Abstract
Objectives
To report the reduction in new neovascular age-related macular degeneration (nAMD) referrals during the COVID-19 pandemic and estimate the impact of delayed treatment on visual outcomes at 1 year.
Design
Retrospective clinical audit and simulation model.
Setting
Multiple UK National Health Service (NHS) ophthalmology centres.
Participants
Data on the reduction in new nAMD referrals were obtained from four NHS Trusts comparing April 2020 with April 2019. To estimate the potential impact on 1-year visual outcomes, a stratified bootstrap simulation model was developed drawing on an electronic medical records dataset of 20 825 nAMD eyes from 27 NHS Trusts.
Main outcome measures
Simulated mean visual acuity and proportions of eyes with vision ≤6/60, ≤6/24 and ≥6/12 at 1 year under four hypothetical scenarios: 0-month, 3-month, 6-month and 9-month treatment delays. Estimated additional number of eyes with vision ≤6/60 at 1 year nationally.
Results
The number of nAMD referrals dropped on average by 72% (range 65%–87%). Simulated 1-year visual outcomes for 1000 nAMD eyes with a 3-month treatment delay suggested an increase in the proportion of eyes with vision ≤6/60 from 15.5% (13.2%–17.9%) to 23.3% (20.7%–25.9%), and a decrease in the proportion of eyes with vision ≥6/12 (driving vision) from 35.1% (32.1%–38.1%) to 26.4% (23.8%–29.2%). Outcomes worsened incrementally with longer modelled delays. Assuming nAMD referrals are reduced to this level for 1 month nationally, these simulated results suggest an additional 186–365 eyes with vision ≤6/60 at 1 year.
Conclusions
We report a large decrease in nAMD referrals during the COVID-19 lockdown and provide an important public health message regarding the risk of delayed treatment. As a conservative estimate, a treatment delay of 3 months could lead to a >50% relative increase in the number of eyes with vision ≤6/60 and 25% relative decrease in the number of eyes with driving vision at 1 year.
Keywords: COVID-19, public health, ophthalmology
Strengths and limitations of this study.
Audit data regarding the drop in neovascular age-related macular degeneration (AMD) referrals were collected from multiple UK specialist ophthalmology centres.
The simulation model was based on a combination of high-quality randomised control trial data and real-world electronic medical records data from 27 National Health Service Trusts.
An assumption underlying the treatment delay models is that eyes receiving treatment late in the course of the disease will respond as well as if there had been no treatment delay.
Conservative analysis that probably underestimates the true burden of additional visual loss from treatment delay as untreated neovascular AMD causes retinal scarring.
Introduction
Healthcare services responding to the burden of the COVID-19 pandemic have had to institute policies to limit the number of patients attending hospital for other conditions. In addition, members of the public have altered their own health behaviour and reports have appeared of greatly reduced attendance at accident and emergency and significantly lowered referral rates for some conditions.1 2As the primary burden of treating patients with COVID-19 has begun to ease in many countries, the implication of the epidemic for patients indirectly affected has become a focus of concern.
Ophthalmic conditions are the major source of outpatient appointments in the UK3 and many, including neovascular age-related macular degeneration (nAMD), are diseases of older adults, a population at risk of developing severe COVID-19 complications.4 5 In England, this led to a recommendation that individuals over 70 years old, the population most at risk of nAMD, should self-isolate.6 The Royal College of Ophthalmologists produced guidance, updated on 30 March 2020, on how to triage and care for patients during the pandemic, reflecting the requirement to minimise patient contact and the associated risk for patients and staff.7 They advised that patients with nAMD already under review continue with all preplanned visits and that new patients be investigated and treatment started as required. Nevertheless, as we report here, once the epidemic started, ophthalmology clinics began to experience a drop in referrals and high rates of missed appointments.
As ophthalmology clinics are now planning for the resumption of services, assessing the impact of delays to different categories of patients becomes increasingly urgent.8 In this paper, we focus on nAMD, the leading cause of blindness in high-income countries.9 Since patients who initiate treatment for nAMD with a better baseline visual acuity (VA) are more likely to maintain a high level of vision and achieve good long-term outcomes10 11 if COVID-19 delays the initiation of treatment, the outcome may have a long-term impact on the burden of sight impairment and associated social independence and health consequences.
The objectives of this paper are to report the change in the number of patients referred to hospital eye services with nAMD at the start of the COVID-19 pandemic compared with the previous year, and to estimate the potential impact of delayed nAMD treatment initiation.
Methods
Study design
Cross-sectional study with simulation.
Audit of nAMD referrals
The lead clinicians for ophthalmology at multiple National Health Service (NHS) Trusts across England were contacted to provide audit data on the number of nAMD referrals during April 2020 compared with April 2019. The difference in referral numbers was divided by the number of referrals in April 2020 to calculate a percentage change. At the time of writing, four Trusts had responded: Moorfields Eye Hospital, King’s College London Hospital, University Hospital Southampton and Whipps Cross Hospital.
Simulation model
Overview
To estimate the potential impact of delayed treatment on 1-year visual outcomes, a simulation model based on a large electronic medical records (EMR) dataset of treated nAMD eyes was designed (summarised in figure 1). The key visual outcomes of interest included VA at 1 year and the proportions of eyes with VA ≤35 letters (6/60 Snellen), ≤55 letters (6/24 Snellen) and ≥70 letters (6/12 Snellen) that map approximately to the UK criteria for severe sight impairment and sight impairment (group 2), and the UK criteria for driving vision, respectively.12 13 These outcomes comply with recommendations from the International Consortium for Health Outcomes Measurement AMD working group and were compared for four modelled scenarios: no treatment delay, and 3-month, 6-month and 9-month treatment delays (figure 1).14 Of note, the UK criteria for vision impairment are defined in Snellen, whereas in our UK EMR Group dataset, VA is recorded in Early Treatment Diabetic Retinopathy Study (ETDRS) letters. A recent study supported 35 letters mapping over to about 3/60 Snellen (UK criteria for severe sight impairment, group 1), and 70 letters to 6/12 Snellen.13 In addition, visual field deficits may also alter vision impairment status. These factors guided our choice of ETDRS letter values for sight impairment.12
Figure 1.
(A) Summary of the simulation process. (B) Modelling process for estimating the effect of vision loss during delayed treatment on baseline VA. Letter losses for the delayed treatment models are based on data from the Marina randomised control trial control arm. anti-VEGF, anti-vascular endothelial growth factor; EMR, electronic medical records; nAMD, neovascular age-related macular degeneration; NICE, National Institute for Health and Care Excellence; VA, visual acuity.
EMR data
Thirty-six NHS Trusts known to make comprehensive use of the Medisoft EMR system (http://www.medisoft.co.uk) to store detailed structured clinical records of patients were invited to contribute data to studies of the treatment of retinal diseases, including nAMD. Twenty-seven (75%) agreed to supply data. Patients who underwent anti-vascular endothelial growth factor (VEGF) treatment for nAMD between 2009 and 2018, and also had VA measurements recorded at first injection (baseline) and 1 year, were included. Patients with missing age and gender data were excluded. Patients who were not treatment naïve or were receiving one of the treatments of interest for another condition were excluded. VA measurement was performed as a part of routine clinical practice using an ETDRS chart to give an ETDRS letter score.
Simulation model
No treatment delay
The simulation process was based on a stratified bootstrapping procedure. First, all eyes in the EMR nAMD dataset were pooled into the four baseline VA bins (<35, 35–49, 50–64, ≥65 letters). Second, to simulate a range of year visual outcomes for nAMD with no treatment delay, we sampled with replacement a total of 1000 eyes from these pools with probabilities equal to the proportion of eyes in each category (table 1). One-year visual outcome measures for the sampled eyes were extracted and this process was repeated 1000 times. Finally, the range of visual outcome values generated was summarised.
Table 1.
Summary of patient demographics and baseline visual acuity (VA) distribution for the full EMR nAMD cohort of 20 825 eyes (18 340 patients) treated for nAMD
| Demographics | |
| Age years (±SD) | 80.5 years (±7.8) |
| Male, % (n) | 35.5 (7398) |
| Right eye, % (n) | 50.8 (10 580) |
| Baseline VA | |
| Mean baseline VA (±SD) | 55.6 letters (±15.9) |
| % ≥65 letters (n) | 35.3 (7342) |
| % 50–64 letters (n) | 33.0 (6876) |
| % 35–49 letters (n) | 22.3 (4652) |
| % <35 letters (n) | 9.4 (1955) |
| One-year outcomes | |
| Mean 1-year VA (±SD) | 58.2 letters (±18.6) |
| % severe sight impairment (≤35 letters) (n) | 14.5 (3027) |
| % sight impairment (≤55 letters) (n) | 39.0 (8129) |
| % driving vision (≥70 letters) (n) | 35.2 (7326) |
EMR, electronic medical records; nAMD, neovascular age-related macular degeneration; SD, standard deviation.
Delayed treatment: 3, 6 and 9 months
Visual outcomes for the three delayed treatment models were estimated by varying the sampling probabilities in the simulation process described above. Baseline VA is a strong predictor of visual outcome, with good baseline VAs tending to have better outcomes and vice versa (figure 1Ai). nAMD causes progressive visual decline and delayed treatment would therefore result in a poorer range of baseline VAs leading to poorer visual outcomes.
To estimate the distribution of baseline VAs after 3-month, 6-month and 9-month treatment delay periods, we first ascertained the approximate mean letter loss and associated standard deviations (SD) (derived from digitised standard error bars (https://automeris.io/WebPlotDigitizer)) at these time points from the MARINA trial untreated control arm, that represents the natural history of untreated nAMD. These values are tabled in figure 1Bi. We then transformed the proportions of eyes in each baseline VA category for the no delay model in the following way. For each eye in the full cohort (n=20 825), a randomly generated letter loss was applied to the baseline VA. The number of letters subtracted was sampled from a normal distribution based on the mean and SD values obtained from the MARINA trial. We set a lower limit of zero letters loss (ie, if a negative number of letters was sampled, then this reset to zero) as untreated nAMD eyes rarely improve vision.15 The new proportions of eyes fitting each baseline VA category were then calculated and these were applied as sampling probabilities in the simulation. This method is illustrated in figure 1Bii for a 3-month delay model. A new set of sampling probabilities was generated in this way for all 1000 iterations in each of the delayed treatment models.
Modelling by this method implicitly assumes that eyes with a given baseline VA would respond to treatment in the same way as if there had been no treatment delay. This important assumption is revisited in the Discussion section.
Eyes with vision below National Institute for Health and Care Excellence (NICE) treatment criteria
A period of treatment delay would result in some eyes falling below standard NICE treatment criteria (≤6/96 Snellen). To reflect this in our simulation, we identified eyes with a baseline VA of ≤25 letters and modelled them to remain at the same level of VA at 1 year, as if they had not received treatment. This rule was applied to all models, including the no treatment delay model, to allow a fair comparison of results. The simulation was also performed without this additional rule as a sensitivity analysis (online supplemental materials).
bmjopen-2021-057269supp001.pdf (478.7KB, pdf)
National estimates of delayed nAMD referrals
The reduction in new nAMD referrals audited at the four aforementioned NHS Trusts was projected to the whole of the UK and results from the simulation model were then applied to estimate the potential national impact of delayed nAMD treatment.
Statistical analysis
The simulation model and analyses of its output were implemented in R V.3.6.2.16 Visual outcomes were compared between the delayed treatment models with the no treatment delay model using the z-test. Graphs were generated using the ggplot2 package. All analysis code is available at https://github.com/rmgpanw/nAMD_tx_delay_simulation. VAs are presented in ETDRS letter format unless stated otherwise.
Patient and public involvement
Given the urgency of the COVID-19 situation, there were no funds or time allocated for patient and public involvement and we were unable to involve patients. We have invited the patient support group, the Macular Society, and patients associated with this society to help us develop our dissemination strategy.
Results
New nAMD referrals during the COVID-19 pandemic
Figures from the first four hospitals that responded to our request for data showed a change in the number of referrals for nAMD from 142 in April 2019 to 40 in April 2020, representing a drop of 72%. The number of referrals dropped at all four hospitals (range 65%–87%).
Simulation model
EMR nAMD cohort
From the EMR dataset, we identified 20 825 eyes (18 340 patients) that underwent anti-VEGF treatment for nAMD between 2009 and 2018 and had VA measurements recorded at first injection (baseline) and 1 year. The demographics, baseline VA distribution and 1-year visual outcomes for this cohort are described in table 1.
Simulated visual outcomes at 1 year
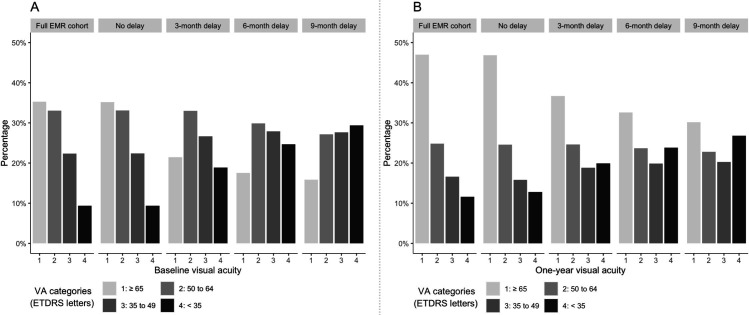
The average simulated mean baseline VAs for the no delay, 3-month, 6-month and 9-month delay models were 55.6 letters (±0.6 SD), 49.9 letters (±0.6 SD), 47.2 letters (±0.6 SD) and 45.5 letters (±0.6 SD), respectively. Figure 2 summarises the average baseline and 1-year VA distributions generated for each model, as well as those for the full EMR nAMD cohort. Results for the no delay model resemble those of the whole cohort, whereas both the baseline and 1-year VA distributions worsen incrementally with increasing treatment delay.
Figure 2.
The average distribution of visual acuities (VAs) across all iterations in the simulation process at baseline (A) and at 1 year (B) for the full EMR nAMD cohort and under four modelled conditions: no, 3-month, 6-month and 9-month treatment delay. EMR, electronic medical records; ETDRS, Early Treatment Diabetic Retinopathy Study.
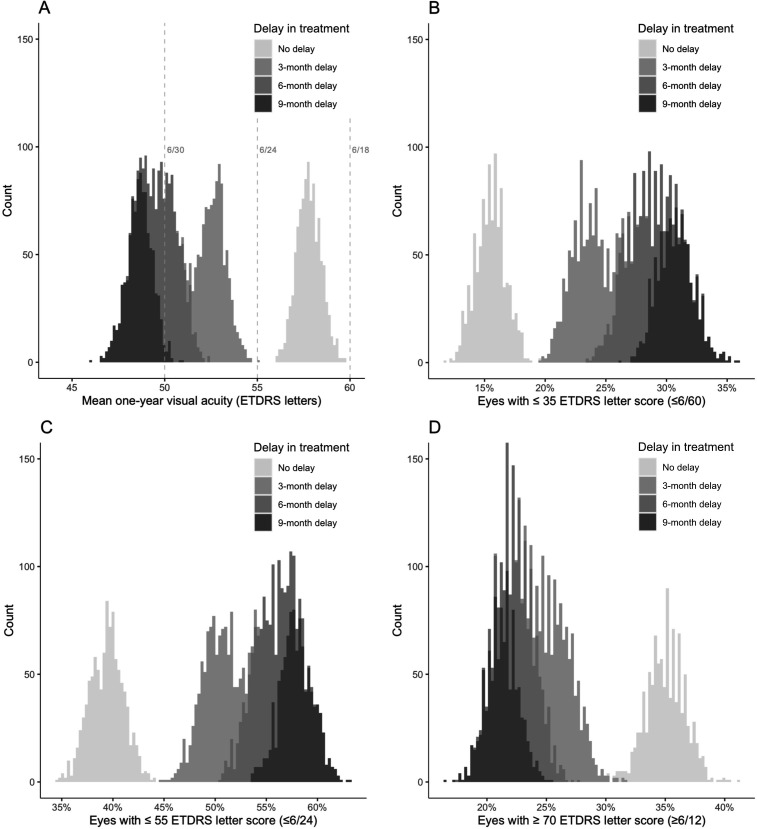
Average simulated 1-year visual outcomes for the four models are presented in table 2. These results are complemented by figure 3 which graphically depicts the range of estimates generated across 1000 iterations for each model, showing minimal overlap between the no delay model and any of the treatment delay models.
Table 2.
Average (95% bootstrap confidence interval) simulated 1-year visual outcomes under each of the four modelled conditions
| No treatment delay | 3-month delay | 6-month delay | 9-month delay | |
| Mean 1-year VA | 57.8 letters (56.6–59.1) |
52.7 letters (51.4–54.1) (−5.1 letters ; p<0.001) |
50.3 letters (48.8–51.6) (−7.5 letters; p<0.001) |
48.6 letters (47.2–49.9) (−9.2 letters; p<0.001) |
| % severe sight impairment (≤35 letters) |
15.5 (13.2–17.9) |
23.3 (20.7–25.9) (+7.8; p<0.001) |
27.6 (24.7–30.6) (+12.1; p<0.001) |
30.8 (28.0–33.7) (+15.3; p<0.001) |
| % sight impairment (≤55 letters) |
39.4 (36.1–42.5) |
50.0 (46.9–53.1) (+10.6; p<0.001) |
54.8 (51.5–58.2) (+15.4; p<0.001) |
57.9 (54.7–61.1) (+18.5; p<0.001) |
| % driving vision (≥70 letters) |
35.1 (32.1–38.1) |
26.4 (23.8–29.2) (−8.7; p<0.001) |
23.1 (20.6–25.7) (−12.0; p<0.001) |
21.3 (18.7–23.9) (−13.8; p<0.001) |
Comparisons with the no treatment delay model are provided in italics.
VA, visual acuity.
Figure 3.
Overlaid histograms showing the range of simulated 1-year visual acuity (VA) outcomes across 1000 iterations for the no delay, 3-month, 6-month and 9-month treatment delay models. ‘Count’ on the y-axis refers to the number of iterations that returned a visual outcome estimate at the values on the x-axis. (A) Mean 1-year VA (dashed grey vertical lines illustrate Snellen equivalents). (B) Percentage of eyes with ≤35 letters (≤6/60 Snellen). (C) Percentage of eyes with ≤55 letters (≤6/24 Snellen). (D) Percentage of eyes with ≥70 letters (≥6/12 Snellen). ETDRS, Early Treatment Diabetic Retinopathy Study.
Results of the sensitivity analysis, where eyes with a baseline VA of ≤25 letters were not modelled to remain at the same level of VA at 1 year, returned slightly reduced estimated differences in visual outcomes between the no treatment delay and treatment delay models (online supplemental materials). The differences in visual outcomes between the delayed treatment models and no treatment delay model remained significant in all cases.
Estimated national impact of delayed nAMD treatment
Owen et al estimate the annual incidence of nAMD to be 39 700.17 If the COVID-19 pandemic affects patient referrals for 3 months, and over that period they are reduced by 72%, 7146 patients will delay initiating treatment. We estimate that if these patients did not delay treatment, then 1108 eyes would have a VA ≤35 letters (severe sight impairment) after 12 months. This rises to between 1665 and 2201 if treatment initiation is delayed by 3–9 months. The number of people who would meet the legal driving limit would be 2508 with no delay but fall to between 1522 and 1887 under the different modelled delays.
Discussion
Principal findings
During the COVID-19 lockdown, people over 70 years old have been advised to self-isolate. We report a 72% reduction in the expected number of referrals for nAMD at four UK nAMD treatment centres, suggesting there is a substantial number of patients with new nAMD who will suffer from delayed treatment. We also estimate the potential impact of this by simulating 1-year visual outcomes following various periods of treatment delay and projecting these results to a national level. It has been well documented that AMD negatively affects daily living tasks including mobility, face recognition, computer use, meal preparation, cleaning, watching TV, reading, driving and, in some cases, self-care.18 The consequences of untreated nAMD are likely to exacerbate the problems of COVID-19 and non-COVID-19-induced social isolation on the mental and physical health of the elderly.19
Simulation results
The aim of our simulation model was not to provide an absolute estimate of the additional burden of VA loss from delayed nAMD treatment during the COVID-19 pandemic, but rather to provide conservative estimates with some indication of variability due to chance. The simulation is based on a large dataset of 20 825 eyes treated at multiple sites across the UK. It is reasonable therefore to assume that the range of presentations and visual outcomes is representative of the UK population as a whole. While the dataset represents treatment outcomes spanning a 10-year period, VA at 1 year remained stable over this time when adjusting for baseline VA (unpublished results). In reality, the presenting characteristics and visual outcomes of nAMD cases fluctuate randomly between shorter time periods. We mirror this by adopting a stratified bootstrapping approach with 1000 iterations for each simulation model, predicting the degree of possible variation around average visual outcome estimates. It is important to note that our approach estimates monocular but not binocular visual outcomes. The latter would have required speculative estimations for additional parameters including pre-existing sight impairment in the fellow eye as well as the proportion of patients presenting with bilateral nAMD.
The method used to estimate vision loss during a period of delayed treatment was based on high-quality data from the pivotal MARINA trial control arm.15 This represents the best available natural history data for nAMD. Natural history studies of untreated nAMD from before the anti-VEGF era are heterogeneous and unrepresentative of the presenting VA in patients by today’s standards.20 A lower limit of zero letters lost was applied during this process since it is improbable for untreated nAMD to result in improved VA. The average differences in mean baseline VA between the no delay and 3-month, 6-month and 9-month delay models (5.7, 8.4 and 10.1 letters, respectively) were therefore inflated slightly above the corresponding mean letter losses reported in MARINA (approximately 4, 7 and 9 letters, respectively). However, the mean baseline VA in the MARINA trial (53.6 letters) was lower than that of the EMR cohort (55.6 letters). While a better baseline VA is predictive of better visual outcomes, it also carries an increased chance of higher degrees of letter loss. This suggests that the hypothetical mean letter losses for this EMR cohort at 3, 6 and 9 months if they had been left untreated are indeed likely to have been higher than those reported in MARINA.
The EMR dataset includes 725 treated eyes (3.5% of the full EMR nAMD cohort) with a baseline VA of ≤25 letters (≤6/96 Snellen), which falls outside standard NICE treatment criteria. The decision to treat in these cases may have been made either on compassionate grounds or based on VA measurements that fell within NICE treatment criteria at the time of listing for treatment. These eyes were modelled to remain at the same level of vision at 1 year, as if they had not received treatment. We believe this more accurately represents reality, as visual loss during a period of treatment delay would result in a higher proportion of new nAMD eyes falling below NICE treatment criteria by the time they reached clinic. This rule was also applied to the no delay model to allow fair comparison with delayed treatment scenarios. Despite this, visual outcome results from the no delay scenario still closely resembled those for the whole EMR cohort. Running the simulation without applying this rule returned similar results, although with slightly reduced differences between the modelled scenarios.
A key assumption underlying the treatment delay models is that eyes receiving treatment late in the course of the disease will respond as well as if there had been no treatment delay. This underestimates the true burden of additional visual loss from treatment delay as untreated nAMD causes retinal scarring, which would lead to progressively worse treatment responses with longer delays. It follows that the degree to which our simulation process underestimates visual loss would also therefore increase with longer modelled delays.
Certain patient groups may have a higher chance than others of suffering from delayed nAMD treatment during the COVID-19 period. For example, patients with good vision in the fellow eye may be less likely to seek medical attention than patients where nAMD develops in their better seeing eye. This could bias estimates of binocular but not monocular vision impairment. It is also conceivable that elderly patients would be more likely to experience treatment delays possibly due to transport difficulties or even COVID-19 illness. As older age is associated with poorer visual outcomes,11 21 this would bias our predictions towards overestimating vision impairment if mortality from COVID-19 affected a significant proportion of this age group, or the opposite if these patients did eventually attend the ophthalmology clinic.
Overall, the presented figures are most likely to underestimate the true additional burden of vision impairment that would be incurred if treatment were delayed by 3–9 months.
Estimating the national impact
Following guidelines from the Royal College of Ophthalmologists, treating centres for nAMD have endeavoured to continue treating this condition for patients already diagnosed, and delaying follow-up of other less urgent conditions to allow capacity for safe spacing for this high COVID-19 risk group.22 Despite these interventions, many patients (between 5% and 25%) have not attended their appointment in the four centres surveyed. What is of more concern is the large drop in patients presenting with new nAMD, which, according to data from four large treating centres, is about 72% less than expected. While this represents a relatively small sample of ophthalmology units in the UK as a whole, all four centres reported a similarly large drop in nAMD referrals. Projecting this nationally, using an accepted estimate of the incidence rate of nAMD, then approximately 2382 patients will have delayed treatment in the month of April alone. If treatment were delayed by 3–9 months for all these cases, we estimate that this will lead to between 186 and 365 additional eyes with severe sight impairment (≤6/60 Snellen) at 1 year. However, this is a conservative estimate, derived from a 1-month reduction in presentation with a 3-month delay before treatment and assuming that treatment, once initiated on the delayed eyes, has a similar benefit as when initiated promptly. If patients with other sight-threatening diseases behave in a similar manner to those studied here, the increase in cases with severe sight impairment might be distinctly higher.
Summary
In summary, adopting a conservative model, our estimates still indicate a substantial increase in visual loss (severe sight impairment, loss of driving vision) from delayed nAMD treatment, lending strong support to an important public health message. Isolating the elderly might reduce mortality, however this will lead to visual loss in those at risk of nAMD, predominantly elderly women. From a purely ocular standpoint, it is therefore imperative that those at risk are advised to seek care in a timely fashion, before irreversible vision loss occurs. Equally and more holistically, safe transport and socially distanced diagnostic and treatment environments must be provided and instructions on how to access these resources communicated effectively to patients, general practitioners, optometrists and patient support groups. A continuing reduction in patients presenting for treatment will add to the cumulative indirect healthcare burden that COVID-19 is already having on health, well-being and social care costs. Public health messaging via national and local agencies and patient support groups may be warranted to allow for timely treatment to prevent visual loss, and help for preparations should further waves of infection and lockdown occur.
Footnotes
Twitter: @anthonykhawaja, @paul3548, @adnan_tufail1
DST and AW contributed equally.
Collaborators: UK EMR Users Group: Ms T Akerele (Hinchingbrooke Healthcare NHS Trust), Mr R Antcliff (Royal United Hospital Bath NHS Trust), Ms C Bailey (University Hospitals Bristol NHS Foundation Trust), Mr C Brand (Sheffield Teaching Hospitals NHS Foundation Trust), Professor U Chakravarthy (Belfast Health and Social Care Trust), Ms A Davis (Moorfields Eye Centre at Croydon University Hospital), Mr N Dhingra (Mid Yorkshire Hospitals NHS Trust), Ms L Downey (Hull and East Yorkshire Hospitals NHS Foundation Trust), Ms S George (The Hillingdon Hospital NHS Foundation Trust), Mr F Ghanchi (Bradford Teaching Hospitals NHS Foundation Trust), Mr C Jones (Norfolk and Norwich University Hospitals NHS Foundation Trust), Mrs R Khan (Calderdale and Huddersfield NHS Foundation Trust), Mr V Kumar Wirral (University Teaching Hospital NHS Foundation Trust), Mrs P Lip (Sandwell and West Birmingham Hospitals NHS Trust), Mr A Lobo (Moorfields Eye Centre at Bedford Hospital), Mr S Mahmood (Central Manchester University Hospitals NHS Foundation Trust), Professor G Menon (Frimley Park Hospital NHS Foundation Trust), Mr R Mukherjee (Leeds Teaching Hospitals NHS Trust), Mr S Natha (Wrightington, Wigan and Leigh NHS Foundation Trust), Ms H Palmer (University Hospitals Birmingham NHS Foundation Trust), Mr A Patwardhan (Royal Cornwall Hospitals NHS Trust), Mr B Paul (Barking, Havering and Redbridge University Hospitals NHS Trust), Mr J Talks (The Newcastle On Tyne Hospitals NHS Foundation Trust), Dr E Wilkinson (Northern Devon Healthcare NHS Trust).
Contributors: PT, DST, AW, AO-B, CE and AT designed the study and/or raised funding. PT, AO-B, AT, CE, AL, EP and AK were involved in aggregation of data for the study. AT, AW, AO-B, PT and DST undertook data management and analysed the data. The writing committee (PT, CE, AO-B, AW, DT, AT, SP, HE, AK, AL, PLM, RS and RH) wrote the first draft of the report, which was critically appraised by all authors. UK EMR Users Group collected data and appraised the final manuscript. The final draft was approved by all authors. PT and AT are responsible for data integrity. AT accepts full responsibility for the work and conduct of the study, had access to the data, and controlled the decision to publish.
Funding: AT, CE and RS received a proportion of their funding from the Department of Health’s NIHR Biomedical Research Centre for Ophthalmology at Moorfields Eye Hospital and UCL Institute of Ophthalmology. AW is supported by the UCL Wellcome Trust PhD Programme for Clinicians (220558/Z/20/Z). PLM is supported by the German Research Foundation (grant # MU4279/2-1). Data collection was funded by a grant from Novartis Pharmaceutical.
Disclaimer: The views expressed in the publication are those of the authors and not necessarily those of the Department of Health.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Contributor Information
UK EMR Users Group:
T Akerele, R Antcliff, C Bailey, C Brand, U Chakravarthy, A Davis, N Dhingra, L Downey, S George, F Ghanchi, C Jones, R Khan, V Kumar, P Lip, A Lobo, S Mahmood, G Menon, R Mukherjee, S Natha, H Palmer, A Patwardhan, B Paul, J Talks, and E Wilkinson
Data availability statement
No data are available. The terms and governance of our data access do not permit us to share the data.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
Ethical approval was not required for the analysis of anonymised EMR data. Signed permission was returned from the lead clinician and Caldicott guardian (who oversees data protection) at each clinical site allowing anonymised data to be extracted. The study is registered on a list of studies using anonymised data at the lead clinician’s home institution.
References
- 1. West D. Some hospitals left “quiet” as covid-19 sparks huge fall in attendances. Health Service Journal 2020. [Google Scholar]
- 2. Lai AG, Pasea L, Banerjee A, et al. Estimated impact of the COVID-19 pandemic on cancer services and excess 1-year mortality in people with cancer and multimorbidity: near real-time data on cancer care, cancer deaths and a population-based cohort study. BMJ Open 2020;10:e043828. 10.1136/bmjopen-2020-043828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. NHS Digital . Hospital outpatient activity 2018-19.
- 4. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–62. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. European Centre for Disease Prevention and Control . Rapid Risk Assessment: Coronavirus disease 2019 (COVID-19) in the EU/EEA and the UK – eighth update [Internet] 2020.
- 6. Prime Minister’s Office DS . PM statement on coronavirus: 16 March 2020;2020. [Google Scholar]
- 7. Ophthalmologists RCo . Protecting patients, protecting staff 2020.
- 8. Ophthalmologists. RCo . Reopening and redeveloping ophthalmology services during Covid recovery – interim guidance 2020.
- 9. Flaxman SR, Bourne RRA, Resnikoff S, et al. Global causes of blindness and distance vision impairment 1990-2020: a systematic review and meta-analysis. Lancet Glob Health 2017;5:e1221–34. 10.1016/S2214-109X(17)30393-5 [DOI] [PubMed] [Google Scholar]
- 10. Tufail A, Margaron P, Guerin T, et al. Visual benefit versus visual gain: what is the effect of baseline covariants in the treatment arm relative to the control arm? A pooled analysis of anchor and marina. Br J Ophthalmol 2020;104:672–7. 10.1136/bjophthalmol-2018-313682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Writing Committee for the UK Age-Related Macular Degeneration EMR Users Group . The neovascular age-related macular degeneration database: multicenter study of 92 976 ranibizumab injections: report 1: visual acuity. Ophthalmology 2014;121:1092–101. 10.1016/j.ophtha.2013.11.031 [DOI] [PubMed] [Google Scholar]
- 12. UK DoHaSC . Certificate of vision impairment. explanatory notes for consultant ophthalmologists and hospital eye clinic staff in England 2017.
- 13. Chen FK, Agelis LE, Peh KK, et al. Factors contributing to discrepancy between visual acuity fractions derived from a Snellen chart and letter scores on the early treatment diabetic retinopathy study chart. Asia Pac J Ophthalmol 2014;3:277–85. 10.1097/APO.0000000000000007 [DOI] [PubMed] [Google Scholar]
- 14. Rodrigues IA, Sprinkhuizen SM, Barthelmes D, et al. Defining a minimum set of standardized patient-centered outcome measures for macular degeneration. Am J Ophthalmol 2016;168:1–12. 10.1016/j.ajo.2016.04.012 [DOI] [PubMed] [Google Scholar]
- 15. Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2006;355:1419–31. 10.1056/NEJMoa054481 [DOI] [PubMed] [Google Scholar]
- 16. Team RC . R: a language and environment for statistical computing 2020.
- 17. Owen CG, Jarrar Z, Wormald R, et al. The estimated prevalence and incidence of late stage age related macular degeneration in the UK. Br J Ophthalmol 2012;96:752–6. 10.1136/bjophthalmol-2011-301109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Taylor DJ, Hobby AE, Binns AM, et al. How does age-related macular degeneration affect real-world visual ability and quality of life? A systematic review. BMJ Open 2016;6:e011504. 10.1136/bmjopen-2016-011504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Armitage R, Nellums LB. COVID-19 and the consequences of isolating the elderly. Lancet Public Health 2020;5:e256. 10.1016/S2468-2667(20)30061-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wong TY, Wong T, Chakravarthy U, et al. The natural history and prognosis of neovascular age-related macular degeneration: a systematic review of the literature and meta-analysis. Ophthalmology 2008;115:116–26. 10.1016/j.ophtha.2007.03.008 [DOI] [PubMed] [Google Scholar]
- 21. Lanzetta P, Cruess AF, Cohen SY, et al. Predictors of visual outcomes in patients with neovascular age-related macular degeneration treated with anti-vascular endothelial growth factor therapy: post hoc analysis of the view studies. Acta Ophthalmol 2018;96:e911–8. 10.1111/aos.13751 [DOI] [PubMed] [Google Scholar]
- 22. Ophthalmologists RCo . Medical retinal management plans during COVID-19 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-057269supp001.pdf (478.7KB, pdf)
Data Availability Statement
No data are available. The terms and governance of our data access do not permit us to share the data.