Abstract
The COVID-19 pandemic has affected the global socioeconomic and healthcare infrastructure. Vaccines have been the cornerstone in limiting the global spread of the pandemic. However, the mass scale vaccination has resulted in some unanticipated adverse events. Arguably the most serious of these has been the development of widespread thrombosis with viral-vectored vaccines. We present a case of extensive thrombosis associated with the messenger RNA (m-RNA) vaccine.
Keywords: VTE, Thrombosis, Thromboembolism, COVID-19 vaccine, SARS-CoV-2, BNT162b2, Pfizer, mRNA-1273, Moderna, AZD1222, Oxford–AstraZeneca
1. Introduction
Vaccination against SARS-CoV-2 is arguably the single most effective preventive strategy against COVID-19 [1]. With the short time to development and unprecedented scale of vaccination, one of the reasons behind vaccine hesitancy is the lack of knowledge regarding the safety and side effects of the vaccine [2]. Arguably, the single adverse effect that has produced widespread hysteria and led to a temporary halt of vaccinations in certain countries is thrombosis associated with the viral vectored ‘vaccines AZD1222/ChAdOx1 nCoV-19 and Ad26.COV2.S [[3], [4], [5]]. This phenomenon was initially labeled as “vaccine-induced pro-thrombotic immune thrombocytopenia (VIPIT)" or “vaccine-induced immune thrombotic thrombocytopenia (VITT)” [6]. The CDC and FDA recently renamed it as “thrombosis with thrombocytopenia syndrome (TTS)” [7].
Even though thrombosis is commonly reported in association with the viral vectored vaccines, the phenomenon is rare with mRNA vaccines. Thromboembolism has been observed with all mainstream mRNA vaccines, including mRNA-1273, BNT162b2, and AZD1222 COVID-19 Vaccines [8]. We report a middle-aged lady with no risk factors or history of thrombosis who presented with extensive lower limb superficial and deep thrombosis.
2. Case report
A 57-year-old non-smoker Filipino lady with a history of dyslipidemia presented with the first episode of progressive severe left leg pain, erythema, and swelling for two weeks. She received amoxicillin/clavulanate from primary care 11 days before presentation for suspected cellulitis, but the symptoms progressed. Relevant history of recent travel, trauma, surgery, prolonged immobilization, use of hormonal medications, or oral contraceptives was negative. Family or personal history of thrombosis, malignancy, a pro-thrombotic state, or bleeding diathesis was negative. Age-appropriate malignancy screen including PAP-smear, mammogram, and the fecal immunochemical test was negative. She received the second dose of BNT162b2 three weeks before this presentation.
Upon presentation, her blood pressure was 145/88, not tachycardiac (70 beats/min), not tachypneic (19/min), afebrile (37.3C), and maintaining saturation on room air. The examination was normal except for an erythematous, tender, hot, and swollen left lower limb with the left calf 3cm larger than the right. Complete blood count, peripheral smear, electrolyte panel, renal function test, liver function tests, and coagulation profile, were normal except for a D-dimer above the detection range of assay i.e., >35.2 mg/L (0.00–0.46 mg/L.)
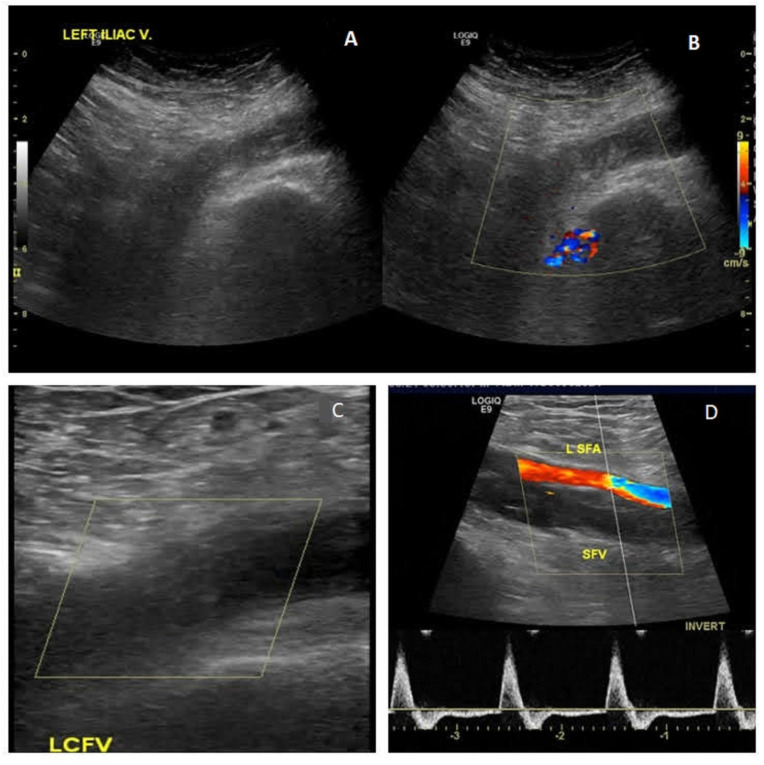
Due to a high Well's score for deep venous thrombosis (DVT), a duplex and doppler ultrasound of the lower limbs was performed and showed left popliteal, posterior tibial, superficial femoral, common femoral, and external iliac thrombosis extending up to the infrarenal inferior vena cava. Thrombus was also noted in the great saphenous vein and short saphenous vein (Fig. 1 ). The right lower extremity was normal. A bedside transthoracic echocardiogram was performed which ruled out right ventricular dysfunction, with no visible thrombi in heart chambers.
Fig. 1.
1A. Echogenic filling defect in the left iliac vein
1B. Echogenic filling defect in left iliofemoral vein with no flow. 1C. Echogenic filling defect in left common femoral vein (LCFV). 1D. Normal flow in the left superficial femoral artery (LSFA) but no flow in superficial femoral vein (SFV).
An ultrasound of the abdomen and pelvis looking for any compressive etiology predisposing to thrombosis was negative. Screening for thrombophilia, including factor V Leiden and JAK2 V617F mutation, was negative. An antiphospholipid antibody screen for anticardiolipin, anti-β2 glycoprotein, and lupus anticoagulant, was negative. A comprehensive autoimmune workup including extractable nuclear antigen (ENA), antinuclear antibody (ANA), ANCA, C3, C4, and anti-Ds-DNA was also negative. No relevant trigger could be identified in the patient as the inciting factor for the DVT. A COVID-19 nasopharyngeal reverse transcription-polymerase chain reaction (RT-PCR) was negative. She had no history of COVID-19.
The patient did well during her hospital stay and received subcutaneous therapeutic enoxaparin for 2 days followed by rivaroxaban. Three months later the patient was followed up in the medicine clinic. She was asymptomatic and her thrombophilia workup (normal protein C and S, negative for antithrombin and factor V Leiden mutation) did not reveal any coagulation disorder. As the patient was at low risk for bleeding, the plan was made to continue anticoagulation for 6 months and to decide on lifelong anticoagulation versus discontinuation at the 6-month follow-up visit (which is still due).
3. Discussion
Thrombosis associated with COVID-19 vaccination is a major concern with the recent mass vaccination drive. A literature review on PubMed using Boolean operator strategy of: ((“BNT162b2"[Title/Abstract] OR “Pfizer"[Title/Abstract]) AND (“thromb*"[All Fields] OR “embol*"[All Fields])) AND ((english[Filter]) AND (2020:2022[pdat])) on 20/01/2022 yielded 133 results (Fig. 1.) 37 articles could be identified which described various forms of thrombotic events concerning BNT162b2 vaccine. The most common adverse event was immune thrombocytopenia purpura (ITP), followed by thrombocytopenic purpura (TTP), and Cerebral Venous Thrombosis (CVT). The proposed mechanism was a heparin-induced thrombocytopenia-like phenomenon- TTS.
There are 16 cases of ITP, 12 cases of TTP reactivation, 11 cases of suspected intracerebral hemorrhage (ICH), 6 cases of cerebral venous sinus thrombosis, 1 case of pulmonary embolism, 1 case of renal thrombotic microangiopathy, a case of ST-elevation myocardial infarction (STEMI), 1 case of stroke, 1 case of central retinal artery occlusion, and a case of lower limb DVT associated with BNT162b2(Fig. 1) [[9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48]]. The STEMI reported in an elderly gentleman with multiple cardiovascular and pro-thrombotic risk factors 30mins post first dose of BNT162b2 is attributed to vaccination-induced “Kounis syndrome” or allergic myocardial infarction likely secondary to mast cell activation from an allergic or anaphylactoid reaction [11]. Another case of Kounis syndrome associated with inactivated SARS-CoV-2 vaccine (CoronaVac, Sinovac Life Sciences, Beijing, China) is reported [49]. The lower limb DVT post-second dose of vaccination reported by Carli G et al. [12] was not as extensive as reported in our case, and superficial veins were not involved. The authors report successful management of the DVT using direct oral anticoagulants (DOAC), albeit a different one from the case we present; apixaban vs. rivaroxaban, respectively [12].
Of interest is that most of the adverse reactions to AZD1222 were reported after the first dose. However, the DVT in the case under discussion and the case reported by Carli G. et al. were diagnosed after the second dose. This fact, along with the report of the absence of thrombocytopenia in both cases, points towards a different mechanism of thrombosis in BNT162b2 compared to the TTS associated with AZD1222.
It is yet unclear if the difference in the proposed mechanism of thrombosis is related to the inherent differences in the vaccines' genetic component (double-stranded vs. single-stranded) or a carrier medium, or difference in vectors (viral vectored vs. m-RNA.) However, Marshall M. et al. have suggested a role of “increased systemic reactogenicity and immunogenicity” post-second dose of mRNA-based vaccination in vaccine-associated-myocarditis [50]. This is in keeping with the trend of side effects associated with BNT162b2 being a common post-second dose [51]. A similar mechanism may be implicated in thrombosis associated with BNT162b2. As the mechanism seems to be different from TTS as reported with AZD 1222, these patients may still be able to take heparin formulations safely, as we did in our case, without any bleeding complications. However, more data is required, preferably from prospectively designed studies to assess the safety of heparin formulations in various SARS-CoV-2-vaccine-induced thromboembolic events.
The diagnosis of TTS is based on SARS-CoV-2 vaccination in the preceding 4–30 days, venous or arterial thrombosis, thrombocytopenia, and a positive PF4 “HIT” (heparin-induced thrombocytopenia) ELISA. Agents recommended by experts for use in TTS treatment are intravenous immunoglobulin (IVIG), non-heparin anticoagulation, and replacement of fibrinogen in patients with bleeding. Plasma exchange is recommended in case of treatment failure and platelet transfusion after assessing individual risk. There is no consensus on corticosteroid use [52]. The thrombosis associated with BNT162b2 is not yet recognized as a separate entity; no clear guidelines/expert opinion exists. Regarding the duration of anticoagulation, it should currently be managed like any other provoked episode of venous thromboembolism (VTE). VTE provoked by a reversible inciting factor can be started on anticoagulation initially for three months, and further continuation versus discontinuation of anticoagulation largely depends on the risk of bleeding compared to the risk of recurrent VTE [53]. In our case, we initially started the patient on rivaroxaban with a plan to decide on continuing or discontinuation at a follow-up clinic appointment.
4. Conclusion
-
1.
Association of thrombosis with BNT162b2 is a rare yet patho-physiologically distinct entity from TTS.
-
2.
With mass vaccinations, clinicians should be aware of this phenomenon and the treatment options, which do not conform to the current recommendations for vaccine-induced TTS.
-
3.
Thrombocytopenia and positive PF4 “HIT” (heparin-induced thrombocytopenia) ELISA are essential components for diagnosing vaccine-induced TTS, which are absent in BNT162b2 associated thrombosis.
-
4.
The BNT162b2 associated thrombosis can be treated with either heparin or non-heparin anticoagulation e.g., DOACs.
Financial support and sponsorship
The publication of this manuscript was supported by Qatar National Library (QNL).
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Author contributions
Zohaib Yousaf contributed towards analyzing the case, literature review, writing original draft, and critically revised the manuscript to the final form. Fateen Ata contributed towards the literature review and manuscript writing. Riyadh Hammamy was involved in the clinical management of the case, case identification, obtaining informed consent, and critically revised the draft. All authors approved the final version for submission.
Consent
Written informed consent was obtained from the patient for publication of this case report. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request. The medical research council (MRC) at Hamad Medical Corporation (HMC) approved the manuscript (proposal ID MRC-04-21-551.)
Ethics approval
Ethical approval for this study was obtained from the medical research council at Hamad Medical Corporation approved the study (Proposal ID MRC-04-21-551.)
Informed consent
Written informed consent was obtained from all subjects before the study.
Trial registration
Not applicable.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The publication of this article was funded by Qatar National Library.
References
- 1.Doroshenko A. The combined effect of vaccination and nonpharmaceutical public health interventions-ending the COVID-19 pandemic. JAMA Netw. Open. 2021;4(6) doi: 10.1001/jamanetworkopen.2021.11675. [DOI] [PubMed] [Google Scholar]
- 2.Latkin C.A., Dayton L., Yi G., Konstantopoulos A., Boodram B. Trust in a COVID-19 vaccine in the U.S.: a social-ecological perspective. Soc. Sci. Med. 2021;270:113684. doi: 10.1016/j.socscimed.2021.113684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montastruc J.L., Lafaurie M., de Canecaude C., Montastruc F., Bagheri H., Durrieu G., Sommet A. COVID-19 vaccines: a perspective from social pharmacology. Therapie. 2021;76(4):311–315. doi: 10.1016/j.therap.2021.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muir K.L., Kallam A., Koepsell S.A., Gundabolu K. Thrombotic thrombocytopenia after Ad26.COV2.S vaccination. N. Engl. J. Med. 2021;384(20):1964–1965. doi: 10.1056/NEJMc2105869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dyer O. Covid-19: EMA defends AstraZeneca vaccine as Germany and Canada halt rollouts. BMJ. 2021;373:n883. doi: 10.1136/bmj.n883. [DOI] [PubMed] [Google Scholar]
- 6.Choi P.Y., Grace R.F., Therese Ahlen M., Nazy I., Sachs U.J., Arnold D.M., McKenzie S.E., Althaus K., Sharma R., Bakchoul T. The SSC platelet immunology register of VITT and VIITP: toward standardization of laboratory and clinical parameters. J. Thromb. Haemostasis. 2021;19(8):2094–2095. doi: 10.1111/jth.15402. [DOI] [PubMed] [Google Scholar]
- 7.CDC . TTS); 2021. Updates on Thrombosis with Thrombocytopenia Syndrome.https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-12-16/02-COVID-See-508.pdf [Google Scholar]
- 8.Tobaiqy M., MacLure K., Elkout H., Stewart D. Thrombotic adverse events reported for moderna, pfizer and oxford-AstraZeneca COVID-19 vaccines: comparison of occurrence and clinical outcomes in the EudraVigilance database. Vaccines. 2021;9(11):1326. doi: 10.3390/vaccines9111326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shimazawa R., Ikeda M. Potential adverse events in Japanese women who received tozinameran (BNT162b2, Pfizer-BioNTech) J. Pharm. Policy Pract. 2021;14(1):46. doi: 10.1186/s40545-021-00326-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sissa C., Al-Khaffaf A., Frattini F., Gaiardoni R., Mimiola E., Montorsi P., Melara B., Amato M., Peyvandi F., Franchini M. Relapse of thrombotic thrombocytopenic purpura after COVID-19 vaccine. Transfus. Apher. Sci. 2021;60(4):103145. doi: 10.1016/j.transci.2021.103145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tajstra M., Jaroszewicz J., Gąsior M. Acute coronary tree thrombosis after vaccination for COVID-19. JACC Cardiovasc. Interv. 2021;14(9):e103–e104. doi: 10.1016/j.jcin.2021.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carli G., Nichele I., Ruggeri M., Barra S., Tosetto A. Deep vein thrombosis (DVT) occurring shortly after the second dose of mRNA SARS-CoV-2 vaccine. Intern. Emerg. Med. 2021;16(3):803–804. doi: 10.1007/s11739-021-02685-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alislambouli M., Veras Victoria A., Matta J., Yin F. EJHaem; 2021. Acquired Thrombotic Thrombocytopenic Purpura Following Pfizer COVID-19 Vaccination. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chamarti K., Dar K., Reddy A., Gundlapalli A., Mourning D., Bajaj K. Thrombotic thrombocytopenic purpura presentation in an elderly gentleman following COVID vaccine circumstances. Cureus. 2021;13(7) doi: 10.7759/cureus.16619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng N. Cerebral venous sinus thrombosis after pfizer-BioNTech COVID-19 (BNT162b2) vaccination. J. Clin. Neurol. 2021;17(4):573–575. doi: 10.3988/jcn.2021.17.4.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collins E.C., Carr M.J., Kim J.S., Lewis J., Jr., Maleque N., Desai K., Chen L., Sevransky J., Gaddh M., Rouphael N., Hubbard J., Pendley A.M. Immune thrombocytopenia in 2 healthy young women after the Pfizer-BioNTech BNT16B2b2 messenger RNA coronavirus disease 2019 vaccination. J. Am. Coll. Emerg. Physicians Open. 2021;2(5) doi: 10.1002/emp2.12531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Bruijn S., Maes M.B., De Waele L., Vanhoorelbeke K., Gadisseur A. First report of a de novo iTTP episode associated with an mRNA-based anti-COVID-19 vaccination. J. Thromb. Haemostasis. 2021;19(8):2014–2018. doi: 10.1111/jth.15418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Fabritiis M., Angelini M.L., Fabbrizio B., Cenacchi G., Americo C., Cristino S., Lifrieri M.F., Cappuccilli M., Spazzoli A., Zambianchi L., Mosconi G. Renal thrombotic microangiopathy in concurrent COVID-19 vaccination and infection. Pathogens. 2021;10(8):1045. doi: 10.3390/pathogens10081045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dias L., Soares-Dos-Reis R., Meira J., Ferrão D., Soares P.R., Pastor A., Gama G., Fonseca L., Fagundes V., Carvalho M. Cerebral venous thrombosis after BNT162b2 mRNA SARS-CoV-2 vaccine. J. Stroke Cerebrovasc. Dis. 2021;30(8):105906. doi: 10.1016/j.jstrokecerebrovasdis.2021.105906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fan B.E., Shen J.Y., Lim X.R., Tu T.M., Chang C.C.R., Khin H.S.W., Koh J.S., Rao J.P., Lau S.L., Tan G.B., Chia Y.W., Tay K.Y., Hameed S., Umapathi T., Ong K.H., Prasad B. Cerebral venous thrombosis post BNT162b2 mRNA SARS-CoV-2 vaccination: a black swan event. Am. J. Hematol. 2021;96(9):e357–e361. doi: 10.1002/ajh.26272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finsterer J., Nics S. Venous sinus thrombosis after the second jab of an mRNA-based SARS-CoV-2 vaccine. Brain Hemorrhages. 2021 doi: 10.1016/j.hest.2021.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Finsterer J., Redzic Z. Symptomatic peduncular, cavernous bleeding following SARS-CoV-2 vaccination induced immune thrombocytopenia. Brain Hemorrhages. 2021;2(4):169–171. doi: 10.1016/j.hest.2021.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fueyo-Rodriguez O., Valente-Acosta B., Jimenez-Soto R., Neme-Yunes Y., Inclán-Alarcón S.I., Trejo-Gonzalez R., García-Salcido M. Secondary immune thrombocytopenia supposedly attributable to COVID-19 vaccination. BMJ Case Rep. 2021;14(5) doi: 10.1136/bcr-2021-242220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fujita M., Ureshino H., Sugihara A., Nishioka A., Kimura S. Immune thrombocytopenia exacerbation after COVID-19 vaccination in a young woman. Cureus. 2021;13(9) doi: 10.7759/cureus.17942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ganzel C., Ben-Chetrit E. Immune thrombocytopenia following the pfizer-BioNTech BNT162b2 mRNA COVID-19 vaccine. Isr. Med. Assoc. J. 2021;23(6):341. [PubMed] [Google Scholar]
- 26.Giuffrida G., Condorelli A., Di Giorgio M.A., Markovic U., Sciortino R., Nicolosi D., Di Raimondo F. Immune-mediated thrombotic thrombocytopenic purpura following Pfizer-BioNTech COVID-19 vaccine. Haematologica. 2021;107(4):1008–1010. doi: 10.3324/haematol.2021.279535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Güney T., Can F., Akıncı S., Soyer Ö., Kösemehmetoğlu, Dilek İ. 1st. Vol. 39. Turk J Haematol; 2021. İmmune-mediated Thrombotic Thrombocytopenic Purpura after BNT162b2 Vaccine; pp. 74–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Innao V., Urso S., Insalaco M., Borraccino A., Consoli U. Immune Thrombotic Thrombocytopenic Purpura following Pfizer-BioNTech anti-COVID-19 vaccination in a patient healed from lymphoma after allogeneic hematopoietic stem cell transplantation. Thromb. Res. 2022;210:91–93. doi: 10.1016/j.thromres.2021.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jasaraj R.B., Shrestha D.B., Gaire S., Kassem M. Immune thrombocytopenic purpura following pfizer-BioNTech COVID-19 vaccine in an elderly female. Cureus. 2021;13(8) doi: 10.7759/cureus.16871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jawed M., Khalid A., Rubin M., Shafiq R., Cemalovic N. Acute immune thrombocytopenia (ITP) following COVID-19 vaccination in a patient with previously stable ITP. Open Forum Infect. Dis. 2021;8(7) doi: 10.1093/ofid/ofab343. ofab343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.King E.R., Towner E. A case of immune thrombocytopenia after BNT162b2 mRNA COVID-19 vaccination. Am. J. Case Rep. 2021;22 doi: 10.12659/AJCR.931478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kirpalani A., Garabon J., Amos K., Patel S., Sharma A.P., Ganesan S.L., Barton M., Cacciotti C., Leppington S., Bakovic L., Huang S.S., Knauer M.J., Tole S. Thrombotic thrombocytopenic purpura temporally associated with BNT162b2 vaccination in an adolescent successfully treated with caplacizumab. Br. J. Haematol. 2022;196(1):e11–e14. doi: 10.1111/bjh.17782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krajewski P.K., Szepietowski J.C. Immune thrombocytopenic purpura associated with COVID-19 Pfizer-BioNTech BNT16B2b2 mRNA vaccine. J. Eur. Acad. Dermatol. Venereol. 2021;35(10):e626–e627. doi: 10.1111/jdv.17444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malayala S.V., Papudesi B.N., Sharma R., Vusqa U.T., Raza A. A case of idiopathic thrombocytopenic purpura after booster dose of BNT162b2 (Pfizer-Biontech) COVID-19 vaccine. Cureus. 2021;13(10) doi: 10.7759/cureus.18985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pavenski K. Relapse of immune thrombotic thrombocytopenic purpura following vaccination with COVID19 mRNA vaccine. TH Open. 2021;5(3):e335–e337. doi: 10.1055/s-0041-1732342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qasim H., Ali E., Yassin M.A. Immune thrombocytopenia relapse post covid-19 vaccine in young male patient. IDCases. 2021;26 doi: 10.1016/j.idcr.2021.e01344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodríguez C., Pérez-Nieva A., Máiz L., Meijón M.D.M., Llamas P., Monreal M., Bikdeli B., Jiménez D. Vaccine-induced immune thrombotic thrombocytopenia after the BNT162b2 mRNA Covid-19 vaccine: a case study. Thromb. Res. 2021;208:1–3. doi: 10.1016/j.thromres.2021.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruhe J., Schnetzke U., Kentouche K., Prims F., Baier M., Herfurth K., Schlosser M., Busch M., Hochhaus A., Wolf G. Acquired thrombotic thrombocytopenic purpura after first vaccination dose of BNT162b2 mRNA COVID-19 vaccine. Ann. Hematol. 2021:1–3. doi: 10.1007/s00277-021-04584-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shah P.P., Gelnick S., Jonisch J., Verma R. Retin Cases Brief Rep; 2021. Central Retinal Vein Occlusion Following BNT162b2 (Pfizer-BioNTech) COVID-19 Messenger RNA Vaccine. [DOI] [PubMed] [Google Scholar]
- 40.Shah S.R.A., Dolkar S., Mathew J., Vishnu P. COVID-19 vaccination associated severe immune thrombocytopenia. Exp. Hematol. Oncol. 2021;10(1):42. doi: 10.1186/s40164-021-00235-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thomas W., Albano A., Kirkel D., Rouhizad N., Arinze F. Immune thrombocytopenic purpura following administration of mRNA-based SARS-CoV-2 and mmr vaccinations: a cautionary tale. Case Rep Infect Dis. 2021;2021:2704249. doi: 10.1155/2021/2704249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vaira L.A., Podda L., Doneddu P., Careddu M.G., Fozza C., De Riu G. Secondary thrombocytopenia after SARS-CoV-2 vaccine: report of a case of hemorrhage and hematoma after minor oral surgery. J. Stomatol Oral Maxillofac Surg. 2021;123(2):95–97. doi: 10.1016/j.jormas.2021.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Waqar S.H.B., Khan A.A., Memon S. Thrombotic thrombocytopenic purpura: a new menace after COVID bnt162b2 vaccine. Int. J. Hematol. 2021;114(5):626–629. doi: 10.1007/s12185-021-03190-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wiest N.E., Johns G.S., Edwards E. A case of acute pulmonary embolus after mRNA SARS-CoV-2 immunization. Vaccines (Basel) 2021;9(8):903. doi: 10.3390/vaccines9080903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamaguchi Y., Kimihira L., Nagasawa H., Seo K., Wada M. Cerebral venous sinus thrombosis after BNT162b2 mRNA COVID-19 vaccination. Cureus. 2021;13(10) doi: 10.7759/cureus.18775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoshida K., Sakaki A., Matsuyama Y., Mushino T., Matsumoto M., Sonoki T., Tamura S. Acquired thrombotic thrombocytopenic purpura following BNT162b2 mRNA coronavirus disease vaccination in a Japanese patient. Intern. Med. 2021;61(3):407–412. doi: 10.2169/internalmedicine.8568-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoshida K., Tanaka K., Suto Y., Fukuda H. Repeated cardioembolic stroke after COVID-19 mRNA vaccination: a case report. J. Stroke Cerebrovasc. Dis. 2021;31(2):106233. doi: 10.1016/j.jstrokecerebrovasdis.2021.106233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zakaria Z., Sapiai N.A., Ghani A.R.I. Cerebral venous sinus thrombosis 2 weeks after the first dose of mRNA SARS-CoV-2 vaccine. Acta Neurochir. 2021;163(8):2359–2362. doi: 10.1007/s00701-021-04860-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Özdemir İ H., Özlek B., Özen M.B., Gündüz R., Bayturan Ö. Type 1 Kounis syndrome induced by inactivated SARS-COV-2 vaccine. J. Emerg. Med. 2021;61(4):e71–e76. doi: 10.1016/j.jemermed.2021.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marshall M., Ferguson I.D., Lewis P., Jaggi P., Gagliardo C., Collins J.S., Shaughnessy R., Caron R., Fuss C., Corbin K.J.E., Emuren L., Faherty E., Hall E.K., Di Pentima C., Oster M.E., Paintsil E., Siddiqui S., Timchak D.M., Guzman-Cottrill J.A. Symptomatic acute myocarditis in 7 adolescents after pfizer-BioNTech COVID-19 vaccination. Pediatrics. 2021;148(3) doi: 10.1542/peds.2021-052478. [DOI] [PubMed] [Google Scholar]
- 51.CDC 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/pdfs/321466-A_FS_What_Expect_COVID-19_Vax_Final_12.13.20.pdf
- 52.Bussel J.B., Connors J.M., Cines D.B., Dunbar C.E., Michaelis L.C., Kreuziger L.B., Lee A.Y.Y., Ingrid Pabinger-Fasching M. 2021. Thrombosis with Thrombocytopenia Syndrome (Also Termed Vaccine-Induced Thrombotic Thrombocytopenia)https://www.hematology.org/covid-19/vaccine-induced-immune-thrombotic-thrombocytopenia [Google Scholar]
- 53.Kearon C., Akl E.A. Duration of anticoagulant therapy for deep vein thrombosis and pulmonary embolism. Blood. 2014;123(12):1794–1801. doi: 10.1182/blood-2013-12-512681. [DOI] [PubMed] [Google Scholar]