Abstract
Background
Associations between vitamin D (VD) deficiency and the risk of SARS-CoV-2 infection have been documented in cross-sectional population studies. Intervention studies in patients with moderate to severe COVID-19 have failed to consistently document a beneficial effect.
Objective
To determine the efficacy and safety of VD-supplementation in the prevention of SARS-CoV-2 infection in highly exposed individuals.
Methods
A double-blind, parallel, randomized trial was conducted. Frontline healthcare workers from four hospitals in Mexico City, who tested negative for SARS-CoV-2 infection, were enrolled between July 15 and December 30, 2020. Participants were randomly assigned to receive 4,000 IU VD (VDG) or placebo (PG) daily for 30 d. RT-PCR tests were taken at baseline and repeated if COVID-19 manifestations appeared during follow-up. Serum 25-hydroxyvitamin D3 and antibody tests were measured at baseline and at day 45. Per-protocol and intention-to-treat analysis were conducted.
Results
Of 321 recruited subjects, 94 VDG and 98 PG completed follow-up. SARS-CoV-2 infection rate was lower in VDG than in PG (6.4 vs. 24.5%, p <0.001). The risk of acquiring SARS-CoV-2 infection was lower in the VDG than in the PG (RR: 0.23; 95% CI: 0.09–0.55) and was associated with an increment in serum levels of 25-hydroxyvitamin D3 (RR: 0.87; 95% CI: 0.82–0.93), independently of VD deficiency. No significant adverse events were identified.
Conclusions
Our results suggest that VD-supplementation in highly exposed individuals prevents SARS-CoV-2 infection without serious AEs and regardless of VD status.
Key Words: SARS-CoV-2, COVID-19, Vitamin D, Healthcare workers, 25-hydroxyvitamin D3
Introduction
SARS-CoV-2, the cause of COVID-19, has led to more than 338 million cases and over 5.71 million deaths worldwide as for February 4, 2022. Mexico is one of the most affected countries, with more than 5.03 million infections and more than 307,000 deaths (1). COVID-19 pandemic coexists with vitamin D (VD) deficiency, which is also a major public health problem, both globally (2., 3., 4.) and in Mexico (5). This is relevant because of the well-known immunomodulatory role of VD as it attenuates Th1 cell and stimulates Th2 cell proliferation, favoring the synthesis and secretion of anti-inflammatory cytokines, and limiting the production of pro-inflammatory mediators (6). Therefore, it is expected that VD deficiency increases the susceptibility to bacterial and viral infections, including SARS-CoV-2.
Very active research regarding VD and COVID-19 has been conducted in the last two years. Cross sectional, as well as large population-based studies have found an inverse correlation between serum 25-hydroxyvitamin D3 concentrations and SARS-Cov-2 positivity rate, severity, and mortality (7., 8., 9., 10., 11., 12.). Furthermore, meta-analyses of case-control and cohort studies conclude that individuals with lower 25-hydroxyvitamin D3 concentrations not only have an increased risk of SARS-CoV-2 positivity but are also more likely to require admission to intensive care units and to dye from the disease. However, most of these meta-analyses report a high degree of heterogeneity, and low precision or certainty of the analyzed studies (13., 14., 15.).
The effect of VD supplementation to COVID-19 patients has been evaluated in intervention studies with inconsistent results probably due to differences in doses, duration of supplementation, evaluated outcomes, and sample size. For instance, Murai IH, et al. (16) failed to detect differences in hospital length-of-stay of patients with COVID-19 who received a high single VD dose compared to those who did not. In contrast, Quesada and his team reported that weekly high-dose calcifediol, a potent VD metabolite, decreased intensive care requirement and mortality in COVID-19 patients (17,18). Rastogi A, et al. found that patients with mild COVID-19 and VD deficiency, who received high oral doses of VD, tested negative for SARS-CoV-2 earlier than controls (19).
Thus, although it appears that VD supplementation of COVID-19 patients has a positive impact in disease outcomes, there is scarce information regarding the role of VD as a preventive measure to reduce the rate of SARS-Cov-2 infection in subjects with varying degrees of susceptibility. Therefore, we aimed to determine the efficacy and safety of VD supplementation to prevent SARS-CoV-2 infection in highly exposed individuals.
Subjects and Methods
Design and Study Participants
Our primary outcomes were the rate of SARS-CoV-2 infection and the severity of the disease. Secondary endpoints included the reduction of VD deficiency prevalence and the frequency of treatment-associated adverse events (AEs). A multicenter, double-blind, parallel placebo-controlled, randomized clinical trial was conducted in Mexico City. Frontline healthcare workers from four tertiary-care hospitals converted to COVID-19 units were enrolled between July 15 and December 30, 2020, prior to vaccination. The protocol was approved by the institutional review boards of the Instituto Mexicano del Seguro Social (IMSS) system, which includes three hospitals (CNSI # R-2020-785-090) and the Hospital Infantil de México Federico Gómez (HIM 2020-045). All participants provided written informed consent. The study follows the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
We included SARS-Cov-2-free healthcare workers caring for hospitalized patients with COVID-19, who were not receiving supplementation with VD or other vitamins. Subjects with an immunocompromising condition, such as cancer or autoimmune disorders, were excluded. Subjects were randomly assigned to receive VD (VDG) or placebo (PG) in a 1:1 allocation fashion for 30 d. Randomization was conducted using a web software (Research Randomizer; https://www.randomizer.org/) by a senior researcher who had no contact with participants (MAVK). VD was administered as daily 4,000 IU cholecalciferol capsules (histofil®; Medix S.A.de CV; Mexico City, Mexico) or placebo (capsules with 450 mg cornstarch. ISAAQUIM, Mexico City, Mexico) with identical appearance.
Invitation posters were placed in the work areas of the participant hospitals explaining the goals and procedures of the study. An appointment was given to individuals who agreed to participate, to sign informed consent and for screening procedures. A full medical history and physical exam was obtained in all participating subjects upon recruitment, paying particular attention to the presence of chronic conditions such as hypertension, diabetes mellitus, dyslipidemia, and coronary artery disease. During this initial visit a nasopharyngeal swab specimen for the identification of SARS-Cov-2 by RT-PCR as well as blood for analytical purposes were obtained. Laboratory determinations included serum IgG antibodies against SARS-Cov-2 and blood chemistry, as well as serum concentrations of 25-hydroxyvitamin D3. Overweight and obesity were defined by a body mass index between 25–30 and above 30, respectively. Follow up visits took place on days 7, 14, 21, 28 and 45. Serum IgG antibodies against SARS-Cov-2 were monitored at baseline and on day 45. All investigators remained blinded for the entire duration of the study.
AEs were registered as per local regulatory requirements, including nausea, loss of appetite, vomiting, abdominal pain, diarrhea, headache, dizziness, drowsiness, constipation, muscle weakness, and rash. Information on whether the AEs were related to the study medication or resulting in medication discontinuation was also recorded.
Laboratory Procedures
The presence of the virus was established in accordance with the Standardized Guidelines for Epidemiological Surveillance by a certified COVID-19 Laboratory (20). SARS-CoV-2 was determined by RT-PCR. A result was considered positive if the sample showed amplification for any of the viral markers (Ct <37) and the RP control, and as negative a sample without amplification for viral markers, but with amplification for the RP control. Qualitative detection of IgG antibodies to SARS-CoV-2 in serum was determined with a chemiluminescent microparticle immunoassay (Alinity i and ARCHITECT i1000SR Abbott Systems, and LIAISON® SARS-CoV-2 S1/S2 IgG, with LIAISON® XL Analyzer of DiaSorin). A result was registered as positive if IgG antibodies to SARS-CoV-2 were higher than 5.0 AU/mL and S1/S2 IgG antibodies were higher than 15.0 AU/mL (21).
Serum concentration of 25-hydroxyvitamin D3 was determined in the Unit of Research in Medical Nutrition, whose lab technicians are certified in such measurements. Concentration of 25-hydroxyvitamin D3 was determined with the method described by Van Den Ouweland KM, et al. (22), using a Waters ACQUITYH UPLC Class coupled to a Xevo TQD (Waters, Milford, MA, USA), with an APCI Ion SABRE II probe. A solid phase extraction cartridge (Strata C18-E; 55 µm, 70 A, Phenomenex CA, USA) was used. Chromatographic analyses were performed with a C18-column at 50°C (Kinetex 1.7 µm XB-C18 100 A LC Column 50 × 2.1 mm, Phenomenex, CA, USA). Data aquistion and analyses were performed using a Masslynx version 4.1 software (Waters). The 25-hydroxyvitamin D3 was analyzed in duplicate in a subsample of serum specimens, the coefficient of variation was 5.16%. Values <20 ng/mL were considered as VD deficiency (23).
Statistical Analysis
Sample size was calculated based on a binary result. An 11% probability of acquiring COVID-19 in healthcare workers (Mexican Government. https://coronavirus.gob.mx/2020/05/11/conferencia-11-de-mayo/) and 50% reduction in the likelihood of infection with VD supplementation, were considered for calculation, with 90% statistical power and an α-level <0.05. A sample of 156 subjects per group was estimated.
Statistical analyses were performed with the statistical software STATA v.12.0. A p-value <0.05 was considered for statistical significance. Qualitative variables are described as proportions and quantitative variables as medians and interquartile ranges (IQR). To assess changes in 25-hydroxyvitamin D3 concentration, delta values were calculated as the difference between final and basal concentrations. Fisher, χ2, Mann-Whitney U or Wilcoxon tests were used as appropriate.
To test the hypotheses that VD supplementation can effectively to prevent SARS-CoV-2 infection and to improve VD status, relative risks (RR) and 95% CI were estimated. Two multivariate logistic regression models were used to identify explanatory variables. While in the first model the intervention (VD or placebo) was introduced as a covariate, in the second model we introduced the deltas of 25-hydroxyvitamin D3 concentration. Both models were adjusted by age, morbidity, and basal VD deficiency. Per protocol and intention-to-treat (ITT) analyses were conducted for primary and secondary outcomes; missing values of VD concentrations and AEs were assigned by multiple imputation.
Results
Baseline Characteristics
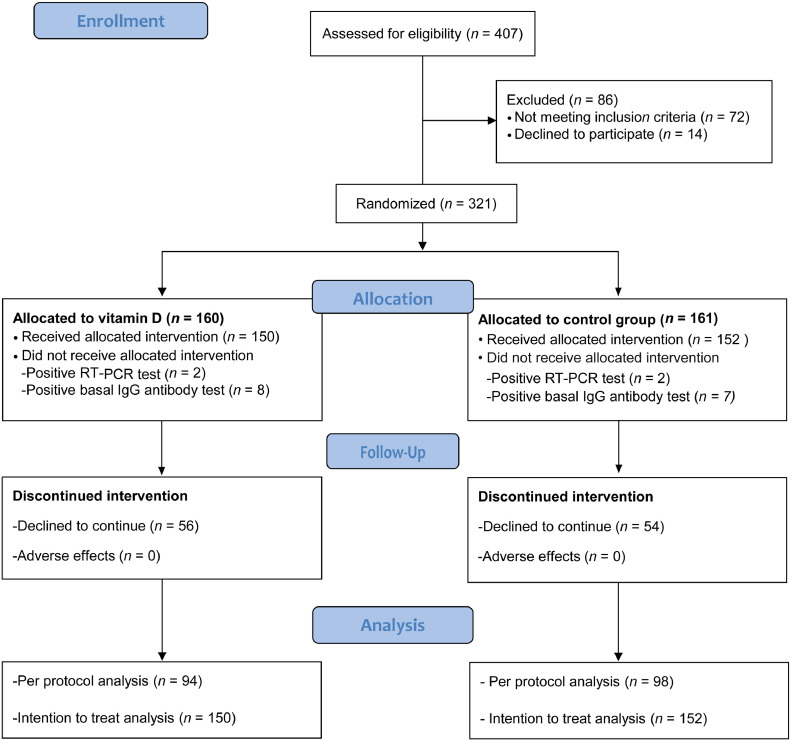
Of 407 screened healthcare workers, 321 met the inclusion criteria and were randomized (160 VDG, 161 PG). Ten VDG and 9 PG participants were excluded because of positive SARS-CoV-2 test; 56 VDG and 54 PG did not finish follow-up. The main reason for not completing the study was lack of time due to heavy workload. None of the participants decided to leave because of adverse events. Thus, 94 VDG and 98 PG were included in per protocol analysis, and 150 and 152 respectively for ITT analysis (Figure 1 ).
Figure 1.
Flow diagram according to the consolidated standards of reporting clinical trials. VDG, vitamin D-supplemented group; PG placebo group.
Comparisons between dropouts and completers showed that VD deficiency was more frequent among dropouts (71 vs. 64% p = 0.008), but all the other studied variables were comparable. Most of the participants were physicians or nurses (55.4%), followed by laboratory technicians involved in blood handling of COVID-19 patients (23.7%); this distribution was comparable among the four studied hospitals and comparable between VDG and PG. PG subjects were older and exhibited a higher frequency of diabetes than VDG. The median 25-hydroxyvitamin D3 concentration (18.3 [14.6, 22.9] vs. 17.1 [13.6, 21.3 ng/mL], p = 0.105), and the frequency of VD deficiency (102 [63.8%] vs. 113 [70.2%]; p = 0.423), was comparable between VDG and PG respectively groups (Table 1 ).
Table 1.
Baseline subject characteristics stratified by treatmenta
| Characteristic | Total n = 321 | VDG n = 160 | PG n = 161 | pb |
|---|---|---|---|---|
| Age, years | ||||
| Median (interquartile range) | 37.5 (30,46) | 36.0 (30,43) | 39.0 (31,48) | 0.019 |
| Sex | ||||
| Female | 224 (70.0) | 114 (71.0) | 110 (68.0) | 0.568 |
| Male | 97 (30.0) | 46 (29.0) | 51 (32.0) | |
| Type of personnel | ||||
| Physicians | 115 (35.8) | 59 (36.9) | 56 (34.8) | 0.649 |
| Laboratory technicians | 76 (23.7) | 39 (24.4) | 37 (23.0) | |
| Nurses | 63 (19.6) | 26 (16.2) | 37 (23.0) | |
| Technical radiologist | 46 (14.3) | 25 (15.6) | 21 (13.0) | |
| Orderlies and cleaning staff | 21 (6.6) | 11 (6.9) | 10 (6.2) | |
| Hospitals | ||||
| IMSS Specialty Hospital | 111 (34.6) | 57 (35.6) | 54 (33.5) | 0.897 |
| IMSS Pediatric Hospital | 82 (25.5) | 41 (25.6) | 41 (25.5) | |
| IMSS Cardiology Hospital | 45 (14.0) | 23 (14.4) | 22 (13.7) | |
| Hospital Infantil de Mexico | 83 (25.9) | 39 (24.4) | 44 (27.3) | |
| Body mass index, kg/m2 | ||||
| Median (interquartile range) | 26.2 (23.6,30.0) | 26.5 (23.9,30.1) | 25.9 (23.5,29.6) | 0.435 |
| ≤25 (normal) | 125 (39.0) | 56 (35.0) | 69 (43.0) | 0.321 |
| 25.1–29.9 (overweight) | 114 (36.0) | 62 (39.0) | 52 (32.0) | |
| ≥30 (obesity) | 82 (25.0) | 42 (26.0) | 40 (25.0) | |
| Risk factors | ||||
| Hypertension | 95 (29.6) | 43 (26.9) | 52 (32.3) | 0.287 |
| Type 2 Diabetes | 13 (4.1) | 3 (1.9) | 10 (6.2) | 0.044 |
| Obesity | 82 (25.6) | 42 (26.3) | 40 (24.8) | 0.773 |
| Otherc | 38 (11.9) | 19 (11.9) | 19 (11.8) | 0.984 |
| 25-hydroxyvitamin D3, ng/ml | ||||
| Median (interquartile range) | 17.6 (14.0,21.7) | 18.3 (14.6,22.9) | 17.1 (13.6,21.3) | 0.105 |
| <20 (deficiency) | 215 (67.0) | 102 (63.8) | 113 (70.2) | 0.423 |
| 20–29.9 (insufficiency) | 86 (26.8) | 48 (30.0) | 38 (23.6) | |
| ≥30 (normal) | 20 (6.2) | 10 (6.2) | 10 (6.2) |
VDG, vitamin D group; PG placebo group.
Values are n (%) or median (interquartile range).
Statistics was conducted with χ2 or Mann-Whitney U test as appropriate.
Other includes rheumatoid arthritis and migraine.
End of Follow-up
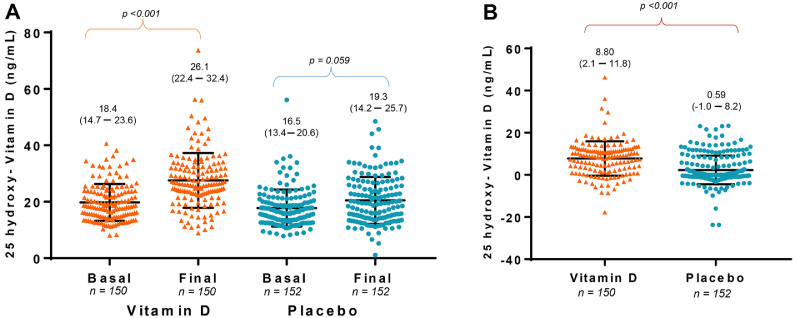
A significant increase in the concentration of 25-hydroxyvitamin D3 was detected in VDG, while it did not change in PG. Therefore, deltas 25-hydroxyvitamin D3 were higher in VDG than in PG (Median [IQR] 8.8 [2.1, 11.8] vs. 0.59 [–1.0, 8.2 ng/mL], p <0.001) (Figure 2 A and 2B). Accordingly, the risk of VD deficiency at the end of follow-up was lower in VDG than in PG (Table 2 ). Six VDG and 24 PG tested positive for SARS-CoV-2 during follow-up (12 to RT-PCR and 18 to IgG antibody tests). The frequency of positive SARS-CoV-2 was 17.1% in VD deficient and 15.6% in non-deficient individuals (χ2, p = 0.799). Nevertheless, the risk of acquiring SARS-CoV-2 infection was lower in VDG compared with PG in both, per-protocol, and ITT analyses (Table 2). While no subject in VDG required hospitalization or died, one PG participant was hospitalized with severe COVID-19 for 23 d and discharged home with mild respiratory symptoms.
Figure 2.
A. Within comparisons of 25-hydroxyvitamin D3 concentration in vitamin D supplemented and placebo groups. Medians and 95% confidence intervals are presented. Statistical analysis was conducted with Wilcoxon. B. Deltas of 25-hydroxyvitamin D3 concentration are compared between vitamin D supplemented and placebo groups. Medians and 95% confidence intervals are presented. Statistical analysis was performed with Mann-Whitney U test.
Table 2.
Efficacy and safety of vitamin D supplementation to prevent SARS-CoV-2 infection and vitamin D deficiency in frontline healthcare workers
| Outcome | Intention-to-treat analysis |
Per-protocol analysis |
||||
|---|---|---|---|---|---|---|
| n | n (%) | RR (95% CI) | n | n (%) | RR (95% CI) | |
| SARS-CoV-2 infection | ||||||
| VDG | 150 | 7 (4.7) | 0.23 (0.09, 0.55) | 94 | 6 (6.4) | 0.22 (0.08, 0.59) |
| PG | 152 | 26 (17.1) | Reference | 98 | 24 (24.5) | Reference |
| Vitamin D deficiency | ||||||
| VDG | 150 | 29 (19.3) | 0.20 (0.12, 0.34) | 94 | 10 (10.6) | 0.05 (0.02, 0.12) |
| PG | 152 | 82 (54.0) | Reference | 98 | 66 (67.3) | Reference |
| Adverse event | ||||||
| VDG | 150 | 46 (30.7) | 0.95 (0.58, 1.56) | 94 | 21 (22.3) | 0.49 (0.26, 0.93) |
| PG | 152 | 48 (31.6) | Reference | 98 | 36 (36.7) | Reference |
RR, relative risk; 95% CI, 95% confidence interval; VDG, vitamin D supplemented group; PG, placebo group.
Multivariate analysis demonstrated that the risk of acquiring SARS-CoV-2 infection was lower in VDG than in the non-supplemented control group, independently of basal VD status (Table 3 ). Another multivariate analysis revealed that increases of 25-hydroxyvitamin D3 predicted the risk of acquiring SARS-CoV-2 infection (Table 3). Both models were adjusted for age, comorbidities, vitamin D deficiency at baseline, as well as by site of study (hospital) and type of personnel.
Table 3.
Predictors of SARS-CoV-2 infectiona
| Model 1 |
Model 2 |
|||||
|---|---|---|---|---|---|---|
| Characteristic | RR | 95%CI | p | RR | 95%CI | p |
| Age, years | 1.02 | 0.97, 1.06 | 0.332 | 1.03 | 0.98, 1.07 | 0.140 |
| Hypertension | 1.32 | 0.83, 2.96 | 0.420 | 1.33 | 0.44, 1.89 | 0.470 |
| Type 2 diabetes | 1.98 | 0.40, 9.76 | 0.401 | 1.17 | 0.23, 6.04 | 0.843 |
| Obesity | 2.01 | 0.82, 4.88 | 0.122 | 1.82 | 0 .74, 4.48 | 0.190 |
| Basal VD deficiency | 0.86 | 0.35, 2.10 | 0.756 | 1.42 | 0.56, 3.56 | 0.450 |
| VDGb | 0.22 | 0.09, 0.56 | 0.001 | - | - | - |
| 25-hydroxyvitamin D3, deltasc | - | - | - | 0.87 | 0.82, 0.93 | 0.001 |
Logistic regression analysis, adjusted by type of personnel and hospital.
Compared with PG.
Deltas are the difference between final and basal concentration.
95%CI, 95% confidence interval; VD, vitamin D; VDG, vitamin D supplemented group; PG, placebo group.
Thirty one percent of VDG and 32% of PG subjects reported at least one AE. The more frequently reported were headache, constipation, and diarrhea. While headache was more frequently reported in PG, constipation tended to be more common in VDG (Table 4 ). The risk of presenting at least one AE was lower in VDG than in PG (Table 2), but in the ITT analysis that association was no longer significant. None of the AEs led to treatment discontinuation.
Table 4.
Treatment-related adverse events stratified by treatment groupsa
| Account (%) |
|||
|---|---|---|---|
| Adverse event | VDG n = 150 | PG n = 152 | pb |
| Any treatment-related adverse event | 46 (30.7) | 48 (31.6) | 0.864 |
| Headache | 8 (5.3) | 18 (11.8) | 0.044 |
| Constipation | 15 (10.0) | 7 (4.6) | 0.071 |
| Diarrhea | 7 (4.7) | 8 (5.3) | 0.811 |
| Nausea | 7 (4.7) | 3 (2.0) | 0.191 |
| Abdominal pain | 5 (3.3) | 2 (1.3) | 0.244 |
| Dizziness | 3 (2.0) | 6 (3.9) | 0.320 |
| Drowsiness | 9 (6.0) | 9 (5.9) | 0.977 |
| Muscular weakness | 6 (4.0) | 8 (5.2) | 0.602 |
| Vomiting | 1 (0.7) | 0 (0) | 0.313 |
Values are n (%);
Statistics was conducted with χ2 analysis.
Discussion
The results of our double-blind, placebo-controlled, prospective study demonstrate that VD supplementation is effective in preventing SARS-CoV-2 infection in high risk, frontline healthcare personnel. Furthermore, the protective effect of VD supplementation was independent of basal serum concentrations of 25-hydroxyvitamin D3. We have thus confirmed and extended the results of previous cross-sectional and intervention studies whereby vitamin sufficiency was found to be associated with better COVID-19 outcomes, including a lower requirement for intensive care unit admission and mortality rate (7,17). To our knowledge, this is the first controlled study evaluating the role of VD supplementation as a prophylactic measure to prevent SARS-Cov-2 infection and therefore has profound clinical and public health implications.
VD is known to modulate the immune system (6,24). Both, the nuclear vitamin D receptor (VDR) and the vitamin D metabolizing enzymes are expressed in virtually all cells of the innate and adaptive immune system, including macrophages, dendritic cells and activated B and T cells. All these cells are thus, capable of locally synthesizing the active VD metabolite 1,25(OH)2 D3, which exerts genomic and nongenomic actions. Exogenous VD reduces the production of inflammatory mediators and of reactive oxygen species in neutrophils by activating the 5-lipoxygenase gene and by suppressing the cyclooxygenase gene. Locally produced 1,25(OH)2 D3 promotes macrophage differentiation and activation and reduces the production of proinflammatory mediators such as interleukin (IL)-1β, IL-6 and TNF-α. Active VD interferes with the maturation and differentiation of dendritic cells promoting a tolerogenic phenotype characterized by a reduced secretion of proinflammatory cytokines (IL-1B, IL-6, IL23) and an increased production of IL-10 and TNF-α. 1,25(OH)2 D3 promotes B-cell apoptosis, inhibits memory B-cell formation, and prevents B-cell differentiation into immunoglobulin-producing plasma cells. Both, the VDR and the VD metabolizing enzymes gene expression is up regulated in human CD4+ and CD8+ T cells. In general, vitamin D deficiency is associated with an increased risk for various autoimmune and infectious diseases (24). Thus, VD potentially interferes with some of the pathways used by SARS-CoV-2 to enter and replicate in the bronchial epithelium. It is well known that VD inhibits renin expression and the angiotensin converting enzyme (25). It also favors an anti-inflammatory over a pro-inflammatory immune state, potentially preventing progression to more severe forms of the disease and promotes an antioxidant environment in the lungs impairing the replication capacity of the virus, which may result in a reduction of the viral load (26). Thus, the above pathophysiological considerations pertaining the relationship between VD and SARS-Cov-2 are in line with our findings. VD supplementation was equally effective in preventing SARS-Cov-2 infection in high-risk healthcare workers, regardless of the basal concentrations of 25-hydroxyvitamin D3. Our results show that the protective effect of VD was achieved with a medium dose (4,000 IU/d), during a short period of time (one-month), and with only a mild increase of the vitamin concentration (8.8 ng/mL), suggesting that the intake of VD plays a role in preventing SARS-CoV-2 infection in high exposure periods, even though the adequate VD status is not reached. Nevertheless, these results highlight the need for further studies to identify the appropriate dose required to provide the optimal protective effect (27., 28., 29., 30., 31.).
The more frequently reported AEs were headache, intestinal transit alterations, nausea, abdominal pain, and fatigue, coinciding with VD-associated collateral effects. Nevertheless, the frequency and type of AEs were comparable between groups, which suggests that the perception of such symptoms may have been related to the acute stress derived from long working periods, or with the fear of acquiring COVID-19 as proposed by others (32).
The main strengths of our study are the randomized and experimental design, the inclusion of a highly exposed population, the use of a gold standard method to determine 25-hydroxyvitamin D3, and the fact that it was conducted before vaccines against SARS-CoV-2 were available. Nevertheless, we recognize that the high proportion of participants who did not complete follow-up could lead to misinterpretation of the results by not identifying subjects with the disease among those who dropped the study. To deal with such situation, we performed an active surveillance on the hospitals’ epidemiological records systems searching for dropouts among the confirmed SARS-CoV-2 positive, but none was identified. In addition, the association persisted in the ITT analysis, and confirmed after including confounders in the analysis.
In conclusion, the findings of our study indicate that VD protects against SARS-CoV-2 infection in highly exposed individuals, regardless VD status and with relatively few side effects.
Data described in the manuscript, code book, and analytic code will not be made available because it belongs to the Institutions where the study was conducted.
Conflict of Interest
Mardia G López-Alarcón, is the Editor-in-Chief of Archives of Medical Research. All other authors do not have any Conflict of Interest.
Acknowledgments
The study was supported with grants from the Instituto Mexicano del Seguro Social (FIS/IMSS/PROT/PRIO/18/085) and Hospital Infantil de México Federico Gómez (HIM-2020-045/SSA1666). ClinicalTrials.gov Identifier: NCT04535791.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.arcmed.2022.04.003.
Appendix. Supplementary materials
References
- 1.The World Health Organization (WHO). https://covid19.who.int/. (Access February 4, 2022).
- 2.Roth DE, Abrams SA, Aloia J, et al. Global prevalence and disease burden of vitamin D deficiency: a roadmap for action in low- and middle-income countries. Ann N Y Acad Sci. 2018;1430:44–79. doi: 10.1111/nyas.13968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cashman KD, Dowling KG, Škrabáková Z, et al. Vitamin D deficiency in Europe: pandemic? Am J Clin Nutr. 2016;103:1033–1044. doi: 10.3945/ajcn.115.120873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herrick KA, Storandt RJ, Afful J, et al. Vitamin D status in the United States, 2011–2014. Am J Clin Nutr. 2019;110:150–157. doi: 10.1093/ajcn/nqz037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gutiérrez JP, Rivera-Dommarco J, Shamah-Levy T, et al. Instituto Nacional de Salud Pública; Cuernavaca, México(MX): March 20, 2022. Encuesta Nacional de Salud y Nutrición 2012. Resultados Nacionales.https://ensanut.insp.mx/encuestas/ensanut2012/doctos/informes/ENSANUT2012ResultadosNacionales.pdf 2012. Available at. Accessed. [Google Scholar]
- 6.Vanherwegen AS, Gysemans C, Mathieu C. Regulation of immune function by vitamin D and its use in diseases of immunity. Endocrinol Metab Clin North Am. 2017;46:1061–1094. doi: 10.1016/j.ecl.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 7.Kaufman HW, Niles JK, Kroll MH, et al. SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D levels. PLoS One. 2020;15 doi: 10.1371/journal.pone.0239252. eCollection 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ali N. Role of vitamin D in preventing COVID-19 infection, progression and severity. J Infect Public Health. 2020;13:1373–1380. doi: 10.1016/j.jiph.2020.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alguwaihes AM, Sabico S, Hasanato R, et al. Severe vitamin D deficiency is not related to SARS-CoV-2 infection but may increase mortality risk in hospitalized adults: a retrospective case-control study in an Arab Gulf country. Aging Clin Exp Res. 2021;33:1415–1422. doi: 10.1007/s40520-021-01831-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Daghri NM, Amer OE, Alotaibi NH, et al. Vitamin D status of Arab Gulf residents screened for SARS-CoV-2 and its association with COVID-19 infection: a multi-centre case-control study. J Transl Med. 2021;19:166. doi: 10.1186/s12967-021-02838-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campi I, Gennari L, Merlotti D, et al. Vitamin D and COVID-19 severity and related mortality: a prospective study in Italy. BMC Infect Dis. 2021;21:566. doi: 10.1186/s12879-021-06281-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiodini I, Gatti D, Soranna D, et al. Vitamin D Status and SARS-CoV-2 Infection and COVID-19 Clinical Outcomes. Front Public Health. 2021;9 doi: 10.3389/fpubh.2021.736665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petrelli F, Luciani A, Perego G, et al. Therapeutic and prognostic role of vitamin D for COVID-19 infection: A systematic review and meta-analysis of 43 observational studies. J Steroid Biochem Mol Biol. 2021;211 doi: 10.1016/j.jsbmb.2021.105883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu N, Sun J, Wang X, et al. Low vitamin D status is associated with coronavirus disease 2019 outcomes: a systematic review and meta-analysis. Int J Infect Dis. 2021;104:58–64. doi: 10.1016/j.ijid.2020.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghasemian R, Shamshirian A, Heydari K, et al. The role of vitamin D in the age of COVID-19: A systematic review and meta-analysis. Int J Clin Pract. 2021:e14675. doi: 10.1111/ijcp.14675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murai IH, Fernandes AL, Sales LP, et al. Effect of a single high dose of vitamin D3 on hospital length of stay in patients with moderate to severe COVID-19: A randomized clinical trial. JAMA. 2021;325:1053–1060. doi: 10.1001/jama.2020.26848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Entrenas Castillo M, Entrenas Costa LM, Vaquero Barrios JM, et al. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study. J Steroid Biochem Mol Biol. 2020;203 doi: 10.1016/j.jsbmb.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nogues X, Ovejero D, Pineda-Moncusí M, et al. Calcifediol treatment and COVID-19-related outcomes. J Clin Endocrinol Metab. 2021;106:e4017–e4027. doi: 10.1210/clinem/dgab405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rastogi A, Bhansali A, Khare N, et al. Short term, high-dose vitamin D supplementation for COVID-19 disease: a randomised, placebo-controlled, study (SHADE study) Postgrad Med J. 2022;98:87–90. doi: 10.1136/postgradmedj-2020-139065. [DOI] [PubMed] [Google Scholar]
- 20.Corman VM, Landt O, Kaiser M, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abbott. ARCHITECT SARS-CoV-2 IgG instructions for use. H14806R03. Available at: https://www.corelaboratory.abbott/int/en/offerings/segments/infectious-disease/sars-cov-2- (Accessed March 20, 2022)
- 22.Van den Ouweland JM, Beijers AM, Demacker PN, van Daal H. Measurement of 25-OH-vitamin D in human serum using liquid chromatography tandem-mass spectrometry with comparison to radioimmunoassay and automated immunoassay. J Chromatogr B Analyt Technol Life Sci. 2010;878:1163–1168. doi: 10.1016/j.jchromb.2010.03.035. [DOI] [PubMed] [Google Scholar]
- 23.Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53–58. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vanherwegen AS, Gysemans C, Mathieu C. Regulation of Immune Function by Vitamin D and Its Use in Diseases of Immunity. Endocrinol Metab Clin North Am. 2017;46:1061–1094. doi: 10.1016/j.ecl.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 25.Malek MA. A brief review of interplay between vitamin D and angiotensin-converting enzyme 2: Implications for a potential treatment for COVID-19. Rev Med Virol. 2020;30:e2119. doi: 10.1002/rmv.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferder L, Martín Giménez VM, Inserra F, et al. Vitamin D supplementation as a rational pharmacological approach in the COVID-19 pandemic. Am J Physiol Lung Cell Mol Physiol. 2020;319:L941–L948. doi: 10.1152/ajplung.00186.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jolliffe DA, Camargo CA, Jr, Sluyter JD, et al. Vitamin D supplementation to prevent acute respiratory infections: a systematic review and meta-analysis of aggregate data from randomised controlled trials. Lancet Diabetes Endocrinol. 2021;9:276–292. doi: 10.1016/S2213-8587(21)00051-6. [DOI] [PubMed] [Google Scholar]
- 28.Ma W, Nguyen LH, Yue Y, et al. Associations between predicted vitamin D status, vitamin D intake, and risk of SARS-CoV-2 infection and Coronavirus Disease 2019 severity. Am J Clin Nutr. 2022;115:1123–1133. doi: 10.1093/ajcn/nqab389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crafa A, Cannarella R, Condorelli RA, et al. Influence of 25-hydroxy-cholecalciferol levels on SARS-CoV-2 infection and COVID-19 severity: A systematic review and meta-analysis. E Clinical Medicine. 2021;37 doi: 10.1016/j.eclinm.2021.100967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sabico S, Enani MA, Sheshah E, et al. Effects of a 2-Week 5000 IU versus 1000 IU Vitamin D3 supplementation on recovery of symptoms in patients with mild to moderate COVID-19: A randomized clinical trial. Nutrients. 2021;13:2170. doi: 10.3390/nu13072170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pal R, Banerjee M, Bhadada SK, et al. Vitamin D supplementation and clinical outcomes in COVID-19: a systematic review and meta-analysis. J Endocrinol Invest. 2022;45:53–68. doi: 10.1007/s40618-021-01614-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chapa-Koloffon GDC, Jean-Tron MG, Ávila-Hernández AV, et al. Frequency of acute stress disorder in health care workers of a tertiary level pediatric hospital during the National Safe Distance Strategy for COVID-19 prevention. Bol Med Hosp Infant Mex. 2021;78:10–17. doi: 10.24875/BMHIM.20000226. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.