Abstract
Background
Toll-like receptors are implicated in the pathophysiology of the severe acute respiratory syndrome coronavirus (SARS-CoV) and the Middle East respiratory disease (MERS), according to several studies. The whole-genome sequencing of SARS-CoV-2 revealed that the TLR7 gene could be implicated in the virus's pathogenesis since the virus includes ssRNA patterns that could bind to TLR7.
Aim
The purpose of this study was to look into the function of the TLR7 (rs3853839) C/G polymorphism and the expression of TLR7 mRNA transcript in the development, severity and progression of COVID-19.
Subjects and methods
A case-control study included 285 participants who were divided into two groups: 150 middle-aged people with COVID 19 who had no previous co-morbidities and 135 healthy volunteers who served as controls. TaqMan test was used to genotype the TLR7 (rs3853839) C/G polymorphism, and real-time PCR was used to determine the relative expression of its mRNA transcript. The level of IL-6 in serum was determined using the ELISA method as an indicator of cytokine storm and COVID-19 severity.
Results
The GG genotype was shown to be much more common in COVID-19 patients (38.7%) than controls (4.4%), with an OR of 19.86 (95% CI: 7.85; 50.22) and was linked to disease severity and poor clinical outcomes (hospitalization, respiratory failure, cardiac complications, ICU admission and mechanical ventilation).
As a result, the G allele was considerably higher in cases (57.0%), while the C allele was significantly higher in controls (p = 0.001). The GG genotype was found to be substantially more common in patients who were severely/critically unwell. TLR7 mRNA expression levels were significantly higher in COVID-19 patients (2.44 ± 0.89) than in controls (1.06 ± 0.46) (p = 0.001). TLR7 mRNA levels were highest in COVID 19 patients with the GG genotype (rs3853839). Patients with the GG genotype had considerably lower WBC counts, but significantly higher serum ferritin, CRP, IL-6 and D dimer levels (P = 0.045, 0.001, 0.023, 0.033, 0.001, respectively).
Conclusion
The GG form of the TLR7 SNP (rs3853839) could be a genetic risk factor for COVID-19 infection, severe illness and poor clinical outcome. TLR7 mRNA expression was also elevated in COVID-19 patients who were severely/critically unwell and had a bad outcome, suggesting that they could be used as COVID-19 prognostic biomarkers.
Abbreviations: ARDS, Acute respiratory distress syndrome; CBC, complete blood count; CRP, C-reactive protein; MERS, Middle East respiratory disease, according to several studies; RT-PCR, reverse transcriptase polymerase chain reaction; SARS-CoV, Severe acute respiratory syndrome coronavirus; ssRNA, single-strand RNA; TLR7, Toll Like Receptor 7; WHO, World Health Organization
Keywords: COVID-19, TLR7, PCR, IL-6, mRNA, SNP
Graphical abstract
1. Introduction
The World Health Organization (WHO) confirmed a pandemic caused by a newly emerged coronavirus known as severe acute respiratory syndrome coronavirus 2 in March 2020 (SARS-CoV-2) (Cascella et al., 2022). The infection quickly spread over the world, wreaking havoc in all areas and posing a serious threat to healthcare systems. This virus can cause a variety of mild to severe symptoms, which are collectively known as coronavirus disease-2019 (COVID-19) (Coronaviridae Study Group of the International Committee on Taxonomy of Viruses, 2020). SARS-CoV-2 is a member of the Betacoronavirus genus, which belongs to the Coronaviridae family. This encapsulated virus contains a single-stranded RNA genome that is between 29.8 and 29.9 kb, making it the largest human RNA virus (Khailany et al., 2020). The SARS-CoV-2 epidemic has become one of the world's most critical public health issues. Despite the recent approval of various vaccines for the prevention of coronavirus illness 2019 (COVID-19), a viable treatment is still needed (Hashemi et al., 2021a). Age, gender, smoking, immunological state, diabetes, hypertension, cardiovascular disease, chronic respiratory illness, cancer and genetic background may all influence the clinical signs and fate of infection (Zheng et al., 2020). Toll-like receptors (TLRs) are involved in the detection of microbial infections as well as the recognition of viral particles. TLRs initiate innate immune responses and also induce adaptive immune responses. TLR3 detects double-strand RNA, TLR4 detects lipopolysaccharide (LPS), TLR7 detects single-strand RNA (ssRNA) (Kawasaki et al., 2011) and TLR9 is essential for the recognition of unmethylated CpG DNA (Ashkar & Rosenthal, 2002). TLR7 is a protein that recognizes ssRNA and synthesized oligoribonucleotides and is encoded by the TLR7 gene, which is produced in innate immune cells and situated on the short arm of X chromosome locus number 22.3 in the endosome membrane. As a result, they may play a role in the identification of the SARSCoV2 genome (de Groot & Bontrop, 2020a). TLR7 may be more implicated in the pathogenesis of SARSCoV2 than SARS-CoV and MERS-CoV, according to whole genome sequencing of SARSCoV, MERSCoV and SARSCoV2. SARSCoV2 includes more ssRNA motifs that potentially bind to TLR7 than SARS-CoV and MERS-CoV (van der Made et al., 2020a). TLR7 expression is linked to the TLR7 rs3853839 C/G polymorphism, which influences TLR7 mRNA turnover, according to genetic studies (Wang et al., 2019). In COVID-19-infected patients, TLR7 may be the major pathogen recognition receptor involved in the development of cytokine storm (Safaei & Karimi-Googheri, 2021). We hypothesize a link between COVID-19 susceptibility, severity and clinical outcome and the TLR7 gene polymorphism (rs3853839) and TLR7 mRNA expression.
2. Subjects and methods
2.1. Subjects
From March 2020 to October 2021, a case control research was conducted by collaboration between the departments of Chest Disease, Medical Biochemistry & Molecular Biology, Clinical Pathology, Public Health and Microbiology at Menoufia Faculty of Medicine in Egypt. The study included 285 volunteers who were separated into two groups: 150 COVID-19 patients and 135 age and gender matched healthy subjects who served as controls. We included PCR verified cases of COVID-19 with a positive result from a nasopharyngeal swab specimen using a real-time reverse transcriptase polymerase chain reaction (RT-PCR) assay. Our study comprised adult patients (18–50 years old) with mild, moderate, severe, or critical COVID-19 infection, as defined by WHO criteria for COVID-19 clinical care. Mild disease was classified as having any of the COVID-19 signs and symptoms (fever, cough, sore throat, malaise, headache, muscle pain, nausea, vomiting, diarrhea TLRs both initiate and instigate innate and adaptive immune responses, loss of taste and smell) but no shortness of breath, dyspnea, or abnormal chest imaging without evidence of viral pneumonia or hypoxia. Patients with moderate illness had clinical indications of pneumonia (fever, cough, dyspnea, rapid breathing) but no signs of severe pneumonia, such as a SpO2 of less than 90% on room air. Patients with severe disease had clinical indications of pneumonia (fever, cough, dyspnea, rapid breathing) as well as one or more of the following: respiratory rate > 30 breaths/min; significant respiratory distress; or SpO2 90% on room. Acute respiratory distress syndrome (ARDS), sepsis, septic shock and other critical diseases were characterized as patients who met the criteria for ARDS, sepsis and septic shock or various disorders that would ordinarily necessitate life-sustaining interventions such as invasive or non-invasive mechanical breathing or vasopressor therapy (World Health Organization, 2021). Old age, smokers, pregnancy, obesity, diabetes mellitus, hypertension, cardiovascular illnesses, chronic respiratory diseases, cancer, chronic kidney diseases and recipients of transplant or immunosuppressive therapy were among the patient group's exclusion criteria. Laboratory investigations in the form of complete blood count (CBC) with differential, as well as inflammatory markers such D-dimer, serum ferritin and C-reactive protein (CRP), interleukin-6 (IL-6) (as an indicator of cytokine storm and COVID-19 severity) were assayed. Chest X-rays and computed tomography were used to evaluate these patients' chest imaging. CO-RADS or COVID-19 Reporting and Data System is a categorical radiological (CT) assessment scheme for patients suspected of having COVID-19. CO-RADS (1) mean that COVID-19 is highly unlikely. The CT is normal or there are findings that indicate a non-infectious disease like congestive heart failure, sarcoid, histoplasmosis, malignancy, usual interstitial pneumonia or fibrotic nonspecific interstitial pneumonia. CO-RADS (2) mean that level of suspicion of COVID-19 infection is low. Findings are consistent with other infections like typical bronchiolitis with tree-in-bud and thickened bronchus walls. CO-RADS (3) mean that COVID-19 unsure or indeterminate. CT abnormalities are indication of infection, but unsure whether COVID-19 is involved, like widespread bronchopneumonia, lobar pneumonia, and septic emboli with ground glass opacities. CO-RADS (4) mean that the level of suspicion is high. Mostly these are suspicious CT findings but not extremely typical. Unilateral ground glass, multifocal consolidations without any other typical finding, and findings doubtful of COVID- 19 in underlying pulmonary disease are the abovementioned suspicious CT findings which are not extremely typical. CO-RADS (5) mean multifocal areas of ground glass and consolidation. CO-RADS (6) mean that the patient with positive PCR and bilateral ground glass opacity (Prokop et al., 2020).
2.2. Ethical approval
All subjects signed a consent form approved by “the Local Ethics & Human Rights committee in Research at Faculty of Medicine, Menoufia University” before taking blood samples.
2.3. Blood sample collection and preparation
Using sterile venipuncture, six milliliters of fresh venous blood were taken. 2 ml of blood were immediately transferred to an EDTA tube and divided into two aliquots, one for CBC (WBC and lymphocyte relative count) and the other for genetic testing. For the D-dimer test, 1.8 ml was transferred to a sodium citrate tube. The remaining 2.2 ml was transferred to a plain tube and centrifuged at 4000 rpm for 20 min; the serum was then refrigerated at −20 °C until serum ferritin, C-reactive protein (CRP), and interleukin 6 (IL-6) were measured.
2.4. Methods
Mispa-i2 was used to calculate CRP using the nephelometric method (Agape Diagnostics, Switzerland). The Architect plus i1000SR immunoassay analyzer was used to measure serum ferritin using a chemoilumincence immunoassay (Abbott, Illinois, USA). The stago -STA Compact Max Analyzer, Fully Automated Coagulation System - was used to measure the D-dimer (Diagnostic Stago, France).
The Sysmex XN-1000 Automated Hematology Analyzer was used to measure WBCs and lymphocytes (Sysmex Corporation, Kobe, Japan). Quantikine, Canada, USA, provided enzyme-linked immunosorbent assay (ELISA) human kits for measuring serum IL-6 levels.
2.5. Genotyping of rs3853839 C/G polymorphism of TLR7 gene
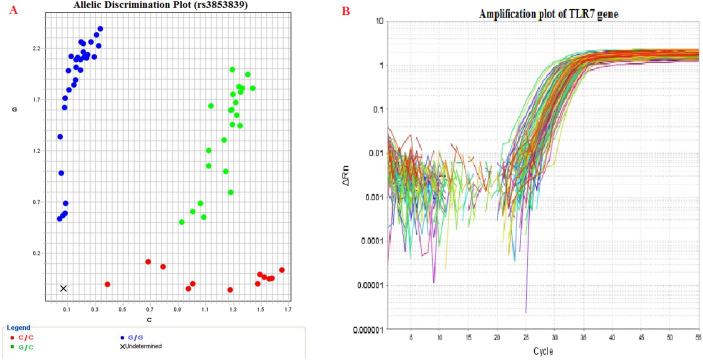
Thermo Fisher Scientific's GeneJET Whole Blood Genomic DNA Purification extraction kit was used to extract DNA from peripheral blood. The C/G polymorphism at the TLR7 gene was genotyped using a real-time PCR approach with a TaqMan probe from Applied Biosystems in the United States. Thermo Scientific also donated the primers, probes and Master Mix (40×). The probe sequences were designed as: [VIC/FAM] TGCTTCAGTGCTTCCTGCTCTTTTT[C/G]CTTGGGCCTGCTTCTGGGTTCC TA. Each reaction contained 1.25 μl of primer/probe mixture, 10 μl of Master Mix, 3.75 μl of nuclease-free water and 5 μl of purified DNA. The cycling conditions were: 10 min at 94 °C for the first denaturation, then 50 cycles (30 s at 95 °C for the second denaturation, 60 s at 50 °C for primer annealing and 1.5 min at 72 °C for primer extension) and finally 1 min at 72 °C for the final extension step. The ABI 7500 real-time PCR software, version 2.0.1, was used to analyse the data (Fig. 1A).
Fig. 1.
A - Allelic discrimination plot showing different genotypes of TLR 7 Gene SNP (rs3853839). An allelic discrimination plot or “cluster plot” shows three clusters. The points in each cluster are grouped closely together and express one genotype. At the lower right corner of the plot the cluster represents allele C (homozygote), labelled with VIC® dye. At the Upper left corner of the plot the cluster represents allele G (homozygote), labelled with FAM™ dye. Approximately midway between the allele C and allele G clusters represent both (allele C and allele G—heterozygote).
B - Amplification plot of TLR7 gene expression displaying fluorescence versus cycle number. Amplification plots are created when the fluorescent signal from each sample is plotted against cycle number; therefore, amplification plots represent the accumulation of product over the duration of the real-time PCR experiment.
2.6. Measurement of TLR7 mRNA expression by real-time PCR technique
The total RNA in venous blood was freshly extracted using Thermo Scientific's Gene-JET Blood RNA Purification Kit. The NanoDropTM 2000 technology was used to determine the concentrations and purity of RNA (Thermo Scientific, USA). Until the reverse transcription process, the RNA extract was stored frozen at −80 °C. For cDNA synthesis, Thermo Scientific's RevertAid First Strand cDNA Synthesis Kit was employed. On ice, with a total volume of 20 μl, the reaction was prepared in two steps as follows: To make a total volume of 12 μl, 10 μl of RNA was mixed with 1 μl of hexamer primer and 1 μl of nuclease-free water. After that, the samples were incubated at 65 °C for 5 min before being chilled on ice. Second, we added 4 μl of 5 X reaction buffer, 1 μl of Ribolock RNase inhibitor, 2 μl of 10 mM dNTPs and 1 μl of Revertaid RT to the above-mentioned mix to make a total volume of 20 μl.
The 2720 thermal cycler (ABI systems, Singapore) was used for a single cycle of incubation as follows: 5 min at 25 °C, 60 min at 42 °C and 5 min at 70 °C.
The cDNA was stored at −20 °C until the real-time PCR stage. SensiFASTTM SYBR Lo-ROX Kit, USA, was used for real-time PCR. The 20 μl total volume comprised of 10 μl of SYBR green dye, 1 μl of nuclease-free water, 6 μl of cDNA and 1.5 μl of each forward and reverse primers. The National Center for Biotechnology Information confirmed the primer sequence (NCBI). The primers used were as follows: Forward primer for the TLR7 gene: 5′-CCTTGAGGCCAACAACATCT-3′, Reverse 5′-GTAGGGACGGCTGTGACATT-3'and housekeeping gene (GAPDH): forward 5′-GAAGGTGAAGGTCGGAGTC-3′, Reverse: 5′-GAAGATGGTGATGGGATTTC-3′.
Three phases were used for gene amplification: an initial denaturation step at 95 °C for 10 min, followed by 50 cycles at 95 °C for 15 s, 63 °C for 1 min, and 72 °C for 1 min and a final extension step at 72 °C for 10 min.
Finally, 7500 (v.2.0.1; Applied Biosystems, USA) was used to finish the fluorescence detection and data analysis (Fig. 1B and 2A). The relative expression of the TLR7 gene was determined using the 2−ΔΔCt technique, which was normalized to the endogenous housekeeping gene (GAPDH) and compared to the control, with ΔCt = Ct target – Ct reference, ΔΔCt = (ΔCt sample –ΔCt control) (Dorak & Real-time, 2004).
Fig. 2.
A - Melting curve of TLR7 gene showing the specificity of the chosen primers. Melting curve can detect the presence of nonspecific products, such as primer-dimer, if they present, will appear as additional peaks to the left and right of the main peak for the amplified product in the melt curve.
B - Association between TLR7 mRNA expression and SNP rs3853839 genotyping among cases.
3. Statistical analysis
The Wilks-Shapiro test was used to determine the normality of various variables. Number (No), percentage (%), mean (x) and standard deviation (SD) were used to express various variables. For comparisons of quantitative normally distributed variables between two groups, the Student's t-test was employed, whereas Mann–Whitney U's test was used for non-normally distributed variables. The ANOVA test with homogeneity of variance was used to compare quantitative variables between more than two groups of normally distributed data, whereas the Kruskal-Wallis test was used for not normally distributed data. To investigate the relationship between qualitative variables, the Chi-square test (2) was utilized, along with the Z-test to compare column proportions. Fischer's exact test was employed whenever any of the anticipated cells were fewer than five. To determine the effects of potential risk factors on the ultimate outcome, a univariate logistic regression analysis was used. Statistical significance was defined as a two-sided P value of less than 0.05. All statistical analyses were carried out with the statistical package for social science (SPSS) version 23 (SPSS Inc., 2015). Version 23.0 of IBM SPSS Statistics for Windows (Armnok, NY: IBM Corp).
4. Results
Cases and controls were matched in age and gender. The mean age of cases was 38.24 ± 8.42 years and for controls was 38.68 ± 9.86 years (p = 0.992). Males represented 53.3% of cases and 59.3% of controls (p 0.314). Cases had significantly higher mean serum IL6 and TLR7 mRNA expression than controls. The mean serum IL6 of the cases was 15.29 ± 7.67 pg/ml and the mean for controls was 5.15 ± 1.42 pg/ml (p < 0.001) (Table 1 ).
Table 1.
Genotype frequency & allele distribution and TLR7 mRNA expression in the studied groups:
| Variables | Cases (n = 150) No. (%) |
Controls (n = 135) No. (%) |
P value | OR (95% CI) |
|---|---|---|---|---|
| Age (mean ± SD) | 38.70 ± 9.60 | 38.68 ± 9.86 | 0.992† | – |
| Gender | ||||
| Male | 81 (54.0) | 80 (59.3) | 0.371 | – |
| Female | 69 (46.0) | 55 (40.7) | ||
| SNP rs3853839 genotyping | ||||
| GG | 58 (38.7)⁎ | 6 (4.4) | <0.001 | 19.86 (7.85; 50.22) |
| GC | 55 (36.7) | 53 (39.3) | 2.13 (1.24;3.68) | |
| CC | 37 (24.7) | 76 (56.3)⁎ | ||
| Allele | ||||
| G | 171 (57.0) | 65 (24.1) | <0.001 | 4.18 (2.91;6.00) |
| C | 129 (43.0) | 205 (75.9) | ||
| TLR7 mRNA expression (mean ± SD) | 2.44 ± 0.89 | 1.06 ± 0.46 | <0.001 | – |
| TLR7 mRNA expression | ||||
| GG | 3.33 ± 0.40 | 1.18 ± 0.43 | <0.001 | |
| GC | 2.23 ± 0.38 | 1.05 ± 0.47 | <0.001 | – |
| CC | 1.37 ± 0.57 | 1.05 ± 0.46 | 0.005 | |
Significantly higher than their corresponding in the other groups.
Significantly lower than their corresponding in the other groups.
The prevalence of genotype GG was significantly higher among cases (38.7%) than controls (4.4%) with OR 19.86 (95% CI: 7.85; 50.22), while the genotype CC was significantly higher among controls (56.3%) than cases (24.7%). Consequently, the G allele was significantly higher among cases (57.0%) and the C allele was significantly higher among controls (p < 0.001). The TLR7 mRNA expression was significantly higher among cases (2.44 ± 0.89) than controls (1.06 ± 0.46) (p < 0.001) (Table 1).
Table 2a provides a detailed description of the characteristics of COVID-19 patients: 120 case (80.0%) complained of fever, 51 case (34.0%) of anosmia, 93 case (62.0%) of muscle aches, 84 case (56.0%) of fatigue, 141 case (94.0%) of cough, 54 case (36.0%) of dyspnea, 84 case (56.0%) of headache, 93 case (62.0%) of anorexia and 51 case (34.0%) of diarrhea. Their mean respiratory rate was 21.80 ± 6.55, SO2% was 92.58 ± 6.25, temperature was 37.92 ± 0.71, WBCs (106/ml) was 5.05 ± 2.23, Lymphocytes (%) was 22.11 ± 9.35, hemoglobin was (12.40 ± 1.36) g/dL, HCT (%) was 39.95 ± 3.45, platelets was 198.64 ± 50.68, serum ferritin was (227.40 ± 279.41) ng/ml, CRP was (59.11 ± 49.03) mg/dl and D dimer was (0.93 ± 0.75) μg/ml. Thirty-two percent had CORAD grade 1, (6%) had grade 2, (6%) had grade 3, (14%) had grade 4 and (42%) had grade 5.
Table 2a.
Clinical features, vital signs, laboratory investigations of patient group.
| Clinical features | No. (%) |
| Fever | 120 (80.0) |
| Anosmia | 51 (34.0%) |
| Myalgia | 93 (62.0%) |
| Headache | 84 (56.0%) |
| Fatigue | 84 (56.0%) |
| Cough | 141 (94.0%) |
| Dyspnea | 54 (36.0) |
| Anorexia | 93 (62.0%) |
| Diarrhea | 51 (34.0%) |
| Vital signs | Mean ± SD, median |
| Respiratory rate | 21.80 ± 6.55, 19.0 |
| SO2% | 92.58 ± 6.25, 95.0 |
| Temperature | 37.92 ± 0.71, 37.95 |
| Laboratory investigations | Mean ± SD, median |
| WBCs (106/ml) | 5.05 ± 2.23, 4.5 |
| Lymphocytes % | 22.11 ± 9.35, 19.0 |
| Hemoglobin (g/dl) | 12.40 ± 1.36, 13.0 |
| HCT | 39.95 ± 3.45, 40.0 |
| Platelets | 198.64 ± 50.68, 189.5 |
| Ferritin | 227.40 ± 279.41, 67.0 |
| CRP | 59.11 ± 49.03, 39.50 |
| D dimer | 0.93 ± 0.75, 0.60 |
| Radiological features (CORADS) | No. (%) |
| 1 | 48 (32.0) |
| 2 | 9 (6.0) |
| 3 | 9 (6.0) |
| 4 | 21 (14.0) |
| 5 | 63 (42.0) |
Table 2b provides a detailed description of the clinical outcome of COVID-19 patients: 34% of cases had mild disease, (28.0%) had moderate, (22.7%) had severe and (15.3%) had critically ill grade of COVID-19 (11.3%). Ten percent of cases had complications in the form of thromboembolic complications, (10%) had renal complications, (10%) had cardiac complications and (1.3%) had fungal infection. About (15.3%) needed ICU admission and (9.3%) needed mechanical ventilation. Six cases (4.0%) died and 57 (38.0%) suffered from post COVID-19 symptoms.
Table 2b.
Clinical outcome of the patient group.
| Outcome | No. (%) |
|---|---|
| Severity | |
| 1 - Mild | 51 (34.0) |
| 2 - Moderate | 42 (28.0) |
| 3 - Severe | 34 (22.7) |
| 4 - Critically ill | 23 (15.3) |
| Thromboembolic complications | 15 (10.0) |
| Renal complications | 15 (10.0) |
| Cardiac complications | 15 (10.0) |
| Fungal infection | 2 (1.3) |
| ICU admission | 23 (15.3) |
| Mechanical ventilation | 14 (9.3) |
| Death | 6 (4.0) |
| Post COVID symptoms | 57 (38.0) |
Patients' age did not show any significant difference among different genotypes (p = 0.056). Percentage of genotype GC was significantly lower among males than other genotypes (p = 0.001). Clinical manifestations did not show any significant difference among different genotypes except dyspnea which was significantly higher among patients with GG genotype (p = 0.014). The mean respiratory rate was significantly higher and the mean oxygen saturation was significantly lower among patients with GG genotype (p < 0.001 for any). The mean WBCs count was significantly lower, while the mean serum ferritin, CRP, IL6 and D dimer were significantly higher among patients with GG genotype (P = 0.045, <0.001, 0.023, 0.033, <0.001 respectively). Patients with GC genotype had significantly lower prevalence of advanced CO-RADS classification than other genotypes (p < 0.001) Table 3a .
Table 3a.
Personal characters and clinical manifestations among patients with different genotypes.
| Variables | GG (n = 58) No. (%) |
GC (n = 55) No. (%) |
CC (n = 37) | P value |
|---|---|---|---|---|
| Age in years (mean ± SD) | 40.08 ± 6.76 | 36.56 ± 9.10 | 37.56 ± 9.16 | 0.056 |
| Gender | ||||
| Male | 34 (58.6) | 19 (34.5)† | 27 (73.0) | 0.001 |
| Female | 24 (41.4) | 36 (65.5) | 10 (27.0) | |
| Clinical manifestation | ||||
| Fever | 43 (74.1) | 49 (89.1) | 28 (75.7) | 0.117 |
| anosmia | 18 (31.0) | 15 (27.3) | 18 (48.6) | 0.087 |
| Anorexia | 36 (62.1) | 36 (65.5) | 21 (56.8) | 0.701 |
| fatigue | 37 (63.8) | 28 (50.9) | 19 (51.4) | 0.312 |
| Cough | 52 (89.7)† | 52 (94.5) | 37 (100.0) | 0.115 |
| Dyspnea | 28 (48.3)⁎ | 19 (34.5) | 7 (18.9) | 0.014 |
| myalgia | 36 (62.1) | 36 (65.5) | 21 (56.8) | 0.701 |
| Diarrhea | 18 (31.0) | 15 (27.3) | 18 (48.6) | 0.087 |
| headache | 37 (63.8) | 28 (50.9) | 19 (51.4) | 0.312 |
| Mean ± SD | Mean ± SD | Mean ± SD | ||
| Vital signs | ||||
| RR | 24.65 ± 6.83⁎ | 19.56 ± 4.66 | 20.64 ± 7.00 | <0.001 |
| SO2 (%) | 88.70 ± 7.15† | 95.34 ± 3.48 | 94.54 ± 4.81 | <0.001 |
| Temperature (°C) | 37.86 ± 0.79 | 37.98 ± 0.61 | 37.91 ± 0.72 | 0.655 |
| Laboratory parameters | ||||
| WBCs (106/ml) | 4.48 ± 2.25† | 5.39 ± 2.27 | 5.45 ± 1.99 | 0.045 |
| Lymphocytes (%) | 21.32 ± 10.60 | 22.58 ± 7.65 | 22.64 ± 9.73 | 0.101 |
| S.Ferritin (ng/ml) | 380.51 ± 329.71⁎ | 88.18 ± 127.78 | 182.97 ± 243.65 | <0.001 |
| CRP (mg/dl) | 76.82 ± 59.32⁎ | 49.84 ± 41.18 | 45.13 ± 31.55 | 0.023 |
| D dimer (μg/ml) | 1.15 ± 0.79⁎ | 0.86 ± 0.80 | 0.68 ± 0.46 | 0.033 |
| S.IL6 (pg/ml) | 21.43 ± 7.67⁎ | 8.64 ± 4.21 | 6.06 ± 2.09 | <0.001 |
| CORADs | ||||
| 1 | 12 (20.7) | 27 (49.1)⁎ | 9 (24.3) | <0.001 |
| 2 | 3 (5.2) | 6 (10.9)⁎ | 0 (0.0) | |
| 3 | 0 (0.0) | 6 (10.9)⁎ | 3 (8.1) | |
| 4 | 12 (20.7) | 3 (5.5) † | 6 (16.2) | |
| 5 | 31 (53.4) | 13 (23.6)† | 19 (51.4) | |
RR: respiratory rate SO2: oxygen saturation.
Significantly higher than their corresponding in the other groups.
Significantly lower than their corresponding in the other groups.
There was a significant association between TLR7 mRNA expression and different genotypes of SNP rs3853839 among cases (p < 0.001). Patients carrying GG genotype had the highest mRNA expression levels, while CC carriers had the lowest levels (Fig. 2B).
About (74%) of patients with GG genotype were hospitalized, (61.8%) developed respiratory failure (26.3%) of cases had thromboembolic complications and (22.4%) had cardiac complications (p < 0.001 for any). The need for ICU admission was significantly lower among patients with GC genotype (p < 0.001), while the need for mechanical ventilation was significantly higher among patients with GG genotype (p < 0.001). Death rate did not show any significant difference among the 3 genotypes (p = 0.505) (Table 3b ).
Table 3b.
Clinical outcome among patients with different genotypes.
| Variables | GG (n = 58) No. (%) |
GC (n = 55) No. (%) |
CC (n = 37) | P value |
|---|---|---|---|---|
| Hospitalized | 43 (74.1)⁎ | 22 (40.0) | 13 (35.1) | <0.001 |
| Respiratory failure (So2 < 90) | 34 (61.8)⁎ | 1 (1.9) | 4 (11.8) | <0.001 |
| Complications | ||||
| Thromboembolic | 15 (26.3) | 1 (1.8) | 1 (2.7) | <0.001 |
| Renal complications | 7 (12.1) | 7 (12.7) | 1 (2.7) | 0.225 |
| Cardiac complications | 13 (22.4)† | 1 (1.8) | 1 (2.7) | <0.001 |
| ICU admission | 16 (27.6) | 1 (1.8)† | 6 (16.2) | 0.001 |
| Mechanical ventilation | 12 (21.1)⁎ | 1 (1.8) | 1 (2.7) | 0.001 |
| Severity categorization | ||||
| Mild | 12 (21.1) | 27 (49.1)⁎ | 12 (32.4) | <0.001 |
| Moderate | 12 (21.1) | 15 (27.3) | 15 (40.5)⁎ | |
| Severe | 18 (31.6) | 12 (21.8) | 4 (10.8)† | |
| Critically ill | 15 (26.3) | 1 (1.8)† | 6 (16.2) | |
| Mortality | 4 (6.9) | 1 (1.8) | 1 (2.7) | 0.505 |
Significantly higher than their corresponding in the other groups.
Significantly lower than their corresponding in the other groups.
There was no significant difference in age or gender between cases with mild/moderate and severe/critically ill COVID-19 disease (p = 0.068 and 0.121 respectively). About (40%) of severe/critically ill cases were admitted to the ICU and (24.6%) of them needed mechanical ventilation (p < 0.001 for any). Genotype GG was significantly higher among severe/critically ill cases, while GC genotype was significantly higher among mild/moderate cases (p < 0.001). The TLR7 mRNA expression was also significantly higher among severe/critically ill cases (p < 0.001) (Table 4 ). The univariate regression analysis showed that each of dyspnea, respiratory rate, SO2, high serum levels of IL6, D Dimer, ferritin, CRP, up-regulation of TLR7 mRNA expression and GG genotype were independent risk factors for the development of severe disease (Table 5 ).
Table 4.
Comparison of personal and clinical characters between patients between mild/moderate and severe/critically ill cases.
| Mild/Moderate (n = 93) No. (%) |
Severe/critically ill (n = 57) No. (%) |
P value | |
|---|---|---|---|
| Age (mean ± SD) | 37.25 ± 8.17 | 39.84 ± 8.64 | 0.068 |
| Gender | |||
| Male | 45 (48.4) | 35 (61.4) | 0.121 |
| Female | 48 (51.6) | 22 (38.6) | |
| Genotype | |||
| GG | 24 (25.8) | 34 (59.6)⁎ | <0.001 |
| GC | 42 (45.2)⁎ | 13 (22.8) | |
| CC | 27 (29.0) | 10 (17.5) | |
| TLR7 mRNA expression (mean ± SD) | 2.15 ± 0.71 | 2.92 ± 0.96 | <0.001† |
Significantly higher than their corresponding in the other groups.
Significantly lower than their corresponding in the other groups.
Table 5.
Uni-variate logistic regression analysis of risk factors associated with the occurrence of severe disease.
| Variables | Univariate |
|||
|---|---|---|---|---|
| P value | OR | 95% CI |
||
| Lower | Upper | |||
| Dyspnea | <0.001 | 4.71 | 2.304 | 9.647 |
| RR | <0.001 | 1.576 | 1.382 | 1.798 |
| SO2 | <0.001 | 0.580 | 0.490 | 0.687 |
| S. IL6 | <0.001 | 1.115 | 1.063 | 1.170 |
| D dimer | <0.001 | 3.236 | 1.963 | 5.332 |
| S. ferritin | <0.001 | 1.005 | 1.003 | 1.007 |
| CRP | 0.001 | 1.013 | 1.005 | 1.020 |
| TLR7 mRNA expression | <0.001 | 31.00 | 1.946 | 4.936 |
| Genotypea | <0.001 | |||
| GG | 0.003 | 3.825 | 1.564 | 9.353 |
| GC | 0.713 | 0.836 | 0.321 | 2.163 |
CC is the reference.
5. Discussion
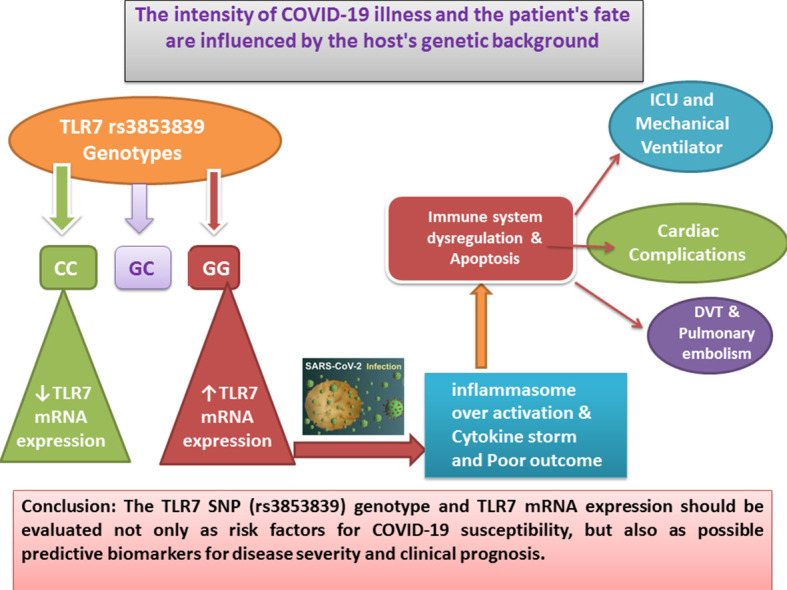
Why some COVID-19 infected people remain asymptomatic while others have severe symptoms is still unknown. The answer to this question, as well as the identification of the parameters that determine SARS-CoV-2 pathogenicity, will aid in the formulation of effective treatment programs and infection management (Hashemi et al., 2021b). The intensity of the illness and the patient's fate appear to be influenced by the host's genetic background (Ogishi et al., 2020). The purpose of this study was test a hypothesis that TLR7 gene polymorphism (rs3853839) and its mRNA expression might be used to predict COVID-19 susceptibility, severity and clinical outcome. In a study of more than 1.3 million PCR-proven COVID-19 cases in the United States, they found that individuals with past co-morbidities had a greater rate of hospitalization, ICU admission and mortality than those without previous co-morbidities (Stokes et al., 2020; Zhou et al., 2020; Harrison et al., 2020). As a result, we excluded the elderly, smokers, pregnant women, obese patients and patients with co-morbidities from our study because they are all known risk factors for patient deterioration and a poor clinical outcome, to concentrate on TLR7 (rs3853839) genetic variants and TLR7 transcript as a risk factor for severe COVID-19 illness and poor prognosis. In the current investigation, the GG genotype and G allele were shown to be significantly more common in COVID-19 cases than controls, whereas the CC genotype and C allele were found to be significantly more common in controls than cases. Furthermore, patients with the GG genotype had the highest levels of TLR7 mRNA expression, whereas those with the CC genotype had the lowest. TLR7 is involved in the detection of viral genomic RNA, which results in the creation of an antiviral response (Urcuqui-Inchima et al., 2017). Fakhir et al. (2017) and Lauhkonen et al. (2016) reported that TLR7 SNP (rs179008) has been linked to an elevated risk of disease progression in Moroccan patients with HCV-mediated hepatic illness and to post-bronchiolitis pulmonary function insufficiency in the Finnish population. Similarly, Mukherjee & Tripathi (2019) stated that TLR7 SNPs (rs3853839 & rs179008) had significant relationship with Dengue virus infection. Also (Zhang et al., 2020), in Chinese Han patients, SNPs at TLR7 were investigated for their relevance in HIV-1 infection and prognosis. TLR7 SNP (rs3853839) was also found to be substantially linked to chikungunya virus infection in Indians (Dutta & Tripathi, 2017). In Chinese patients, the TLR7 SNP (rs3853839) has also been linked to HCV persistence and predisposition to enterovirus-71-mediated hand, foot and mouth infection (Yue et al., 2014).
All of these investigations found that the TLR7 SNP (rs3853839) plays a role in viral infection and pathogenesis in different ethnic groups.
The significant elevation of TLR7 mRNA transcripts in individuals carrying the G mutant allele, as well as the higher level of G allele-containing TLR7 mRNA in heterozygous participants supported a physiological role for TLR7 SNP (rs3853839) in the adjustment of TLR7 mRNA expression, according to a study on systemic lupus erythematosus patients (Shen et al., 2010). Also, Raafat et al. (2018) reported that TLR signaling can be regulated by miRNAs through direct effects on expression or by modulation of downstream regulators, adaptor molecules, and cytokines. The TLR7 SNP (rs3853839) has the potential to impact miRNAs binding and as a result, TLR7 mRNA expression and/or sensitivity. The wild C allele features a binding site for miR-3148 (miR-3148), which causes fast transcript breakdown and reduces TLR7 mRNA levels. Similarly, Shen et al. (2010) concluded the link between the risk allele G of the TLR7 SNP (rs3853839) and higher TLR7 transcript expression. The GG genotype was shown to be substantially more common among severe/critically sick patients in the current investigation.
TLR7 mRNA expression was also significantly increased in severe/critically unwell patients. van der Made et al. (2020b) stated that TLRs are thought to play a dual role in COVID-19; activation of TLRs by SARSCoV2 causes inflammasome stimulation and the release of IL1, which causes IL-6 to be produced (de Rivero Vaccari et al., 2020). Furthermore, TLR-induced activation of Janus kinase transducers (JAK/STAT) may result in macrophage triggering syndrome (Alnefaie & Albogami, 2020). TLR7 could trigger a cytokine storm in SARSCoV1 and result in a variety of adverse effects (de Groot & Bontrop, 2020b). As TLR7 is required for the generation of type I IFN, the most likely involvement of TLR7 SNPs in COVID-19 pathogenesis is likely to be explained by increased type I IFN production (Kyogoku & Tsuchiya, 2007). Zhao et al. also revealed that TLR activation-induced cytokine storm is dramatically reduced by sunitinib, a tyrosine kinase inhibitor (Zhao et al., 2019).
This study showed that dyspnea which was significantly higher among patients with GG genotype. Duan et al. (2020) reported that Dyspnea was linked to a poor prognosis and mortality. According to the current study, 74% of individuals with the GG genotype were hospitalized, with 61.8% having respiratory failure. Petrilli et al. (2020) reported a substantial link between hypoxia and poor clinical outcomes.
The current investigation found that patients with the mutant GG genotype had significantly lower mean WBC counts, but their mean blood ferritin, IL6, CRP and D dimer levels were significantly higher. Kyttaris (2019) stated that, increased production of inflammatory cytokines causes immune system dysregulation and immunopathology.
In persons with COVID-19, the cytokine storm syndrome plays a critical role in the infection's progression. COVID-19 can be cured with a treatment strategy aimed at preventing over-activation of the immune system and controlling cytokine production in patients with autoimmune diseases (ADs), due to the undeniable involvement of inflammatory cytokines in patients with ADs (Valencia et al., 2019). Cytokine storms suppress the immune system and promote lymphopenia (a reduction in CD8 and CD4 T cells) by inducing T cell apoptosis mediated by IFN-I (Channappanavar et al., 2016). IFN-activation also reduces ATP in T cells and causes their death (Perl et al., 2004). In persons with COVID-19, the beginning of cytokine storms is linked to a rise in apoptosis in the lung and kidney. Apoptosis reduces the sensitivity of receptors to recognize the virus by overproducing nucleic acids (Devaux et al., 2020).
Del Valle et al. (2020) found that elevated D-dimer levels, CRP levels and IL-6 levels have all been recognized as risk factors for severity. Certain medications, like as anti-IL-6, are hoped to open up new techniques and therapeutic avenues in the fight against this dreadful illness (Cao, 2020).
This study showed that, Patients with the GG genotype were more likely to require ICU admission and mechanical breathing 22.4% of patients with the GG genotype experienced cardiac issues such as arrhythmias, myocardial damage, heart failure and shock, while 26.3% experienced thromboembolic consequences such as widespread deep vein thrombosis (DVT) and pulmonary embolism (PE). Klok et al. (2020) reported that thromboembolic complications are common in COVID-19 patients who are critically unwell, especially in the ICU. Also Galloway et al. (2020) reported that the need for ICU admission and mechanical ventilation was much higher in COVID-19 patients who were critically unwell. In COVID19 patients, excessive inflammasome augmentation is connected to a bad result (de Rivero Vaccari et al., 2020). The suppression of inflammasome over activation and the development of neutrophil extracellular traps could likewise be considered a therapeutic target. Several experiments are being undertaken on the pathways of TLRs in COVID19, which could lead to the development of a novel medicine or vaccine to treat the infection (Khanmohammadi & Rezaei, 2021).
6. Conclusion
The TLR7 SNP (rs3853839) could be a genetic risk factor for COVID-19 infection, severe sickness and poor clinical outcomes. When compared to CC genotype carriers, GG genotype carriers had the greatest levels of TLR7 mRNA expression, which was related with severe infection and poor prognosis. As a result, the TLR7 SNP (rs3853839) genotype and TLR7 mRNA expression should be evaluated not only as risk factors for COVID-19 susceptibility, but also as possible predictive biomarkers for disease severity and clinical prognosis.
Role of funding sources
There was no funding for this study.
CRediT authorship contribution statement
SME: Investigation, Methodology. HAE: Sample and Data collection. RGM: Validation, Visualization. SSS: Conceptualization, editing, TAO: Writing Original draft preparation, RMA: Investigation, Methodology.
Availability of data and materials
Not applicable.
Declaration of competing interest
The authors state that they have no conflicts of interest.
Acknowledgements
We would like to express our gratitude to all of the patients and volunteers who took part in our research. We also thank Professor Dr. Eman Badr (General Manager of Central Laboratory) and all of the associates of the Menoufia Faculty of Medicine's Central Laboratory for their technical assistance with this study.
References
- Cascella M., Rajnik M., Aleem A., Dulebohn S.C., Di Napoli R. StatPearls. StatPearls Publishing; Treasure Island (FL): 2022. Features, evaluation, and treatment of coronavirus (COVID-19) January 5. [PubMed] [Google Scholar]
- Coronaviridae Study Group of the International Committee on Taxonomy of Viruses The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khailany R.A., Safdar M., Ozaslan M. Genomic characterization of a novel SARS-CoV-2. Gene Rep. 2020;19 doi: 10.1016/j.genrep.2020.100682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashemi S.M.A., Thijssen M., Hosseini S.Y., Tabarraei A., Pourkarim M.R., Sarvari J. Human gene polymorphisms and their possible impact on the clinical outcome of SARS-CoV-2 infection. Arch. Virol. 2021;166(8):2089–2108. doi: 10.1007/s00705-021-05070-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z., Peng F., Xu B., et al. Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis. J. Infect. 2020;81(2) doi: 10.1016/j.jinf.2020.04.021. e16-e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki A., Furukawa H., Kondo Y., et al. TLR7 single-nucleotide polymorphisms in the 3' untranslated region and intron 2 independently contribute to systemic lupus erythematosus in Japanese women: a case-control association study. Arthritis Res. Ther. 2011;13(2) doi: 10.1186/ar3277. Published 2011 Mar 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashkar A.A., Rosenthal K.L. Toll-like receptor 9, CpG DNA and innate immunity. Curr. Mol. Med. 2002;2(6):545–556. doi: 10.2174/1566524023362159. [DOI] [PubMed] [Google Scholar]
- de Groot N.G., Bontrop R.E. COVID-19 pandemic: is a gender-defined dosage effect responsible for the high mortality rate among males? Immunogenetics. 2020;72(5):275–277. doi: 10.1007/s00251-020-01165-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Made C.I., Simons A., Schuurs-Hoeijmakers J., et al. Presence of genetic variants among young men with severe COVID-19. JAMA. 2020;324(7):663–673. doi: 10.1001/jama.2020.13719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., Marken J., Chen J. High TLR7 expression drives the expansion of CD19+CD24hiCD38hi transitional B cells and autoantibody production in SLE patients. Front. Immunol. 2019;10:1243. doi: 10.3389/fimmu.2019.01243. Published 2019 Jun 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safaei S., Karimi-Googheri M. Toll-like receptor antagonists as a potential therapeutic strategy against cytokine storm in COVID-19-infected patients. Viral Immunol. 2021;34:361–362. doi: 10.1089/vim.2020.0074. [DOI] [PubMed] [Google Scholar]
- World Health Organization . Clinical Management of COVID-19: Patients: Living Guidance. v0.16. World Health Organization; 2021. pp. 13–15. [Google Scholar]
- Prokop M., van Everdingen W., van Rees Vellinga T., et al. CO-RADS: a categorical CT assessment scheme for patients suspected of having COVID-19-definition and evaluation. Radiology. 2020;296(2) doi: 10.1148/radiol.2020201473. E97-E104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorak M., Real-time P.C.R. Clin. Chem. 2004;50:1680–1682. [Google Scholar]
- Hashemi S., Thijssen M., Hosseini S., et al. Human gene polymorphisms and their possible impact on the clinical outcome of SARS-CoV-2 infection. Arch. Virol. 2021;166:2089–2108. doi: 10.1007/s00705-021-05070-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogishi M., Sabli I.K., Hodeib S., Korol C. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science. 2020;370(6515) doi: 10.1126/science.abd4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes E.K., Zambrano L.D., Anderson K.N. Coronavirus disease 2019 case surveillance—United States, January 22–May 30, 2020. MMWR Morb. Mortal. Wkly Rep. 2020:69. doi: 10.15585/mmwr.mm6924e2. https://www.cdc.gov/mmwr/volumes/69/wr/pdfs/mm6924e2-H.pdf Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison S.L., Fazio-Eynullayeva E., Lane D.A., et al. Comorbidities associated with mortality in 31,461 adults with COVID-19 in the United States: a federated electronic medical record analysis. PLoS Med. 2020;17 doi: 10.1371/journal.pmed.1003321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urcuqui-Inchima S., Cabrera J., Haenni A.L. Interplay between dengue virus and Toll-like receptors, RIG-I/MDA5 and microRNAs: implications for pathogenesis. Antivir. Res. 2017;147:47–57. doi: 10.1016/j.antiviral.2017.09.017. [DOI] [PubMed] [Google Scholar]
- Fakhir F.Z., Lkhider M., Badre W., et al. Genetic variations in toll-like receptors 7 and 8 modulate natural hepatitis C outcomes and liver disease progression. Liver Int. 2017;38:432–442. doi: 10.1111/liv.13533. [DOI] [PubMed] [Google Scholar]
- Lauhkonen E., Koponen P., Vuononvirta J. 2016. Gene Polymorphism of Toll- Like Receptors and Lung Function at Five to Seven Years of Age After Infant. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S., Tripathi A. Contribution of Toll like receptor polymorphisms to dengue susceptibility and clinical outcome among eastern Indian patients. Immunobiology. 2019;224(6):774–785. doi: 10.1016/j.imbio.2019.08.009. [DOI] [PubMed] [Google Scholar]
- Zhang T., Zhu J., Su B. Effects of TLR7 polymorphisms on the susceptibility and progression of HIV-1 infection in Chinese MSM population. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.589010. Published 2020 Oct 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta S.K., Tripathi A. Association of toll-like receptor polymorphisms with susceptibility to chikungunya virus infection. Virology. 2017;511:207–213. doi: 10.1016/j.virol.2017.08.009. [DOI] [PubMed] [Google Scholar]
- Yue M., Feng L., Tang S.D., et al. Sex-specific association between X-linked Toll like receptor 7 with the outcomes of hepatitis C virus infection. Gene. 2014;548(2):244–250. doi: 10.1016/j.gene.2014.07.040. 15. [DOI] [PubMed] [Google Scholar]
- Shen N., Fu Q., Deng Y., Qian X., Zhao J., Kaufman K.M., et al. Sex-specific association of X-linked Toll-like receptor 7 (TLR7) with male systemic lupus erythematosus. Proc. Natl. Acad. Sci. U. S. A. 2010;107:15838–15843. doi: 10.1073/pnas.1001337107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raafat I.I., El Guindy N., Shahin R.M.H., Samy L.A., El Refai R.M. Toll-like receptor 7 gene single nucleotide polymorphisms and the risk for systemic lupus erythematosus: a case-control study. Einzelnukleotidpolymorphismen im Toll-like-receptor-7-Gen (TLR7) und das Risiko eines systemischen Lupus erythematodes: eine Fall-Kontroll-Studie. Z. Rheumatol. 2018;77(5):416–420. doi: 10.1007/s00393-017-0283-7. [DOI] [PubMed] [Google Scholar]
- van der Made C.I., Simons A., Schuurs-Hoeijmakers J., et al. Presence of genetic variants among young men with severe COVID-19. JAMA. 2020;324:663. doi: 10.1001/jama.2020.13719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rivero Vaccari J.C., Dietrich W.D., Keane R.W., de Rivero Vaccari J.P. The inflammasome in times of COVID-19. Front. Immunol. 2020;11(2474) doi: 10.3389/fimmu.2020.583373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alnefaie A., Albogami S. Current approaches used in treating COVID-19 from a molecular mechanisms and immune response perspective. Saudi Pharm. J. 2020;28:1333–1352. doi: 10.1016/j.jsps.2020.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot N.G., Bontrop R.E. COVID-19 pandemic: is a gender-defined dosage effect responsible for the high mortality rate among males? Immunogenetics. 2020;72(5):275–277. doi: 10.1007/s00251-020-01165-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyogoku C., Tsuchiya N. A compass that points to lupus: genetic studies on type I interferon pathway. Genes Immun. 2007;8:445–455. doi: 10.1038/sj.gene.6364409. [DOI] [PubMed] [Google Scholar]
- Zhao S., Gao N., Qi H., et al. Suppressive effects of sunitinib on a TLR activation-induced cytokine storm. Eur. J. Pharmacol. 2019;854:347–353. doi: 10.1016/j.ejphar.2019.04.045. [DOI] [PubMed] [Google Scholar]
- Duan J., Wang X., Chi J., et al. Correlation between the variables collected at admission and progression to severe cases during hospitalization among patients with COVID-19 in Chongqing. J. Med. Virol. 2020 doi: 10.1002/jmv.26082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrilli C.M., Jones S.A., Yang J., et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369 doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyttaris V.C. Targeting cytokines to treat autoimmunity. Clin. Immunol. (Orlando, Fla) 2019;206 doi: 10.1016/j.clim.2019.108251. [DOI] [PubMed] [Google Scholar]
- Valencia J.C., Egbukichi N., Erwin-Cohen R.A. Autoimmunity and Cancer, the paradox comorbidities challenging therapy in the context of preexisting autoimmunity. J. Interf. Cytokine Res. 2019;39(1):72–84. doi: 10.1089/jir.2018.0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channappanavar R., Fehr A.R., Vijay R., Mack M., Zhao J., Meyerholz D.K., Perlman S. Dysregulated type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host Microbe. 2016;19(2):181–193. doi: 10.1016/j.chom.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perl A., Gergely P., Jr., Nagy G., Koncz A., Banki K. Mitochondrial hyperpolarization: a checkpoint of T-cell life, death and autoimmunity. Trends Immunol. 2004;25(7):360–367. doi: 10.1016/j.it.2004.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaux C.A., Rolain J.M., Colson P., Raoult D. New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19? Int. J. Antimicrob. Agents. 2020;55(5) doi: 10.1016/j.ijantimicag.2020.105938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Valle D.M., Kim-Schulze S., Huang H.H., et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat. Med. 2020;26:1636. doi: 10.1038/s41591-020-1051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W. 2020. Clinical Features and Laboratory Inspection of Novel Coronavirus Pneumonia (COVID-19) in Xiangyang, Hubei. [Google Scholar]
- Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020;191:145. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway J.B., Norton S., Barker R.D., et al. A clinical risk score to identify patients with COVID-19 at high risk of critical care admission or death: an observational cohort study. J. Infect. 2020 doi: 10.1016/j.jinf.2020.05.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanmohammadi S., Rezaei N. (2020): Role of Toll like receptors in the pathogenesis of COVID-19. J. Med. Virol. 2021;93:2735–2739. doi: 10.1002/jmv.26826. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.