Abstract
Commercial antidiabetic polyherbal formulations (APH) are available with claimed hypoglycemic activities; yet they lack systematic scientific studies leading to their limited global acceptance. In the present study, six selected APH from the Indian market were evaluated for their phytochemical contents, anti-hyperglycemic, anti-hyperlipidemic, antioxidant activities and further identifying the major antidiabetic bioactive compound of “MA” by HPLC–ESI–MS/MS. Our results revealed highest TPC (136.97 ± 0.6 µg GAE/mg) and TFC (128.85 ± 0.74 µg QE/mg) in APH-DB and APH-SN, respectively. APH-MA has exhibited highest α-amylase 72.5% (IC50-579.65 μg/ml), α-glucosidase 88.02% (IC50-261.03 μg/ml) and moderate lipase inhibition 57.7% (IC50 159.57 μg/ml). A variable free radical scavenging activity was observed by all the tested APH. Further significant linear positive correlations were observed between TPC-Lipase (r2-0.985****), TFC-α-amylase (r2-0.868**) and DPPH-α-amylase inhibition (r2-0.8098*). HPLC–ESI–MS/MS of MA showed the presence of anti-hyperglycemic compounds, Pheophorbide a and Pyropheophorbide a, as the major peaks. Among the tested extracts, MA exhibited better activities while BG, MH, SN, DB, and DT have showed comparable/mild anti-hyperglycemic, anti-hyperlipidemic and antioxidant potential. Hence the tested APH may be considered effective for DM management which can further be assessed for their other targets of inhibition.
Keywords: Antioxidant, Correlation, Hyperglycemic, Hyperlipidemic, HPLC–ESI–MS/MS, Polyherbal formulations
Introduction
Diabetes mellitus (DM) is an endocrine complex disorder of multiple etiologies, characterized by hyperglycemia. DM is increasing predominantly at an alarming rate, with 415 million affected worldwide which is predicted to increase to 640 million by 2040, further DM was responsible for 1.5 million deaths in 2019 (WHO 2021; Cho et al. 2018). Current therapies include oral hypoglycemic agents, insulin and combinatorial approach, prolonged use of which is related to adverse side effects (Artasensi et al. 2020). This necessitates immediate consideration for complementary sources of medicine without side effects. Polyherbal formulation can be considered as an alternative with its reports of synergistic mechanism of action, numerous bioactive compounds and broader therapeutic strategy (Duraiswamy et al. 2016). Though extensive research on antidiabetic herbs have been reported previously, fewer data on antidiabetic polyherbal formulations (APH) are available (Ojo et al. 2019; Thengyai et al. 2020). The prime factors associated with DM are reflected with hyperglycemia, hyperlipidemia, and generation of disproportionate reactive oxygen species (ROS) (Halim and Halim 2019; Artasensi et al. 2020). This condition can be managed by antioxidant as reducing agents, hydrogen donators, metal chelators, and single oxygen quenchers (Sies 2016; Sadeer et al. 2020). Further hyperlipidemia is an associated complication of DM with uneven ROS and antioxidants. Abnormal pancreatic lipase activity leads to accumulation of triglycerides, which can be controlled by inhibition of lipase (Ojo et al. 2019). Earlier reports on in-vitro mechanism of action have proved the importance of hyperglycemic, hyperlipidemic, and antioxidant studies on various other APH (Sompong et al. 2016; Telapolu et al. 2018; Thengyai et al. 2020).
The complex composition of APH emerges to effect in various ways by interacting with multiple receptor targets to manage DM and its associated complication (Telapolu et al. 2018). However, a large number of APH is available in the Indian market for management of DM, they lack investigation on their in-vitro mechanism of actions which may restrict their global acceptance (Butala et al. 2017). Therefore, the present study is aimed to assess the in-vitro mechanisms of six commercial APH in management of DM coded as follows BG, MA, MH, SN, DB, and DT in their carbohydrases, lipases inhibitory and antioxidant activities. Our work also intend to determine the correlation between phytochemical contents and their bio-activities, followed by HPLC–ESI–MS/MS analysis of APH-MA.
Materials and methods
Materials
Six APH were purchased from local Indian markets, were randomly coded: BG, DB, DT, MA, MH, and SN, composition details provided in Table 1. All other chemicals and solvents used were of analytical grade.
Table 1.
Sample ID and composition of six antidiabetic polyherbal formulation
| Sample ID | Composition |
|---|---|
| BG | Berberis aristata DC, Pterocarpus marsupium Roxb, Gymnema sylvestre (Retz.) R.Br. ex Sm, Rubia cordifolia L, Trigonella foenum-graecum L, Tinospora cardifolia Miers, Berberis Aristata and Tinospora cordifolia |
| DB | Gymnema sylvestre, Pterocarpus marsupium, Yashtimadhu, Glycyrrhiza glabra, Casearia esculenta, Syzygium cumini, Asparagus racemosus, Boerhavia diffusa, Sphaeranthus indicus, Tinospora cordifolia, Swertia chirata, Tribulus terrestris, Phyllanthus amarus, Gmelina arborea, Gossypium herbaceum, Berberis aristata, Vidangadi lauham, Momordica charantia, Piper nigrum, Ocimum sanctum, Abutilon indicum, Rumex maritimus, Curcuma longa |
| DT | Syzigium cumin, Plectranthus Amboinicus, Andropogan Muricatus, Cinanamum zeylanicum, Anacyclus Pyrethrum,Cassia fistula, Strychnos potatorum, Cocculus cordifolius, Gymnema sylvestre |
| SN | Vachellia nilotica, Tinospora cordifolia, Catharanthus roseus, Momordica charantia, Andrographis pinculata, Gymnema sylvestre, Trigonella foenum-graecum, Azadirachta indica, Ficus racemosa, Ocimum tenuiflorum, Phyllanthus niruri, Cinnamomum verum, Curcuma longa, Picrorhiza kurrooa |
| MA | Momordica charantia, Syzygium cuminii, Mangifera indica, Gymnema sylvestre |
| MH | Pterocarpus marsupium, Salacia reticulata, Curcuma longa, Emblica officinalis, Momordica charantia, Tinospora cordifolia |
Extraction
Extraction of APH was carried out with cold maceration technique with slight modification to Gaurav et al. (2020), 5 g of APH was extracted with 150 ml of solvent, i.e., 70% hydro-alcoholic (HAE) and distilled water (AQE) for 24 h. The 70% hydro-alcoholic extract and aqueous extract were filtered with whatman filter paper #1. Filtrate was concentrated, stored in sterile vials at 4 °C for further analysis.
Preliminary phytochemical analysis
Qualitative screening of phytochemical
Qualitative tests have been carried out to screen the phytochemical present in the APH extracts (1 mg/ml) according to standard protocol (Madike et al. 2017).
Test for phenols and tannins: To 2 ml of APH extract 500 µl of 5% FeCl3 was added, development of blueish-black colour revealed presence of phenols and tannins.
Test for flavonoids and Coumarin: Two millilitre of APH extracts was treated with NaOH (10%), colour change to deep yellow was noted.
Test for Alkaloids: APH (3 ml) was heated with 3 ml of HCl (1%) for 20 min. The obtained solution was cooled followed by addition of Wagner’s reagent drop wise, development of reddish-brown precipitate revealed presence of alkaloids.
Test for Saponins: To 2 ml of APH extracts equal volume of distilled water was added and shaken, formation of a steady tenacious foam was considered as positive test.
Test for Glycosides: To 2 millilitre of APH extracts two millilitre of glacial acetic acid and 100 µl of FeCl3 was added, formation of brown/violet/brownish green coloured ring specified existence of glycosides.
Quantitative phytochemical analysis
Estimation of total phenol content (TPC)
TPC content was estimated using folin ciocalteu reagent, 1.5 ml was added and pre-incubated (15 min) with 1 ml of APH extracts (1 mg/ml). Followed by addition of 2 ml Na2CO3 (7.5%), incubated in dark (30 min). Absorbance was read at 765 nm and gallic acid was used as standard (GAE) (Chanthasri et al. 2018).
Estimation of total flavonoid content (TFC)
TFC was measured by adding 0.5 ml of NaNO3 (5%) to 0.5 ml of APH extracts (1 mg/ml), incubated for 5 min followed by addition of AlCl3 (10%) and absorbance was read at 430 nm. Quercetin was considered as standard (QAE) (Chanthasri et al. 2018).
Bioactivity assays
Porcine pancreatic α-amylase inhibitory activity
DNSA method was used to determine α-amylase inhibitory activity with slight modification to Butala et al. (2017). The reaction mixture was pre-incubated for 20 min at 37 °C, composed of 250 µl of extracts (200–1000 µg/ml) and 250 µl of α-amylase enzyme (1 unit). Reaction was initiated by addition of 250 µl of 1% potato soluble starch, incubated for 10 min at 37 °C. Reaction was stopped with the addition of 0.5 µl of DNS reagent, incubated in boiling water bath for 10 min, the tubes were cooled and absorbance was taken at 540 nm, Acarbose was considered as positive control. The percentage of inhibition was calculated (Eq. 1).
| 1 |
Saccharomyces cerevisiae α-glucosidase inhibition activity
α-Glucosidase inhibitory activity was assessed, with pre-incubating reaction mixture containing 50 µl of APH extracts (200–1000 µg/ml) and 240 µl of α-glucosidase enzyme (1 unit/ml) for 20 min at 37 °C.The reaction was initiated with addition of 40 µl PNPG (5 mM), incubated (10 min), followed by addition of 750 µl Na2CO3 (0.2 M). Absorbance was recorded at 405 nm with acarbose as positive control (Butala et al. 2017). Percentage of inhibition was calculated (Eq. 1).
Porcine pancreatic lipase (PPL) inhibitory activity
Lipase inhibitory activity was performed by pre-incubating 20 µl of APH extracts (12.5–200 µg/ml) with 20 µl of lipase (1Unit/ml) for 1 h at room temperature. The reaction was initiated by 5 μl of pNPB (10 mM p-nitrophenyl butyrate in dimethyl formamide), measured at 405 nm and Orlistat was used as positive control (Franco et al. 2017), percentage of inhibition was calculated (Eq. 1).
In vitro antioxidant activities
2; 2-Diphenyl-1-picrylhydrazil (DPPH) free radical scavenging assay
DPPH free radical scavenging activity was measured by adding 200 ml of (0.01 M) of DPPH to 40 µl of APH extracts (12.5–200 µg/ml), incubated in dark for 30 min and absorbance was read at 517 nm. A stable antioxidant, ascorbic acid was used as positive control (Faitanin et al. 2018). Percent of scavenging was calculated (Eq. 2).
| 2 |
ABTS scavenging assay
ABTS scavenging assay was followed according to Faitanin et al. (2018) method with slight revision. To 150 µl of APH extracts (12.5–200 µg/ml), 1850 μl of prepared ABTS solution was added and incubated for 20 min at room temperature. Ascorbic acid was considered as positive control. Absorbance was read at 745 nm and percentage of scavenging were calculated (Eq. 2).
Metal chelating activity
Iron chelating activity of APH was measured by adding 500 μl of ferrous sulfate solution (0.1 mM) with 500 µl APH (12.5–200 µg/ml), the reaction was initiated by adding 1000 µl of Ferrozine reagent (0.25 mM) incubated for 10 min at room temperature. Absorbance was read at 562 nm (Chanthasri et al. 2018). EDTA was considered as positive control and percentage of metal chelation was calculated (Eq. 2.).
FRAP assay (ferric reducing antioxidant power assay)
FRAP assay was determined with 500 μl of the extract (12.5–200 µg/ml), incubated for 30 min with 1000 μl of FRAP reagent at 37 °C. Absorbance was read at 593 nm, values are represented as ascorbic acid equivalents (AAE) (Koch and Deo 2016).
Total antioxidant capacity (TAC)
Total antioxidant capacity was determined by phosphomolybdenum assay (Saleem et al. 2019). 1.2 ml of phosphomolybdenum reagent was incubated with 600 μl of APH extracts at 95 °C for 90 min, further cooled and absorbance was read at 635 nm. Values were expressed in Tannic acid equivalent (TAE).
HPLC–ESI–MS/MS
HPLC–ESI–MS/MS was performed for the analysis of MA (HAE) constituents using a chromatograph agilent poroshell 120 (4.6 × 150 mm). SB-C18, 2.7 µm particle size was used as stationary phase. The mobile phase was taken as water and 0.1% formic acid (A) and acetonitrile with 0.1% formic acid (B). The gradient elution starts with 5% B and is changed to 95% B in 70 min, flow rate was 300 ml/min. Guard cartridge used was 5micron, c8, 4.6 × 12.5 mm, zorbax eclipse. The chromatograms were monitored at 210 nm and column temperature maintained at 25 °C. MS/MS spectra were acquired using electro spray (ESI) with ESI-L-Low conc tuning mix, as ion source at a range of 100–2200 m/z, operating in positive ion mode.
Statistical analysis
Values are represented as mean ± SE (n = 3). Significant differences were analyzed using Two-way analysis of variance (ANOVA), with Tukey’s multiple comparisons test at p < 0.005. Pearson correlation coefficient (r2) between different bioactivities was carried out. Analysis and Figure preparations were carried-out in GraphPad prism 6.
Results
Effective extracting of phytochemicals is a crucial step, in the present study the obtained extractive values were higher in HAE (Table 2) compared to AQE. Further presence of phenols, flavonoids, alkaloids, tannin, coumarin, saponins and glycoside were observed in qualitative phytochemical screening of APH (HAE and AQE). Among the tested formulations, highest quantitative TPC was observed in DB (HAE) 136.97 ± 0.6 µg GAE/mg whereas SN (HAE) 128.85 ± 0.74 µg QE/mg exhibited maximum TFC (Table2).
Table 2.
In-vitro bioactive activities of hydroalcoholic and aqueous extracts of antidiabetic polyherbal formulation
| APH | %f yield (mean ± SD) | TPC µg GAE/mg (mean ± SD) | TFC µg QAE/mg (mean ± SD) | FRAP µmoles AAE/ml (mean ± SE) | TAC µmoles TAE/ml (mean ± SE) | ABTS IC50 μg/ml (mean ± SE) | DPPH IC50 μg/ml (mean ± SE) | Metal chelating IC50 μg/ml (mean ± SE) |
|---|---|---|---|---|---|---|---|---|
| BG(HAE) | 32.94 ± 0.9 | 108.13 ± 0.8 | 88.33 ± 0.26 | 52.03 ± 0.019 | 120.3 ± 0.04 | 1.24 ± 1.01 | 118.39 ± 1.56 | 187.1 ± 1.12 |
| BG(AQE) | 28 ± 0.31 | 94.48 ± 0.1 | 81.89 ± 0.41 | 34.34 ± 0.04 | 49.69 ± 0.01 | 82.08 ± 0.9 | 183.76 ± 8.67 | 294.48 ± 1.6 |
| DB(HAE) | 21.66 ± 0.76 | 136.97 ± 0.6 | 78.85 ± 0.74 | 71.43 ± 0.08 | 86.23 ± 0.06 | 52.59 ± 0.6 | 85.56 ± 0.17 | 134.8 ± 1.09 |
| DB(AQE) | 18.6 ± 0.42 | 84.19 ± 0.49 | 46.23 ± 0.1 | 54.28 ± 0.04 | 44.45 ± 0.005 | 106.8 ± 1.055 | 119.42 ± 0.98 | 235.67 ± 0.96 |
| DT(HAE) | 11.25 ± 0.8 | 99.99 ± 0.22 | 56.94 ± 0.4 | 124.95 ± 0.2 | 52.35 ± 0.04 | 8.29 ± 1.36 | 121.94 ± 0.61 | 180.94 ± 1.2 |
| DT(AQE) | 4 ± 0.98 | 45.2 ± 0.26 | 22.29 ± 0.6 | 35.38 ± 0.15 | 46.39 ± 0.08 | 113.57 ± 0.89 | 432.10 ± 14.9 | 364.27 ± 1.8 |
| MA(HAE) | 14.02 ± 0.23 | 89.36 ± 0.5 | 73.18 ± 0.18 | 191.76 ± 0.06 | 174.5 ± 0.06 | 9.1 ± 0.432 | 83.75 ± 83.76 | 143.28 ± 1.5 |
| MA(AQE) | 11.2 ± 0.703 | 88.52 ± 0.9 | 8.63 ± 0.2 | 123.57 ± 0.06 | 143.16 ± 0.015 | 64.87 ± 0.11 | 113.17 ± 107.73 | 227.43 ± 1.06 |
| MH(HAE) | 48.3 ± 0.42 | 95.44 ± 0.22 | 86 ± 0.15 | 116.54 ± 0.08 | 41.7 ± 0.008 | 39.16 ± 0.9 | 120.05 ± 0.83 | 438.06 ± 3.6 |
| MH(AQE) | 36.46 ± 0.769 | 47.87 ± 0.29 | 38.82 ± 0.15 | 86.48 ± 0.05 | 35.98 ± 0.005 | 37.42 ± 0.15 | 365.25 ± 12.48 | 488.83 ± 2.65 |
| SN(HAE) | 22.7 ± 0.93 | 83.19 ± 0.59 | 128.85 ± 0.7 | 100.32 ± 0.16 | 186.6 ± 0.05 | 4.08 ± 0.48 | 48.18 ± 0.78 | 148.67 ± 1.5 |
| SN(AQE) | 20.22 ± 0.39 | 36.14 ± 0.43 | 11.46 ± 0.4 | 23.9 ± 0.11 | 35.34 ± 0.04 | 155.6 ± 0.6 | 64.61 ± 0.714 | 352.02 ± 1.6 |
Table illustrates IC50 values (IC50 μg/ml ± SE) for ABTS, DPPH and Metal chelating whereas FRAP and TAC is represented as µmoles AAE/ml (Mean ± SE) and µmoles TAE/ml (mean ± SE) respectively at 200 μg/ml of APH (HAE and AQE)
Bold indicates the highest values among the tested extracts
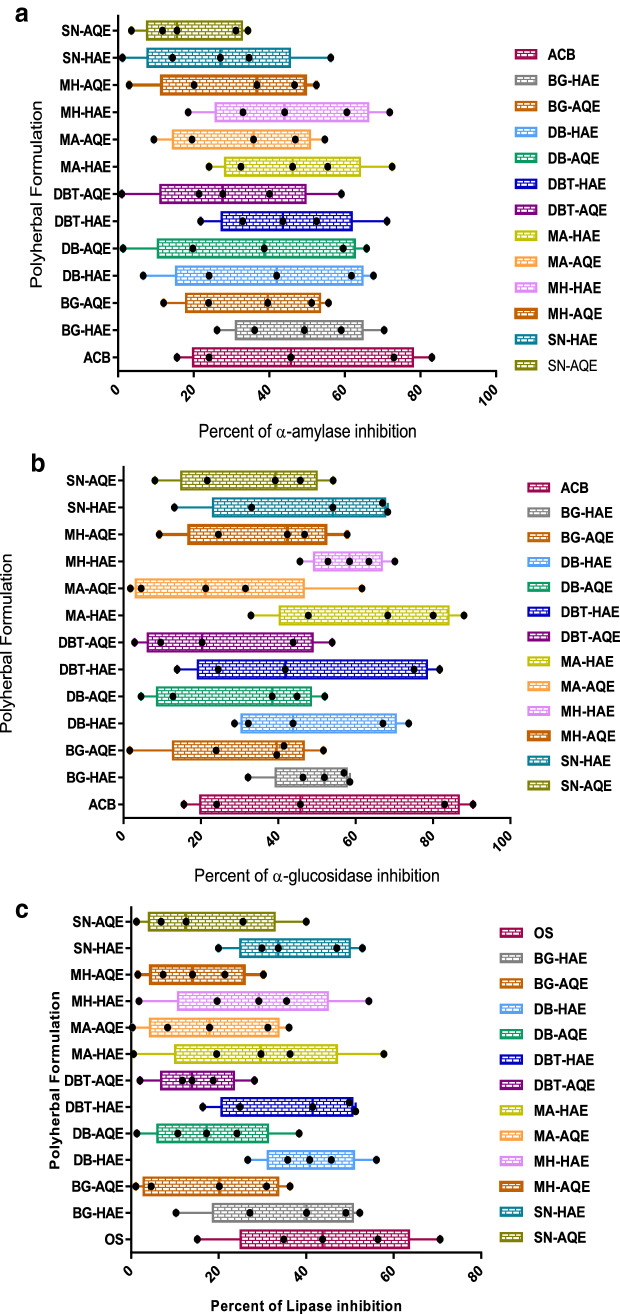
Evaluation of α-amylase and α-glucosidase inhibitory activities were carried out in a dose-dependent manner, which revealed HAE were better inhibitor for both carbohydrases (Fig. 1A, B). Among the tested APH, HAE–MA (α-amylase IC50—579.65 μg/ml and α-glucosidase IC50—261.03 μg/ml) exhibited comparable anti-hyperglycemic activity with acarbose (α-amylase IC50 523.12 μg/ml and α-glucosidase IC50 471.61 μg/ml). Significant difference was observed between the positive control and the tested APH (p > 0.0001). Our findings on PPL inhibition were determined for six APH which exhibited mild lipase inhibition compared to the standard drug orlistat (IC50−100.54 μg/ml) (Fig. 1C). Among the tested APH, maximum inhibitory activity was unveiled by MA (HAE; IC50−159.57 μg/ml).
Fig. 1.
a–c Dose dependent anti-hyperglycemic and anti-hyperlipidemic activities of APH a Percentage of α-amylase inhibition. b Percentage of α-glucosidase inhibition and c percentage of pancreatic lipase inhibition. Values represent mean ± SE each with three independent replicates. Statistical significance was calculated at (p > 0.005) two-way ANOVA performed by following Tukey’s multiple comparisons test using graph pad prism 6. ACB represent acarbose and OS represent orlistat, in the graph
Five different antioxidant assays were involved in the present study (Table 2). All six APH were evaluated for its ability to scavenge DPPH, with variable range, i.e., from 87.82% (SN-HAE) to 32.53% DT-AQE). Significantly higher antioxidant properties were observed in SN (HAE) IC50 − 48.18 ± 0.78 µg/ml compared to standard ascorbic acid IC50-75.7 ± 0.77 µg/ml. Evaluating metal chelating activity is an important aspect, our data showed appreciable metal chelating activity in the following order DB > MA > SN > DT > BG > MH. Obtained values for all the tested APH were observed to be lower than the standard positive control, EDTA (Table 2). In FRAP assay maximum activity was observed in MA (HAE) whereas SN-HAE (186.63 ± 0.05 μmoles TAE/ml) and MA-HAE (174.45 ± 0.06 μmoles TAE/ml), showed highest TAC values.
Correlation studies were carried out with HAE as it has exhibited satisfactory bioactivities compared to AQE (Table 3). A strong positive and highly significant correlation was established between TPC—Lipase inhibition (r2 − 0.9850), TFC—α-amylase (r2 − 0.8684) and DPPH—α-amylase inhibition (r2 − 0.8098). No significant correlation was observed for α-glucosidase inhibition, further vast range of positive mild to low correlation activity were observed in the present study, tabulated in Table 3.
Table 3.
Correlation coefficient (r2) between different antioxidant activity parameters (DPPH, ABTS, FRAP, Metal chelating activity and TAC), total phenol, total flavonoid contents, antihyperglycemic (α-amylase and α-glucosidase inhibition) and lipid inhibition of APH-HAE
| TPC | TFC | DPPH | ABTS | TAC | Metal chelating | FRAP | α-Amylase inhibition | α-Glucosidase inhibition | Lipase inhibition | |
|---|---|---|---|---|---|---|---|---|---|---|
| TPC | – | 0.127 | 0.049 | 0.445 | 0.193 | 0.02722 | 0.2062 | 0.07122 | 0.09879 | 0.9850**** |
| TFC | – | 0.509 | 0.569 | 0.401 | 0.06410 | 0.0731 | 0.8684** | 0.03650 | 0.07584 | |
| DPPH | – | 0.009 | 0.38 | 0.5699 | 0.0049 | 0.8098* | 0.06028 | 0.01759 | ||
| ABTS | – | 0.088 | 0.3856 | 0.0232 | 0.002042 | 0.09549 | 0.5211 | |||
| TAC | – | 0.1399 | 0.0035 | 0.3369 | 0.2193 | 0.1650 | ||||
| Metal chelating | – | 0.1746 | 0.2885 | 0.1520 | 0.03510 | |||||
| FRAP | – | 0.007152 | 0.4134 | 0.1790 | ||||||
| α-amylase inhibition | – | 0.04621 | 0.02758 | |||||||
| α-glucosidase inhibition | – | 0.09406 | ||||||||
| Lipase inhibition | – |
Statistical significance was calculated at (p > 0.005) two-way ANOVA using graph pad prism 6
* denotes the level of significance
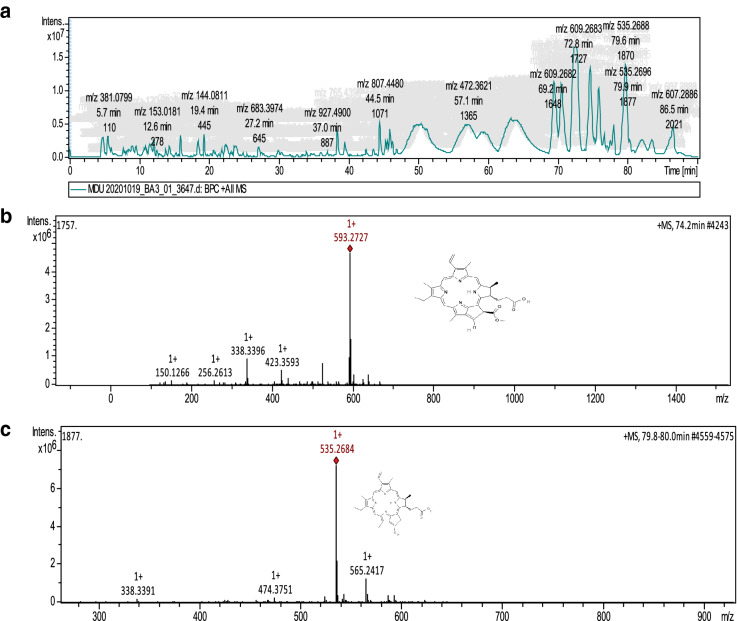
MA(HAE) exhibited better bioactivities, hence the observed values strongly proposed identification of the active chemical constituents (Fig. 2a). HPLC–ESI–MS/MS of MA(HAE) revealed presence of anti-hyperglycemic compounds, Pheophorbide a (C35H36N4O5), retention time 74.57 min and m/z 593.2727 and Pyropheophorbide a, (C33H34N4O3) retention time 79.8–80.0 min and m/z 535.2684 amongst the major peaks obtained (Fig. 2b–c).
Fig. 2.
a–c Chromatogram of HPLC–ESI–MS/MS a full view of chromatogram for MA. b Antidiabetic compound Pheophorbide a structure and chromatogram, Peak 1 [retention time (RT) 74.2 min] c antidiabetic compound Pyropheophorbide a structure and chromatogram, Peak 2 [RT 79.8–80.0 min]
Discussion
Effective extraction of phytochemicals is a crucial step which is based on various factors, i.e., chemical nature of compounds, solvent polarity, etc. (Truong et al. 2019; Nawaz et al. 2020). A possible reason for the obtained high-extractive value in HAE can be the combined solvent system used, i.e., 70% methanol, which might have enabled extraction of compounds soluble in water as well as alcohol. Our study also supports previous reports, which suggest use of combined solvents, i.e., polar and non-polar solvent for better extractive value (Truong et al. 2019; Nawaz et al. 2020). Further percent yield of BG was found to be higher (HAE: 15.44% and AQE: 10.58) when compared to previous reports on BG (8.4%) methanolic extract, which may be due to the dissimilarity in the solvent, time, temperature and batch of APH used (Gaurav et al. 2020).
Presence of phytochemicals in qualitative analysis may be due to the number of herbs used in a single formulation and polarity of solvents used for extraction (Madike et al. 2017; Truong et al. 2019). The obtained values for quantitative TPC and TFC for APH; DB and SN are in agreement with previous report on commercial antidiabetic polyherbal juice (Salunke et al. 2015). In the current study, higher TPC was observed in HAE compared to AQE for all the APH except for MA where similar results were obtained, which might be due to the solubility and structure of phenolic compounds present in the formulation (Truong et al. 2019; Ojo et al. 2019).
α-Amylase and α-glucosidase are the two vital enzymes involved in digestion of polysaccharides to disaccharides and finally monosaccharides thereby increasing the blood glucose level (Butala et al. 2017). Hence, delay in digestion of polysaccharides can be considered as one of the primary mechanisms to control hyperglycemia (Duraiswamy et al. 2016). Therefore, in the present study, α-amylase and α-glucosidase inhibitory activities were carried out in a concentration-dependent manner. Present results showed higher inhibitory activities when compared to previous reports on “ADPHF6” for α-amylase and α-glucosidase (Duraiswamy et al. 2016). Data from the present study also specified that MA(HAE) exhibited a significant α-glucosidase inhibitory activity compared to the standard. Our results are in agreement to the earlier reports which emphasizes that α-glucosidase inhibitors are capable of altering hyperglycemia much better than alpha-amylase inhibitors (Bhatia et al. 2019). An underlying reason for differences in values amongst the tested APH might be due to the position of hydroxyl and methoxyl steric side on the phytocompounds (Oyedemi et al. 2017). Additionally, studies are scarce with commercially available antidiabetic formulations, although extensive studies have been reported on their clinical evaluation (Panda 2017). Our results are in agreement with earlier reports on in vitro evaluation of commercial herbal formulations MD-1 and Lodhrasavam exhibiting significant inhibition of both enzymes (Telapolu et al. 2018; Butala et al. 2017). Advantage of APH over the widely used synthetic drugs, acarbose includes no/few side-effects and it works by synergistic multimode mechanism allowing high potency (Duraiswamy et al. 2016).
Insulin plays an important role in lipid metabolism thus, hyperlipidemia is one of the major complications in DM (Schofield et al. 2016). Fat deposition into adipose tissue can be interrupted/ delayed by inhibiting pancreatic lipase, thus can be considered as a preliminary treatment approach (Unuofin et al. 2018). Our findings corroborate with the previous studies (Jaradat et al. 2017; Unuofin et al. 2018). Literature revealed competitive binding of phenolic compounds to the active site of PPL initiates PPL inhibition, hence might be an underlying reason for observed results (Ojo et al. 2019). An important reson of why PPL inhibitors are attracting wide research interest includes their protective effect of pancreas, which will restore regular insulin production from the β cells. Additionally erlier reports shows despite of numerous studies orlistat is the only commercial PPL inhibitor (Jaradat et al. 2017). Therefore inhibition of lipase by the APH extracts is considered as a vital screening.
Glucose oxidation, followed by oxidative degradation of glycated proteins leads to imbalance in ROS and antioxidant, initiating various complications in DM. Hence maintaining the redox balance can be a therapeutic approach in holistic management of DM and its related complications (Halim and Halim 2019; Sadeer et al. 2020). Five different antioxidant assays were involved in the present study (Table 2), with diverse mechanism of actions, which might assess the overall antioxidant capacity of the APH (Sadeer et al. 2020).
DPPH is a free radical that works by proton/electron transfer by antioxidant to form non-radical DPPH-H (Sadeer et al. 2020). The dissimilarity in obtained DPPH values, might be due to the content of phytochemicals present in different formulations and also due to the steric blockage of the available phenols (Faitanin et al. 2018; Gaurav et al. 2020). SN (AQE, HAE) showed significant reduction in DPPH+ radical at 200 µg/ml, an underlying reason for better SN(HAE) values can be due to synergistic action of the phytocompounds present in the APH and their accessibility to scavenge free radicals (Gaurav et al. 2020).
Mono-cation ABTS+ is a stable long-life protonated radical which can be reduced by hydrophilic or lipophilic free radical (Faitanin et al. 2018). The ABTS+ scavenging potential in the present investigation were higher in contrast to other reported studies on herbs/herbal formulation (Majumder and Paridhavi 2018; Ojo et al. 2019). ABTS+ scavenging requires a chain breaking or hydrogen donating antioxidant hence presence of the same might be an underlying reason for the obtained scavenging potential of APH (Ojo et al. 2019; Thengyai et al. 2020). The difference in DPPH and ABTS+ assays depends on the system, where DPPH requires a hydrophobic system and Mono-cation ABTS+ can be reduced by hydrophilic or lipophilic free radicals (Abramovĭ et al. 2018; Sadeer et al. 2020). Further earlier data revealed individual components of MA exhibiting lower radical quenching capacity, i.e., Mangifera indica (71.6%), Momordica charanti (6.38%) and Syzygium cumini (53%) suggesting importance of synergistic effects of APH (Hwang 2018; Ngo et al. 2019; Ojo et al. 2019; Yadav et al. 2020). An underlying reason for the same can be due to the presence of compounds having proton transfer capacity such as carotenoids, phenolic acids, such as gallic acid and ellagic acid (Rodríguez-González et al. 2017).
Iron is an essential trace element which might have adverse effect when present in excess in human body, as it is converted to lethal hydroxyl free radicals by fenton reaction. Hence evaluating metal chelating activity is an important aspect which act as a preventive antioxidant by halting initiation method (Sadeer et al. 2020). Obtained results in the present study are in agreement with previous reports on 20 polyherbal formulation from Thailand and commercial herbal formulations (Chanthasri et al. 2018; Kumar et al. 2019). As stated in literature: functional group, polysaccharides and ligand with three bidentate are few of the important factors required for effective chelating property of ferric ion (Adjimani and Asare 2015).
Reducing power of Fe3+-TPTZ to Fe2+-TPTZ, by donating H-atom at low pH can be estimated by high intensity color developed. In FRAP assay maximum activity was observed in MA (HAE) which revealed that the polyphenolic compound present in APH might have high affinity towards reducing Fe3+-TPTZ even at lower pH (Kumar et al. 2019; Danet 2020). According to literature, compounds known as reductones with redox potential < 0.7 V are detected in FRAP assay, thus FRAP assay can be estimated to maintain redox status of cells/tissues (Kaur et al. 2019).
Further TAC measures the reducing capability of the extracts of Mo (IV) to Mo(V) at low pH and high temperature. Our observation revealed formation of considerable green colored complex by all the tested extracts our results accord with previous observations (Sadeer et al. 2020). Preceding reports has stated phenolic and non-phenolic compounds accountable in reducing Mo (IV) (Mehwisha et al. 2019; Saleem et al. 2019), hence might be the basis for observed values in present study. Thus, the values obtained in the present study imply that these APH might be useful in management of oxidative stress associated pathological complications. The variability in composition and structure following alteration in the sequence of redox reactions leads to difference in electron/hydrogen donating pattern of the tested APH (Batool et al. 2019). Obtained results are in accordance with previous findings (Batool et al. 2019; Saleem et al. 2019).
Correlation studies were carried out with HAE. The values obtained for Pearson correlation coefficient showed phenolic compound/s as major contributor in lipase inhibition which was in accordance to the previous reports on herbal formulation and black legumes (Sompong et al. 2016; Tan et al. 2017). TFC may be responsible for α-amylase inhibition due to the presence of favorable structures of flavonoids in the APH tested, a comparable result was reported by Trung et al. (2018). The strong positive correlation of DPPH-α-amylase may interpret the interrelationship of free radicals and hyperglycemia, further stating the importance of antioxidant in management of DM (Koch and Deo 2016; Tan et al. 2017). No significant correlation was observed for α-glucosidase inhibition, which was in consistence with the earlier literature (Koch and Deo 2016). As enzyme activities are not solely dependent on TPC and TFC, but also due to the structure of the bioactive compound and its availability for the in-vitro experiment chosen (Koch and Deo 2016). Further vast range of positive mild to low correlation activity were observed, an underlying reason for difference in correlation results might be due to stereoselectivity of radical with the extracts, chemical structure, polarities and solubility in the experimental medium (Oyedemi et al. 2017).
MA(HAE) exhibited inclusive better bioactivities, hence the observed values strongly proposed identification of the active chemical constituents of MA(HAE). A combined HPLC–ESI–MS/MS following MetFrag search from KEGG database was incorporated in the present work. Further generating empirical formula and structural conformity from the fragmented data (Thengyai et al. 2020).
Pheophorbide a has previous reports on its enhanced insulin secretion, further improved phosphorylation of Akt, FoxO1, PKA and inhibition of Rat lense aldose reductase, α‐glucosidase and α‐amylase (Li et al. 2015; Madike et al. 2017; Son et al. 2011). Thus, might contribute to the anti-hyperglycemic activity of APH-MA.
Several reports suggested the presence of Pyropheophorbide a in various antidiabetic herbs such as Ficus exasperata Vahl, Moringa oleifera Lam (Baforn et al. 2012; Ezuruike and Prieto 2014; Igbo et al. 2019). A strong positive correlation with α-amylase (r2 0.704*) and lipase (r2 0.766*) inhibitory activities have also been reported in the literature (Feng et al. 2020). Therefore, the observed α-amylase and lipase inhibitory activities might be partly due to the presence of Pyropheophorbide a in APH-MA.
Conclusion
In the present study, commercial APH were assessed which exhibited different levels of inhibitory activities, i.e., α-amylase, α-glucosidase, lipase and antioxidant activities, where some APH were observed to be more effective. The inhibition of α-amylase, α-glucosidase and lipase also prevent ROS formation which may finally protects the β pancreatic cells. Correlation studies showed no significant values for α-glucosidase inhibition, suggesting that other compounds might be responsible for the observed activity, whereas α-amylase and lipase inhibitory activity have indicated presence of polyphenol might be the primary reason. Further HPLC–ESI–MS/MS analysis of MA(HAE) revealed important antidiabetic compounds, Pheophorbide a and Pyropheophorbide a which might be responsible for the anti-hyperglycemic activity for MA. APH can be considered as good source of alternative for management of DM due to their multiple bioactive compounds exerting holistic biological effects. Previous reports on APH have showed blood glucose reducing ability, however limited data were available about their mechanism of actions, therefore, our study can contribute evidence for the same.
Acknowledgements
The authors are thankful to JAIN University School of Sciences, Bangalore for infrastructural support.
Author contributions
Authors confirms all research are done by authors. SP carried out the experimental, data analysis and has written the manuscript; MM supervised the work and edited the manuscript.
Declarations
Conflict of interest
Author declare no conflict of interest.
Contributor Information
Saptadipa Paul, Email: saproses@yahoo.com.
Mala Majumdar, Email: mala.majumdar@jainuniversity.ac.in.
References
- Abramovĭc H, Grobin B, Ulrih PN, Cigí B. Relevance and standardization of in vitro antioxidant assays: ABTS, DPPH, and Folin-Ciocalteu. J Chem. 2018;2018:1–9. doi: 10.1155/2018/4608405. [DOI] [Google Scholar]
- Adjimani PJ, Asare P. Antioxidant and free radical scavenging activity of iron chelators. Toxicol Rep. 2015;2:721–728. doi: 10.1016/j.toxrep.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artasensi A, Pedretti A, Vistoli G, Fumagalli L. Type 2 diabetes mellitus: a review of multi-target drugs. Molecules. 2020;25:1987. doi: 10.3390/molecules25081987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baforn EE, Lim CV, Rowan GE, Edrada-Ebel R. The leaves of Ficus exasperata Vahl (Moraceae) generates uterine active chemical constituents. J Ethanopharmacol. 2012;145:803–812. doi: 10.1016/j.jep.2012.12.020. [DOI] [PubMed] [Google Scholar]
- Batool R, Khan RM, Sajid M, Ali S, Zahra Z. Estimation of phytochemical constituents and in vitro antioxidant potencies of Brachychiton populneus (Schott & Endl.) R.Br. BMC Chem. 2019;13:32. doi: 10.1186/s13065-019-0549-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia A, Singh B, Arora R, Arora S. In vitro evaluation of the α-glucosidase inhibitory potential of methanolic extracts of traditionally used antidiabetic plants. BMC Complement Altern Med. 2019;19:74. doi: 10.1186/s12906-019-2482-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butala MA, Kukkupuni KS, Vishnuprasad NC. Ayurvedic anti-diabetic formulation lodhrasavam inhibits alpha amylase, alpha glucosidase and suppresses adipogenic activity in vitro. J Ayurveda Integr Med. 2017;8:145–151. doi: 10.1016/j.jaim.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanthasri W, Puangkeaw N, Kunworarath N, Jaisamut P, Limsuwan S, Maneenoon K, Choochana P, Chusri S. Antioxidant capacities and total phenolic contents of 20 polyherbal remedies used as tonics by folk healers in Phatthalung and Songkhla provinces, Thailand. BMC Complement Altern Med. 2018;18:73. doi: 10.1186/s12906-018-2131-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, Malanda B. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–281. doi: 10.1016/j.diabres.2018.02.023. [DOI] [PubMed] [Google Scholar]
- Danet AF. Recent advances in antioxidant capacity assays. IntechOpen; 2020. pp. 1–36. [Google Scholar]
- Duraiswamy A, Shanmugasundaram D, Sasikumar SC, Cherian MS, Cherian MK. Development of an antidiabetic formulation (ADJ6) and its inhibitory activity against α-amylase and α-glucosidase. J Tradit Complement Med. 2016;28:204–208. doi: 10.1016/j.jtcme.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezuruike UF, Prieto JM. The use of plants in the traditional management of diabetes in Nigeria: pharmacological and toxicological considerations. J Ethanopharmacol. 2014;11:857–924. doi: 10.1016/j.jep.2014.05.055. [DOI] [PubMed] [Google Scholar]
- Faitanin DR, Gomes DVJ, Rodrigues MP, de Menezes TFL, Neto CA, Gonçalves RCR, Kitagawa RR, Silveira D, Jamal CM. Chemical study and evaluation of antioxidant activity and α-glucosidase inhibition of Myrciaria strigipes O. Berg (Myrtaceae) J Appl Pharm Sci. 2018;8:120–125. doi: 10.7324/JAPS.2018.8317. [DOI] [Google Scholar]
- Feng L, Liu P, Zheng P, Zhang L, Zhou J, Gong Z, Yu Y, Gao S, Zheng L, Wang X, Wan X. Chemical profile changes during pile fermentation of Qingzhuan tea affect inhibition of α-amylase and lipase. Sci Rep. 2020;10:3489. doi: 10.1038/s41598-020-60265-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco RR, da Carvalho DS, de Moura BFR, Justino BA, Silva GCH, Peixoto GL, Espindola SF. Evaluation of α-amylase, α-glucosidase and lipase inhibitory activities of some medicinal plants used in type-2 Diabetes Mellitus and its anti-glycation and antioxidant roles. J Ethnopharmacol. 2017;17:32990–32992. doi: 10.1002/cbdv.201800025. [DOI] [Google Scholar]
- Halim M, Halim A. The effects of inflammation, aging and oxidative stress on the pathogenesis of diabetes mellitus (type 2 diabetes) Diabetes Metab Syndr. 2019;13:1165–1172. doi: 10.1016/j.dsx.2019.01.040. [DOI] [PubMed] [Google Scholar]
- Hwang E. Comparison of antioxidant capacity and α-glucosidase inhibitory activity between bitter melon (Momordica charanti) fruit and leaf extract. Asian Pac J Trop Biomed. 2018;8:189–193. doi: 10.4103/2221-1691.231280. [DOI] [Google Scholar]
- Igbo EU, Igoli JO, Onyiriuka SO, Ogukwe CE, Ayuk AIA, Gray A. Isolation and characterization of Pyropheophorbide-a from Moringa oleifera Lam. Trop J Nat Prod Res. 2019;3:314–318. doi: 10.26538/tjnpr/v3i10.3. [DOI] [Google Scholar]
- Jaradat N, Zaid NA, Hussein F, Zaqzouq M, Aljammal H, Ayesh O. Anti-lipase potential of the organic and aqueous extracts of ten traditional edible and medicinal plants in palestine; a comparison study with orlistat. Medicines. 2017;4:89. doi: 10.3390/medicines4040089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur K, Grewal KS, Gill SP, Singh S. Comparison of cultivated and wild chickpea genotypes for nutritional quality and antioxidant potential. J Food Sci Technol. 2019;56:1864–1876. doi: 10.1007/s13197-019-03646-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch RE, Deo P. Nutritional supplements modulate fluorescent protein-bound advanced glycation endproducts and digestive enzymes related to type 2 diabetes mellitus. BMC Complement Altern Med. 2016;6:1–7. doi: 10.1186/s12906-016-1329-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V, Singh S, Singh A, Subhose V, Prakash O. Assessment of heavy metal ions, essential metal ions, and antioxidant properties of the most common herbal drugs in Indian Ayurvedic hospital: for ensuring quality assurance of certain Ayurvedic drugs. Biocatal Agric Biotechnol. 2019;18:101018. doi: 10.1016/j.bcab.2019.01.056. [DOI] [Google Scholar]
- Li H, Ji H-S, Kang J-H, Shin D, Park H, Choi M-S, Lee C-H, In-K L, Yun B-S, Jeon T-S. Soy leaf extract containing kaempferol glycosides and pheophorbides improves glucose homeostasis by enhancing pancreatic β-Cell function and suppressing hepatic lipid accumulation in db/db Mice. J Agric Food Chem. 2015;63:7198–7210. doi: 10.1021/acs.jafc.5b01639. [DOI] [PubMed] [Google Scholar]
- Madike NL, Takaidza S, Pillay M. Preliminary phytochemical screening of crude extracts from the leaves, stems, and roots of Tulbaghia violacea. Int J Pharmacogn Phytochem. 2017;9:1300–1308. doi: 10.25258/phyto.v9i10.10453. [DOI] [Google Scholar]
- Majumder P, Paridhavi M. A novel polyherbal formulation hastens diabetic wound healing with potent antioxidant potential: a comprehensive pharmacological investigation. Nat Prod Chem Res. 2018;6:1–9. doi: 10.5530/pj.2019.11.48. [DOI] [Google Scholar]
- Mehwisha S, Islamb A, Ullahc I, Wakeelc A, Qasimd M, Khana AM, Ahmada A, Ullah N. In vitro antileishmanial and antioxidant potential, cytotoxicity evaluation and phytochemical analysis of extracts from selected medicinally important plants. Biocatal Agri Biotechnol. 2019;19:101117. doi: 10.1016/j.bcab.2019.101117. [DOI] [Google Scholar]
- Nawaz H, Shad AM, Rehman N, Andaleeb H, Ullah N. Effect of solvent polarity on extraction yield and antioxidant properties of phytochemicals from bean (Phaseolus vulgaris) seeds. Braz J Pharm Sci. 2020;56:1–9. doi: 10.1590/s2175-97902019000417129. [DOI] [Google Scholar]
- Ngo D, Ngo D, Vo NTT, Vo ST. Mechanism of action of Mangifera indica leaves for anti-diabetic activity. Sci Pharm. 2019;87:13. doi: 10.3390/scipharm87020013. [DOI] [Google Scholar]
- Ojo MC, Osunsanmi FO, Zaharare GE, Mosa RA, Cele ND, Oboh MO, Opoku AR. In-vitro anti-diabetic and antioxidant efficacy of methanolic extract of Encephalartos feroxleaves. Pharmacogn J. 2019;11:455–460. doi: 10.5530/pj.2019.11.71. [DOI] [Google Scholar]
- Oyedemi OS, Oyedemi OB, Ijeh II, Ohanyerem EP, Coopoosamy RM, Aiyegoro AO. Alpha-amylase inhibition and antioxidative capacity of some antidiabetic plants used by the traditional healers in southeastern nigeria. Sci World J. 2017;2017:1–11. doi: 10.1155/2017/3592491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda KA. Efficacy of ayurveda formulation Ayush-82 (IME-9) in newly diagnosed type 2 diabetics: retrospective analysis of individual data. J Tradit Med Clin Natur. 2017;6:1–3. doi: 10.4172/2573-4555.1000250. [DOI] [Google Scholar]
- Rodríguez-González S, Gutiérrez-Ruíz MI, Pérez-Ramírez IF, More O, Ramos-Gomez M, Reynoso-Camacho R. Mechanisms related to the anti-diabetic properties of mango (Mangifera indica L.) juice by-product. J Funct Foods. 2017;37:190–199. doi: 10.1016/j.jff.2017.07.058. [DOI] [Google Scholar]
- Sadeer BN, Montesano D, Albrizio S, Zangin G, Mahomoodally MF. The versatility of antioxidant assays in food science and safety—chemistry, applications, strengths, and limitations. Antioxidant. 2020;9:709. doi: 10.3390/antiox9080709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem H, Htara TT, Naiduc R, Nawawid SN, Ahmade I, Ashraff M, Ahemad N. Biological, chemical and toxicological perspectives on aerial and roots of Filago germanica (L.) huds: functional approaches for novel phyto-pharmaceuticals. Food Chem Toxicol. 2019;123:363–373. doi: 10.1016/j.fct.2018.11.016. [DOI] [PubMed] [Google Scholar]
- Salunke S, Pande V, Kendre P, Vibhute S. Effect of standardized polyherbal formulations on blood glucose, body weight, food and water consumption of rat. Pharm Sci. 2015;21:56–63. doi: 10.15171/PS.2015.18. [DOI] [Google Scholar]
- Schofield DJ, Liu Y, Rao-Balakrishna P, Malik AR, Soran H. Diabetes dyslipidemia. Diabetes Ther. 2016;7:203–219. doi: 10.1007/s13300-016-0167-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sies H. The concept of oxidative stress after 30 years. In: Gelpi R, Boveris A, Poderoso J, editors. Advances in biochemistry in health and disease. New York: Springer; 2016. pp. 3–11. [Google Scholar]
- Sompong W, Muangngam N, Kongpatpharnich A, Manacharoenlarp C, Amorworasin C, Suantawee T, Thilavech T, Adisakwattana S. The inhibitory activity of herbal medicines on the keys enzymes and steps related to carbohydrate and lipid digestion. BMC Complement Altern Med. 2016;16:439. doi: 10.1186/s12906-016-1424-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son KY, Jin ES, Kim H-R, Woo CH, Jung AH, Cho SJ. Inhibitory activities of the edible brown alga Laminaria japonica on glucose-mediated protein damage and rat lens aldose reductase. Fish Sci. 2011;77:1069–1079. doi: 10.1007/s12562-011-0406-z. [DOI] [Google Scholar]
- Tan Y, Chang KCS, Zhang Y. Comparison of α-amylase, α-glucosidase and lipase inhibitory activity of the phenolic substances in two black legumes of different genera. Food Chem. 2017;214:259–268. doi: 10.1016/j.foodchem.2016.06.100. [DOI] [PubMed] [Google Scholar]
- Telapolu S, Kalachavedu M, Punnoose MA, Bilikere D. MD-1, a poly herbal formulation indicated in diabetes mellitus ameliorates glucose uptake and inhibits adipogenesis—an invitro study. BMC Complement Altern Med. 2018;18:1–11. doi: 10.1186/s12906-018-2177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thengyai S, Thiantongin P, Sontimuang C, Ovatlarnporn C, Puttarak P. α-glucosidase and α-amylase inhibitory activities of medicinal plants in Thai antidiabetic recipes and bioactive compounds from Vitex glabrata R.Br. stem bark. J Herb Med. 2020;19:100302. doi: 10.1016/j.hermed.2019.100302. [DOI] [Google Scholar]
- Trung QN, Luyen TN, Nam DV, Dat TN. Chemical composition and in vitro biological activities of white mulberry syrup during processing and storage. J Food Nutr Res. 2018;6:660–664. doi: 10.12691/jfnr-6-10-7. [DOI] [Google Scholar]
- Truong D, Nguyen DH, Ta ATN, Bui VA, Do TH, Nguyen CH. Evaluation of the use of different solvents for phytochemical constituents, antioxidants, and in vitro anti-inflammatory activities of Severinia buxifolia. J Food Qual. 2019;2019:8178294. doi: 10.1155/2019/8178294. [DOI] [Google Scholar]
- Unuofin OJ, Otunola AG, Afolayan JA. In vitro a-amylase, a-glucosidase, lipase inhibitory and cytotoxic activities of tuber extracts of Kedrostis africana (L.) Cogn Heliyon. 2018;2018:2405–2844. doi: 10.1016/j.heliyon.2018.e00810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2021) Diabetes Program fact sheet. WHO. https://www.who.int/news-room/fact-sheets/detail/diabetes. Accessed 14 Jan 2022
- Yadav N, Pal A, Sihag S, Nagesh CR. Antioxidant activity profiling of acetonic extract of jamun (Syzygium cumini L.) seeds in different in-vitro models. Open Food Sci J. 2020;12:3–8. doi: 10.2174/1874256402012010003. [DOI] [Google Scholar]
- Zahiruddin S, Parveen B, Ibrahim M, Sharma I, Sharma S, Sharma KA, Praveen R, Ahmad S. TLC–MS bioautography-based identification of free-radical scavenging, α-amylase, and α-glucosidase inhibitor compounds of antidiabetic tablet BGR-34. ACS Omega. 2020;5:29688–29697. doi: 10.1021/acsomega.0c02995. [DOI] [PMC free article] [PubMed] [Google Scholar]