did not occur in patients with a BNP level of less than 40 pg/mL and NT-proBNP level of less than 120 pg/mL before TIPS insertion, but developed in 51.4% of patients with echocardiographic findings, suggesting diastolic dysfunction [76]. Collectively, a significant proportion of LC patients have combined cardiac dysfunction, and many of them can develop cardiac decompensation after TIPS. To date, there is no consensus regarding the use of echocardiography to predict cardiac complications after TIPS. The EASL guideline recommends performing routine echocardiography before elective TIPS, while the AASLD guideline does not take a clear stance on the role of the examination [14,16]. A recent guideline suggests performing echocardiography if abnormal findings are found in preprocedure evaluations, such a 12-lead electocardiogram or cardiac markers, including NT-proBNP [8]. Further research is needed to evaluate the role and cost-effectiveness of echocardiography before performing TIPS in LC patients.
Renal function
TIPS restores hemodynamic and neurohormonal derangement, increasing natriuresis within 4 weeks, which may lead to an improvement in renal function [77,78]. TIPS implementation for LC patients with refractory ascites and an estimated glomerular filtration rate (eGFR) of less than 60 mL/min/1.73 m2 at baseline significantly improved the eGFR (the expected change in eGFR after 90 days, TIPS vs. serial LVP as reference, 21; 95% CI, 13–29; P<0.001), while there was no change in the eGFR level in patients with an eGFR of 60 mL/min/1.73 m2 or greater [79]. Other studies have also demonstrated an improvement in renal function after TIPS [80,81]. However, TIPS might not be beneficial in patients who already have intrinsic renal disease [82]. A high creatinine level was significantly associated with post-TIPS HE [83]. In addition, the contrast agents used when performing TIPS can also worsen renal function in these individuals. A recent study that enrolled 673 patients who underwent TIPS showed that 10% of the patients experienced deterioration in renal function at 30 days after TIPS. Patients with nonalcoholic fatty liver disease and diabetes who were susceptible to having intrinsic renal disease were at high risk of post-TIPS renal impairment [81]. A recent guideline does not recommend performing TIPS in patients with intrinsic renal disease of stage 4 or 5 [8]. Collectively, although renal dysfunction caused by portal hypertension usually improves after TIPS, performing TIPS should be carefully decided in patients with intrinsic renal disease.
PROGNOSTIC SCORES AND PATIENT SELECTION
Several prognostic scores for predicting survival after TIPS have been developed, such as the CTP, MELD, or MELD-Na scores [84,85]. A bilirubin-platelet model was developed for LC patients with refractory ascites and introduced in the EASL guideline [16]. The 1-year survival rate was significantly lower at 31.2% in patients with a platelet count of less than 75×109/L or a bilirubin level of greater than 3 mg/dL compared to 73.1% in patients with a platelet count of greater than 75×109/L and a bilirubin of less than 3 mg/dL [86]. Recently, a new score of the Freiburg index of post-TIP survival (FIPS) using age and bilirubin, albumin, and creatinine levels was introduced to improve the prognostic predictability for patients undergoing elective TIPS implementation. FIPS indicated that the median overall survival was significantly shorter as 3.1–5.0 months in patients in the high-risk group compared to the low-risk group [87]. However, the result should be validated in future studies.
CONCLUSIONS
Although clinicians may have concerns about performing TIPS due to the non-negligible complications, recent studies have shown high efficacy of TIPS compared to other treatments and presented an acceptable complication rate, albeit not different from when TIP was not performed. Moreover, the survival benefit of pTIPS was proved in patients who are at high risk for treatment failure of esophageal VH. Early TIPS before patients suffer from repeated LVP for refractory or recurrent ascites showed improved quality of life and cost-effectiveness compared to LVP, as well as a significantly higher 1-year transplant-free survival. In the context of organ shortage for LT, the role of TIPS needs to be actively considered in clinical practice. However, to achieve the benefits of TIPS, proper timing and patient selection are crucial.
Abbreviations
- AASLD
American Association for the Study of Liver Diseases
- ACLF
acute on chronic liver failure
- aHR
adjusted hazard ratio
- BCS
Budd-Chiari syndrome
- BNP
brain natriuretic peptide
- BRTO
balloon occluded retrograde transvenous obliteration
- CI
confidence interval
- CTP
Child-Turcotte-Pugh
- EASL
European Association for the Study of the Liver
- EBL
endoscopic band ligation
- eGFR
estimated glomerular filtration rate
- FIPS
Freiburg index of post-TIP survival
- GOV1
gastroesophageal varices type 1
- HCC
hepatocellular carcinoma
- HE
hepatic encephalopathy
- HRS
hepatorenal syndrome
- HVPG
hepatic venous pressure gradient
- LC
liver cirrhosis
- LT
liver transplantation
- LVP
large-volume paracentesis
- MELD
model for end-stage liver disease
- NSBB
nonselective beta-blockers
- OR
odds ratio
- PPG
porto-systemic pressure gradient
- PTFE
polytetrafluoroethylene
- pTIPS
preemptive transjugular intrahepatic portosystemic shunt
- RCT
randomized controlled trial
- SMI
skeletal muscle index
- TACE
transarterial chemoembolization
- TIPS
transjugular intrahepatic portosystemic shunt
Footnotes
Authors’ contribution
Guarantor of the article: Sung Won Lee
Specific author contributions: Study concept and design: Sung Won Lee and Hae Lim Lee; Wrote the paper: Sung Won Lee and Hae Lim Lee. All authors have approved this final version of the manuscript.
Conflicts of Interest: The authors have no conflicts to disclose.
REFERENCES
- 1.Trebicka J. Emergency TIPS in a Child-Pugh B patient: when does the window of opportunity open and close? J Hepatol. 2017;66:442–450. doi: 10.1016/j.jhep.2016.10.025. [DOI] [PubMed] [Google Scholar]
- 2.Rössle M, Haag K, Ochs A, Sellinger M, Nöldge G, Perarnau JM, et al. The transjugular intrahepatic portosystemic stent-shunt procedure for variceal bleeding. N Engl J Med. 1994;330:165–171. doi: 10.1056/NEJM199401203300303. [DOI] [PubMed] [Google Scholar]
- 3.Casado M, Bosch J, García-Pagán JC, Bru C, Bañares R, Bandi JC, et al. Clinical events after transjugular intrahepatic portosystemic shunt: correlation with hemodynamic findings. Gastroenterology. 1998;114:1296–1303. doi: 10.1016/s0016-5085(98)70436-6. [DOI] [PubMed] [Google Scholar]
- 4.Bureau C, Garcia-Pagan JC, Otal P, Pomier-Layrargues G, Chabbert V, Cortez C, et al. Improved clinical outcome using polytetrafluoroethylene-coated stents for TIPS: results of a randomized study. Gastroenterology. 2004;126:469–475. doi: 10.1053/j.gastro.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 5.Bosch J. Small diameter shunts should lead to safe expansion of the use of TIPS. J Hepatol. 2021;74:230–234. doi: 10.1016/j.jhep.2020.09.018. [DOI] [PubMed] [Google Scholar]
- 6.Trebicka J, Bastgen D, Byrtus J, Praktiknjo M, Terstiegen S, Meyer C, et al. Smaller-diameter covered transjugular intrahepatic portosystemic shunt stents are associated with increased survival. Clin Gastroenterol Hepatol. 2019;17:2793–2799.e1. doi: 10.1016/j.cgh.2019.03.042. [DOI] [PubMed] [Google Scholar]
- 7.Wang Q, Lv Y, Bai M, Wang Z, Liu H, He C, et al. Eight millimetre covered TIPS does not compromise shunt function but reduces hepatic encephalopathy in preventing variceal rebleeding. J Hepatol. 2017;67:508–516. doi: 10.1016/j.jhep.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 8.Tripathi D, Stanley AJ, Hayes PC, Travis S, Armstrong MJ, Tsochatzis EA, et al. Transjugular intrahepatic portosystemic stent-shunt in the management of portal hypertension. Gut. 2020;69:1173–1192. doi: 10.1136/gutjnl-2019-320221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017;65:310–335. doi: 10.1002/hep.28906. [DOI] [PubMed] [Google Scholar]
- 10.LaBerge JM, Ferrell LD, Ring EJ, Gordon RL. Histopathologic study of stenotic and occluded transjugular intrahepatic portosystemic shunts. J Vasc Interv Radiol. 1993;4:779–786. doi: 10.1016/s1051-0443(93)71972-7. [DOI] [PubMed] [Google Scholar]
- 11.Saxon RR, Mendel-Hartvig J, Corless CL, Rabkin J, Uchida BT, Nishimine K, et al. Bile duct injury as a major cause of stenosis and occlusion in transjugular intrahepatic portosystemic shunts: comparative histopathologic analysis in humans and swine. J Vasc Interv Radiol. 1996;7:487–497. doi: 10.1016/s1051-0443(96)70789-3. [DOI] [PubMed] [Google Scholar]
- 12.Tang Y, Zheng S, Yang J, Bao W, Yang L, Li Y, et al. Use of concomitant variceal embolization and prophylactic antiplatelet/anticoagulative in transjugular intrahepatic portosystemic shunting: a retrospective study of 182 cirrhotic portal hypertension patients. Medicine (Baltimore) 2017;96:e8678. doi: 10.1097/MD.0000000000008678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steib CJ, Li H, Zhang J, Mayerle J, Ricke J, Gerbes AL, et al. Transjugular intrahepatic portosystemic shunt for patients with liver cirrhosis: survey evaluating indications, standardization of procedures and anticoagulation in 43 German hospitals. Eur J Gastroenterol Hepatol. 2020;32:1179–1185. doi: 10.1097/MEG.0000000000001628. [DOI] [PubMed] [Google Scholar]
- 14.Boyer TD, Haskal ZJ, American Association for the Study of Liver Diseases The role of transjugular intrahepatic portosystemic shunt (TIPS) in the management of portal hypertension: update 2009. Hepatology. 2010;51:306. doi: 10.1002/hep.23383. [DOI] [PubMed] [Google Scholar]
- 15.García-Pagán JC, Caca K, Bureau C, Laleman W, Appenrodt B, Luca A, et al. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med. 2010;362:2370–2379. doi: 10.1056/NEJMoa0910102. [DOI] [PubMed] [Google Scholar]
- 16.European Association for the Study of the Liver EASL clinical practice guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406–460. doi: 10.1016/j.jhep.2018.03.024. [DOI] [PubMed] [Google Scholar]
- 17.de Franchis R, Baveno VI Faculty Expanding consensus in portal hypertension: report of the Baveno VI consensus workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743–752. doi: 10.1016/j.jhep.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 18.Monescillo A, Martínez-Lagares F, Ruiz-del-Arbol L, Sierra A, Guevara C, Jiménez E, et al. Influence of portal hypertension and its early decompression by TIPS placement on the outcome of variceal bleeding. Hepatology. 2004;40:793–801. doi: 10.1002/hep.20386. [DOI] [PubMed] [Google Scholar]
- 19.Lv Y, Yang Z, Liu L, Li K, He C, Wang Z, et al. Early TIPS with covered stents versus standard treatment for acute variceal bleeding in patients with advanced cirrhosis: a randomised controlled trial. Lancet Gastroenterol Hepatol. 2019;4:587–598. doi: 10.1016/S2468-1253(19)30090-1. [DOI] [PubMed] [Google Scholar]
- 20.Korean Association for the Study of the Liver (KASL) KASL clinical practice guidelines for liver cirrhosis: varices, hepatic encephalopathy, and related complications. Clin Mol Hepatol. 2020;26:83–127. doi: 10.3350/cmh.2019.0010n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rudler M, Cluzel P, Corvec TL, Benosman H, Rousseau G, Poynard T, et al. Early-TIPSS placement prevents rebleeding in high-risk patients with variceal bleeding, without improving survival. Aliment Pharmacol Ther. 2014;40:1074–1080. doi: 10.1111/apt.12934. [DOI] [PubMed] [Google Scholar]
- 22.Garcia-Pagán JC, Di Pascoli M, Caca K, Laleman W, Bureau C, Appenrodt B, et al. Use of early-TIPS for high-risk variceal bleeding: results of a post-RCT surveillance study. J Hepatol. 2013;58:45–50. doi: 10.1016/j.jhep.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 23.Augustin S, Altamirano J, González A, Dot J, Abu-Suboh M, Armengol JR, et al. Effectiveness of combined pharmacologic and ligation therapy in high-risk patients with acute esophageal variceal bleeding. Am J Gastroenterol. 2011;106:1787–1795. doi: 10.1038/ajg.2011.173. [DOI] [PubMed] [Google Scholar]
- 24.Nicoară-Farcău O, Han G, Rudler M, Angrisani D, Monescillo A, Torres F, et al. Effects of early placement of transjugular portosystemic shunts in patients with high-risk acute variceal bleeding: a meta-analysis of individual patient data. Gastroenterology. 2021;160:193–205.e10. doi: 10.1053/j.gastro.2020.09.026. [DOI] [PubMed] [Google Scholar]
- 25.Halabi SA, Sawas T, Sadat B, Jandali A, Halabi HA, Halabi FA, et al. Early TIPS versus endoscopic therapy for secondary prophylaxis after management of acute esophageal variceal bleeding in cirrhotic patients: a meta-analysis of randomized controlled trials. J Gastroenterol Hepatol. 2016;31:1519–1526. doi: 10.1111/jgh.13303. [DOI] [PubMed] [Google Scholar]
- 26.Holster IL, Tjwa ET, Moelker A, Wils A, Hansen BE, Vermeijden JR, et al. Covered transjugular intrahepatic portosystemic shunt versus endoscopic therapy + β-blocker for prevention of variceal rebleeding. Hepatology. 2016;63:581–589. doi: 10.1002/hep.28318. [DOI] [PubMed] [Google Scholar]
- 27.Sauerbruch T, Mengel M, Dollinger M, Zipprich A, Rössle M, Panther E, et al. Prevention of rebleeding from esophageal varices in patients with cirrhosis receiving small-diameter stents versus hemodynamically controlled medical therapy. Gastroenterology. 2015;149:660–668.e1. doi: 10.1053/j.gastro.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 28.Escorsell A, Bañares R, García-Pagán JC, Gilabert R, Moitinho E, Piqueras B, et al. TIPS versus drug therapy in preventing variceal rebleeding in advanced cirrhosis: a randomized controlled trial. Hepatology. 2002;35:385–392. doi: 10.1053/jhep.2002.30418. [DOI] [PubMed] [Google Scholar]
- 29.Lo GH, Liang HL, Chen WC, Chen MH, Lai KH, Hsu PI, et al. A prospective, randomized controlled trial of transjugular intrahepatic portosystemic shunt versus cyanoacrylate injection in the prevention of gastric variceal rebleeding. Endoscopy. 2007;39:679–685. doi: 10.1055/s-2007-966591. [DOI] [PubMed] [Google Scholar]
- 30.Luo X, Xiang T, Wu J, Wang X, Zhu Y, Xi X, et al. Endoscopic cyanoacrylate injection versus balloon-occluded retrograde transvenous obliteration for prevention of gastric variceal bleeding: a randomized controlled trial. Hepatology. 2021;74:2074–2084. doi: 10.1002/hep.31718. [DOI] [PubMed] [Google Scholar]
- 31.Wang YB, Zhang JY, Gong JP, Zhang F, Zhao Y. Balloon-occluded retrograde transvenous obliteration versus transjugular intrahepatic portosystemic shunt for treatment of gastric varices due to portal hypertension: a meta-analysis. J Gastroenterol Hepatol. 2016;31:727–733. doi: 10.1111/jgh.13248. [DOI] [PubMed] [Google Scholar]
- 32.Park JK, Saab S, Kee ST, Busuttil RW, Kim HJ, Durazo F, et al. Balloon-occluded retrograde transvenous obliteration (BRTO) for treatment of gastric varices: review and meta-analysis. Dig Dis Sci. 2015;60:1543–1553. doi: 10.1007/s10620-014-3485-8. [DOI] [PubMed] [Google Scholar]
- 33.Rössle M, Ochs A, Gülberg V, Siegerstetter V, Holl J, Deibert P, et al. A comparison of paracentesis and transjugular intrahepatic portosystemic shunting in patients with ascites. N Engl J Med. 2000;342:1701–1707. doi: 10.1056/NEJM200006083422303. [DOI] [PubMed] [Google Scholar]
- 34.Ginès P, Uriz J, Calahorra B, Garcia-Tsao G, Kamath PS, Del Arbol LR, et al. Transjugular intrahepatic portosystemic shunting versus paracentesis plus albumin for refractory ascites in cirrhosis. Gastroenterology. 2002;123:1839–1847. doi: 10.1053/gast.2002.37073. [DOI] [PubMed] [Google Scholar]
- 35.Sanyal AJ, Genning C, Reddy KR, Wong F, Kowdley KV, Benner K, et al. The North American study for the treatment of refractory ascites. Gastroenterology. 2003;124:634–641. doi: 10.1053/gast.2003.50088. [DOI] [PubMed] [Google Scholar]
- 36.Salerno F, Merli M, Riggio O, Cazzaniga M, Valeriano V, Pozzi M, et al. Randomized controlled study of TIPS versus paracentesis plus albumin in cirrhosis with severe ascites. Hepatology. 2004;40:629–635. doi: 10.1002/hep.20364. [DOI] [PubMed] [Google Scholar]
- 37.Narahara Y, Kanazawa H, Fukuda T, Matsushita Y, Harimoto H, Kidokoro H, et al. Transjugular intrahepatic portosystemic shunt versus paracentesis plus albumin in patients with refractory ascites who have good hepatic and renal function: a prospective randomized trial. J Gastroenterol. 2011;46:78–85. doi: 10.1007/s00535-010-0282-9. [DOI] [PubMed] [Google Scholar]
- 38.Bureau C, Thabut D, Oberti F, Dharancy S, Carbonell N, Bouvier A, et al. Transjugular intrahepatic portosystemic shunts with covered stents increase transplant-free survival of patients with cirrhosis and recurrent ascites. Gastroenterology. 2017;152:157–163. doi: 10.1053/j.gastro.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 39.Benmassaoud A, Roccarina D, Arico F, Leandro G, Yu B, Cheng F, et al. Sarcopenia does not worsen survival in patients with cirrhosis undergoing transjugular intrahepatic portosystemic shunt for refractory ascites. Am J Gastroenterol. 2020;115:1911–1914. doi: 10.14309/ajg.0000000000000959. [DOI] [PubMed] [Google Scholar]
- 40.Shen NT, Schneider Y, Congly SE, Rosenblatt RE, Namn Y, Fortune BE, et al. Cost effectiveness of early insertion of transjugular intrahepatic portosystemic shunts for recurrent ascites. Clin Gastroenterol Hepatol. 2018;16:1503–1510.e3. doi: 10.1016/j.cgh.2018.03.027. [DOI] [PubMed] [Google Scholar]
- 41.Piecha F, Radunski UK, Ozga AK, Steins D, Drolz A, Horvatits T, et al. Ascites control by TIPS is more successful in patients with a lower paracentesis frequency and is associated with improved survival. JHEP Rep. 2019;1:90–98. doi: 10.1016/j.jhepr.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Surani SR, Mendez Y, Anjum H, Varon J. Pulmonary complications of hepatic diseases. World J Gastroenterol. 2016;22:6008–6015. doi: 10.3748/wjg.v22.i26.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raevens S, Boret M, De Pauw M, Fallon MB, Van Vlierberghe H. Pulmonary abnormalities in liver disease: relevance to transplantation and outcome. Hepatology. 2021;74:1674–1686. doi: 10.1002/hep.31770. [DOI] [PubMed] [Google Scholar]
- 44.Hou F, Qi X, Guo X. Effectiveness and safety of pleurodesis for hepatic hydrothorax: a systematic review and meta-analysis. Dig Dis Sci. 2016;61:3321–3334. doi: 10.1007/s10620-016-4260-9. [DOI] [PubMed] [Google Scholar]
- 45.Gordon FD, Anastopoulos HT, Crenshaw W, Gilchrist B, McEniff N, Falchuk KR, et al. The successful treatment of symptomatic, refractory hepatic hydrothorax with transjugular intrahepatic portosystemic shunt. Hepatology. 1997;25:1366–1369. doi: 10.1002/hep.510250611. [DOI] [PubMed] [Google Scholar]
- 46.Jeffries MA, Kazanjian S, Wilson M, Punch J, Fontana RJ. Transjugular intrahepatic portosystemic shunts and liver transplantation in patients with refractory hepatic hydrothorax. Liver Transpl Surg. 1998;4:416–423. doi: 10.1002/lt.500040506. [DOI] [PubMed] [Google Scholar]
- 47.Siegerstetter V, Deibert P, Ochs A, Olschewski M, Blum HE, Rössle M. Treatment of refractory hepatic hydrothorax with transjugular intrahepatic portosystemic shunt: long-term results in 40 patients. Eur J Gastroenterol Hepatol. 2001;13:529–534. doi: 10.1097/00042737-200105000-00011. [DOI] [PubMed] [Google Scholar]
- 48.Spencer EB, Cohen DT, Darcy MD. Safety and efficacy of transjugular intrahepatic portosystemic shunt creation for the treatment of hepatic hydrothorax. J Vasc Interv Radiol. 2002;13:385–390. doi: 10.1016/s1051-0443(07)61741-2. [DOI] [PubMed] [Google Scholar]
- 49.Wilputte JY, Goffette P, Zech F, Godoy-Gepert A, Geubel A. The outcome after transjugular intrahepatic portosystemic shunt (TIPS) for hepatic hydrothorax is closely related to liver dysfunction: a long-term study in 28 patients. Acta Gastroenterol Belg. 2007;70:6–10. [PubMed] [Google Scholar]
- 50.Dhanasekaran R, West JK, Gonzales PC, Subramanian R, Parekh S, Spivey JR, et al. Transjugular intrahepatic portosystemic shunt for symptomatic refractory hepatic hydrothorax in patients with cirrhosis. Am J Gastroenterol. 2010;105:635–641. doi: 10.1038/ajg.2009.634. [DOI] [PubMed] [Google Scholar]
- 51.Jindal A, Mukund A, Kumar G, Sarin SK. Efficacy and safety of transjugular intrahepatic portosystemic shunt in difficult-to-manage hydrothorax in cirrhosis. Liver Int. 2019;39:2164–2173. doi: 10.1111/liv.14200. [DOI] [PubMed] [Google Scholar]
- 52.Saab S, Kim NG, Lee EW. Practical tips on TIPS: when and when not to request it. Am J Gastroenterol. 2020;115:797–800. doi: 10.14309/ajg.0000000000000611. [DOI] [PubMed] [Google Scholar]
- 53.Qiu B, Zhao MF, Yue ZD, Zhao HW, Wang L, Fan ZH, et al. Combined transjugular intrahepatic portosystemic shunt and other interventions for hepatocellular carcinoma with portal hypertension. World J Gastroenterol. 2015;21:12439–12447. doi: 10.3748/wjg.v21.i43.12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yan H, Wang G, Zhu W, Feng K, Zhu W, Wu X, et al. Feasibility and clinical value of TIPS combined with subsequent antitumor treatment in HCC patients with refractory ascites. Transl Oncol. 2020;13:100864. doi: 10.1016/j.tranon.2020.100864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fan W, Guo J, Zhu B, Wang S, Yu L, Huang W, et al. Drug-eluting beads TACE is safe and non-inferior to conventional TACE in HCC patients with TIPS. Eur Radiol. 2021;31:8291–8301. doi: 10.1007/s00330-021-07834-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guevara M, Ginès P, Bandi JC, Gilabert R, Sort P, Jiménez W, et al. Transjugular intrahepatic portosystemic shunt in hepatorenal syndrome: effects on renal function and vasoactive systems. Hepatology. 1998;28:416–422. doi: 10.1002/hep.510280219. [DOI] [PubMed] [Google Scholar]
- 57.Brensing KA, Textor J, Perz J, Schiedermaier P, Raab P, Strunk H, et al. Long term outcome after transjugular intrahepatic portosystemic stent-shunt in non-transplant cirrhotics with hepatorenal syndrome: a phase II study. Gut. 2000;47:288–295. doi: 10.1136/gut.47.2.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Charilaou P, Devani K, Petrosyan R, Reddy C, Pyrsopoulos N. Inpatient mortality benefit with transjugular intrahepatic portosystemic shunt for hospitalized hepatorenal syndrome patients. Dig Dis Sci. 2020;65:3378–3388. doi: 10.1007/s10620-020-06136-2. [DOI] [PubMed] [Google Scholar]
- 59.European Association for the Study of the Live EASL clinical practice guidelines: vascular diseases of the liver. J Hepatol. 2016;64:179–202. doi: 10.1016/j.jhep.2015.07.040. [DOI] [PubMed] [Google Scholar]
- 60.Chen H, He C, Lv Y, Fan J, Tang S, Niu J, et al. Long-term results of variceal bleeding management in 302 patients with chronic extrahepatic portal vein obstruction. J Gastroenterol Hepatol. 2020;35:1049–1056. doi: 10.1111/jgh.14902. [DOI] [PubMed] [Google Scholar]
- 61.Lv Y, Li K, He C, Luo B, Zhang B, Liu H, et al. TIPSS for variceal bleeding in patients with idiopathic non-cirrhotic portal hypertension: comparison with patients who have cirrhosis. Aliment Pharmacol Ther. 2019;49:926–939. doi: 10.1111/apt.15186. [DOI] [PubMed] [Google Scholar]
- 62.Sarwar A, Zhou L, Novack V, Tapper EB, Curry M, Malik R, et al. Hospital volume and mortality after transjugular intrahepatic portosystemic shunt creation in the United States. Hepatology. 2018;67:690–699. doi: 10.1002/hep.29354. [DOI] [PubMed] [Google Scholar]
- 63.Depaire M, Larrue H, Rudler M, Nault JC, Bureau C. Futility criteria for preemptive TIPS in patients with cirrhosis and variceal bleeding are still missing in most severe patients! J Hepatol. 2021;74:997–999. doi: 10.1016/j.jhep.2020.10.009. [DOI] [PubMed] [Google Scholar]
- 64.Trebicka J, Gu W, Ibáñez-Samaniego L, Hernández-Gea V, Pitarch C, Garcia E, et al. Rebleeding and mortality risk are increased by ACLF but reduced by pre-emptive TIPS. J Hepatol. 2020;73:1082–1091. doi: 10.1016/j.jhep.2020.04.024. [DOI] [PubMed] [Google Scholar]
- 65.Luca A, Miraglia R, Maruzzelli L, D’Amico M, Tuzzolino F. Early liver failure after transjugular intrahepatic portosystemic shunt in patients with cirrhosis with model for end-stage liver disease score of 12 or less: incidence, outcome, and prognostic factors. Radiology. 2016;280:622–629. doi: 10.1148/radiol.2016151625. [DOI] [PubMed] [Google Scholar]
- 66.Berlioux P, Robic MA, Poirson H, Métivier S, Otal P, Barret C, et al. Pre-transjugular intrahepatic portosystemic shunts (TIPS) prediction of post-TIPS overt hepatic encephalopathy: the critical flicker frequency is more accurate than psychometric tests. Hepatology. 2014;59:622–629. doi: 10.1002/hep.26684. [DOI] [PubMed] [Google Scholar]
- 67.Jindal A, Jagdish RK. Sarcopenia: ammonia metabolism and hepatic encephalopathy. Clin Mol Hepatol. 2019;25:270–279. doi: 10.3350/cmh.2019.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nardelli S, Lattanzi B, Torrisi S, Greco F, Farcomeni A, Gioia S, et al. Sarcopenia is risk factor for development of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt placement. Clin Gastroenterol Hepatol. 2017;15:934–936. doi: 10.1016/j.cgh.2016.10.028. [DOI] [PubMed] [Google Scholar]
- 69.Gioia S, Merli M, Nardelli S, Lattanzi B, Pitocchi F, Ridola L, et al. The modification of quantity and quality of muscle mass improves the cognitive impairment after TIPS. Liver Int. 2019;39:871–877. doi: 10.1111/liv.14050. [DOI] [PubMed] [Google Scholar]
- 70.Riggio O, Masini A, Efrati C, Nicolao F, Angeloni S, Salvatori FM, et al. Pharmacological prophylaxis of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt: a randomized controlled study. J Hepatol. 2005;42:674–679. doi: 10.1016/j.jhep.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 71.Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, et al. Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60:715–735. doi: 10.1002/hep.27210. [DOI] [PubMed] [Google Scholar]
- 72.Bureau C, Thabut D, Jezequel C, Archambeaud I, D’Alteroche L, Dharancy S, et al. The use of rifaximin in the prevention of overt hepatic encephalopathy after transjugular intrahepatic portosystemic shunt : a randomized controlled trial. Ann Intern Med. 2021;174:633–640. doi: 10.7326/M20-0202. [DOI] [PubMed] [Google Scholar]
- 73.Razpotnik M, Bota S, Wimmer P, Hackl M, Lesnik G, Alber H, et al. The prevalence of cirrhotic cardiomyopathy according to different diagnostic criteria. Liver Int. 2021;41:1058–1069. doi: 10.1111/liv.14769. [DOI] [PubMed] [Google Scholar]
- 74.Chahal D, Liu H, Shamatutu C, Sidhu H, Lee SS, Marquez V. Review article: comprehensive analysis of cirrhotic cardiomyopathy. Aliment Pharmacol Ther. 2021;53:985–998. doi: 10.1111/apt.16305. [DOI] [PubMed] [Google Scholar]
- 75.Armstrong MJ, Gohar F, Dhaliwal A, Nightingale P, Baker G, Greaves D, et al. Diastolic dysfunction on echocardiography does not predict survival after transjugular intrahepatic portosystemic stent-shunt in patients with cirrhosis. Aliment Pharmacol Ther. 2019;49:797–806. doi: 10.1111/apt.15164. [DOI] [PubMed] [Google Scholar]
- 76.Billey C, Billet S, Robic MA, Cognet T, Guillaume M, Vinel JP, et al. A prospective study identifying predictive factors of cardiac decompensation after transjugular intrahepatic portosystemic shunt: the toulouse algorithm. Hepatology. 2019;70:1928–1941. doi: 10.1002/hep.30934. [DOI] [PubMed] [Google Scholar]
- 77.Wong F, Sniderman K, Liu P, Allidina Y, Sherman M, Blendis L. Transjugular intrahepatic portosystemic stent shunt: effects on hemodynamics and sodium homeostasis in cirrhosis and refractory ascites. Ann Intern Med. 1995;122:816–822. doi: 10.7326/0003-4819-122-11-199506010-00002. [DOI] [PubMed] [Google Scholar]
- 78.Wong F, Sniderman K, Liu P, Blendis L. The mechanism of the initial natriuresis after transjugular intrahepatic portosystemic shunt. Gastroenterology. 1997;112:899–907. doi: 10.1053/gast.1997.v112.pm9041252. [DOI] [PubMed] [Google Scholar]
- 79.Allegretti AS, Ortiz G, Cui J, Wenger J, Bhan I, Chung RT, et al. Changes in kidney function after transjugular intrahepatic portosystemic shunts versus large-volume paracentesis in cirrhosis: a matched cohort analysis. Am J Kidney Dis. 2016;68:381–391. doi: 10.1053/j.ajkd.2016.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Anderson CL, Saad WE, Kalagher SD, Caldwell S, Sabri S, Turba UC, et al. Effect of transjugular intrahepatic portosystemic shunt placement on renal function: a 7-year, single-center experience. J Vasc Interv Radiol. 2010;21:1370–1376. doi: 10.1016/j.jvir.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 81.Ge J, Lai JC, Boike JR, German M, Jest N, Morelli G, et al. Nonalcoholic fatty liver disease and diabetes mellitus are associated with post-transjugular intrahepatic portosystemic shunt renal dysfunction: an advancing liver therapeutic approaches group study. Liver Transpl. 2021;27:329–340. doi: 10.1002/lt.25949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Somberg KA, Lake JR, Tomlanovich SJ, LaBerge JM, Feldstein V, Bass NM. Transjugular intrahepatic portosystemic shunts for refractory ascites: assessment of clinical and hormonal response and renal function. Hepatology. 1995;21:709–716. [PubMed] [Google Scholar]
- 83.Riggio O, Angeloni S, Salvatori FM, De Santis A, Cerini F, Farcomeni A, et al. Incidence, natural history, and risk factors of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt with polytetrafluoroethylene-covered stent grafts. Am J Gastroenterol. 2008;103:2738–2746. doi: 10.1111/j.1572-0241.2008.02102.x. [DOI] [PubMed] [Google Scholar]
- 84.Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864–871. doi: 10.1053/he.2000.5852. [DOI] [PubMed] [Google Scholar]
- 85.Guy J, Somsouk M, Shiboski S, Kerlan R, Inadomi JM, Biggins SW. New model for end stage liver disease improves prognostic capability after transjugular intrahepatic portosystemic shunt. Clin Gastroenterol Hepatol. 2009;7:1236–1240. doi: 10.1016/j.cgh.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bureau C, Métivier S, D’Amico M, Péron JM, Otal P, Pagan JC, et al. Serum bilirubin and platelet count: a simple predictive model for survival in patients with refractory ascites treated by TIPS. J Hepatol. 2011;54:901–907. doi: 10.1016/j.jhep.2010.08.025. [DOI] [PubMed] [Google Scholar]
- 87.Bettinger D, Sturm L, Pfaff L, Hahn F, Kloeckner R, Volkwein L, et al. Refining prediction of survival after TIPS with the novel Freiburg index of post-TIPS survival. J Hepatol. 2021;74:1362–1372. doi: 10.1016/j.jhep.2021.01.023. [DOI] [PubMed] [Google Scholar]
- 88.Korean Association for the Study of the Liver (KASL) KASL clinical practice guidelines for liver cirrhosis: ascites and related complications. Clin Mol Hepatol. 2018;24:230–277. doi: 10.3350/cmh.2018.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]