Abstract
ASABF is a CSαβ-type antimicrobial peptide that contains four intramolecular disulfide bridges (Y. Kato and S. Komatsu, J. Biol. Chem. 271:30493–30498, 1996). In the present study, a recombinant ASABF was produced by using a yeast expression system, and its antimicrobial activity was characterized in detail. The recombinant ASABF was active against all gram-positive bacteria tested (7 of 7; minimum bactericidal concentration [MBC], 0.03 to 1 μg/ml) except Leuconostoc mesenteroides, some gram-negative bacteria (8 of 14; MBC, >0.5 μg/ml), and some yeasts (3 of 9; MBC >3 μg/ml). Slight hemolytic activity (4.2% at 100 μg/ml) against human erythrocytes was observed only under low-ionic-strength conditions. Less than 1 min of contact was enough to kill Staphylococcus aureus ATCC 6538P. The bactericidal activity against S. aureus was inhibited by salts.
CSαβ-type antimicrobial peptides contain a single α helix and a pair of antiparallel β sheets stabilized by three or four intramolecular disulfide bridges (9). Insect defensins that contain three intramolecular disulfide bridges (7, 12), drosomycin (isolated from the fruit fly [Drosophila melanogaster]) (16), and plant defensin (3), which contains four intramolecular disulfide bridges, have been experimentally demonstrated to be members of a CSαβ-type antimicrobial peptide by three-dimensional structural analyses. Some antimicrobial peptides that contain the consensus sequence of insect defensins (6) have been isolated from other arthropods and mollusks (4, 6, 10, 13), suggesting that they should also be CSαβ-type antimicrobial peptides. Although these peptides form similar three-dimensional structures, the CSαβ-type antimicrobial peptides exhibit diversified antimicrobial spectra. For instance, insect defensins are active against gram-positive bacteria but not against gram-negative bacteria or eukaryotic microbes (9), despite some exceptions (16). In contrast, drosomycin is active against fungi but not against bacteria (14). Plant defensins are categorized into four groups with different antimicrobial spectra, i.e., group I (active against gram-positive bacteria and fungi), group II (active against fungi but inactive against bacteria), group III (active against gram-positive and gram-negative bacteria but inactive against fungi), and group IV (active against gram-positive and gram-negative bacteria and fungi) (11). In addition, the antimicrobial activities of some CSαβ-type antimicrobial peptides are inhibited by salts (5, 17), but those of others are not (15). However, the structure-activity relationship that can explain such diversified antimicrobial characteristics has not been well elucidated. Characterization of novel CSαβ-type antimicrobial peptides has been providing novel antimicrobial substances with unique properties (15).
ASABF is a novel CSαβ-type antimicrobial peptide that contains four intramolecular disulfide bridges isolated from the nematode Ascaris suum (14). In this study, a recombinant ASABF was produced with a yeast expression system, and the antimicrobial activity was characterized in detail.
MATERIALS AND METHODS
Construction of ASABF expression vector.
The mature peptide region of ASABF was amplified by a high-fidelity PCR with the full-length cDNA clone of ASABF (14) as a template and the following set of primers: a sense primer (44-mer) that includes the region of ASABF from Ala19 to Cys25 and the XhoI site (underlined) (5′-CCTTAGGGCCCCTCGAGAAAAGAGCAGTCGACTTTTCATCATGC-3′) and an antisense primer (45-mer) that included the region from the terminal codon to Lys83 and the NotI site (5′-GCCGAGCTCTGCAGCGGCCGCCTATCCACGTGAACTTCGCCCTTT-3′). This product was ligated to the pPIC9 vector by using the XhoI-NotI sites. By using this construct, the recombinant ASABF was expected to be produced as a secretory fusion peptide flanking the α-factor secretory signal at the N terminus.
Expression of recombinant ASABF using a yeast expression system.
After linearization by digestion with SacI, the ASABF expression vector was transformed into Picha pastoris GS115. The P. pastoris transformant was inoculated into 40 ml of BMGY medium (1% yeast extract, 2% peptone, 100 mM potassium phosphate [pH 6.0], 1.34% yeast nitrogen broth, 4 × 10−5% biotin, 1% glycerol), and the culture was grown at 30°C until the culture reached an optical density at 600 nm of 6. The transformants were harvested by centrifugation and resuspended to an optical density at 600 nm of 1 in 400 ml of BMMY medium (1% yeast extract, 2% peptone, 100 mM potassium phosphate [pH 6.0], 1.34% yeast nitrogen broth, 4 × 10−5% biotin, 1% methanol) to induce recombinant ASABF expression. The culture was grown for 6 days at 30°C in a shaking incubator by adding 100% methanol to a final concentration of 1% every 24 h to maintain the induction. After the incubation, the expression culture was centrifuged, and the supernatant was mixed with Hyflo Super-cell and filtered through a nitrocellulose membrane filter.
Purification of recombinant ASABF. (i) Step 1. Positive-ion-exchange chromatography.
The supernatant was diluted fivefold to obtain a lower ionic strength and was applied to a SP-Sepharose FF column (Amersham Pharmacia Biotech) which had been equilibrated with 20 mM potassium phosphate (pH 6.0) at 4°C. Recombinant ASABF was eluted with 0.5 M NaCl. The A280 was monitored, and the ASABF-containing fractions were collected and dialyzed against deionized water.
(ii) Step 2. Positive-ion-exchange high-pressure liquid chromatography.
The dialyzed fractions were applied to a HiTrap SP column (Amersham Pharmacia Biotech) connected to a Pharmacia fast-protein liquid chromatography system. The recombinant ASABF was eluted with a linear concentration gradient (0 to 300 mM) of NaCl containing 50 mM potassium phosphate buffer (pH 6.0). The fractions containing ASABF were collected.
(iii) Step 3. Reversed-phase high-pressure liquid chromatography.
The final purification was carried out with an Asahipak C4P-90 2F column (Asahi Chemical Industry Co., Ltd.). A linear gradient elution was used (20 to 40% acetonitrile in water containing 1% trifluoroacetic acid). The purified recombinant ASABF was lyophilized and stored at 4°C.
Microorganisms. (i) Bacterial strains.
Escherichia coli JM109 was purchased from Takara. Staphylococcus aureus ATCC 6538P was a gift from Masanori Yamamoto. Bacillus subtilis IFO3134 was purchased from the Institute for Fermentation (IFO), Osaka, Japan.
Curtobacterium flaccumfacuens pv. ooritt MAFF301198, Kocuria varians MAFF118076, Leuconostoc mesenteroides subsp. mesenteroides MAFF117201, Pediococcus sp. strain MAFF516018, Staphylococcus saprophyticus MAFF118078, Azorhizobium caulinodans MAFF210459, Bdellovibrio bacteriovorus MAFF106101, Bradyrhizobium elkanii MAFF303126, Edowardsiella tarda MAFF130044, Klebsiella pneumoniae MAFF519002, Ochrobacterium anthropi MAFF520011, Rhizobium huakuii MAFF210250, and Salmonella agona MAFF910335 were obtained from the National Institute of Agrobiological Resources, Tsukuba, Japan. Clavibacter michiganensis pv. michiganensis, Agrobacterium tumefaciens, Erwinia carotobora subsp. carotovora, Pseudomonas marginalis, Pseudomonas syringae pv. mori, and Xanthomonas campestris pv. campestris were isolated by Akira Shirata, National Institute of Sericultural and Entomological Science, Tsukuba, Japan.
(ii) Yeast strains.
Candida krusei MAFF114085, Debaryomyces hansenii MAFF113836, Kloeckera apiculata MAFF114302, Kluyveromyces thermotolerans MAFF113848, Picha anomala MAFF113717, Saccharomyces cerevisiae MAFF113011, Sporobolomyces sp. strain MAFF425173, Torulaspora delbrueckii MAFF113811, and Zygosaccharomyces rouxii MAFF113405 were obtained from the National Institute of Agrobiological Resources, Tsukuba, Japan.
Microbicidal assay.
Each microbial strain in the logarithmic phase of growth was suspended in 10 μl of 10 mM Tris-HCl (pH 7.5) containing a series of purified recombinant ASABF for a threefold increase in concentration. The optical density of the microbial suspension was adjusted to 0.02 at 650 nm. After 2 h of incubation, the test suspension (5 μl) was diluted 1,000 times. The diluted sample (200 μl) was inoculated onto an optimum medium. The numbers of colonies were counted, and the minimum bactericidal concentration (MBC) was determined.
Hemolytic assay.
Human type A erythrocytes were used for the hemolytic assay. Hemolysis was estimated as the leakage of hemoglobin. The erythrocytes were washed in 10 mM Tris-HCl (pH 7.6) containing 154 mM NaCl or 308 mM sucrose (308 mosM) and were resuspended in the same buffer containing recombinant ASABF. After 0.5 h of incubation, the test suspension was centrifuged to remove the intact erythrocytes. The supernatant was diluted, and the A540 was measured.
RESULTS
Expression of recombinant ASABF.
To characterize the antimicrobial activity in detail, recombinant ASABF was expressed by using the yeast P. pastoris (see Materials and Methods). The recombinant ASABF was designed as a fusion peptide with an α-factor secretory signal at the N terminus with a downstream-flanking mature region of ASABF. The recombinant ASABF was collected as a secretory peptide from the culture supernatant. The purified peptide was analyzed by N-terminal sequencing and mass spectrometry (Fig. 1). The N-terminal sequence of the recombinant ASABF was AVDFSSCARM, which is completely identical to that of natural ASABF (14), suggesting that the α-factor secretory signal was cleaved as expected. The recombinant ASABF was estimated to be a mixture of two peptides whose molecular masses were 7,415.8 and 7,358.4 Da, respectively, on the basis of mass spectrometry. These peptides are thought to be the processed peptides in which a four-residue peptide (calculated mass, 7,412.4 Da) and a five-residue peptide (calculated mass, 7,355.5 Da) at the C terminus were eliminated. It is noteworthy that a four-residue peptide at the C terminus of natural ASABF was also removed by processing (14). Because the differently processed recombinant ASABF was only slightly separated, the mixture of peptides was directly subjected to characterization of its antimicrobial properties.
FIG. 1.
Amino acid sequence of recombinant ASABF. The recombinant ASABF was analyzed by N-terminal sequencing and mass spectrometry. The sequence obtained by N-terminal sequencing is underlined. Numbers indicate molecular masses (in Daltons). The experimentally determined molecular mass is followed by its theoretical value in parentheses. The filled triangle indicates the cleavage site of processing in native ASABF.
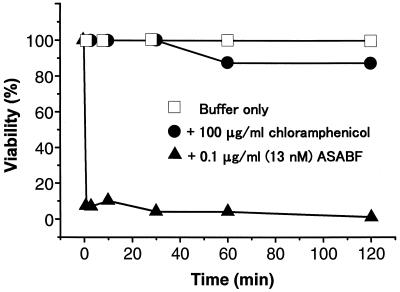
Time dependence of bactericidal activity.
S. aureus ATCC 6538P, which was the bacterium the most sensitive to natural ASABF (14), was treated with 0.1 μg of recombinant ASABF per ml (13 mM) in 10 mM Tris-HCl buffer (pH 7.6) for various times, and the viability was tested (Fig. 2). When the viability of S. aureus was tested in the presence of 100 μg chloramphenicol per ml or in buffer with no antibiotics, no significant decrease in viability was observed. In contrast, 93% of the bacteria were killed within 1 min of contact with ASABF, suggesting that ASABF is bactericidal.
FIG. 2.
Rate of killing of S. aureus. Bacteria were incubated for various times, and their viabilities were tested. Most of the bacteria (93%) were killed within 1 min of contact with ASABF.
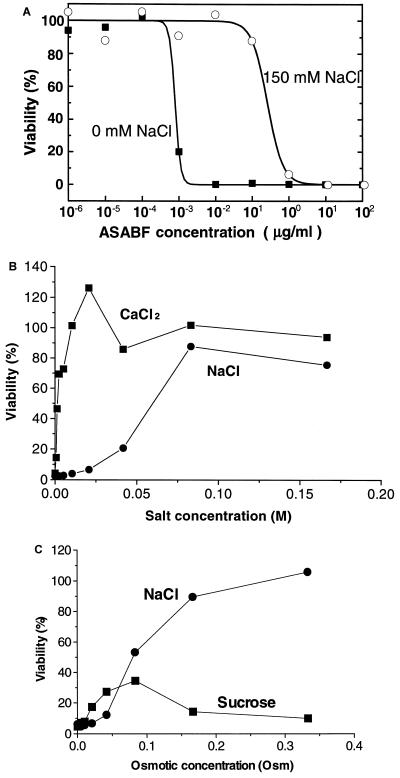
Inhibition by salts.
The antimicrobial activities of some known CSαβ-type antimicrobial peptides, such as insect defensins or plant defensins, are inhibited by salts (see the introduction). The bactericidal activity of recombinant ASABF (10 ng/ml [0.13 nM]) against S. aureus ATCC 6538P was tested in 10 mM Tris-HCl buffer (pH 7.6) with or without 150 mM NaCl (Fig. 3A). The bactericidal activity decreased 1/400 in 150 mM NaCl, suggesting that NaCl inhibits the activity of ASABF. Next, the bactericidal activity of recombinant ASABF in the presence of various concentrations of NaCl was explored (Fig. 3B). The inhibitory effect of NaCl was concentration dependent and was saturated at about 40 to 80 mM. The bactericidal activity was more strongly inhibited by CaCl2 (Fig. 3B). In addition, 0 to 330 mM sucrose slightly affected the bactericidal activity of recombinant ASABF, but the influence of sucrose was far different from the influence (inhibition) of NaCl (Fig. 3C), suggesting that the inhibition by salts should be attributed to an electrostatic interaction.
FIG. 3.
Inhibition of bactericidal activity of ASABF by salts. S. aureus ATCC 6538P was treated with 10 ng of ASABF per ml in 10 mM Tris-HCl (pH 7.6) for 2 h. (A) Dose-effect relationship in the presence or absence of 150 mM NaCl. The bactericidal activity decreased 1/400 in 150 mM NaCl. (B) Influence of NaCl and CaCl2. CaCl2 more strongly inhibited bactericidal activity than NaCl did. (C) Influence of osmotic pressure. Sucrose did not strongly affect the activity of ASABF. Osm, osmolar.
Antimicrobial spectrum.
The antimicrobial spectrum of recombinant ASABF was tested under an optimum condition, i.e., in 10 mM Tris-HCl buffer (Table 1). Due to the good reproducibility, we estimated the activity intensity as the MBC. All (seven of seven) gram-positive bacteria tested were sensitive. The MBC for gram-positive bacteria was estimated to be 0.03 to 1 μg/ml (4 to 130 nM) except only for that for L. mesenteroides (10 μg/ml [130 nM]). Although some (8 of 14) gram-negative bacteria were also sensitive, a higher concentration was required (i.e., MBC, >0.5 μg/ml [70 nM]). Interestingly, recombinant ASABF was also active against some (three of nine) yeasts (i.e., MBC, >3 μg/ml [400 nM]).
TABLE 1.
Antimicrobial spectrum and MBCs of recombinant ASABF
| Organism | MBC (μg/ml) |
|---|---|
| Gram-positive bacteria | |
| Bacillus subtilis IFO3134 | 0.03 |
| Clavibacter michiganensis pv. michiganensis | 0.1 |
| Curtobacterium flaccumfacuens pv. ooritt MAFF301198 | 0.01 |
| Kocuria varians MAFF118076 | 0.5 |
| Leuconostoc mesenteroides subsp. mesenteroides MAFF117201 | 10 |
| Staphylococcus aureus ATCC 6538P | 0.02 |
| Staphylococcus saprophyticus MAFF118078 | 1 |
| Gram-negative bacteria | |
| Agrobacterium tumefaciens | 10 |
| Azorhizobium caulinodans MAFF210459 | NDa |
| Bdellovibrio bacteriovorus MAFF106101 | 0.5 |
| Bradyrhizobium elkanii MAFF303126 | ND |
| Edowardsiella tarda MAFF130044 | ND |
| Klebsiella pneumoniae MAFF519002 | 70 |
| Erwinia carotobora subsp. carotovora | 10 |
| Escherichia coli JM109 | 200 |
| Ochrobacterum anthropi MAFF520011 | 300 |
| Pseudomonas marginalis | ND |
| Pseudomonas syringae pv. mori | ND |
| Rhizobium huakuii MAFF210250 | 10 |
| Salmonella agona MAFF910335 | ND |
| Xanthomonas campestris pv. campestris | 10 |
| Yeasts | |
| Candida krusei MAFF114085 | 10 |
| Debaryomyces hansenii MAFF113836 | ND |
| Kloeckera apiculata MAFF114302 | ND |
| Kluyveromyces thermotolerans MAFF113848 | 3 |
| Pichia anomala MAFF113717 | 30 |
| Saccharomyces cerevisiae MAFF113011 | ND |
| Sporobolomyces sp. strain MAFF425173 | ND |
| Torulaspora delbrueckii MAFF113811 | ND |
| Zygosaccharomyces rouxii MAFF113405 | ND |
ND, not detected.
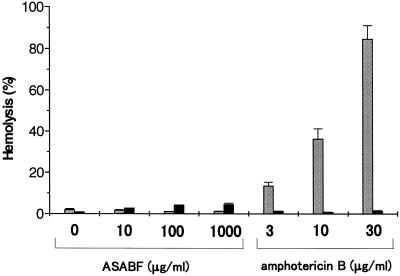
Hemolytic activity.
Recombinant ASABF is effective not only against bacteria but also against some yeasts, suggesting that the toxicity of ASABF is not limited to prokaryotes. A CSαβ-type antimicrobial peptide, an insect defensin, is reported to disrupt the permeability barrier of the cytoplasmic membranes of gram-positive bacteria (5). To test the toxic activity of ASABF against the cytoplasmic membranes of higher animals, the hemolytic activity against human red blood cells was explored (Fig. 4). No hemolytic activity was detected in 150 mM NaCl, although amphotericin B caused severe hemolysis (19). The substitution of 300 mM sucrose for 150 mM NaCl caused a slight hemolysis (4.2%) in the presence of 100 μg of recombinant ASABF per ml (13 μM) (note that 0.8% hemolysis was observed even for the ASABF-free control). No prominent increase in hemolysis was observed at a higher concentration (4.5% at 1,000 μg of ASABF per ml). These results suggest that ASABF is much less harmful to the cytoplasmic membranes of higher animals, and its limited toxicity is inhibited by salts.
FIG. 4.
Hemolytic activity of ASABF. The hyposmotic hemolysis in 10 mM Tris-HCl (pH 7.6) was defined as 100% hemolysis. The hemolysis was measured in 10 mM Tris-HCl (pH 7.6) containing isotoinc NaCl (stippled column) or sucrose (filled column). Amphotericin B was used as a positive control. Slight hemolysis (4.2% at 100 μg/ml) was observed only under low-ionic strength conditions.
DISCUSSION
In this study, ASABF, a CSαβ-type antimicrobial peptide isolated from the nematode A. suum, was produced as a recombinant peptide with a yeast expression system, and its antimicrobial activity was explored in detail.
A four-residue peptide at the C terminus of natural ASABF was removed by processing (14). The removed peptide contains a dibasic site thought to be a recognition site for the processing of enzymes. Although the recombinant ASABF was also processed in a similar way, we observed recombinant peptides from which not only the four-residue peptide but also the five-residue peptide had been removed. Many proteases which recognize dibasic sites have been reported, and their cleavage sites are diversified (18). The recombinant ASABF could be processed by multiple proteases which recognized the dibasic site and cleaved it at different positions.
In this study, we tested the bactericidal activity of ASABF against 31 species of various microbes. The results suggest that ASABF is effective against gram-positive and gram-negative bacteria and yeasts. To the best of our knowledge, only a group IV plant defensin, So-D2, and an insect defensin, Phormia defensin, have also been reported to be CSαβ-type antimicrobial peptides active against both prokaryotic and eukaryotic microbes. Further analysis of ASABF will provide a new key to revealing the structure-activity relationships of the CSαβ-type antimicrobial peptides. Since recombinant ASABF could be obtained with a yeast expression system, the use of mutational analysis should be possible (8).
The hemolytic activity of ASABF is limited and is observed only under lower-ionic-strength conditions, suggesting that ASABF is much less harmful to the cytoplasmic membranes of higher vertebrates. On the other hand, ASABF was active against some yeasts, suggesting that the effective site of ASABF is not specific in prokaryotes. It remains to be elucidated whether the selective toxicity of ASABF is attributed to resistance factors in higher animals or sensitivity factors that may be common in both prokaryotic and eukaryotic microbes.
ASABF killed S. aureus within 1 min after exposure. Similar results have been reported for some membrane-disrupting antimicrobial peptides such as insect defensins (5) and cecropins (1). It is possible to argue that ASABF could also kill microbes by disrupting the cytoplasmic membrane. The exact bactericidal mechanism of ASABF remains to be elucidated.
The bactericidal activity of ASABF was inhibited by salts. The salt inhibition was also reported in other known CSαβ-type antimicrobial peptides such as insect defensins, plant defensins, and drosomycin (see introduction). The antimicrobial activities of cationic lantibiotics (e.g., nisin) are also inhibited by salts (2). The inhibition by salts seems to be a hallmark of antimicrobial peptides as a class. The calcium ion was more inhibitory than the sodium ion. In addition, osmotic pressure did not strongly affect the bactericidal activity of ASABF. These results suggest that inhibition by salts is attributed to the inhibition of the electrostatic interaction between microbial target molecules presumably charged negative and ASABF charged positive at neutral pH. Although the hemolytic activity of ASABF was faint, the inhibition by salts was also observed, suggesting that the nature of the toxicity of ASABF may be a membrane-disrupting activity.
In conclusion, ASABF is a good candidate as a clinically applicable antimicrobial agent because of its wide antimicrobial spectrum and confirmed activity against some pathogens, especially S. aureus. The inhibition by salts both of the microbicidal activity and of the toxicity toward human erythrocytes should be considered for effective clinical applications.
ACKNOWLEDGMENTS
We are grateful to Akira Matsui, University of Tsukuba, for permission for collaboration.
This work was supported by a grant-in-aid (Bio Design Program) from the Ministry of Agriculture, Forestry and Fisheries of Japan (BDP-00-V-2-1).
The first three authors contributed equally to this work.
REFERENCES
- 1.Boman H G, Agerberth B, Boman A. Mechanisms of action on Escherichia coli of cecropin P1 and PR-39, two antibacterial peptides from pig intestine. Infect Immun. 1993;61:2978–2984. doi: 10.1128/iai.61.7.2978-2984.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bouttefroy A, Mansour M, Linder M, Milliere J B. Inhibitory combinations of nisin, sodium chloride, and pH on Listeria monocytogenes ATCC 15313 in broth by an experimental design approach. Int J Food Microbiol. 2000;54:109–115. doi: 10.1016/s0168-1605(99)00171-3. [DOI] [PubMed] [Google Scholar]
- 3.Broekaert W F, Terras F R, Cammue B P, Osborn R W. Plant defensins: novel antimicrobial peptides as components of the host defense system. Plant Physiol. 1995;108:1353–1358. doi: 10.1104/pp.108.4.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Charlet M, Chernysh S, Philippe H, Hetru C, Hoffmann J A, Bulet P. Innate immunity. Isolation of several cysteine-rich antimicrobial peptides from the blood of a mollusc, Mytilus edulis. J Biol Chem. 1996;271:21808–21813. doi: 10.1074/jbc.271.36.21808. [DOI] [PubMed] [Google Scholar]
- 5.Cociancich S, Ghazi A, Hetru C, Hoffmann J A, Letellier L. Insect defensin, an inducible antibacterial peptide, forms voltage-dependent channels in Micrococcus luteus. J Biol Chem. 1993;268:19239–19245. [PubMed] [Google Scholar]
- 6.Cociancich S, Goyffon M, Bontems F, Bulet P, Bouet F, Menez A, Hoffmann J. Purification and characterization of a scorpion defensin, a 4kDa antibacterial peptide presenting structural similarities with insect defensins and scorpion toxins. Biochem Biophys Res Commun. 1993;194:17–22. doi: 10.1006/bbrc.1993.1778. [DOI] [PubMed] [Google Scholar]
- 7.Cornet B, Bonmatin J M, Hetru C, Hoffmann J A, Ptak M, Vovelle F. Refined three-dimensional solution structure of insect defensin A. Structure. 1995;3:435–448. doi: 10.1016/s0969-2126(01)00177-0. [DOI] [PubMed] [Google Scholar]
- 8.De Samblanx G W, Goderis I J, Thevissen K, Raemaekers R, Fant F, Borremans F, Acland D P, Osborn R W, Patel S, Broekaert W F. Mutational analysis of a plant defensin from radish (Raphanus sativus L.) reveals two adjacent sites important for antifungal activity. J Biol Chem. 1997;272:1171–1179. doi: 10.1074/jbc.272.2.1171. [DOI] [PubMed] [Google Scholar]
- 9.Dimarcq J-L, Bulet P, Hetru C, Hoffmann J. Cysteine-rich antimicrobial peptides in invertebrates. Biopolymers. 1998;47:465–477. doi: 10.1002/(SICI)1097-0282(1998)47:6<465::AID-BIP5>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 10.Ehret-Sabatier L, Loew D, Goyffon M, Fehlbaum P, Hoffmann J A, van Dorsselaer A, Bulet P. Characterization of novel cysteine-rich antimicrobial peptides from scorpion blood. J Biol Chem. 1996;271:29537–29544. doi: 10.1074/jbc.271.47.29537. [DOI] [PubMed] [Google Scholar]
- 11.García-Olmedo F, Molina A, Alamillo J M, Rodríguez-Palenzuéla P. Plant defense peptides. Biopolymers. 1998;47:479–491. doi: 10.1002/(SICI)1097-0282(1998)47:6<479::AID-BIP6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 12.Hanzawa H, Shimada I, Kuzuhara T, Komano H, Kohda D, Inagaki F, Natori S, Arata Y. 1H nuclear magnetic resonance study of the solution conformation of an antibacterial protein, sapecin. FEBS Lett. 1990;269:413–420. doi: 10.1016/0014-5793(90)81206-4. [DOI] [PubMed] [Google Scholar]
- 13.Hubert F, Noel T, Roch P. A member of the arthropod defensin family from edible Mediterranean mussels. Eur J Biochem. 1996;240:302–306. doi: 10.1111/j.1432-1033.1996.0302h.x. [DOI] [PubMed] [Google Scholar]
- 14.Kato Y, Komatsu S. ASABF, a novel cysteine-rich antibacterial peptide isolated from the nematode Ascaris suum: purification, primary structure, and molecular cloning of cDNA. J Biol Chem. 1996;271:30493–30498. doi: 10.1074/jbc.271.48.30493. [DOI] [PubMed] [Google Scholar]
- 15.Lamberty M, Ades S, Uttenweiler-Joseph S, Brookhart G, Bushey D, Hoffmann J A, Bulet P. Insect immunity. Isolation from the lepidopteran Heliothis virescens of a novel insect defensin with potent antifungal activity. J Biol Chem. 1999;274:9320–9326. doi: 10.1074/jbc.274.14.9320. [DOI] [PubMed] [Google Scholar]
- 16.Landon C, Sodano P, Hetru C, Hoffmann J, Ptak M. Solution structure of drosomycin, the first inducible antifungal protein from insects. Protein Sci. 1997;6:1878–1884. doi: 10.1002/pro.5560060908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osborn R W, De Samblanx G W, Thevissen K, Goderis I, Torrekens S, van Leuven F, Attenborough S, Rees S B, Broekaert W F. Isolation and characterisation of plant defensins from seeds of Asteraceae, Fabaceae, Hippocastanaceae and Saxifragaceae. FEBS Lett. 1995;368:257–262. doi: 10.1016/0014-5793(95)00666-w. [DOI] [PubMed] [Google Scholar]
- 18.Siezen R J, Leunissen J A. Subtilases: the superfamily of subtilisin-like serine proteases. Protein Sci. 1997;6:501–523. doi: 10.1002/pro.5560060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Etten E W, van Vianen W, Roovers P, Frederik P. Mild heating of amphotericin B-desoxycholate: effects on ultrastructure, in vitro activity and toxicity, and therapeutic efficacy in severe candidiasis in leukopenic mice. Antimicrob Agents Chemother. 2000;44:1598–1603. doi: 10.1128/aac.44.6.1598-1603.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]