Abstract
Background/Aims
Non-alcoholic fatty liver disease (NAFLD) has become a major concern in Korea since its emergence as a dominant cause of chronic liver disease. However, no study has explored its prevalence in adults under 30 years of age. Therefore, we performed a cross-sectional study to investigate the prevalence of NAFLD in Korean men in their early 20s.
Methods
We collected data of 596,359 Korean soldiers who participated in a health examination between January 2015 and July 2021. A total of 571,872 individuals were analyzed after excluding those with missing data and hepatitis B antigen positivity. Hepatic steatosis was determined using the hepatic steatosis index (HSI). Participants with HSI >36 were considered to have NAFLD.
Results
All participants were men, and the mean age was 20.9±1.3 years. Of the 571,872 participants screened, 77,020 (13.47%) were classified as having NAFLD. The prevalence of NAFLD consistently increased from 2015 to 2021 (10.66% vs. 16.44%, P<0.001). Increases from 2015 to 2021 were also noted in the prevalence of hypercholesterolemia, hyperglycemia, and hypertension (P<0.001 for all). The mean body mass index also increased from 23.3±3.0 kg/m2 to 23.9±3.1 kg/m2 between 2015 and 2021 (P<0.001).
Conclusions
The prevalence of NAFLD and of other metabolic dysfunctions in Korean men in their early 20s increased from 2015 to 2021.
Keywords: Non-alcoholic fatty liver disease, Metabolic syndrome, Prevalence, Young adult, Korea
Graphical Abstract
Introduction
Non-alcoholic fatty liver disease (NAFLD) has become a major global concern since its emergence as a dominant cause of chronic liver disease [1]. In the Republic of Korea, various studies have reported that the prevalence of NAFLD ranges from 15% to 50% [2-4]. A recent study noted that the prevalence of NAFLD in the Korean adult population is rising, and is predicted to reach 44% by 2035 [5]. This has increased the awareness of the risk of NAFLD in the Korean society. NAFLD is defined as the accumulation of fat in the liver in the absence of alcohol consumption. The prevalence of NAFLD is higher in patients with diabetes mellitus or obesity compared to the general population [6]. Moreover, the awareness of its association with metabolic disorders has also increased, and the term “metabolic-associated fatty liver disease (MAFLD)” has recently been described in an international consensus [7,8]. MAFLD is diagnosed by “positive criteria,” which do not preclude an alcohol consumption history. The proposed positive criteria are based on the evidence of hepatic steatosis with any of the following: type 2 diabetes mellitus, obesity, or other metabolic dysregulations.
Regarding the NAFLD prognosis, a meta-analysis of 11 studies which performed paired liver biopsies revealed that the annual fibrosis progression rates were 0.07 and 0.14 stages in NAFLD and NASH patients, respectively [9]. In our institute, a study involving a national population-based cohort demonstrated that NAFLD had a significantly higher hazard ratio for the incidence of gastrointestinal tract malignancies [10]. A recent Swedish study demonstrated that the mortality rate in young patients with NAFLD was five times higher than that in the general population, thereby emphasizing the importance of NAFLD development at a young age [11].
There have been several studies on the prevalence and comorbidities of NAFLD in the Korean population [12-15]. However, the cohort sizes in some of these studies were insufficient to represent the entire population. Moreover, most of the enrolled participants were limited to the elderly, and no study has yet explored the prevalence of NAFLD in young adults aged under 30 years.
In Korea, men in their early 20s are obligated to serve in the armed forces and are forbidden from consuming alcohol in the military facilities. We accessed the Korean military database to evaluate the prevalence of NAFLD in conscripted young male individuals. To evaluate liver steatosis, we used the hepatic steatosis index (HSI). Developed by a Korean study group, the HSI represents a non-invasive modality for assessing hepatic steatosis [16]. It has a high predictive value for NAFLD, as validated in the original and subsequent studies [17,18].
We performed the present cross-sectional study in a Korean military population consisting of 596,359 men in their early 20s. Our study can be considered as a nearly complete enumeration survey of men in their early 20s, since almost all men in Korea are required to serve in the military. We aimed to determine the trends in the prevalence of NAFLD over the last 7 years in this population and the association of NAFLD with other metabolic disorders, such as dyslipidemia, hypertension, and hyperglycemia.
MATERIALS AND METHODS
Study population
In Korea, young men aged between 18 and 31 years are obligated to serve in the military for 19–23 months; and when promoted to the rank of a corporal, soldiers are required to undergo medical examinations. Data collected at these examinations are recorded in the Korean armed forces database (new defense medical information system, NDEMIS). In this study, we extracted the medical data of 596,359 men, which were recorded in the NDEMIS between January 2015 and July 2021. After excluding the subjects who were positive for the hepatitis B surface antigen and those with missing data, 571,872 men were included in this cross-sectional study (Fig. 1). This study was approved by the Institutional Review Board of the Korean Armed Forces Medical Command (AFMC-202106-HR-041-01). Informed consent was not required due to the retrospective nature of this study.
Figure 1.
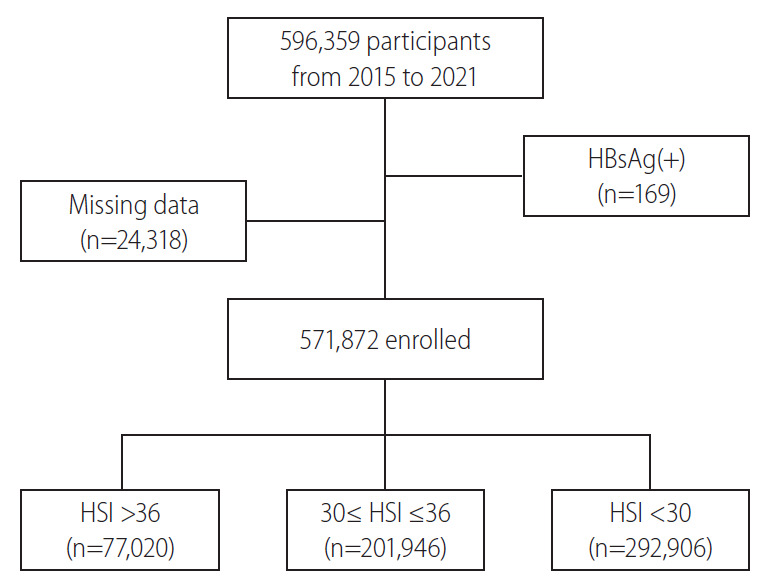
Flow chart of patient selection. HBsAg, hepatitis B surface antigen; HSI, hepatic steatosis index.
Measurements
With the participants barefoot and wearing light clothing, the height and weight were measured to the nearest 0.1 cm and 0.1kg, respectively. The body mass index (BMI; kg/m2) was calculated from the measured height and weight. The systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured to the nearest 2 mmHg using a sphygmomanometer (BPBIO330N; InBody Co. Ltd., Seoul, Korea) after 5 minutes of rest. Blood samples were collected from the antecubital vein after overnight fasting. The levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma-glutamyl transpeptidase (γGTP), triglycerides (TG), total cholesterol (TC), low-density lipoprotein (LDL), high-density lipoprotein (HDL), glucose, and creatinine were measured using a TBA-2000 FR Chemistry Analyzer (Toshiba, Tokyo, Japan). The complete blood count, hemoglobin level, hematocrit, and platelet count were measured using an XN-1000TM Hematology Analyzer (Sysmex, Kobe, Japan). Hepatitis B surface antigen and antibody levels were analyzed using electrochemiluminescence immunoassays with a Cobas 6000 analyzer (Roche Diagnostics, Basel, Switzerland).
Definitions
Hepatic steatosis was determined using the HSI, which was calculated by the following formula: HSI = 8 × (ALT/AST) + BMI (kg/m2) (+2, if female; +2, if having diabetes mellitus). Participants with HSI >36 were considered to have liver steatosis. Since the population enrolled in this study served in the armed forces, they were prohibited from drinking alcohol in the military facilities, where they spent most of their time during the period of service. Therefore, considering the circumstances wherein access to alcohol was extremely limited, NAFLD was defined as the presence of hepatic steatosis without any evidence of viral hepatitis in this study. Hypercholesterolemia and hypertriglyceridemia were defined in accordance with the Korean guidelines for management of dyslipidemia (4th edition) [19]. Accordingly, hypercholesterolemia was defined as TC ≥240 mg/dL, while hypertriglyceridemia was defined as TG ≥200 mg/dL. Dyslipidemia was diagnosed if any of the following criteria were met: TC ≥240 mg/dL, TG ≥200 mg/dL, HDL <40 mg/dL, and LDL ≥160 mg/dL. Hypertension was defined as either SBP ≥140 mmHg or DBP ≥90 mmHg, in accordance with the 2018 guidelines [20] of the Korean Society of Hypertension. Participants who had impaired fasting glucose levels or diabetes mellitus were considered as having hyperglycemia; an 8-hour fasting glucose level >100 mg/dL was considered for the diagnosis.
The fibrosis-4 (FIB-4) index and the AST-to-platelet ratio index (APRI) were used to evaluate fibrosis among those who were classified as having NAFLD [21,22]. The FIB-4 index is a widely used noninvasive predictive score for hepatic fibrosis. It is based on serologic tests, and is calculated using the following formula: (age × AST level in IU/L) / (platelet counts in 109/L × ALT level in IU/L). An FIB-4 index of 1.3 was considered as the cut-off for excluding hepatic fibrosis. The APRI was calculated using the variables AST level, the upper normal limit of AST, and the platelet count. The upper normal limit of AST was set at 32 U/L by a previous study on a Korean population [23]. The APRI was calculated as follows: APRI = (AST level in IU/L) / (the upper normal limit of AST in IU/L) / (platelet counts in 109/L). An APRI score >1.0 was considered to indicate severe fibrosis [24].
Statistical analyses
All statistical analyses were performed using SPSS version 23.0 (IBM Corp., Armonk, NY, USA) and R version 4.0.3 (R Foundation Inc.; http://cran.r-project.org). Continuous variables were expressed as means with standard deviations (SDs). The standardized mean difference (SMD), which is the difference between two means divided by a pooled SD, was calculated when comparing two different groups with continuous variables. Student’s t-test was performed to compare the continuous variables between two different groups, while an analysis of variance was performed to compare the continuous variables between three or more different groups. Categorical variables were compared using the chi-square test. The Cochran-Armitage trend test was performed to confirm the trends in NAFLD prevalence over time. Moreover, to measure the annual percent change (APC) and the average annual percent changes (AAPC) of prevalence during the 7-year examination period, the joinpoint model was applied using Joinpoint Regression Program version 4.9.0.0 (Statistical Methodology and Applications Branch, Surveillance Research Program; National Cancer Institute, Rockville, MD, USA). Statistical significance was set at P<0.05.
RESULTS
Characteristics of study participants
A total of 571,872 participants, whose medical data were registered in the NDEMIS from January 2015 to July 2021, were included in the analysis. Table 1 shows the characteristics of the study participants. Among them, 77,020 participants were classified as having NAFLD and were included in the NAFLD cohort. All of the participants were men. The mean ages were 20.9±1.3 and 21.0±1.4 years in the non-NAFLD and NAFLD cohorts, respectively (P<0.001). The mean BMI was higher in the NAFLD cohort (28.4 kg/m2) than in the non-NAFLD cohort (22.9 kg/m2). The mean SBP and DBP were also higher in the NAFLD cohort than in the non-NAFLD cohort (mean SBP, 125.7 vs. 118.2 mmHg; mean DBP, 75.0 vs. 69.7 mmHg; P<0.001). Regarding the lipid profile, the mean TC, LDL-cholesterol, and TG levels were higher in the NAFLD cohort than in the non-NAFLD cohort (TC, 184.8 vs. 168.9 mg/dL; LDL-cholesterol, 117.9 vs. 101.5 mg/dL; TG, 125.9 vs. 79.1 mg/dL; P<0.001 for all). Conversely, the mean HDL-cholesterol level was higher in the non-NAFLD cohort than in the NAFLD cohort (HDL-cholesterol, 55.0 vs. 48.9 mg/dL; P<0.001). A comparison of blood test results revealed higher white blood cell (WBC) counts, hemoglobin levels, hematocrit, and platelet count in the NAFLD cohort compared to the non-NAFLD cohort. The AST, ALT, and γGTP levels were also significantly higher in the NAFLD cohort than in the non-NAFLD cohort (P<0.001 for all).
Table 1.
Baseline characteristics of entire participants
| Non-NAFLD cohort (n=494,852) | NAFLD cohort (n=77,020) | SMD | P-value | |
|---|---|---|---|---|
| Sex, male/female | 494,852 (100.0)/0 (0.0) | 77,020 (100.0)/0 (0.0) | ||
| Age (years) | 20.9±1.3 | 21.0±1.4 | 0.120 | <0.001 |
| Height (cm) | 174.3±5.6 | 174.4±5.8 | 0.017 | <0.001 |
| Weight (kg) | 69.7±8.3 | 86.7±11.2 | 1.725 | <0.001 |
| BMI (kg/cm2) | 22.9±2.3 | 28.4±3.1 | 2.066 | <0.001 |
| SBP (mm/Hg) | 118.2±12.0 | 125.7±12.5 | 0.613 | <0.001 |
| DBP (mm/Hg) | 69.7±9.0 | 75.0±9.8 | 0.563 | <0.001 |
| Lipid profile | ||||
| Total cholesterol (mg/dL) | 168.9±28.0 | 184.8±33.0 | 0.520 | <0.001 |
| HDL-cholesterol (mg/dL) | 55.0±10.5 | 48.9±9.5 | 0.602 | <0.001 |
| LDL-cholesterol (mg/dL) | 101.5±24.0 | 117.9±28.0 | 0.629 | <0.001 |
| Triglyceride (mg/dL) | 79.1±45.7 | 125.9±84.9 | 0.687 | <0.001 |
| Fasting glucose (mg/dL) | 90.4±8.1 | 92.3±8.9 | 0.228 | <0.001 |
| WBC (1,000/μL) | 6.6±1.6 | 7.2±1.7 | 0.384 | <0.001 |
| Hemoglobin (g/dL) | 15.5±0.9 | 15.9±0.9 | 0.393 | <0.001 |
| Hematocrit (%) | 45.6±2.5 | 46.5±2.5 | 0.356 | <0.001 |
| Platelet (109/L) | 241.9±45.4 | 258.2±49.4 | 0.344 | <0.001 |
| Creatinine (mg/dL) | 0.96±0.13 | 0.94±0.13 | 0.142 | <0.001 |
| AST (IU/L) | 23.8±18.7 | 28.9±16.6 | 0.292 | <0.001 |
| ALT (IU/L) | 18.8±10.8 | 46.1±38.9 | 0.958 | <0.001 |
| γGTP (IU/L) | 17.9±8.2 | 33.4±23.4 | 0.885 | <0.001 |
Values are presented as mean±standard deviation or number (%).
NAFLD, non-alcoholic fatty liver disease; SMD, standardized mean difference; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein; WBC, white blood cell; AST, aspartate aminotransferase; ALT, alanine aminotransferase; γGTP, gamma-glutamyl transpeptidase.
Year-wise comparison of NAFLD prevalence
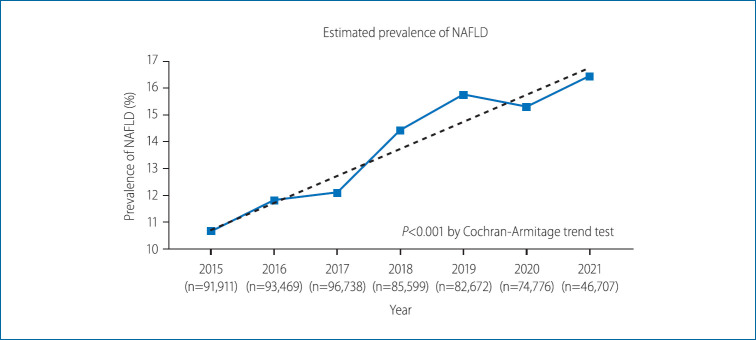
The prevalence of NAFLD from 2015 to 2021 is presented in Figure 2. The overall prevalence of NAFLD over the entire study period was 13.47% (77,020/571,872 participants evaluated as having NAFLD). In 2015, the prevalence was estimated to be 10.66% (9,798/91,911 participants). It continued to rise over the years, with the prevalence rate being reported as 11.81% in 2016 (11,040/93,469 participants), 12.09% in 2017 (11,698/96,378 participants), 14.42% in 2018 (12,345/85,599 participants), and 15.75% in 2019 (13,022/83,483 participants). In 2020, the prevalence seemed to slightly decline at 15.30% (11,439/74,776 participants); however, the upward trend was recovered in 2021 at 16.44% (7,678/46,707). The prevalence of NAFLD showed a tendency to increase over the years, with P for trend <0.001. The absolute change in the prevalence over the 7-year period was 5.78%, indicating an annual increase of approximately 0.83%.
Figure 2.
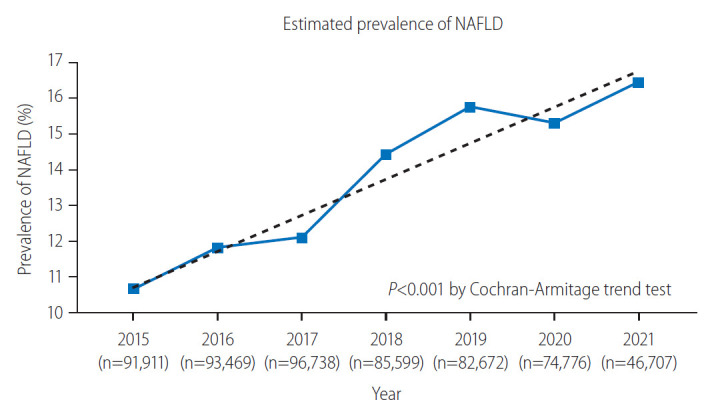
Comparison of estimated non-alcoholic fatty liver disease (NAFLD) prevalence over the study period.
Year-wise comparison of baseline characteristics in the entire cohort
The baseline characteristics of the cohorts in each year are presented in Table 2. The mean BMI, ALT, TC, and platelet count kept rising until 2021; their values were the lowest in 2015 and the highest in 2021 (BMI, 23.3 vs. 23.9 kg/m2; ALT, 21.4 vs. 24.9 mg/dL; TC, 169.7 vs. 174.3 mg/dL; platelet count, 239.1×103/L vs. 251.9×103/L; P<0.001 for all). The fasting glucose levels were also the highest in 2021. However, the mean TG levels and WBC counts were the lowest in 2021 (TG, 85.7 mg/dL in 2015 vs. 82.4 mg/dL in 2021; WBC, 6.9×103/L in 2015 vs. 6.5×103/L in 2021; P<0.001 for both).
Table 2.
Comparison of baseline characteristics by year
| 2015 (n=91,911) | 2016 (n=93,469) | 2017 (n=96,738) | 2018 (n=85,999) | 2019 (n=82,672) | 2020 (n=74,776) | 2021 (n=46,707) | P-value | |
|---|---|---|---|---|---|---|---|---|
| BMI (kg/m2) | 23.3±2.9 | 23.5±3.0 | 23.6±2.9 | 23.8±3.1 | 23.8±3.2 | 23.8±3.1 | 23.9±3.1 | <0.001 |
| ALT (U/L) | 21.4±17.4 | 21.3±18.1 | 21.6±18.1 | 22.4±19.7 | 23.4±23.4 | 24.0±20.9 | 24.9±21.6 | <0.001 |
| FBS (mg/dL) | 90.8±8.6 | 89.9±8.1 | 90.1±8.2 | 90.5±8.4 | 91.2±8.5 | 90.8±7.7 | 91.9±7.9 | <0.001 |
| TC (mg/dL) | 169.7±28.8 | 171.7±29.2 | 171.0±28.8 | 170.6±29.0 | 169.9±29.2 | 171.7±29.7 | 174.3±30.1 | <0.001 |
| TG (mg/dL) | 85.7±55.2 | 85.3±54.9 | 85.1±55.3 | 86.7±56.2 | 87.8±57.6 | 83.3±52.8 | 82.4±51.3 | <0.001 |
| WBC (1,000/μL) | 6.9±1.7 | 6.8±1.7 | 6.8±1.6 | 6.7±1.6 | 6.5±1.6 | 6.5±1.6 | 6.5±1.5 | <0.001 |
| PLT (109/L) | 239.1±45.1 | 240.4±45.6 | 241.9±46.0 | 242.9±46.2 | 247.4±46.7 | 250.7±46.4 | 251.9±46.9 | <0.001 |
Values are presented as mean±standard deviation.
BMI, body mass index; ALT, alanine aminotransferase; FBS, fasting blood sugar; TC, total cholesterol; TG, triglycerides; WBC, white blood cell; PLT, platelet.
Comparison of the prevalence of metabolic dysfunctions between 2015–2017 and 2018–2021
We categorized the participants into group A (n=282,118; medical data collected during 2015–2017) and group B (n=289,754; medical data collected during 2018–2021) to ensure that the group sizes were matched. The prevalence of metabolic dysfunction was compared between these two groups (Fig. 3). The prevalence of hypertriglyceridemia did not significantly differ between group A (3.69%) and group B (3.64%) (P=0.282). Conversely, the prevalence of hypertension was significantly higher in group B (3.00%) than in group A (2.42%) (P<0.001). Hyperglycemia was significantly more prevalent in group B (9.99%) than in group A (8.69%) (P<0.001). The prevalence of hypercholesterolemia was significantly higher in group B (2.08%) than in group A (1.89%) (P<0.001). Overall, the prevalence of hypertension, hyperglycemia, and hypercholesterolemia increased over time.
Figure 3.
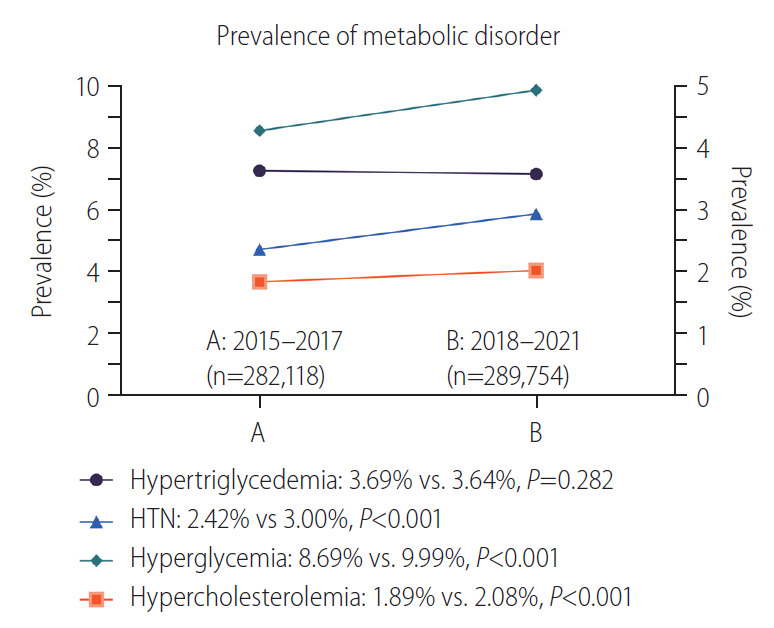
Linear plot on prevalence of metabolic disorder (2015–2017 vs. 2018–2021). HTN, hypertension.
NAFLD prevalence by subgroups
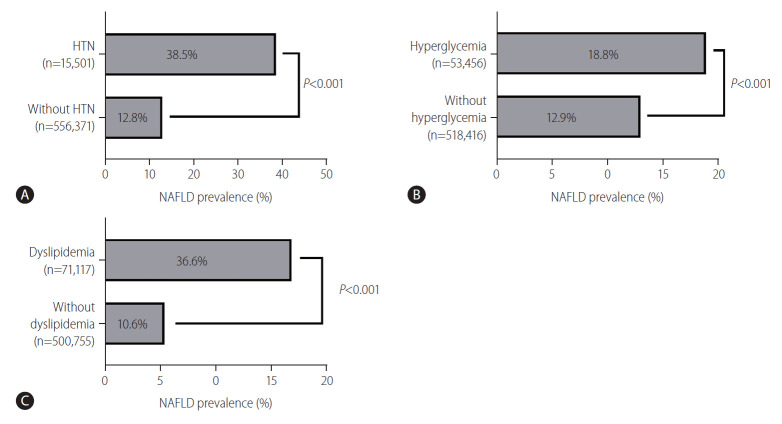
We compared the NAFLD prevalence between the presence and absence of comorbidities. Figure 4 is a bar plot graph that shows the subgroup-wise prevalence of NAFLD. The number of participants with and without hypertension was 15,501 (hypertension group, 2.71%) and 556,371 (non-hypertension group, 97.29%), respectively. The prevalence of NAFLD was higher in the hypertension group (38.9%) than in the non-hypertension group (12.8%) (P<0.001). The number of participants with and without hyperglycemia was 53,456 (hyperglycemia group; 9.35%) and 518,416 (non-hyperglycemia group; 90.65%) respectively. The prevalence of NAFLD was higher in the hyperglycemia group (18.8%) than in the non-hyperglycemia group (12.9%) (P<0.001). The numbers of participants with and without dyslipidemia were 71,117 (dyslipidemia group) and 500,755 (non-dyslipidemia group), respectively. The prevalence of NAFLD was higher in the dyslipidemia group (33.6%) than in the non-dyslipidemia group (10.6%) (P<0.001).
Figure 4.
Non-alcoholic fatty liver disease (NAFLD) prevalence by subgroups. (A) NAFLD prevalence in participants with or without hypertension (HTN). (B) NAFLD prevalence in participants with or without hyperglycemia. (C) NAFLD prevalence in participants with or without dyslipidemia.
Evaluation of fibrosis using serologic tools
First, using the FIB-4 index, the prevalence of fibrosis among patients with NAFLD was found to be only 0.08% (61/77,020) with cut-off values of 1.3. Then, using the APRI, the prevalence of severe fibrosis among patients with NAFLD was found to be 1.64% (1,261/77,020); these patients had an APRI >1.0. Furthermore, we used the same serologic indices to estimate the prevalence of fibrosis among patients without NAFLD. The prevalence was estimated to be 0.69% (3,443/494,852) and 0.98% (4,833/494,852) using the FIB-4 index and the APRI, respectively.
Future prediction of NAFLD prevalence using the joinpoint model
We used the joinpoint regression analysis to calculate the APC and the AAPC of prevalence. The APC was 10.1% from 2015 to 2019 and 1.9% from 2019 to 2021. Overall, the AAPC in the NAFLD prevalence during the study period was 7.9% (4.9–10.9%, 95% confidence interval) per year. We predicted the future prevalence of NAFLD among young men using a formula driven from the joinpoint regression analysis as follows: in (prevalence) = -150.129 + Year × 0.07569.
Using the aforementioned formula, the estimated prevalence in year 2030 was calculated to be 33.91% (Fig. 5).
Figure 5.
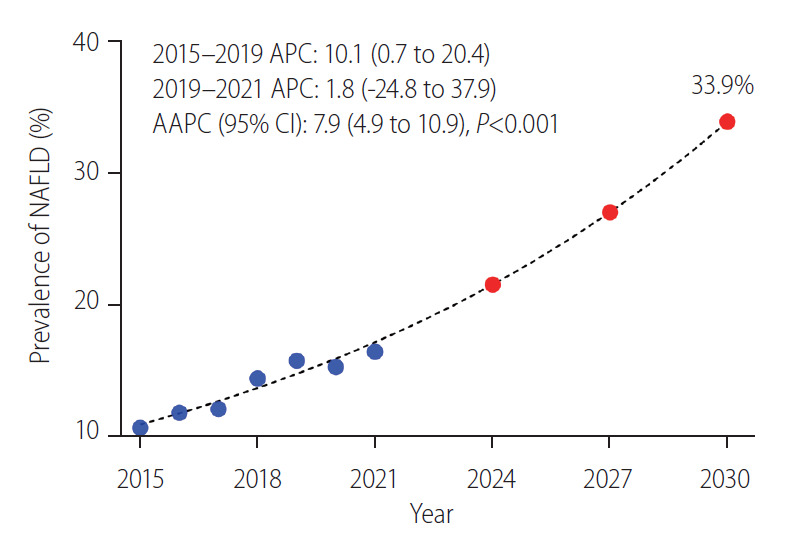
Future prediction of non-alcoholic fatty liver disease (NAFLD) prevalence using the joinpoint model. APC, annual percent change; AAPC, average annual percent changes.
Discussion
The current study analyzed the data of young men in Korea (mean age, 20.9 years), and found that the prevalence of NAFLD increased over time from 10.66% in 2015 to 16.44% in 2021 (annual increase: approximately 0.83%). This trend was statistically significant (P<0.001). Using the joinpoint model, the AAPC of NAFLD prevalence was 7.9%, and the prevalence was expected to reach 33.91% by 2030 using the aforementioned model. The prevalence of metabolic dysfunctions (hypercholesterolemia, hyperglycemia, and hypertension) also increased from 2015 to 2021. The BMI also showed a clear tendency to increase.
These results and the observed tendency were in line with the findings of previous studies conducted in Korea and other parts of the world. Kang et al. [5] showed that the prevalence of NAFLD has increased over time (with an annual increase rate of 2.3%), and is predicted to reach 43.8% by 2035. Many studies in Korea have also reported an increase in NAFLD prevalence over time [25-27]. Outside Korea, Arshad et al. [28] investigated 4,654 young adults (aged 12–29 years) from the USA; they also reached a similar conclusion that the prevalence of NAFLD among individuals aged 18–24 years increased during 2007–2016.
Our study also showed that the prevalence of NAFLD tended to be higher among patients with metabolic dysfunctions, such as hyperglycemia, dyslipidemia, and hypertension. The NAFLD prevalence was 38.5% in the hypertension group and 12.8% in the non-hypertension group. Although lower than in the hypertension group, the prevalence of NAFLD was higher in the hyperglycemia and dyslipidemia groups than in the non-hyperglycemia and nondyslipidemia groups, respectively. In line with our findings, the association between the metabolic syndrome and NAFLD has been widely reported [29,30]. Furthermore, the recently proposed term “MAFLD” encompasses metabolic dysfunctions as criteria for diagnosis, implying that it is crucial to consider metabolic dysfunctions while treating NAFLD.
As the majority of individuals aged >30 years are willing to undergo health examinations, sufficient data have been reported on the prevalence of NAFLD in this age group. However, little is known concerning the prevalence of NAFLD in younger adults not only in Korea, but also worldwide, due to a lack of need for medical surveys. To the best of our knowledge, this study is the first to present the prevalence of NAFLD and metabolic dysfunction in Korean young adults with a sufficiently large sample size. As most Korean men in their early 20s are required to serve in the military, it can be assumed that our study has a credibility close to that of a full-scale survey.
Considering the findings of previous studies, an increase in populations with NAFLD might pose a severe threat to the society. The all-cause mortality was up to five times higher in young adults with NAFLD, and there have been reports that NAFLD might be associated with compromised reproductive health in both men and women [31]. Furthermore, NAFLD is also known to be associated with many other extra-hepatic comorbidities, such as cardiovascular disease and chronic kidney disease [32-35]. Based on these reports, our findings can be crucial to the society, as they indicate that the national health status can be threatened by NAFLD complications in the near future.
Recently, the Korean society underwent rapid changes in lifestyle and social behaviors. According to the Korea National Health and Nutrition Examination Survey, Koreans showed a clear tendency of consuming more high-fat foods and were less likely to perform high-intensity exercise in the last decade [36]. The increasing prevalence of NAFLD may be partly elucidated by the foregoing facts. Nevertheless, the participants in this study were serving in the military, where strict lifestyle is required, raising the question regarding the nutritional balance within the Korean army. Convenience stores in military bases provide various types of food, including instant meals and high-fat food, which might provoke nutritional imbalance and metabolic disorders [37]. Therefore, we propose a routine Korean military nutritional survey, as the Korea National Health and Nutrition Examination Survey has done for decades with Korean populations, to stratify possible risk factors regarding nutrition and to intervene in dietary patterns following the outcomes of the survey. By improving the nutritional balance of young men serving in the military, the Republic of Korea Armed Forces may contribute to the Korean society by lowering the estimated prevalence of NAFLD, which is predicted to reach 33.91% by the year 2030.
This study had some limitations. First, it used a non-invasive method, the HSI, to determine liver steatosis. Although the HSI is primarily designed for Korean populations and has been well-validated in other studies, ultrasonography and biopsy are preferable for defining fatty liver with a higher reliability. Second, we only used serologic tools for evaluating fibrosis. The FIB-4 index and the APRI are among the most commonly used serologic tools for detecting hepatic fibrosis. However, several previous studies have noted that the FIB-4 index had a low predictive value for hepatic fibrosis in patients aged <35 years, and advised using it with caution [38]. In addition, in the case of APRI, it reportedly has a low predictive efficiency for fibrosis in those aged <30 years [39]. Considering the low predictive value and the discrepancy between the results from these tools, further studies using biopsy or magnetic resonance elastography are required to better predict the prevalence of significant fibrosis in patients with NAFLD. Third, selection bias cannot be ruled out. Although most Korean men are required to serve in the military, some with physical disabilities are advised not to. In order to receive military exemption for fatty liver, advanced fibrosis must be proven through biopsy, which is very difficult in real-life practice. Finally, insufficient data were provided to discriminate other chronic liver diseases, such as alcoholic fatty liver and chronic hepatitis C. There was no questionnaire asking regarding alcohol consumption, although we can presume that extremely low percentage of participants would have alcoholic fatty liver disease, given the fact that their mean age was only 21 and that time of examination was 1 year after enlistment. Regarding HCV infection, only selected participants who showed elevated ALT or AST levels were tested for HCV serology, although none of them were positive for HCV antibody. In contrast, according to data from the Korean National Health and Nutritional Examination Survey, only 0.2% of Koreans in their 20s had hepatitis C, which implies that there would be only little portion of participants are HCV antibody-positive [36]. Although such possibilities of having chronic liver diseases are low, complete exclusion of the aforementioned disease would be preferable in evaluating the prevalence of NAFLD.
In conclusion, we used the HSI and showed that the prevalence of NAFLD among young Korean men increased in the last 7 years. The prevalence of metabolic dysfunctions has also increased. These results are critically important, as they imply an increase in socio-economic disease burden for young men with NAFLD [40]. Therefore, the Korean society should remain alert for an increase in NAFLD prevalence and continuously undertake efforts to reduce its complications. In this context, although efficient treatment options are important, active screening of patients with metabolic disorders should also be considered. Further longitudinal studies are required to demonstrate the possible risk and socio-economic costs of NAFLD in young adults.
Acknowledgments
This research was supported by the Korean Military Medical Research Project funded by the ROK Ministry of National Defense (ROK-MND-2021-KMMRP-014).
This research was also supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (2020R1A2C3011569).
Abbreviations
- AAPC
average annual percent change
- ALT
alanine aminotransferase
- APC
annual percent change
- APRI
AST-to-platelet ratio index
- AST
aspartate aminotransferase
- BMI
body mass index
- DBP
diastolic blood pressure
- FIB-4
fibrosis-4
- HDL
high-density lipoprotein
- HSI
hepatic steatosis index
- LDL
low-density lipoprotein
- MAFLD
metabolic-associated fatty liver disease
- NAFLD
non-alcoholic fatty liver disease
- NDEMIS
new defense medical information system
- SBP
systolic blood pressure
- SDs
standard deviations
- SMD
standardized mean difference
- TC
total cholesterol
- TG
triglycerides
- WBC
white blood cell
- γGTP
gamma-glutamyl transpeptidase.
Study Highlights
The overall prevalence of NAFLD in young men during the study period (from 2015 to 2021) was 13.47%. The prevalence of NAFLD continued to rise in the last 7 years, with prevalence rate of 10.66% in 2015 and 16.44% in 2021. The Korean society should be alert for an increase in disease prevalence and continue making efforts to reduce the associated complications.
Footnotes
Authors’ contributions
Study concept and design: Si Hyun Bae and Jaejun Lee; Data collection: Jaejun Lee, Taeyun Kim; Data analysis and interpretation: Jaejun Lee, Hyun Yang, and Si Hyun Bae; manuscript writing: Jaejun Lee and Si Hyun Bae; Final approval of the version to be published: All authors
Conflicts of Interest
The authors have no conflicts to disclose.
REFERENCES
- 1.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 2.Im HJ, Ahn YC, Wang JH, Lee MM, Son CG. Systematic review on the prevalence of nonalcoholic fatty liver disease in South Korea. Clin Res Hepatol Gastroenterol. 2021;45:101526. doi: 10.1016/j.clinre.2020.06.022. [DOI] [PubMed] [Google Scholar]
- 3.Park J, Lee EY, Li J, Jun MJ, Yoon E, Ahn SB, et al. NASH/liver fibrosis prevalence and incidence of nonliver comorbidities among people with NAFLD and incidence of NAFLD by metabolic comorbidities: lessons from South Korea. Dig Dis. 2021;39:634–645. doi: 10.1159/000514953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kang SH, Lee HW, Yoo JJ, Cho Y, Kim SU, Lee TH, et al. KASL clinical practice guidelines: management of nonalcoholic fatty liver disease. Clin Mol Hepatol. 2021;27:363–401. doi: 10.3350/cmh.2021.0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kang SY, Kim YJ, Park HS. Trends in the prevalence of non-alcoholic fatty liver disease and its future predictions in Korean men, 1998-2035. J Clin Med. 2020;9:2626. doi: 10.3390/jcm9082626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhatt HB, Smith RJ. Fatty liver disease in diabetes mellitus. Hepatobiliary Surg Nutr. 2015;4:101–108. doi: 10.3978/j.issn.2304-3881.2015.01.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eslam M, Sanyal AJ, George J, International Consensus Panel MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158:1999–2014.e1. doi: 10.1053/j.gastro.2019.11.312. [DOI] [PubMed] [Google Scholar]
- 8.Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, RomeroGomez M, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73:202–209. doi: 10.1016/j.jhep.2020.03.039. [DOI] [PubMed] [Google Scholar]
- 9.Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, Loomba R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol. 2015;13:643–654.e1-e9. doi: 10.1016/j.cgh.2014.04.014. quiz e39-e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee JM, Park YM, Yun JS, Ahn YB, Lee KM, Kim DB, et al. The association between nonalcoholic fatty liver disease and esophageal, stomach, or colorectal cancer: national population-based cohort study. PLoS One. 2020;15:e0226351. doi: 10.1371/journal.pone.0226351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simon TG, Roelstraete B, Hartjes K, Shah U, Khalili H, Arnell H, et al. Non-alcoholic fatty liver disease in children and young adults is associated with increased long-term mortality. J Hepatol. 2021;75:1034–1041. doi: 10.1016/j.jhep.2021.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim JW, Lee KJ, Yang HR, Chang JY, Moon JS, Khang YH, et al. Prevalence and risk factors of elevated alanine aminotransferase among Korean adolescents: 2001-2014. BMC Public Health. 2018;18:617. doi: 10.1186/s12889-018-5548-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang Y, Park S, Kim S, Koh H. Estimated prevalence of adolescents with nonalcoholic fatty liver disease in Korea. J Korean Med Sci. 2018;33:e109. doi: 10.3346/jkms.2018.33.e109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeong EH, Jun DW, Cho YK, Choe YG, Ryu S, Lee SM, et al. Regional prevalence of non-alcoholic fatty liver disease in Seoul and Gyeonggi-do, Korea. Clin Mol Hepatol. 2013;19:266–272. doi: 10.3350/cmh.2013.19.3.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park SH, Plank LD, Suk KT, Park YE, Lee J, Choi JH, et al. Trends in the prevalence of chronic liver disease in the Korean adult population, 1998-2017. Clin Mol Hepatol. 2020;26:209–215. doi: 10.3350/cmh.2019.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee JH, Kim D, Kim HJ, Lee CH, Yang JI, Kim W, et al. Hepatic steatosis index: a simple screening tool reflecting nonalcoholic fatty liver disease. Dig Liver Dis. 2010;42:503–508. doi: 10.1016/j.dld.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Jung TY, Kim MS, Hong HP, Kang KA, Jun DW. Comparative assessment and external validation of hepatic steatosis formulae in a community-based setting. J Clin Med. 2020;9:2851. doi: 10.3390/jcm9092851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang JW, Lee HW, Kim BK, Park JY, Kim DY, Ahn SH, et al. Hepatic steatosis index in the detection of fatty liver in patients with chronic hepatitis b receiving antiviral therapy. Gut Liver. 2021;15:117–127. doi: 10.5009/gnl19301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rhee EJ, Kim HC, Kim JH, Lee EY, Kim BJ, Kim EM, et al. 2018 Guidelines for the management of dyslipidemia. Korean J Intern Med. 2019;34:723–771. doi: 10.3904/kjim.2019.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim HC, Ihm SH, Kim GH, Kim JH, Kim KI, Lee HY, et al. 2018 Korean Society of Hypertension guidelines for the management of hypertension: part I-epidemiology of hypertension. Clin Hypertens. 2019;25:16. doi: 10.1186/s40885-019-0121-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–526. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 22.Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–1325. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 23.Sohn W, Jun DW, Kwak MJ, Park Q, Lee KN, Lee HL, et al. Upper limit of normal serum alanine and aspartate aminotransferase levels in Korea. J Gastroenterol Hepatol. 2013;28:522–529. doi: 10.1111/j.1440-1746.2012.07143.x. [DOI] [PubMed] [Google Scholar]
- 24.Lin ZH, Xin YN, Dong QJ, Wang Q, Jiang XJ, Zhan SH, et al. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepatology. 2011;53:726–736. doi: 10.1002/hep.24105. [DOI] [PubMed] [Google Scholar]
- 25.Lim S, Shin H, Song JH, Kwak SH, Kang SM, Yoon JW, et al. Increasing prevalence of metabolic syndrome in Korea: the Korean National Health and Nutrition Examination Survey for 1998-2007. Diabetes Care. 2011;34:1323–1328. doi: 10.2337/dc10-2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee SE, Han K, Kang YM, Kim SO, Cho YK, Ko KS, et al. Trends in the prevalence of metabolic syndrome and its components in South Korea: findings from the Korean National Health Insurance Service Database (2009-2013) PLoS One. 2018;13:e0194490. doi: 10.1371/journal.pone.0194490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim MH, Lee SH, Shin KS, Son DY, Kim SH, Joe H, et al. The change of metabolic syndrome prevalence and its risk factors in Korean adults for decade: Korea National Health and Nutrition Examination Survey for 2008–2017. Korean J Fam Pract. 2020;10:44–52. [Google Scholar]
- 28.Arshad T, Paik JM, Biswas R, Alqahtani SA, Henry L, Younossi ZM. Nonalcoholic fatty liver disease prevalence trends among adolescents and young adults in the United States, 2007-2016. Hepatol Commun. 2021;5:1676–1688. doi: 10.1002/hep4.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oikonomou D, Georgiopoulos G, Katsi V, Kourek C, Tsioufis C, Alexopoulou A, et al. Non-alcoholic fatty liver disease and hypertension: coprevalent or correlated? Eur J Gastroenterol Hepatol. 2018;30:979–985. doi: 10.1097/MEG.0000000000001191. [DOI] [PubMed] [Google Scholar]
- 30.Kotronen A, Westerbacka J, Bergholm R, Pietiläinen KH, Yki-Järvinen H. Liver fat in the metabolic syndrome. J Clin Endocrinol Metab. 2007;92:3490–3497. doi: 10.1210/jc.2007-0482. [DOI] [PubMed] [Google Scholar]
- 31.Doycheva I, Watt KD, Alkhouri N. Nonalcoholic fatty liver disease in adolescents and young adults: the next frontier in the epidemic. Hepatology. 2017;65:2100–2109. doi: 10.1002/hep.29068. [DOI] [PubMed] [Google Scholar]
- 32.Rosato V, Masarone M, Dallio M, Federico A, Aglitti A, Persico M. NAFLD and extra-hepatic comorbidities: current evidence on a multi-organ metabolic syndrome. Int J Environ Res Public Health. 2019;16:3415. doi: 10.3390/ijerph16183415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.An JN, Joo SK, Koo BK, Kim JH, Oh S, Kim W. Portal inflammation predicts renal dysfunction in patients with nonalcoholic fatty liver disease. Hepatol Int. 2020;14:798–807. doi: 10.1007/s12072-020-10063-9. [DOI] [PubMed] [Google Scholar]
- 34.Kim HL, Koo BK, Joo SK, Kim W. Association of arterial stiffness with the histological severity of nonalcoholic fatty liver disease. Hepatol Int. 2020;14:1048–1056. doi: 10.1007/s12072-020-10108-z. [DOI] [PubMed] [Google Scholar]
- 35.Park JH, Koo BK, Kim W, Kim WH, Innovative Target Exploration of NAFLD (ITEN) Consortium Histological severity of nonalcoholic fatty liver disease is associated with 10-year risk for atherosclerotic cardiovascular disease. Hepatol Int. 2021;15:1148–1159. doi: 10.1007/s12072-021-10209-3. [DOI] [PubMed] [Google Scholar]
- 36.Ministry of Health and Welfare. Korea Centers for Disease Control and Prevention . Korea Health Statistics 2019: Korea National Health and Nutrition Examination Survey (KNHANES VIII-1) Cheongju: Korea Centers for Disease Control and Prevention; 2020. [Google Scholar]
- 37.Yang JY, Yu JA, Jang JE. A nutritional environment survey in military camps. Korean J Mil Nurs Res. 2016;34:31–40. [Google Scholar]
- 38.McPherson S, Hardy T, Dufour JF, Petta S, Romero-Gomez M, Allison M, et al. Age as a confounding factor for the accurate noninvasive diagnosis of advanced NAFLD fibrosis. Am J Gastroenterol. 2017;112:740–751. doi: 10.1038/ajg.2016.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Q, Lu C, Li W, Huang Y, Chen L. Impact of age on the diagnostic performances and cut-offs of APRI and FIB-4 for significant fibrosis and cirrhosis in chronic hepatitis B. Oncotarget. 2017;8:45768–45776. doi: 10.18632/oncotarget.17470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Younossi ZM, Blissett D, Blissett R, Henry L, Stepanova M, Younossi Y, et al. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology. 2016;64:1577–1586. doi: 10.1002/hep.28785. [DOI] [PubMed] [Google Scholar]