Abstract
Objectives
Healthcare workers (HCWs), at increased risk of coronavirus disease 2019 (COVID-19) were among the primary targets for vaccination, which became mandatory for them on September 15th, 2021 in France. In November they were confronted to the fifth COVID-19 wave despite excellent vaccine coverage. We aimed to estimate the incidence of SARS-CoV-2 infection after complete vaccination among HCWs with different vaccination schemes, and its determinants.
Methods
We enrolled all HCWs in the university hospital of Rennes, France who had received complete vaccination (two doses of COVID-19 vaccine). The delay from last vaccination dose to SARS-CoV-2 infection was computed. Fitted mixed Cox survival model with a random effect applied to exposure risk periods to account for epidemic variation was used to estimate the determinants of SARS-CoV-2 infection after complete vaccination.
Results
Of the 6674 (82%) HCWs who received complete vaccination (36% BNT162b2, 29% mRNA-1273, and 34% mixed with ChAdOx1 nCoV-19) and were prospectively followed-up for a median of 7.0 [6.3–8.0] months, 160 (2.4%) tested positive for SARS-CoV-2 by RT-PCR. Incidence density of SARS-CoV-2 infection after complete vaccination was 3.39 [2.89–3.96] infections per 1000 person-month. Median time from vaccine completion to SARS-CoV-2 infection was 5.5 [3.2–6.6] months. Using fitted mixed Cox regression with the delay as a time-dependent variable and random effect applied to exposure risk periods, age (P < 0.001) was independently associated with the incidence of SARS-CoV-2 infection. Vaccine schemes were not associated with SARS-CoV-2 infection (P = 0.068). A period effect was significantly associated with the incidence of SARS-CoV-2 infection (P < 0.001).
Conclusions
In this real-world study, incidence of SARS-CoV-2 infection increases with time in fully vaccinated HCWs with no differences according to the vaccination scheme. The short delay between complete vaccination and incident SARS-CoV-2 infection highlights the need for sustained barrier measures even in fully vaccinated HCWs.
Keywords: COVID-19, Vaccine, SARS-CoV-2 infection, Complete vaccination, Healthcare workers
1. Introduction
Healthcare workers (HCWs) are at increased risk for exposure to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) due to their interactions with patients in addition to their household and community [1]. In France, COVID-19 vaccines have been available since January 2021, with priority initially given to individuals at high risk of severe coronavirus disease 2019 (COVID-19), and HCWs. At that time, the alpha variant was predominant and responsible for the third wave. From June, the Delta variant progressively replaced the alpha variant to become predominant in July 2020, responsible for the fourth wave followed by the fifth wave (Data extracted from Santé Publique France; https://www.santepubliquefrance.fr/dossiers/coronavirus-covid-19/coronavirus-chiffres-cles-et-evolution-de-la-covid-19-en-france-et-dans-le-monde). Since September 15th, 2021, complete vaccination was set compulsory for all HCWs to protect them, their patients, and their relatives against COVID-19. In our institution, 82% (6674 out of 8165) HCWs received complete vaccination, an increase in the number of positive HCWs for SARS-CoV-2 has been observed concomitant to fifth wave. We performed a prospective monocentric cohort study to evaluated the risk of SARS-CoV-2 infection and assess its determinants among fully vaccinated HCWs.
2. Methods
2.1. Study design
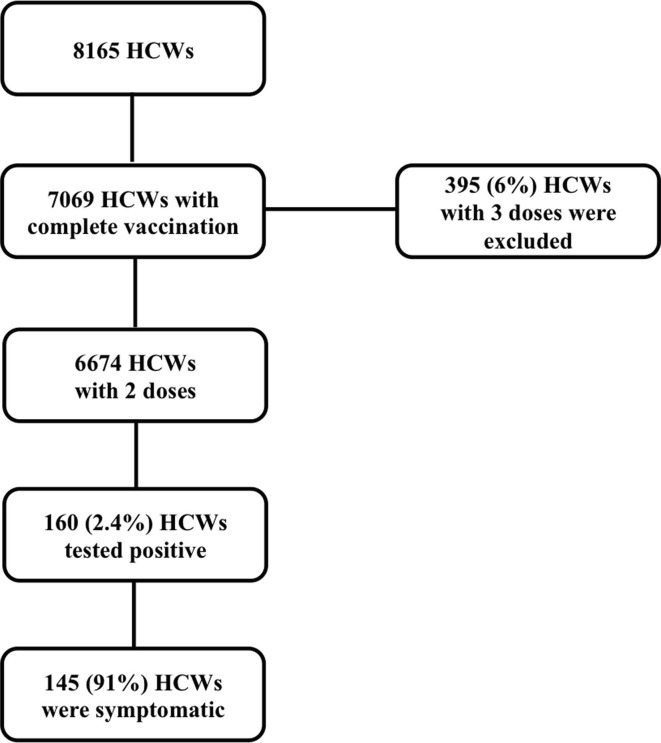
We performed a monocentric prospective cohort study in the university hospital of Rennes, a 1,800-bed tertiary care hospital in Western France, to evaluate the incidence of SARS-CoV-2 infection over time after complete vaccination with different vaccine schemes, in a real-world setting among HCWs. All HCWs who received two doses of COVID-19 vaccines, whether BNT162b2, mRNA-1273 and ChAdOx1 nCoV-19, were defined as completely vaccinated and included in our study. For the purpose of contact tracing, all HCWs are requested to inform the occupational department when they are diagnosed with SARS-CoV-2 infection, and this data was prospectively monitored throughout the study period. Tests for SARS-CoV-2 infection were available in our institution, and in a large number of facilities, with no charge in vaccinated people. Patients who received three doses of vaccines were excluded (n = 395; 6%).
2.2. Definitions
Complete vaccination was defined as the administration of 2 doses of COVID-19 vaccines. Vaccine schemes were defined as follows: ‘BNT162b2′ for administration of two doses of BNT162b2 vaccine, ‘mRNA-1273′ for two doses of mRNA-1273 vaccine, ‘mixed mRNA’ for one dose of BNT162b2 and one dose of mRNA-1273 vaccines and ‘schemes including ChAdOx1 nCoV-19′ for one dose of ChAdOx1 nCoV-19 vaccine and the other with BNT162b2 or mRNA-1273 or ChAdOx1 nCoV-19 vaccines.
Cases were defined as HCWs who tested positive for SARS-CoV-2 by reverse-transcription polymerase-chain-reaction (RT-PCR) from a nasopharyngeal sample, independently of the presence of symptoms. HCWs with SARS-CoV-2 infection prior to complete vaccination and infections within 14 days of complete vaccination were not considered as cases. HCWs were tested in case of any symptom suggestive of COVID-19, or for the purpose of contact tracing when they were identified as contact of someone with SARS-CoV-2 infection.
2.3. Data collection
Data were prospectively collected from January 21st, 2021 (first HCW fully vaccinated) until the December 14th, 2021, to restrict the study period to the circulation of the Alpha and Delta variants, as the first Omicron variant was identified in our hospital on December 14th, 2021. The 31/05/2021 and 31/10/2021 were retained to define the 3rd, 4th and 5th waves respectively.
Two prospective databases systematically recorded in our institution were merged:
-
a)
HCWs who received complete COVID-19 vaccination as previously described [2]. Collected data included baseline demographic data (age), type of vaccine (BNT162b2 vaccine, mRNA-1273 or ChAdOx1 nCoV-19 vaccines), date of last dose, number of doses received.
-
b)
HCWs positive for SARS-CoV-2. SARS-CoV-2 RT-PCR results were retrieved from laboratory data and from patient’s phone interview. All HCWs with positive RT-PCR were contacted within 48 h of diagnosis using a standardized questionnaire. Date of positive RT-PCR, and symptoms by the time RT-PCR was positive were retrieved. Delay between complete vaccination and SARS-CoV-2 infection was systematically computed.
Data were anonymized before analysis, and HCWs were informed of the study and its results through our institution website according to the French law on clinical research. This study was conducted in accordance with the principles of the Declaration of Helsinki.
2.4. Statistical analyses
Data are reported as numbers with percentage for qualitative variables or median with interquartile interval for quantitative variables.
The delay from complete vaccination to SARS-CoV-2 infection was represented according to vaccine schemes over time using box plot and modelled as a time-dependent variable using Kaplan-Meier survival curves. Influence of time from last vaccination to SARS-CoV-2 infection according to the vaccine scheme was assessed using log-rank analysis. Complete vaccination with BNT162b2 vaccine was defined as the reference.
To account for variations in the infection rate and variant circulation throughout the study period, a time-dependent fitted mixed Cox model was performed using i) random effect applied to the different exposure risk periods to account for the following risk throughout the COVID-19 waves, and ii) delay from complete vaccination to SARS-CoV-2 infection as a time-dependent variable, to assess risk factors associated with positivity for SARS-CoV-2 after complete vaccination. Exposure risk periods were defined according to the month last vaccine dose was received. HCWs who received their last dose in November and December were considered as exposed to the same risk (fifth wave). Variables were selected if significant (P < 0.2) in the univariate analysis (Table 1 ). Conditional stepwise regression with, 0.2 as the critical P value for entry, and, 0.1, as the P value for removal, was used. Type of vaccine was planned a priori to be forced into the final model, if not previously selected. Results are presented as hazard risks (adjusted Hazard Ratio, HR) with 95 %CI.
Table 1.
Characteristics of the healthcare workers.
| Overall | No SARS-CoV-2 infection | SARS-CoV-2 infection | P | |
|---|---|---|---|---|
| n | 6674 | 6506 | 160 | |
| Age (years) | 39 [29–49] | 39 [29–49] | 34 [26–47] | 0.015 |
| Follow-up (months) | 7.0 [6.3–8.0] | 7.0 [6.4–8.0] | 5.5 [3.2–6.6] | <0.001 |
| Vaccination strategy | ||||
| Type of vaccine* | 0.244 | |||
| BNT162b2 | 2426 (36) | 2353 (36) | 70 (44) | |
| mRNA-1273 | 1931 (29) | 1886 (29) | 42 (27) | |
| Mixed mRNA | 44 (1) | 42 (1) | 1 (1) | |
| ChAdOx1 nCoV-19 | 50 (0.7) | 40 (0.8) | 0 (0) | |
| Mixed schemes including ChAdOx1 nCoV-19 | 2219 (33) | 2173 (33) | 45 (29) | |
| Delay from complete vaccination to SARS-CoV-2 infection (months) | – | – | 5.5 [3.2–6.6] | |
| COVID-19 waves | ||||
| Third wave | – | – | 9 (6) | |
| Fourth wave | – | – | 49 (31) | |
| Fifth wave | 102 (64) | |||
| Symptoms | – | – | 145 (91) | |
| Headache | – | – | 97 (61) | |
| Cough | – | – | 94 (59) | |
| Thrill | – | – | 55 (35) | |
| Fever | – | – | 52 (33) | |
| Aches | – | – | 62 (39) | |
| Sore Throat | – | – | 76 (48) | |
| Nasal discharge | – | – | 76 (45) | |
| Rhinorrhee | – | – | 114 (72) | |
| Anosmia/agueusia | – | – | 59 (37) |
Data are reported as numbers with percentage for qualitative variables or median with interquartile interval for quantitative variables.
*Vaccine schemes were defined as follows: ‘BNT162b2′ for administration of two doses of BNT162b2 vaccine, ‘mRNA-1273′ for two doses of mRNA-1273 vaccine, ‘mixed mRNA’ for one dose of BNT162b2 and one dose of mRNA-1273 vaccines, ‘ChAdOx1 nCoV-19′ for two doses of ChAdOx1 nCoV-19 vaccines and ‘schemes including ChAdOx1 nCoV-19′ for one dose of ChAdOx1 nCoV-19 vaccine and the other with BNT162b2 or mRNA-1273 or ChAdOx1 nCoV-19 vaccines.
Statistical significance was considered using two-sided tests with a critical alpha risk of 0.05.
Statistical analyses were performed using R version 3.4.4 (R Foundation for Statistical Computing).
3. Results
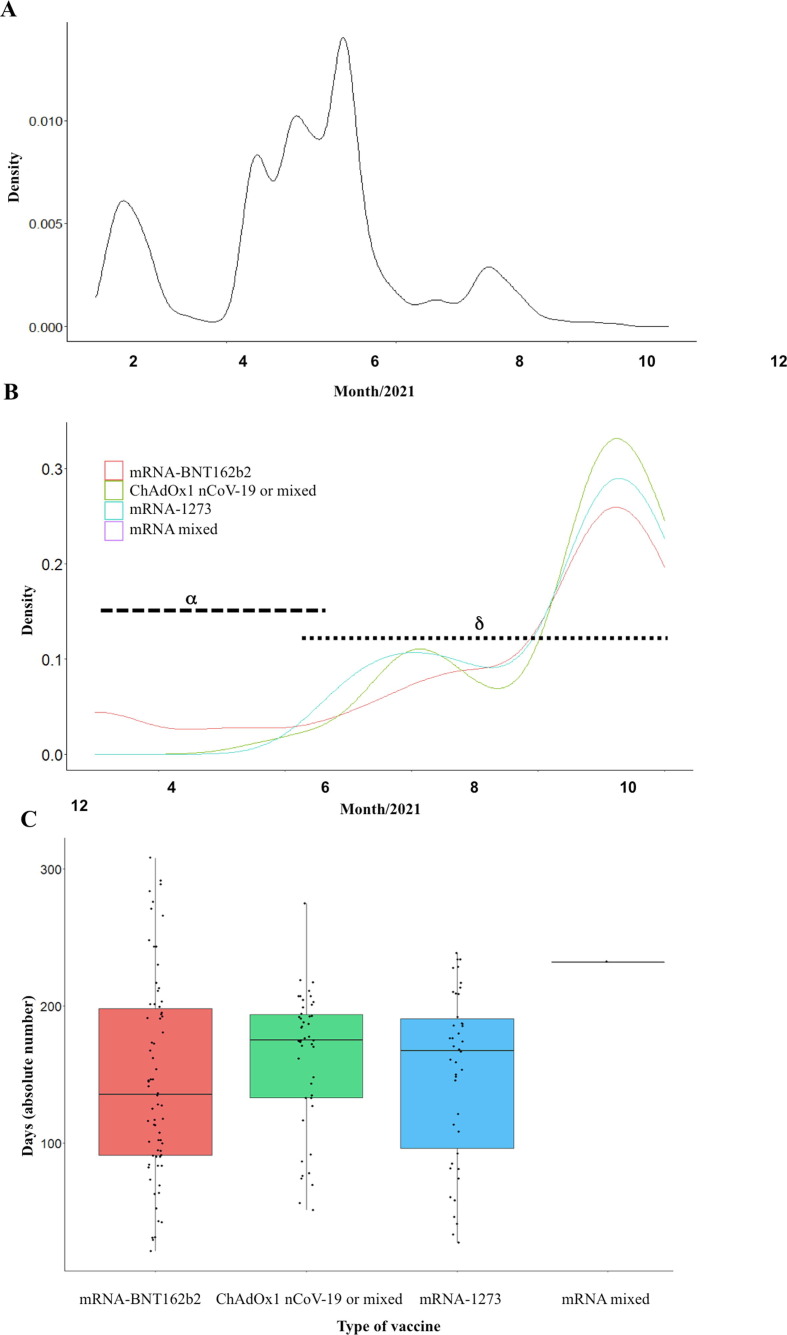
We included the 6674 (82%, out of 8165 HCWs in our institution) HCWs whom received complete vaccination ( Fig. 1 ). Progression of complete vaccination among HCWs is shown in Fig. 2 a, and SARS-CoV-2 infection in HCWs over the different COVID-19 waves in Fig. 2 b. With a median follow-up of 7.0 [6.3–8.0] months, 160 cases (2.4%) tested positive for SARS-CoV-2, with a median time from last vaccination dose of 5.5 [3.2–6.6] months ( Fig. 2 c). Median overall incidence density of SARS-CoV-2 infection during the study period was 3.39 [2.89–3.96] per 1000 person-month (0.19 [0.08–0.36] during the third wave, 1.04 [0.77–1.37] during the fourth wave, and 2.17 [1.77–2.64] during the fifth wave). The incidence at 3 and 6 months of complete vaccination was 7 [5], [6], [7], [8], [9], [10] and 12 [9–15] per 1000 person-month respectively.
Fig. 1.
Flow-chart of the study.
Fig. 2.
SARS-CoV-2 infection after complete vaccination with time. A) Kernel density of fully vaccinated HCWs throughout the study period from January 21 to November 30, 2021. B) Kernel density of SARS-CoV-2 infection in fully vaccinated HCWs throughout the study period from March 4 to December 14, 2021. C) Incidence of SARS-CoV-2 infections according to the delay from complete vaccination for the different vaccine schemes.
HCWs had a median age of 34 [26–47] years when they were diagnosed with SARS-CoV-2 infection, and they were mostly vaccinated with 2 doses of BNT162b2 (44%) or a combination of 2 different vaccines (BNT162b2 or mRNA-1273 with ChAdOx1 nCoV-19; 29%). Among positive HCWs, 145 (91%) were symptomatic with predominant complains being rhinorrhea (72%) followed by headaches (61%) and cough (59%) (Table 1).
In univariate analysis, HCWs with SARS-CoV-2 infection were younger (median age, 34 [26–47] versus 39 [29–49] years, P = 0.015). We found no association between patterns of vaccination and the incidence of SARS-CoV-2 infection (P = 0.244; Table 1 ), or the delay between complete vaccination and SARS-CoV-2 infection (P = 0.245; Fig. 2).
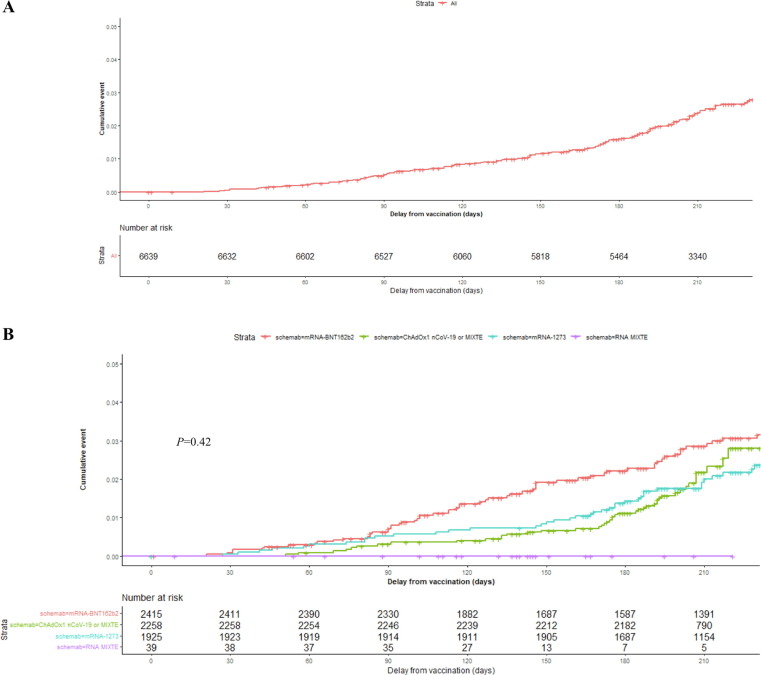
Taking into account the delay between the last dose and SARS-CoV-2 infection as a time-dependent variable, Kaplan-Meier survival curves showed no difference in the incidence of SARS-CoV-2 infection according to the type of received vaccination (P = 0.42; Fig. 3 ). Using fitted mixed Cox regression with random effect applied to exposure risk periods and the delay from complete vaccination to SARS-CoV-2 infection as a time-dependent variable, adjusted on age and vaccine scheme, age (HR 0.96 [0.94–0.98]; P < 0.001) remained independently associated with the incidence of SARS-CoV-2 infection. No difference among vaccines schemes was observed with the incidence of SARS-CoV-2 infection throughout time from complete vaccination (P = 0.108) (Table 2 ). Conversely, the different exposure risk periods effect was significantly associated with the incidence of SARS-CoV-2 infection (P < 0.001).
Fig. 3.
Kaplan-Meier survival curve representing the incidence of SARS-CoV-2 infection with the delay from complete vaccination as a time-dependent variable. A) Kaplan-Meier survival curve representing overall incidence of SARS-CoV-2 infection with time. B) Kaplan-Meier survival curve representing overall incidence of SARS-CoV-2 infection according to vaccine schemes with time. The red curve corresponds to scheme with BNT162b2, the blue to scheme with mRNA-1273, green schemes including ChAdOx1 nCoV-19 and purple mixed mRNA schemes. No difference was found between the different vaccine schemes (P = 0.42) in the incidence of SARS-CoV-2 infection taking the delay as the time-dependent variable, compared using a Log-Rank test. Time is given in days. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Table 2.
Risk factors associated with time to SARS-CoV-2 infection after complete vaccination.
| Fitted mixed cox regression |
|||
|---|---|---|---|
| Variables | HR CI95% | P value | |
| Type of vaccine* | – | 0.068 | |
| BNT162b2 | 1.00 | – | |
| mRNA-1273 | 1.20 [0.47–3.10] | 0.70 | |
| Mixed mRNA | 1.41 [0.19–10.75] | 0.740 | |
| Schemes including ChAdOx1 nCoV-19 | 0.53 [0.26–1.10] | 0.087 | |
| Age (per one year) | 0.96 [0.94–0.98] | <0.001 | |
| Risk exposure periods | – | <0.001 | |
Data are reported as numbers with percentage for qualitative variables or median with interquartile interval for quantitative variables.
*Vaccine schemes were defined as follows: ‘BNT162b2′ for administration of two doses of BNT162b2 vaccine, ‘mRNA-1273′ for two doses of mRNA-1273 vaccine, ‘mixed mRNA’ for one dose of BNT162b2 and one dose of mRNA-1273 vaccines and ‘schemes including ChAdOx1 nCoV-19′ for one dose of ChAdOx1 nCoV-19 vaccine and the other with BNT162b2 or mRNA-1273 or ChAdOx1 nCoV-19 vaccines.
4. Discussion and conclusion
Among fully vaccinated HCWs, we observed an incidence density of SARS-CoV-2 infection after complete vaccination of 3.4 infections per 1000 person-month after a median delay of 5.5 months for all vaccine schemes, which increased over time from complete vaccination. In the multivariate analysis, when accounting for variations in the infections rate throughout the study period, vaccine schemes were not associated with the incidence of SARS-CoV-2.
Effectiveness of vaccines against symptomatic COVID-19 in HCWs was previously assessed in the literature for mRNA vaccines and revealed 88.8% (95% CI, 84.6 to 91.8) and 96.3% (95% CI, 91.3 to 98.4) effectiveness after complete vaccination with BNT162b2 vaccine and mRNA-1273 vaccine respectively according to a 2-week interval follow-up [3]. After a follow-up of 2 months, mRNA-1273 showed 94.1% efficacy after 2 doses of vaccine [4] and ChAdOx1 nCoV-19 62.1% [5]. In addition, complete vaccination with BNT162b2 reduced the risk of COVID-19 and absenteeism among HCWs [6].
During the circulation of the Alpha variant, while only 15% HCWs had received full vaccination, 9 cases of SARS-CoV-2 infections were detected. In mid-June, while 83% of HCWs had received full vaccination, 151 were diagnosed with SARS-CoV-2 infection while the Delta variant was circulating. Since November 2021, an increase in the number of SARS-CoV-2 infections was reported, concomitant to the fifth wave. With a longer follow-up of 7 months, incidence density of SARS-CoV-2 infection with time increased with the delay from full vaccination suggesting reduced vaccines efficiency over time, with a median delay of 5.5 [3.2–6.6] months since last injection, consistent with other reports in the general population [7], [8]. Our observations are in agreement with other studies reporting reduced effectiveness from 82% to 53% after 6 months of two-dose mRNA vaccine in HCWs during Delta variant circulation [9]. Waning immunity might play a crucial role in COVID-19 breakthrough. Other hypotheses have been raised to explain the recent COVID-19 breakthrough including the entry into the winter period, relaxation of barrier measures due to vaccination or reduced efficacy of vaccines against the Delta variant. Of importance, this recent increase cannot be ascribed to the emergence of Omicron variant, as our study was interrupted on December 14th, 2021 just before the first Omicron case was diagnosed in our institution.
In addition, the different exposure risk periods were independently associated with SARS-CoV-2 infection suggesting differences in SARS-CoV-2 infections throughout the different COVID-19 waves. The infection risk increased with time from last vaccination but also along the different variants. Yamamoto and colleagues reported COVID-19 breakthrough infection among fully vaccinated HCWs during the fifth wave, dominated by the delta variant with reduced neutralizing antibodies against COVID-19 variants [10].
Interestingly, age was independently associated with the incidence of SARS-CoV-2 infection with younger HCWs at increased risk of infection in our study. This observation might be explained by the fact that younger HCWs are more exposed and may take more risk with reduced barrier measures.
Our study has limitations. First, as a monocentric study, its findings may not be generalizable to other settings, given the variability of the epidemiology of SARS-CoV-2 variants. In addition, our results might not extrapolate to the Omicron variant. However, our study includes homogeneous data with appropriate follow-up and data were adjusted on the different waves taking into account the circulation of the different strains. Second, we may have slightly underestimated the incidence of SARS-CoV-2 infections, as few HCWs did not communicate their results to the occupational health department when tested outside the institution. However, this underestimate is considered independent from the vaccine strategies, and to only have a slight effect on statistical power without bias. SARS-CoV-2 infections prior to and within 14 days of complete vaccination were not considered as cases but at risk of reinfection and were not excluded from the analysis. Excluding these patients in a sensitivity analysis did not change our results. At last, three COVID-19 vaccines were used in our hospital during the study period, according to authorizations of the French drug agency and to their availability. Following reports of severe thrombotic events related to the ChAdOx1 nCoV-19 vaccine, its administration was interrupted on March 15th in France, and restarted on the 20th March, thereafter restricted to people aged 55 years and older which resulted in imbalance between the vaccine groups. Few HCWs received schemes with mixed mRNA. Consequently, our study was underpowered to identify an association with this vaccine scheme.
To our knowledge, this is the first study estimating the elapsed time since complete vaccination with different vaccine schemes and the incidence of SARS-CoV-2 infection among HCWs in France. Other strengths of this work include the follow-up of 7 months as compared to short follow-up periods in other studies.
In conclusion, our work suggests that independent of COVID-19 vaccination scheme, vaccine efficiency after two doses of either BNT162b2, mRNA-1273, and ChAdOx1 nCoV-19 decreases over time, especially after 3 months, in HCWs. Additional large-scale studies are requested to assess the effect of the third dose regarding the incidence of SARS-CoV-2 infection which might be helpful regarding the current Omicron variant diffusion.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Hall V.J., Foulkes S., Saei A., Andrews N., Oguti B., Charlett A., et al. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study. LancetLond Engl. 2021;397(10286):1725. doi: 10.1016/S0140-6736(21)00790-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paris C., Perrin S., Hamonic S., Bourget B., Roué C., Brassard O., et al. Effectiveness of mRNA-BNT162b2, mRNA-1273, and ChAdOx1 nCoV-19 vaccines against COVID-19 in healthcare workers: an observational study using surveillance data. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis. 2021;27(11):1699.e5–1699.e8. doi: 10.1016/j.cmi.2021.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pilishvili T., Gierke R., Fleming-Dutra K.E., Farrar J.L., Mohr N.M., Talan D.A., et al. Effectiveness of mRNA Covid-19 Vaccine among U.S. Health Care Personnel. N Engl J Med. 2021;385(25):e90. doi: 10.1056/NEJMoa2106599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. LancetLond Engl. 2021 Jan 9;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maltezou H.C., Panagopoulos P., Sourri F., Giannouchos T.V., Raftopoulos V., Gamaletsou M.N., et al. COVID-19 vaccination significantly reduces morbidity and absenteeism among healthcare personnel: A prospective multicenter study. Vaccine. 2021;39(48):7021–7027. doi: 10.1016/j.vaccine.2021.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldberg Y., Mandel M., Bar-On Y.M., Bodenheimer O., Freedman L., Haas E.J., et al. Waning Immunity after the BNT162b2 Vaccine in Israel. N Engl J Med. 2021;385(24):e85. doi: 10.1056/NEJMoa2114228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Israel A., Merzon E., Schäffer A.A., Shenhar Y., Green I., Golan-Cohen A., et al. Elapsed time since BNT162b2 vaccine and risk of SARS-CoV-2 infection: test negative design study. BMJ. 2021;24(375):e067873. doi: 10.1136/bmj-2021-067873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poukka E., Baum U., Palmu A.A., Lehtonen T.O., Salo H., Nohynek H., Leino T. Cohort study of Covid-19 vaccine effectiveness among healthcare workers in Finland. Vaccine. 2022;40(5):701–705. doi: 10.1016/j.vaccine.2021.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamamoto S., Maeda K., Matsuda K., Tanaka A., Horii K., Okudera K., Takeuchi J.S., Mizoue T., Konishi M., Ozeki M., Sugiyama H. COVID-19 breakthrough infection and post-vaccination neutralizing antibody among healthcare workers in a referral hospital in Tokyo: a case-control matching study. Clin Infect Dis Off Publ Infect Dis Soc Am. 2021 doi: 10.1093/cid/ciab1048. [DOI] [PMC free article] [PubMed] [Google Scholar]