Abstract
Background
Short-term side effects related to mRNA vaccines against SARS-CoV-2 are frequent and bothersome, with the potential to disrupt work duties and impact future vaccine decision-making.
Objective
To identify factors more likely to lead to vaccine-associated work disruption, employee absenteeism, and future vaccine reluctance among healthcare workers (HCWs).
Hypothesis
Side effects related to COVID vaccination: 1- frequently disrupt HCW duties, 2- result in a significant proportion of HCW absenteeism, 3- contribute to uncertainty about future booster vaccination, 4- vary based on certain demographic, socioeconomic, occupational, and vaccine-related factors.
Methods
Using an anonymous, voluntary electronic survey, we obtained responses from a large, heterogeneous sample of COVID-19-vaccinated HCWs in two healthcare systems in Southern California. Descriptive statistics and regression models were utilized to evaluate the research questions.
Results
Among 2,103 vaccinated HCWs, 579 (27.5%) reported that vaccine-related symptoms disrupted their professional responsibilities, and 380 (18.1%) missed work as a result. Independent predictors for absenteeism included experiencing generalized and work-disruptive symptoms, and receiving the Moderna vaccine [OR = 1.77 (95% CI = 1.33 – 2.36), p < 0.001]. Physicians were less likely to miss work due to side effects (6.7% vs 21.2% for all other HCWs, p < 0.001). Independent predictors of reluctance toward future booster vaccination included lower education level, younger age, having received the Moderna vaccine, and missing work due to vaccine-related symptoms.
Conclusion
Symptoms related to mRNA vaccinations against SARS-CoV-2 may frequently disrupt work duties, lead to absenteeism, and impact future vaccine decision-making. This may be more common in Moderna recipients and less likely among physicians. Accordingly, health employers should schedule future booster vaccination cycles to minimize loss of work productivity.
Keywords: COVID-19, Vaccine, Side-effects, Healthcare worker, Absenteeism, Hesitancy, Booster
1. Introduction
COVID-19, caused by infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), emerged in late 2019 and reached a global pandemic level by March 2020 [1]. Unprecedented global research efforts produced effective COVID-19 vaccines in record time. The first vaccinations authorized by the US Food and Drug Administration (FDA) for mass-dissemination were the BNT162b2 mRNA (Pfizer-BioNTech) and mRNA-1273 (Moderna) vaccines, with the first doses becoming available for healthcare workers (HCWs) in December 2020 [2]. Real-world analysis demonstrated these vaccines significantly reduced infections, emergency room visits, hospitalizations and death due to COVID-19 [3], [4], [5], [6], [7]. Nevertheless, initial uptake of vaccination was variable, even among HCWs [8], [9]. While vaccine hesitancy is rooted in many factors, concerns over side effects remain most compelling [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21].
Adverse events following injection may also affect daily activities and workplace performance. Effective vaccination enhances work productivity and cost savings by minimizing absenteeism related to the disease itself [22], [23], [24], [25]. However, there is a paucity of data exploring the negative occupational consequences of vaccine-related side effects in the healthcare arena. This is because adverse events due to established vaccinations such as against influenza are infrequent, mild, short-lived, and thus unlikely to substantially impact work performance [26]. In contrast, short-term side effects related to available COVID-19 mRNA vaccines are common and bothersome. Initial safety data for both mRNA vaccines showed that systemic symptoms such as fatigue and headache each occurred in over 50% of vaccination recipients after the second dose [27], [28], [29]. More recent cross-sectional studies reveal at least one generalized symptom is reported in up to 66% [30], [31], [32]. In a study of Moderna vaccine recipients, 25% of HCWs had trouble performing their daily activities shortly after vaccination [31]. Such disruptive symptoms occurring at work have the potential to compromise performance.
Waning immunity to initial vaccination and evolving virus variants have led to implementation of COVID-19 vaccine booster doses, which may become routine for HCWs [33], [34], [35], [36], [37], [38], [39]. Accordingly, our primary goal for this study was to better characterize the practical short-term impacts of COVID-19 vaccination among HCWs, and identify factors more likely to lead to vaccine-associated employee absenteeism. This may help healthcare employers plan the vaccination of their labor personnel and minimize simultaneous truancy which could result in critical shortage of an already depleted workforce. To this end, we surveyed a large, heterogeneous sample of COVID-19-vaccinated healthcare workers in two large health systems in Southern California. We postulated that symptoms related to COVID vaccination would frequently disrupt HCW duties and result in a significant proportion of HCW absenteeism. We further hypothesized that side effect-related worker absenteeism would be influenced by certain demographic, socioeconomic, occupational, and vaccine-related factors, and could also impact future vaccination decision-making.
2. Methods
We conducted an online, cross-sectional survey of HCWs at two large hospital systems (academic and private) in Southern California. The study protocol was reviewed by the respective IRBs of each participating institution and deemed exempt. Both hospitals started vaccinating their HCWs against COVID-19 on December 17, 2020.
2.1. Sample and recruitment strategy
Recruitment occurred relatively early after vaccine dissemination; between February 5–26, 2021 at the academic hospital, and April 3–17, 2021 at the private hospital. We distributed the online Qualtrics survey via institution-wide email listservs, with 8,848 recipients at the academic hospital and 3,062 recipients at the private hospital. To encourage participation, two reminder emails were sent, and the survey was anonymous and voluntary.
2.2. Survey design and measures
We designed the survey to achieve two distinct goals: 1) analyze HCW attitudes toward COVID-19 vaccination to identify clusters or groupings predisposed to vaccine hesitancy, which in turn might aid in tailoring vaccine education programs (reported in another manuscript); [40] and 2) assess the practical work-related impacts of vaccine-associated side effects, which might assist health employers in planning future vaccination booster schedules (the focus of the present report). A working group developed the measures, and the survey was based on previous questionnaires conducted amongst the 2009 H1N1 flu pandemic. It was pilot-tested with seven healthcare professionals and revised to ensure readability and understandability. The final survey included exclusively forced-choice questions to avoid missing data.
The survey instrument relevant to this study was composed of five parts: 1) Demographics : including age, gender, race, ethnicity, education level, self-reported history of chronic illness, income level, household size; 2) Occupational characteristics: including HCW position, hospital system, work setting, medical specialty; 3) Experience with COVID-19 and vaccination: including self-reported infection with SARS-CoV-2 prior to vaccination, type of vaccination received, and frequency, type, timing, and duration of short-term local and generalized side effects, per dose and type of vaccination; 4) Severity and impact of COVID-19 vaccination-related side effects within five days of injection: vaccinated survey recipients were asked if and to what extent they experienced side effect-related work duty disruption (none, mild, moderate, severe), work absenteeism (no, yes, number of days), and need for medical attention (medications to treat symptoms, emergency room visit, need for hospitalization); 5) Reluctance toward future COVID-19 booster vaccination: vaccinated survey recipients were asked if they would take a booster in the future if it was realized that their original vaccination did not provide life-long immunity. We considered the answers ‘unsure,’ ‘probably not,’ and ‘definitely not’ indicative of future booster reluctance. They were then asked to select the reasons for their hesitancy, including concerns about short term side effects, unrealized long-term side effects, efficacy, fertility, and other commonly reported motives.
2.3. Statistics
A parsimonious model was built using the purposeful selection process outlined by Hosmer-Lemeshow. Any covariate having a significant univariate Wald test from univariate logistic regression and p-value cut-off of 0.25 was selected as a candidate for the multivariable analysis. Covariates were removed from the model in an interactive process if they were non-significant or not a confounder. Statistical significance was evaluated at the 0.05 alpha level and confounding as a change in any remaining parameter estimate greater than 15% compared to the full model. Variables that were not selected for the original multivariable model were added back stepwise, with significant covariates and confounders retained in the model.
Logistic regression models were built using generalized linear models with binomial error distributions and logit link functions. Models were compared using partial likelihood ratio tests to evaluate the parsimonious model interactions among all possible pairwise covariates. Hosmer-Lemeshow Goodness-of-Fit tests were conducted as a summary measure of model fit [41].
3. Results
3.1. Sample and vaccine-related symptom characteristics:
Overall, 2,491 HCWs answered the survey (20.9% response rate), with 2,103 (84.4%) being vaccinated. Of the vaccinated sample, 2,002 (95.2%) had taken both available doses and 101 (4.8%) a single dose. A majority (70.1%) received the Pfizer vaccine, and the rest Moderna.
Table 1 presents the descriptive characteristics of the vaccinated sample. The cohort consisted of 32% nurses, 22% physicians, and 7% administrators. The remaining 39% was a heterogeneous mix of other health worker types, each comprising 4% or less of the entire sample. These included, in descending order of representation, advanced practice providers, respiratory therapists, pharmacists, patient care and medical assistants, social workers and case managers, technicians of various types, those providing nutritional, clerical, or custodial services, and speech, physical and occupational therapists. Most HCWs worked in a non-surgical setting (87%) and managed an adult patient population (86%). Twenty-three percent worked in an ICU. The majority of participants was white (72%), non-Hispanic (78%), women (73%), and born after 1965 (73%). At least one chronic medical condition was present in 33% of the sample, a severe allergy to food or medication in 15%, and allergy to previous vaccination in 1%. Ten percent of respondents had been diagnosed with COVID-19 by a PCR test prior to receiving their first vaccination dose.
Table 1.
Respondent characteristics, frequency of vaccine-related work-disruption and work absenteeism due to side effects.
| Total sample, n (%)a |
Experienced side effect-related work disruption | Missed any work due to side effects | |
|---|---|---|---|
| Overall: n (%)b | 2103 (100) | 579 (27.5) | 380 (18.1) |
| Demographics: n (%) | |||
| Female | 1543 (73.4) | 460 (29.8)***c | 309 (20.0)*** |
| Non-female | 560 (26.6) | 119 (21.3)*** | 69 (12.3)*** |
| White | 1514 (72.0) | 425 (28.1) | 258 (17.0) |
| Non-white | 589 (28.0) | 154 (26.2) | 122 (20.7) |
| Hispanic | 462 (22.0) | 128 (27.7) | 99 (21.4)* |
| Non-Hispanic | 1641 (78.0) | 451 (27.5) | 281 (17.1)* |
| Born 1965 or after | 1524 (72.5) | 460 (30.2)*** | 298 (19.6)** |
| Born before 1965 | 579 (27.5) | 119 (20.6)*** | 82 (14.2)** |
| Medical history: n (%) | |||
| Has a chronic medical condition | 694 (33.0) | 183 (26.4) | 127 (18.3) |
| No chronic medical condition | 1409 (67.0) | 396 (28.1) | 253 (18.0) |
| Severe allergy to food or med | 309 (14.7) | 121 (39.2)*** | 67 (21.7) |
| No severe allergy to food or med | 1794 (85.3) | 458 (25.6)*** | 313 (17.4) |
| Prior COVID diagnosis | 210 (10.0) | 64 (30.5) | 39 (18.6) |
| No prior COVID diagnosis | 1893 (90.0) | 515 (27.2) | 341 (18.0) |
| Socioeconomics: n (%) | |||
| Income more than $100,000 | 1421 (67.8) | 377 (26.5) | 243 (17.1)* |
| Income $100,000 or less | 524 (25.0) | 157 (30.0) | 113 (21.6)* |
| Household members more than 2 | 1145 (54.4) | 340 (29.7)* | 225 (19.7)* |
| Household members 2 or less | 958 (45.6) | 239 (25.0)* | 155 (16.2)* |
| Health system: n (%) | |||
| Academic | 1385 (65.9) | 436 (31.4)*** | 279 (20.1)*** |
| Private | 718 (34.1) | 143 (20.0)*** | 101 (14.1)*** |
| Occupation: n (%) | |||
| Nurse | 683 (32.5) | 202 (29.6) | 138 (20.2) |
| Physician | 454 (21.6) | 119 (26.2) | 30 (6.7)*** |
| Other health care provider | 821 (39.0) | 220 (26.8) | 182 (22.2) |
| Administrator | 145 (6.9) | 40 (27.6) | 30 (20.7) |
| Specialty: n (%) | |||
| Surgical | 268 (12.7) | 65 (24.3) | 47 (17.5) |
| Non-surgical | 1835 (87.2) | 496 (27.3) | 333 (18.2) |
| Pediatrics | 289 (13.7) | 83 (28.7) | 63 (21.8) |
| Adult | 1814 (86.3) | 496 (27.3) | 317 (17.5) |
| Work setting: n (%) | |||
| Outpatient – clinic | 725 (34.5) | 209 (28.8) | 124 (17.1) |
| Outpatient – ER / urgent care | 147 (7.0) | 45 (30.6) | 18 (12.2)* |
| Inpatient – ICU | 485 (23.1) | 126 (26.0) | 93 (19.1) |
| Inpatient – non-ICU | 704 (33.5) | 187 (26.6) | 139 (19.7) |
a. Column 2 shows the incidence (n) and proportions (%) of respondents with a given characteristic (row) among the total sample.
b. Columns 3 and 4 show the incidence (n) and proportions (%) of respondents with the given characteristic in that row exhibiting the designated outcome of each column.
c. Comparisons are between proportions of respondents with and without a characteristic type (grouped rows) exhibiting a designated outcome. For occupation and work setting categories, each type is compared to the others in the group. Evaluations were performed using chi-square analysis.
Statistically significant differences: * p < 0.05, ** p < 0.01, *** p < 0.001.
Across the two-dose vaccination cycle, 89% of recipients reported at least one local short-term side effect, such as injection site pain, swelling or extremity weakness. At least one generalized symptom occurred in 77%. Fatigue, body aches, and headache were most common, with all three occurring in 28% of second dose recipients. Most symptoms presented within the first two days after injection, and in 80% of individuals lasted less than three days. Severe side effects or anaphylaxis attributed to vaccination were reported by 1.6%. Only 0.7% required an emergency room or urgent care visit, and none needed hospitalization.
3.2. Impact of vaccine-related side effects
Among the 2103 vaccine recipients, 706 (34%) reported experiencing side effects while at work, 579 (28%) had side effect-related work disruption, and 221 (11%) described their symptoms as moderately or severely disruptive to their professional responsibilities. In addition, 380 HCWs (18%) reported missing work due to vaccine-related side effects. A large majority (93%) were absent for only two or fewer days. Bivariate analysis identified multiple factors associated with side effect-related work disruption and work absenteeism (Table 1).
After adjusting for demographics, medical background, and occupational and socioeconomic variables, we found white race, young age, receiving the Moderna vaccine, and receiving both doses were independently associated with side effect-related work disruption (Table 2 ). Independent predictors for work absenteeism included experiencing generalized symptoms [OR = 26.99 vs local effects only (95% CI = 8.51 – 164.15, p < 0.001)], having side effect-related work disruption [OR = 2.55 (95% CI = 1.92 – 3.38, p < 0.001)], and receiving the Moderna vaccine [OR = 1.77 (95% CI = 1.33 – 2.36, p < 0.001), Table 3 ]. In addition, compared to physicians, nurses were 3.38 times more likely to miss work (95% CI = 2.18 – 5.41, p < 0.001), administrators 3.31 times more likely (95% CI = 1.75 – 6.22, p < 0.001), and all other health providers 3.60 times more likely (95% CI = 2.31 – 5.78, p < 0.001; Table 3). Neither trainee status (5.9% for residents and fellows vs 7.0% attending physicians, p = 0.83) or health system type (6.8% for academic vs 6.4% private, p = 1.0) impacted the likelihood of physician absenteeism.
Table 2.
Parameter estimates showing predictors of experiencing vaccine side effect-related work disruption.
| Variable | Odds Ratio | 95% Confidence Interval | p |
|---|---|---|---|
| Gender and race | |||
| Female | 1.32 | 0.99–1.78 | 0.058 |
| Non-white* | 0.68 | 0.51–0.89 | 0.007 |
| Age (compared to Born before 1965) | |||
| Born 1965–1980* | 1.45 | 1.02–2.06 | 0.039 |
| Born after 1980* | 1.81 | 1.30–2.54 | <0.001 |
| Vaccine | |||
| Moderna (compared to Pfizer)* | 1.66 | 1.27–2.16 | <0.001 |
| 2 doses received (compared to 1)* | 2.02 | 1.10–3.92 | 0.030 |
* Statistically significant.
Table 3.
Parameter estimates showing predictors of work absenteeism.
| Variable | Odds Ratio | 95% Confidence Interval | p |
|---|---|---|---|
| Occupation (compared to Physician) | |||
| Nurse* | 3.38 | 2.18–5.41 | <0.001 |
| Administrator* | 3.31 | 1.75–6.22 | <0.001 |
| Other health provider* | 3.60 | 2.31–5.78 | <0.001 |
| Side effects | |||
| Work-disruptive (compared to not)* | 2.55 | 1.92–3.38 | <0.001 |
| Generalized (compared to localized only)* | 26.99 | 8.51–164.15 | <0.001 |
| Moderna (compared to Pfizer)* | 1.77 | 1.33–2.36 | <0.001 |
* Statistically significant.
In our adjusted model, relevant factors not associated with either side effect-related work disruption or work absenteeism were gender, ethnicity, socioeconomic status, medical specialty, practice setting, underlying medical comorbidities, and previous COVID-19 diagnosis.
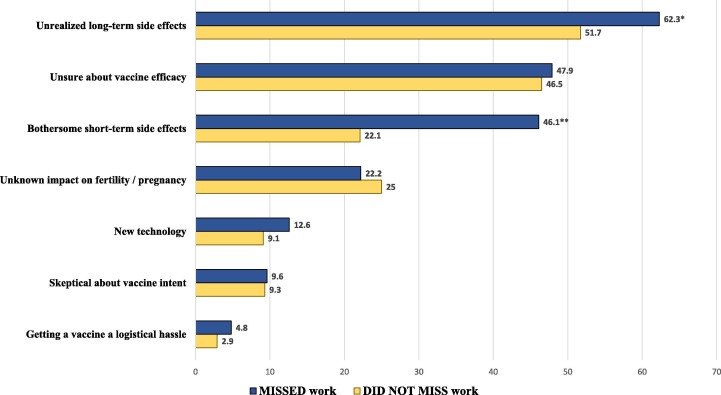
Of the entire vaccinated sample, 683 (33%) had not definitively decided that they would take future booster doses if needed. Those who missed work due to vaccine-related symptoms were more likely to cite concerns over long and short-term side effects as reasons for reluctance toward future vaccination (Fig. 1 ). Work absenteeism did not influence other commonly expressed concerns. Independent predictors for hesitancy about future vaccination included lower education level, younger age, having received the Moderna vaccine, and missing work due to vaccine-related symptoms (Table 4 ).
Fig. 1.
Frequency of reported reasons for reluctance toward future booster vaccination in health care workers who missed work due to vaccine-related symptoms, compared to those who did not miss work. Group comparisons were evaluated using chi-square analysis; * p = 0.02; ** p < 0.001.
Table 4.
Parameter estimates showing predictors of hesitancy for future vaccination.
| Variable | Odds Ratio | 95% Confidence Interval | p |
|---|---|---|---|
| Education (compared to Graduate degree) | |||
| Bachelor’s degree* | 1.66 | 1.31–2.10 | <0.001 |
| Associates degree or less* | 1.81 | 1.41–2.33 | <0.001 |
| Age (compared to Born before 1965) | |||
| Born 1965–1980 | 1.07 | 0.82–1.40 | 0.600 |
| Born after 1980* | 1.60 | 1.25–2.05 | <0.001 |
| Missed work due to vaccine side effects* | 1.37 | 1.06–1.76 | 0.015 |
| Moderna (compared to Pfizer)* | 1.95 | 1.57–2.42 | <0.001 |
| Would not recommend vaccine to family or friends* | 18.73 | 8.59–49.22 | <0.001 |
* Statistically significant.
4. Discussion
We surveyed a large cohort of healthcare workers across two large institutions in southern California and found that COVID-19 vaccination-related side effects have a substantial impact on work-related duties, and lead to absenteeism. HCWs who experienced generalized and work-disruptive side effects, and those who received the Moderna vaccine were more likely to miss work, while physicians were less likely compared to other HCWs. Work absenteeism, in turn, was associated with reluctance to obtain future booster vaccination. To our knowledge, to date this is the largest cohort analyzing the predictors of COVID-19 vaccine-associated symptom impact on work duties, absenteeism, and future booster vaccination.
The motivation for our study arose from multiple anecdotal observations at our institutions soon after the introduction of the initial two-dose vaccination cycle. There was a noticeable rise in HCW absentee rate. Also, HCWs were frequently seen working with ‘flu-like’ symptoms. ICU nurses would report fatigue and body aches throughout a 12-hour shift within a few days post-vaccination. Faculty physicians would attend clinic with high fever. Resident and fellow trainees having received their vaccinations in the morning, by their overnight call shift would appear diaphoretic and in a state of ‘brain fog.’ These occurrences seemed more substantial than during a typical influenza vaccination period. If such side effects were widespread, simultaneous en masse vaccination could not only have a significant impact on workforce productivity, but also potentially affect patient safety.
We found COVID-19 vaccine-related symptoms to be common among and bothersome to HCWs, consistent with previous data analyzing the two available mRNA vaccines [27], [28], [29]. More importantly, over a quarter (28%) of the HCWs in our survey reported experiencing side effects they considered disruptive to their work duties. Almost one out of five (18%) missed work as a result. Not surprisingly, those who had generalized symptoms (rather than local symptoms alone) and experienced work-disruptive side effects were more likely to miss work. These findings are in stark contrast to influenza vaccination. According to the US FDA/CDC Vaccine Adverse Event Reporting System (VAERS), generalized complaints are mild and occur in <10% of individuals after seasonal influenza shots [26]. In a randomized trial, in the week following injection, systemic side effects among healthy adult influenza vaccine recipients were no more common than in those receiving placebo [42]. Another study analyzing both the 2009 pandemic and 2010 seasonal influenza vaccinations reported that systemic symptoms of myalgias and fatigue, with or without fever, resulted in missed work or medical consultation in only about 1% of individuals in the week after injection [43].
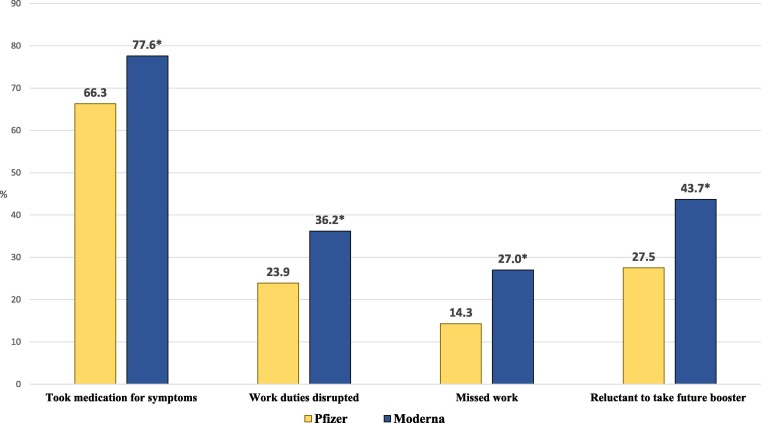
We found the Moderna vaccine was more reactogenic compared to Pfizer. Moderna recipients more frequently self-medicated for post-vaccination side effects and were more likely to report that the symptoms disrupted their work duties (Fig. 2 ). Furthermore, 27.0% of HCWs who took Moderna missed work after vaccination, almost twice the rate of Pfizer recipients. Interestingly, this was independent of the type, frequency, and magnitude of side effects reported, suggesting a possible inherent and unaccounted for characteristic of the Moderna vaccine contributing to absenteeism. For example, Moderna was made available a few weeks after Pfizer. It is possible that Moderna recipients, responding to anecdotal reports of side effects occurring among colleagues receiving Pfizer injections, began preemptively missing work in anticipation of their own symptoms. Our survey did not distinguish between unplanned and planned work absences.
Fig. 2.
Frequency (%) of impactful effects related to vaccine-associated symptoms, by vaccine type. Group comparisons were evaluated using chi-square analysis; * p < 0.001.
Among healthcare worker types, we found physicians to be over three times less likely to report having missed work due to vaccine-related symptoms. This did not seem to vary based on physician type (faculty or trainee) or hospital system (academic or private), and was despite the fact that physicians exhibited work-disruptive symptoms as often as other healthcare workers. These findings may be due to more rigid work schedules, which limit physicians’ time-off capabilities. In addition, they could be explained by the more complex and well-described phenomenon of ‘presenteeism,’ which refers to an inclination to work while feeling physically or mentally unwell [44], [45]. While presenteeism has been reported across multiple HCW occupations [46], [47], [48], physicians are frequent culprits. A combination of cultural and logistical factors, such as perseverance in a competitive environment, fear of ostracism and job-loss due to appearing lazy or unproductive, a lack of practical relief-of-duty systems, and concerns over compromising patient care can all contribute to presenteeism [47], [49], [50], [51], [52], [53], [54], [55]. Negative consequences of presenteeism include reduced work performance, compromised patient safety, and HCW burnout [46], [56], [57], [58]. We alert health employers to consider this important phenomenon in the context of COVID-19 vaccination.
Previous studies have reported a higher incidence of post-vaccine symptoms in younger and female recipients [27], [59], [60]. This represents a large component of the US healthcare workforce, particularly among nurses [61]. In our study, we also found young age to be associated with vaccine symptoms, along with white race. Female gender had a strong trend toward a statistically significant association. However, none of these demographic variables independently predicted work absenteeism. We also did not find work absences to depend on socioeconomic status, work setting, practice specialty, or presence of underlying health conditions. Furthermore, there has been concern that individuals with prior natural SARS-CoV-2 infection may exhibit a more immunogenic response after vaccination, particularly after the first dose, and thus be more likely to experience disruptive symptoms [60], [61], [62]. However, in our cohort those who had a COVID-19 diagnosis prior to vaccination did not miss work more frequently after vaccination.
Our study also underscores how practical consequences of side effects may impact future vaccine acceptance. We found that work-disruptive symptoms themselves were not independently associated with uncertainty about future vaccination. This suggests that HCWs willing to take the initial vaccine are fairly accepting of future doses, despite experiencing short-term side effects. However, individuals who missed work due to vaccine-related symptoms were indeed more averse to receiving future booster shots. In addition, they were also more likely to cite concerns over side effects as the reason for their hesitancy (Fig. 1). Thus, there may be a critical threshold reached when symptoms become severe enough to result in practical adverse consequences. Missing work may produce an anchoring psychological impact, creating an availability bias and predisposing the individual to indecision about future vaccination [21], [63], [64], [65]. While an abundance of literature already highlights vaccine safety concerns as sources of initial vaccine hesitancy [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], our findings are unique in demonstrating the influence of a negative initial vaccine experience on future vaccine decision-making.
Moderna recipients were also more hesitant about future booster vaccination. This was independent of demographics, the type, frequency, and magnitude of experienced side effects, as well as work absenteeism. The reason for this is unclear. It is possible that unaccounted variables in our model, such as secondary sources of vaccine information including social media [66] or ‘word-of-mouth,’ influenced HCWs’ impressions of its effectiveness and/or safety. Also, initial mRNA vaccine data suggesting Moderna’s greater reactogenicity were already available in early 2021 at the time of our survey [27], [28], [67]. HCWs intimately familiar with the vaccine literature could have thus been implicitly biased against Moderna’s effects. Our study did not investigate survey respondents’ a priori opinions, concerns, or skepticism about individual vaccine types. Nevertheless, this bias is likely to persist.
Our findings provide some guidance to health employers on the scheduling and implementation of future COVID-19 booster vaccination. The principal aim should be to minimize collective work absenteeism caused by vaccine-associated symptoms, given its potential impact on both work productivity and future vaccine decision-making. This may be a more substantial consideration for organizations primarily utilizing the Moderna vaccine. Depending on the size, type, infrastructure, and logistical capabilities of a given health system, mechanisms to achieve these goals could include any one or a combination of the following:
-
1-
Rotate or space out vaccine schedules. Implementing regulated on-site vaccination programs may facilitate this goal [68].
-
2-
Avoid simultaneous vaccination of similar HCW types or entire departments, particularly those difficult to replace or already suffering from critical shortages (ie. ICU nurses, operating room staff) [69], [70].
-
3-
Offer voluntary 1–2 days’ duration paid time-off post-vaccination. Such an ‘authorized absence’ is already available to US Department of Veterans Affairs healthcare employees who are unable to work due to an adverse reaction to COVID vaccines [71]. Smaller health systems, such as skilled nursing facilities, can also capitalize on a similar provision for small businesses detailed in the American Rescue Plan Act, 2021 [72].
-
4-
Encourage or mandate that vaccine boosters are taken on days of, or immediately preceding, planned time off (such as weekends or vacation). Partnering with pharmacies or other delivery systems offering vaccination during late evening or weekend hours may assist in this effort [73].
These policies should be made available to and reinforced for the entirety of the healthcare workforce, as vaccine-related work absenteeism occurs across all demographic, socioeconomic, work setting, and practice specialty strata. In parallel, HCW presenteeism should be globally and publicly discouraged and proper relief-of-duty systems implemented to help minimize work inefficiency, medical error, and HCW burnout.
The main limitation of our study is its cross-sectional survey design, relying on self-report of symptoms and experiences. In addition, it is limited to its ‘point in time’ of when vaccinations were fairly new, and detailed information about safety and efficacy were still accruing. We cannot predict how perceptions have since evolved in response to a broader and more comprehensive vaccination knowledge base, and thus extrapolation of our findings to the present and future should proceed with caution. Also, our survey draws conclusions from the initial two-dose vaccination cycle. The reactogenicity of future booster vaccinations is yet unknown, though initial data suggest it may be similar to the initial doses [38], [74]. Finally, our response rate of 20.9% introduces the possibility of non-response bias and thus may not be completely representative of the general HCW population. One reason for this relatively low response could be survey dismissal due to HCW burnout or “COVID fatigue,” as its distribution followed a significant local surge. Other possibilities include incomplete survey delivery resulting from outdated email listservs due to recent workforce turnover. Nevertheless, this response rate mirrors similar surveys systematically reviewed by Li et al. [14].
The main advantage of our study is that it provides a unique analysis of vaccine-related impacts that are thus far inadequately explored in the literature and have important implications for health care employers planning vaccination of their workforce. We employed a large sample size, recruiting from both academic and private hospital systems and across a broad range of HCWs, while offering the survey within a short time after vaccine introduction to minimize recall bias.
In conclusion, our findings collectively support the growing body of evidence that the mRNA vaccinations against SARS-CoV-2 cause bothersome symptoms and may frequently disrupt work duties. A substantial proportion of HCWs may miss work due to vaccine-related effects. Potential impacts include compromised work productivity and secondary indecision over future booster vaccination. Accordingly, health employers should implement vaccine booster schedules to optimize work efficiency and encourage vaccine uptake.
Sources of funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Food and Drug Administration. COVID-19 vaccines. Silver Spring, MD: US Department of Health and Human Services, Food and Drug Administration, 2020. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/covid-19-vaccines/ Accessed: 08/17/2021.
- 3.Pilishvili T., Fleming-Dutra K.E., Farrar J.L., Gierke R., Mohr N.M., Talan D.A., et al. Interim Estimates of Vaccine Effectiveness of Pfizer-BioNTech and Moderna COVID-19 Vaccines Among Health Care Personnel — 33 U.S. Sites, January–March 2021. MMWR Morb Mortal Wkly Rep. 2021;70(20):753–758. doi: 10.15585/mmwr.mm7020e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pawlowski C., Lenehan P., Puranik A., Agarwal V., Venkatakrishnan A.J., Niesen M.J.M., et al. FDA-authorized mRNA COVID-19 vaccines are effective per real-world evidence synthesized across a multi-state health system. Med. 2021;2(8):979–992.e8. doi: 10.1016/j.medj.2021.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tregoning JS, Flight KE, Higham SL, Wang Z, Pierce BF. Progress of the COVID-19 vaccine effort: viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat Rev Immunol. 2021 Aug 9:1–11. https://doi/org/10.1038/s41577-021-00592-1. Epub ahead of print. PMID: 34373623; PMCID: PMC8351583. [DOI] [PMC free article] [PubMed]
- 6.Christie A., Henley S.J., Mattocks L., Fernando R., Lansky A., Ahmad F.B., et al. Decreases in COVID-19 Cases, Emergency Department Visits, Hospital Admissions, and Deaths Among Older Adults Following the Introduction of COVID-19 Vaccine — United States, September 6, 2020–May 1, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(23):858–864. doi: 10.15585/mmwr.mm7023e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Embi P.J., Levy M.E., Naleway A.L., Patel P., Gaglani M., Natarajan K., et al. Effectiveness of 2-Dose Vaccination with mRNA COVID-19 Vaccines Against COVID-19–Associated Hospitalizations Among Immunocompromised Adults — Nine States, January–September 2021. MMWR Morb Mortal Wkly Rep. 2021;70(44):1553–1559. doi: 10.15585/mmwr.mm7044e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization. Coronavirus disease 2019 (COVID-19) situation reports. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/ Accessed: 08/17/2021.
- 9.Goodman, B., and A. Miller. “Huge number of hospital workers still unvaccinated.” WebMD Health News. https://www.webmd.com/vaccines/covid-19-vaccine/news/20210628/huge-number-of-hospital-workers Accessed: 08/17/2021.
- 10.Paterson P., Meurice F., Stanberry L.R., Glismann S., Rosenthal S.L., Larson H.J. Vaccine hesitancy and healthcare providers. Vaccine. 2016;34(52):6700–6706. doi: 10.1016/j.vaccine.2016.10.042. [DOI] [PubMed] [Google Scholar]
- 11.Dror A.A., Eisenbach N., Taiber S., Morozov N.G., Mizrachi M., Zigron A., et al. Vaccine hesitancy: the next challenge in the fight against COVID-19. Eur J Epidemiol. 2020;35(8):775–779. doi: 10.1007/s10654-020-00671-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shekhar R., Sheikh A.B., Upadhyay S., Singh M., Kottewar S., Mir H., et al. COVID-19 Vaccine Acceptance among Health Care Workers in the United States. Vaccines (Basel) 2021 Feb 3;9(2):119. doi: 10.3390/vaccines9020119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirzinger, Ashley, Cailey Munana, and Mollyann Brodie. “Vaccine hesitancy in rural america: 2021. https://www.kff.org/coronavirus-covid-19/poll-finding/vaccine-hesitancy-in-rural-america/ Accessed: 08/17/2021.
- 14.Li M, Luo Y, Watson R, Zheng Y, Ren J, Tang J, Chen Y. Healthcare workers' (HCWs) attitudes and related factors towards COVID-19 vaccination: a rapid systematic review. Postgrad Med J. 2021 Jun 30:postgradmedj-2021-140195. 10.1136/postgradmedj-2021-140195. Epub ahead of print. PMID: 34193545. [DOI] [PubMed]
- 15.Biswas N., Mustapha T., Khubchandani J., Price J.H. The Nature and Extent of COVID-19 Vaccination Hesitancy in Healthcare Workers. J Community Health. 2021;46(6):1244–1251. doi: 10.1007/s10900-021-00984-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burden S., Henshall C., Oshikanlu R. Harnessing the nursing contribution to COVID‐19 mass vaccination programmes: Addressing hesitancy and promoting confidence. J Adv Nurs. 2021;77(8) doi: 10.1111/jan.v77.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.SteelFisher G.K., Blendon R.J., Caporello H. An Uncertain Public - Encouraging Acceptance of Covid-19 Vaccines. N Engl J Med. 2021 Apr 22;384(16):1483–1487. doi: 10.1056/NEJMp2100351. Epub 2021 Mar 3 PMID: 33657291. [DOI] [PubMed] [Google Scholar]
- 18.Luo C., Yang Y., Liu Y., Zheng D., Shao L., Jin J., et al. Intention to COVID-19 vaccination and associated factors among health care workers: A systematic review and meta-analysis of cross-sectional studies. Am J Infect Control. 2021;49(10):1295–1304. doi: 10.1016/j.ajic.2021.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spinewine A., Pétein C., Evrard P., Vastrade C., Laurent C., Delaere B., et al. Attitudes towards COVID-19 Vaccination among Hospital Staff-Understanding What Matters to Hesitant People. Vaccines (Basel) 2021 May 6;9(5):469. doi: 10.3390/vaccines9050469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaplan R.M., Milstein A. Influence of a COVID-19 vaccine's effectiveness and safety profile on vaccination acceptance. Proc Natl Acad Sci U S A. 2021 Mar 9;118(10) doi: 10.1073/pnas.2021726118. e2021726118, PMID: 33619178; PMCID: PMC7958192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Azarpanah H., Farhadloo M., Vahidov R., Pilote L. Vaccine hesitancy: evidence from an adverse events following immunization database, and the role of cognitive biases. BMC Public Health. 2021 Sep 16;21(1):1686. doi: 10.1186/s12889-021-11745-1. PMID: 34530804; PMCID: PMC8444164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olsen G.W., Steinberg M.E., Ley C.A. Worksite influenza immunization programs. Insight into the implementation and cost-benefit. AAOHN J. 2005 Mar;53(3):105–110. PMID: 15789965. [PubMed] [Google Scholar]
- 23.Costantino C., Casuccio A., Caracci F., Bono S., Calamusa G., Ventura G., et al. Impact of Communicative and Informative Strategies on Influenza Vaccination Adherence and Absenteeism from Work of Health Care Professionals Working at the University Hospital of Palermo, Italy: A Quasi-Experimental Field Trial on Twelve Influenza Seasons. Vaccines (Basel) 2019 Dec 24;8(1):5. doi: 10.3390/vaccines8010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferro A., Bordin P., Benacchio L., Fornasiero F., Bressan V., Tralli V., Moretti F., Majori S. Influenza vaccination and absenteeism among healthy working adults: a cost-benefit analysis. Ann Ig. 2020;32(3):234–244. doi: 10.7416/ai.2020.2346. PMID: 32266361. [DOI] [PubMed] [Google Scholar]
- 25.Yaghoubi M., Salimi M., Meskarpour‐Amiri M. Systematic review of productivity loss among healthcare workers due to Covid‐19. Health Planning Manage. 2022;37(1):94–111. doi: 10.1002/hpm.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moro P.L., Woo E.J., Marquez P., Cano M. Monitoring the safety of high-dose, trivalent inactivated influenza vaccine in the vaccine adverse event reporting system (VAERS), 2011–2019. Vaccine. 2020 Aug 18;38(37):5923–5926. doi: 10.1016/j.vaccine.2020.07.007. Epub 2020 Jul 21 PMID: 32709434. [DOI] [PubMed] [Google Scholar]
- 27.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomas SJ, Moreira ED Jr, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. C4591001 Clinical Trial Group. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine through 6 Months. N Engl J Med. 2021 Sep 15:NEJMoa2110345. 10.1056/NEJMoa2110345. Epub ahead of print. PMID: 34525277; PMCID: PMC8461570. [DOI] [PMC free article] [PubMed]
- 30.Kadali R.A.K., Janagama R., Peruru S., Malayala S.V. Side effects of BNT162b2 mRNA COVID-19 vaccine: A randomized, cross-sectional study with detailed self-reported symptoms from healthcare workers. Int J Infect Dis. 2021;106:376–381. doi: 10.1016/j.ijid.2021.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kadali R.A.K., Janagama R., Peruru S., Gajula V., Madathala R.R., Chennaiahgari N., et al. Non‐life‐threatening adverse effects with COVID‐19 mRNA‐1273 vaccine: A randomized, cross‐sectional study on healthcare workers with detailed self‐reported symptoms. J Med Virol. 2021;93(7):4420–4429. doi: 10.1002/jmv.26996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park M.J., Choi Y.J., Choi S. Emergency Department Utilization by In-hospital Healthcare Workers after COVID-19 Vaccination. J Korean Med Sci. 2021 Jul 12;36(27):e196. doi: 10.3346/jkms.2021.36.e196. PMID: 34254475; PMCID: PMC8275460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.The White House Fact Sheet: Biden Administration Announces Details of Two Major Vaccination Policies, November 4, 2021. https://www.whitehouse.gov/briefing-room/statements-releases/2021/11/04/fact-sheet-biden-administration-announces-details-of-two-major-vaccination-policies/ Accessed November 19, 2021.
- 34.Bar-On Y.M., Goldberg Y., Mandel M., Bodenheimer O., Freedman L., Kalkstein N., et al. Protection of BNT162b2 Vaccine Booster against Covid-19 in Israel. N Engl J Med. 2021;385(15):1393–1400. doi: 10.1056/NEJMoa2114255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi A., Koch M., Wu K., Chu L., Ma L.Z., Hill A., et al. Safety and immunogenicity of SARS-CoV-2 variant mRNA vaccine boosters in healthy adults: an interim analysis. Nat Med. 2021;27(11):2025–2031. doi: 10.1038/s41591-021-01527-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kamar N., Abravanel F., Marion O., Couat C., Izopet J., Del Bello A. Three Doses of an mRNA Covid-19 Vaccine in Solid-Organ Transplant Recipients. N Engl J Med. 2021;385(7):661–662. doi: 10.1056/NEJMc2108861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benotmane I., Gautier G., Perrin P., Olagne J., Cognard N., Fafi-Kremer S., et al. Antibody Response After a Third Dose of the mRNA-1273 SARS-CoV-2 Vaccine in Kidney Transplant Recipients With Minimal Serologic Response to 2 Doses. JAMA. 2021;326(11):1063. doi: 10.1001/jama.2021.12339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Falsey A.R., Frenck R.W., Walsh E.E., Kitchin N., Absalon J., Gurtman A., et al. SARS-CoV-2 Neutralization with BNT162b2 Vaccine Dose 3. N Engl J Med. 2021;385(17):1627–1629. doi: 10.1056/NEJMc2113468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Emanuel E.J., Skorton D.J. Mandating COVID-19 Vaccination for Health Care Workers. Ann Intern Med. 2021;174(9):1308–1310. doi: 10.7326/M21-3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dubov A., Distelberg B.J., Abdul-Mutakabbir J.C., Beeson W.L., Loo L.K., Montgomery S.B., et al. Predictors of COVID-19 Vaccine Acceptance and Hesitancy among Healthcare Workers in Southern California: Not Just “Anti” vs “Pro” Vaccine. Vaccines. 2021;9(12):1428. doi: 10.3390/vaccines9121428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bursac Z., Gauss C.H., Williams D.K., Hosmer D.W. Purposeful selection of variables in logistic regression. Source Code Biol Med. 2008 Dec;16(3):17. doi: 10.1186/1751-0473-3-17. PMID: 19087314; PMCID: PMC2633005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nichol K.L., Margolis K.L., Lind A., Murdoch M., McFadden R., Hauge M., et al. Side effects associated with influenza vaccination in healthy working adults. A randomized, placebo-controlled trial. Arch Intern Med. 1996 Jul 22;156(14):1546–1550. PMID: 8687262. [PubMed] [Google Scholar]
- 43.De Serres G., Gariépy M.C., Coleman B., Rouleau I., McNeil S., Benoît M., et al. PHAC-CIHR influenza Research Network (PCIRN). Short and long-term safety of the 2009 AS03-adjuvanted pandemic vaccine. PLoS One. 2012;7(7):e38563. doi: 10.1371/journal.pone.0038563. Epub 2012 Jul 3. PMID: 22802929; PMCID: PMC3389012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Homrich P.H.P., Dantas-Filho F.F., Martins L.L., Marcon E.R. Presenteeism among health care workers: literature review. Rev Bras Med Trab. 2020 Aug 4;18(1):97–102. doi: 10.5327/Z1679443520200478. PMID: 32783010; PMCID: PMC7413686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tartari E., Saris K., Kenters N., Marimuthu K., Widmer A., Collignon P., et al. International Society of Antimicrobial Chemotherapy Infection and Prevention Control (ISAC-IPC) Working Group. Not sick enough to worry? “Influenza-like” symptoms and work-related behavior among healthcare workers and other professionals: Results of a global survey. PLoS One. 2020;15(5) doi: 10.1371/journal.pone.0232168. PMID: 32401751; PMCID: PMC7219706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Letvak SA, Ruhm CJ, Gupta SN. Nurses' presenteeism and its effects on self-reported quality of care and costs. Am J Nurs. 2012 Feb;112(2):30-8; quiz 48, 39. 10.1097/01.NAJ.0000411176.15696.f9. PMID: 22261652. [DOI] [PubMed]
- 47.Szymczak J.E., Smathers S., Hoegg C., Klieger S., Coffin S.E., Sammons J.S. Reasons Why Physicians and Advanced Practice Clinicians Work While Sick: A Mixed-Methods Analysis. JAMA Pediatr. 2015 Sep;169(9):815–821. doi: 10.1001/jamapediatrics.2015.0684. PMID: 26146908. [DOI] [PubMed] [Google Scholar]
- 48.Silva AF, Robazzi MLDCC, Dalri RCMB, Silveira-Monteiro CA, Mendes AMOC. Presenteeism in multiprofessional team workers in the Adult Intensive Care Unit. Rev Bras Enferm. 2019 Feb;72(suppl 1):96-104. English, Portuguese. 10.1590/0034-7167-2017-0779. PMID: 30942350. [DOI] [PubMed]
- 49.McKevitt C., Morgan M., Dundas R., Holland W.W. Sickness absence and 'working through' illness: a comparison of two professional groups. J Public Health Med. 1997 Sep;19(3):295–300. doi: 10.1093/oxfordjournals.pubmed.a024633. PMID: 9347453. [DOI] [PubMed] [Google Scholar]
- 50.Jena A.B., Baldwin D.C., Jr, Daugherty S.R., Meltzer D.O., Arora V.M. Presenteeism among resident physicians. JAMA. 2010 Sep 15;304(11):1166–1168. doi: 10.1001/jama.2010.1315. PMID: 20841527. [DOI] [PubMed] [Google Scholar]
- 51.Jena A.B., Meltzer D.O., Press V.G., Arora V.M. Why physicians work when sick. Arch Intern Med. 2012 Jul 23;172(14):1107–1108. doi: 10.1001/archinternmed.2012.1998. PMID: 22710803. [DOI] [PubMed] [Google Scholar]
- 52.Gustafsson Sendén M., Løvseth L.T., Schenck-Gustafsson K., Fridner A. What makes physicians go to work while sick: a comparative study of sickness presenteeism in four European countries (HOUPE) Swiss Med Wkly. 2013 Aug;22(143):w13840. doi: 10.4414/smw.2013.13840. PMID: 23986177. [DOI] [PubMed] [Google Scholar]
- 53.Chiu S., Black C.L., Yue X., Greby S.M., Laney A.S., Campbell A.P., et al. Working with influenza-like illness: Presenteeism among US health care personnel during the 2014-2015 influenza season. Am J Infect Control. 2017;45(11):1254–1258. doi: 10.1016/j.ajic.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Allemann A., Siebenhüner K., Hämmig O. Predictors of Presenteeism Among Hospital Employees-A Cross-Sectional Questionnaire-Based Study in Switzerland. J Occup Environ Med. 2019 Dec;61(12):1004–1010. doi: 10.1097/JOM.0000000000001721. PMID: 31568102. [DOI] [PubMed] [Google Scholar]
- 55.Imai C., Hall L., Lambert S.B., Merollini K.M.D. Presenteeism among health care workers with laboratory-confirmed influenza infection: A retrospective cohort study in Queensland. Austral Am J Infect Control. 2020 Apr;48(4):355–360. doi: 10.1016/j.ajic.2019.07.024. Epub 2019 Sep 9 PMID: 31515100. [DOI] [PubMed] [Google Scholar]
- 56.Cassie F.J. Nursing research finds presenteeism steps up risk of missed care. Nursing Rev. 2014;14(6):12. [Google Scholar]
- 57.Rainbow J.G. Presenteeism: Nurse perceptions and consequences. J Nurs Manag. 2019 Oct;27(7):1530–1537. doi: 10.1111/jonm.12839. PMID: 31397508. [DOI] [PubMed] [Google Scholar]
- 58.Pei P., Lin G., Li G., Zhu Y., Xi X. The association between doctors' presenteeism and job burnout: a cross-sectional survey study in China. BMC Health Serv Res. 2020 Aug 3;20(1):715. doi: 10.1186/s12913-020-05593-9. PMID: 32746808; PMCID: PMC7398254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Menni C., Klaser K., May A., Polidori L., Capdevila J., Louca P., et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study. Lancet Infect Dis. 2021;21(7):939–949. doi: 10.1016/S1473-3099(21)00224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.d'Arminio Monforte A., Tavelli A., Perrone P.M., Za A., Razzini K., Tomasoni D., et al. Association between previous infection with SARS CoV-2 and the risk of self-reported symptoms after mRNA BNT162b2 vaccination: Data from 3,078 health care workers. EClinicalMedicine. 2021;36:100914. doi: 10.1016/j.eclinm.2021.100914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Data USA: Registered Nurses https://datausa.io/profile/soc/registered-nurses. Accessed November 19, 2021.
- 62.Callegaro A., Borleri D., Farina C., Napolitano G., Valenti D., Rizzi M., et al. Antibody response to SARS‐CoV‐2 vaccination is extremely vivacious in subjects with previous SARS‐CoV‐2 infection. J Med Virol. 2021;93(7):4612–4615. doi: 10.1002/jmv.26982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tversky A., Kahneman D. Judgment under Uncertainty: Heuristics and Biases. Science. 1974 Sep 27;185(4157):1124–1131. doi: 10.1126/science.185.4157.1124. PMID: 17835457. [DOI] [PubMed] [Google Scholar]
- 64.Smith J.C., Appleton M., MacDonald N.E. Building confidence in vaccines. Adv Exp Med Biol. 2013;764:81–98. doi: 10.1007/978-1-4614-4726-9_6. PMID: 23654058. [DOI] [PubMed] [Google Scholar]
- 65.Dubov A., Phung C. Nudges or mandates? The ethics of mandatory flu vaccination. Vaccine. 2015 May 21;33(22):2530–2535. doi: 10.1016/j.vaccine.2015.03.048. Epub 2015 Apr 11 PMID: 25869886. [DOI] [PubMed] [Google Scholar]
- 66.Baines A., Ittefaq M., Abwao M. #Scamdemic, #Plandemic, or #Scaredemic: What Parler Social Media Platform Tells Us about COVID-19 Vaccine. Vaccines (Basel) 2021 Apr 22;9(5):421. doi: 10.3390/vaccines9050421. PMID: 33922343; PMCID: PMC8146829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chapin-Bardales J., Gee J., Myers T. Reactogenicity Following Receipt of mRNA-Based COVID-19 Vaccines. JAMA. 2021 Jun 1;325(21):2201–2202. doi: 10.1001/jama.2021.5374. PMID: 33818592. [DOI] [PubMed] [Google Scholar]
- 68.Gharpure R., Guo A., Bishnoi C.K., Patel U., Gifford D., Tippins A., et al. Early COVID-19 First-Dose Vaccination Coverage Among Residents and Staff Members of Skilled Nursing Facilities Participating in the Pharmacy Partnership for Long-Term Care Program — United States, December 2020–January 2021. MMWR Morb Mortal Wkly Rep. 2021;70(5):178–182. doi: 10.15585/mmwr.mm7005e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wahlster S., Sharma M., Lewis A.K., Patel P.V., Hartog C.S., Jannotta G., et al. The Coronavirus Disease 2019 Pandemic’s Effect on Critical Care Resources and Health-Care Providers. Chest. 2021;159(2):619–633. doi: 10.1016/j.chest.2020.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang D.S., Hastie J., Wagener G., Panzer O. Anesthetic Resource Limitations and Adaptations in Times of Shortage: Experiences from New York Presbyterian Hospital During COVID-19. Anesthesiol Clin. 2021 Jun;39(2):363–377. doi: 10.1016/j.anclin.2021.03.003. Epub 2021 Apr 28 PMID: 34024437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.US Department of Veterans Affairs Office of the Chief Human Capital Officer (OCHCO) Bulletin: “Authorized Absence for Individuals and Family Members who Receive the COVID-19 Vaccine.” VA Central Office, Washington DC. February 5, 2021 (Updated August 5, 2021).
- 72.The White House Fact Sheet: President Biden to call on all employers to provide paid time off for employees to get vaccinated after meeting goal of 200 million shots in first 100 days https://www.whitehouse.gov/briefing-room/statements-releases/2021/04/21/fact-sheet-president-biden-to-call-on-all-employers-to-provide-paid-time-off-for-employees-to-get-vaccinated-after-meeting-goal-of-200-million-shots-in-the-first-100-days/ Accessed November 21, 2021.
- 73.The White House Fact Sheet: President Biden announces new actions to protect Americans against the Delta and Omicron variants as we battle COVID-19 this winter https://www.whitehouse.gov/briefing-room/statements-releases/2021/12/02/fact-sheet-president-biden-announces-new-actions-to-protect-americans-against-the-delta-and-omicron-variants-as-we-battle-covid-19-this-winter/ Accessed December 3, 2021.
- 74.Shapiro Ben David S., Shamir-Stein N., Baruch Gez S., Lerner U., Rahamim-Cohen D., Ekka Zohar A. Reactogenicity of a third BNT162b2 mRNA COVID-19 vaccine among immunocompromised individuals and seniors - A nationwide survey. Clinical Immunology. 2021;232:108860. doi: 10.1016/j.clim.2021.108860. [DOI] [PMC free article] [PubMed] [Google Scholar]