Abstract
Glioblastoma is a type of brain cancer with aggressive and invasive nature. Such features result from increased proliferation and migration and also poor apoptosis of glioma cells leading to resistance to current treatments such as chemotherapy and radiotherapy. In recent studies, micro RNAs have been introduced as a novel target for treating glioblastoma via regulation of apoptotic signaling pathway, remarkably PI3K/AKT, which affect cellular functions and blockage or progression of the tumor. In this review, we focus on PI3K/AKT signaling pathway and other related apoptotic processes contributing to glioblastoma and investigate the role of micro RNAs interfering in apoptosis, invasion and proliferation of glioma through such apoptotic processes pathways. Databases NCBI, PubMed, and Web of Science were searched for published English articles using keywords such as 'miRNA OR microRNA', 'Glioblastoma', 'apoptotic pathways', 'PI3K and AKT', 'Caspase signaling Pathway' and 'Notch pathway'. Most articles were published from 7 May 2015 to 16 June 2020. This study focused on PI3K/AKT signaling pathway affecting glioma cells in separated subparts. Also, other related apoptotic pathways as the Caspase cycle and Notch have been also investigated. Nearly 40 miRNAs were found as tumor suppressors or onco-miRNA, and their targets, which regulated subcomponents participating in proliferation, invasion, and apoptosis of the tumoral cells. Our review reveals that miRNAs affect key molecules in signaling apoptotic pathways, partly PI3K/AKT, making them potential therapeutic targets to overcome the tumor. However, their utility as a novel treatment for glioblastoma requires further examination and investigation.
Key Words: Glioblastoma, Micro RNA, PI3K/AKT pathway
Introduction
The most invasive and aggressive subtype of brain cancer in humans is glioblastoma, globally showing the highest incidence in Western Europe, Northern Ame-rica, and Australia; its prevalence in the United States has been more than 9 per 100,000 people (1-3). According to WHO classification, glioblastoma appears as grade I to IV; nevertheless, patients with different levels of glioblastoma have an average survival from 3 to only one year (4, 5). It is difficult to completely remove the tumor in surgery, and also there is a resis-tance to other treatments such as chemo- and radio-therapy (6, 7). This refers to some characteristics of glioblastomas: fast growth, high proliferation, the potential of self-renew, and absence of apoptosis (8), which may result from anti-apoptotic proteins overexpression (9). As recent statistics, the probability of patient survival with glioblastoma seems low. Thus, it is needed to find novel targets for quick diagnosis and efficient treatments (10).
Based on many studies on various cancers, including glioblastoma, changes in the expression level of some miRNAs could significantly alter tumor progression, and they contribute to biological processes of such cells as proliferation, differentiation, migration, and apoptosis (11, 12). These molecules are single-stranded RNAs with 18 to 24 nucleotides long being conserved highly (13, 14). They can bind to the 3′ untranslated region (UTR) of target genes and inhibit mRNA translation to regulate the protein expression related to a specific gene (15). As findings, increased or decreased levels of numerous miRNAs can induce apoptosis in tumor cells, suppressing their growth and inhibiting cancer development (12, 16).
Deregulation of the apoptotic pathway is the most crucial strategy of glioblastoma cells to fight current treatments (17). Hence, research into effective markers inducing apoptosis against cancer cells can be a novel treatment method. miRNAs participate in various pathways by targeting different genes and inducing oncogenic and anti-apoptotic effects on the function of glioblastoma (18). In this review, we summarize the main apoptotic pathways that affect glioblastoma and investigate the role of miRNAs in the mechanisms, including apoptosis.
Material and Methods
Search Strategy
We conducted an electronic search for published articles in PubMed, NCBI, Scopus, and Google Scholar with no restrictions on publication date. The search of the literature was done independently by two first authors who applied no limitation in the language of the literature. The initial search was conducted using the following terms: ("micro RNAs" OR "miRNAs" OR "MiRNA") AND ("glioblastoma"), ("PI3K" AND "AKT"), in the title and/or abstract.
Inclusion and Exclusion Criteria
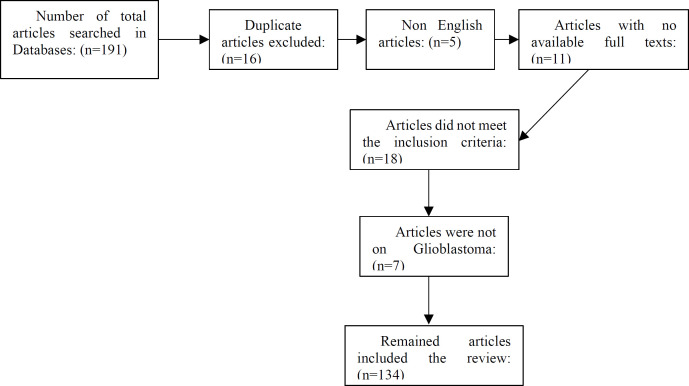
Our search initially yielded 191 records in used databases that were managed in the EndNote X7.2.1 software. A total of 16 duplicate references were removed. After screening the remained articles, 5 of them were not in English, and 11 articles were not found in full text, so those were excluded. The full texts of the remaining 159 articles were carefully read. Then, 18 studies were excluded because there were no data on pathways of miRNA regulation. Additionally, another 7 case studies were not related to glioblastoma and those were also excluded. Figure 1 displays the detailed search results.
Fig. 1.
The flow diagram of excluding articles
1. AKT Signaling Pathway
The AKT pathway is known to play an effective role in the biological functions of cells (19). In particular, it has been involved in tumorigenic active-ties in various cancers like glioblastoma (20, 21). AKT signaling pathway is a key link correlating with growth, proliferation, and invasion of tumor cells (22). It has been suggested that inhibition of AKT and its related signaling pathways can increase apoptosis in glioblastoma (23). Numerous miRNAs affect these pathways via direct and/or indirect targets resulting in suppressing glioblastoma or promoting oncogenic activities of the disease (24).
Correlation of AKT Pathway with Oncogenic EGFR and c-MET
According to results, overexpression of the Epidermal growth factor receptor (EGFR) gene is one of the most common changes that result in glioblas-toma (25). The EGFR has a crucial role in the up-regulation of PI3K/AKT/mTOR pathway, known as an effective downstream of EGFR, correlating with cell proliferation and tumor-forming (26). On the other hand, EGFR induces cell survival and invasion through other downstream pathways RAS/MEK/ERK (Figure 2) (27).
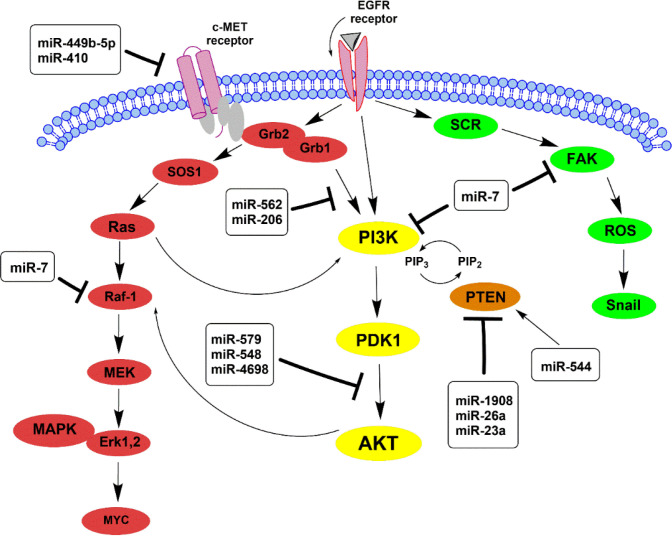
Fig 2.
A schematic representation of PI3K/AKT, RAS/MAPK, and FAK/ROS signaling pathways participates in glioblastoma. They can activate by EGFR (Epidermal Growth Factor Receptor) and c-MET (Mesenchymal epithelial transition factor). The main pathway is PI3K/AKT, which activates other downstream glioma cells and affects apoptosis. The activation of PI3K results in phosphorylation of the PIP2 to generate PIP3. PIP3 activates PDK1 and, in turn, phosphorylates AKT. PTEN dephosphorylates PIP3 to PIP2 and acts as an antagonist of the PIP3 pathway. miRNAs indicating in frame affect their particular targets in these pathways, whether inhibition (showed by ┴) or inducing (showed by →).
Some miRNAs can impact tumor activity by targeting such receptors and their downstream pathways; for example, miR-7 can suppress the expre-ssion of EGFR and inhibit the AKT pathway leading to a reduction in viability of gliomas (28). It has also been reported that miR-7 targets two EGFR downstream, PI3K and Raf-1, simultaneously suppressing both PI3K/ATK and Raf/MEK/ERK pathways (29). In addition, focal adhesion kinase (FAK) is identified as another target of miR-7 that negatively relates to this miRNA; miR-7 can reduce the invasion of glioblas-toma via directly targeting FAK (30).
Furthermore, previous studies have reported that blockage of the c-MET expression can induce apoptosis in glioblastoma (31). The overexpression of the c-MET receptor results in tumor growth, and the c-MET/AKT signaling pathway can impact apoptosis and prolifer-ation in glioma cells (32, 33). As reported, miRNAs are closely correlated with c-MET, including miR-449b-5p and miR-410 that c-MET has been suggested as their direct target in glioblastoma (34, 35). Since the co-activation of c-MET and AKT can affect the apoptosis pathway in tumor cells, suppressing the c-MET/AKT pathway inhibits cell proliferation and promotes apoptosis by activating caspase3 downstream compo-nents (36, 37). Both tumor suppressors miR-206 and miR-562 regulate proliferation and induce apoptosis by inhibiting the c-MET/AKT signaling pathway (37, 38).
1.2. PI3K/PDK1/AKT Pathway and PTEN Regulation
Activation of AKT is mainly correlated with PI3K and PTEN. PI3K is a kinase that phosphorylates PIP2 to produce PI3P, which in turn activates the AKT through direct binding to PDK1 (Figure 2) (39). Instead, PTEN, a tumor suppressor, applies reverse phosphorylation (PI3P is converted to PIP2), thus preventing AKT activation (40). As extensive evidence, there is frequent loss of PTEN in glioblastoma leading to a reduction in apoptosis (41, 42). Some miRNAs via PTEN regulation can affect growth, proliferation, and apoptosis in glioma cells; for example, oncomiR-26a and miR-1908 enhance the AKT pathway activity by down-regulation of PTEN level; besides, miR-23a as an oncogenic effector, targets PTEN to activate the PI3K/AKT pathway (43-45). A high level of miR-554 not only inhibits proliferation and invasion ability in gliomas but also increases cell apoptosis. miR-544 directly targets PARK7 protein to suppress its expression (46). PARK7 plays an important role in tumor development by binding to PTEN, and p53 leading to cell apoptosis inhibition (47, 48).
1.3. Oncogenic PI3K/AKT/mTOR Signaling Pathway
As the results of previous studies, PI3K/AKT pathway is altered in 80% of glioma cells (49, 50). PI3K, a kinase controlling survival, proliferation, and apoptosis of the cells, can activate the AKT that in turn regulates its downstream targets as mTOR complex (39, 51-53). Mammalian target of rapamycin (mTOR) is a protein kinase including two complexes (mTOR1 and mTOR2) with different functions; either up- or down-regulation of some effectors in gliomas (54, 55). It has been suggested that mTOR2 is required for full activation of AKT and is known as the upstream kinase for it (56). The mTOR2 is formed via binding mTOR to RICTOR which is considered as an important oncogene in glioblastoma and its depletion leads to inactive mTOR2 complex (57, 58). Upregulation of AKT and overexpression of RICTOR in glioblastoma result in over-activation of mTOR2 that promotes proliferation and migration of tumor cells (55, 59).
The increase in expression of some miRNAs can modulate PI3K/AKT pathway in gliomas; for instance, miR-579, miR-548, and miR-4698 inhibit proliferation and invasion of glioblastoma through affecting this pathway (60, 61). miR-6071 can bind to ULBP2 as the target gene in glioma cells and can inhibit the PI3K/AKT/mTOR pathway resulting in repress cell proliferation; thus, it promotes apoptosis (62). In addition, miR-489 and miR-326 via targeting SPIN1 and PKM2, respectively, can modulate the PI3K/AKT-/mTOR pathway to increase apoptosis and decrease invasion (63, 64). Interestingly, miR-652 plays an influential role in reducing tumor size in a patient with glioblastoma; miR-652 deactivates the AKT/mTOR pathway through its direct target FOXK1 (65).
As a study, miR-153 is purposed as a tumor suppresser that affects RICTOR as the primary target. It has been suggested that the upregulation of miR-153 is negatively correlated with the downregulation of RICTOR and reduction of AKT activity (66). The results showed that miR-153 significantly inhibits cell growth and activates apoptosis in gliomas (66). Also, overex-pression of miR-128 has been reported to enhance apoptosis significantly in gliomas. Interestingly, miR-128 directly inhibits various targets, including mTOR, RICTOR, IGF1, and PIK3R1, which are members of mTOR signaling. Moreover, miR-128 targets P70S6K1, leading to repress the level of this protein and its downstream effectors, which are HIF-1 and VGEF (67). P70S6K1 protein is a tumorigenic downstream target of mTOR, which is activated through PI3K/PDK1/AKT pathway induced by insulin-like growth factor (IGF)-1 (Figure 3) (68).
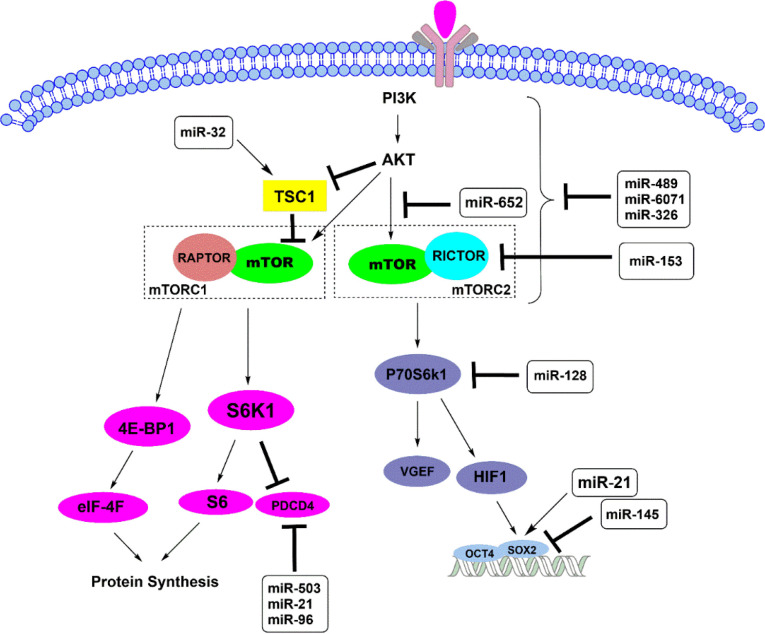
Fig. 3.
Schematic highlighting the mTOR complexes pathways and their downstream affect apoptosis in glioblastoma. TSC1 (Tuberous Sclerosis protein 1) and TSC2 can form a heterodimeric complex that inhibits the mTOR signaling pathway. RAPTOR and RICTOR are the main components of mTORC1 and mTORC2, respectively. The mTORC1 regulates protein synthesis by phosphorylating S6K1 and 4E-BP1, while mTOR2 affects gene expression, including SOX2, leading to cellular survival and tumor development
Following mTOR signaling and P70S6K1 elevation, HIF-1 is activated, affecting Sox2; one of the two gene targets resulting in proliferation, migration, and tumorigenesis (Figure 3). It has been reported that upon overexpression of miR-145 as a tumor suppresser, Oct4 and Sox2 are targeted directly, leading to a decrease in the growth and migration of human glioma cells (68). In contrast, such a process is promoted via Sox2 activation by onco-miR-21 in gliomas (69).
Additionally, miR-21 exerts its oncogenic effects targeting other downstream of mTORC1 as S6K and 4E-BP1(70, 71). Activation of AKT leads to inhibit TSC1-TSC2 complex; thus, it provides conditions for binding of two components, mTOR and RAPTOR, that form the mTORC1 complex (72). Two downstream of mTORC1 interfere in cell growth and protein syn-thesis; S6K/S6 through blockage PDCD4 (program-med cell death) and 4E-BP1 via formation and activation of eIF-4F complex, can affect glioblastoma progression (73). The onco-miRNAs, including miR-21, miR-503, and miR-96 inhibit PDCD4 as the direct target that improves glioma viability and survival (70, 74, 75). On the other hand, miR-32 directly targets TSC1 and suppresses mTOR pathway leading to the reduction of angiogenesis levels (76).
1.4. AKT Inhibits the Pro-apoptosis Downstream
In glioblastoma, the AKT pathway is identified to promote tumor development due to the inhibition of apoptotic effectors and activation of anti-apoptotic components (77). Upon AKT activation, the down-stream, including GSK3, FOXO3, and BAD, are inhibited, resulting in blockage of the apoptotic pathways (Figure 4) (77, 78). Some miRNAs can affect the AKT signaling via regulation FOXO3 and its downstream effectors as Bim, p27, and cyclin D1; for instance, overexpression of miR-184 promotes the capacity of glioma proliferation by downregulating of FOXO3 (79). Also, as reported, the level of p27, an apoptotic effector, can be decreased by both miR-221 and miR-222 (80). These miRNAs suppress apoptosis by reducing P27kip-1 expression, which is known as a CDK (cyclin-dependent kinase) inhibitor (81). Additionally, according to the reports, oncomiR-10b prevents gliomas' death through targeting Homeobox D10 (HOXD10), pro-apoptotic gene, and Bim protein (82, 83).
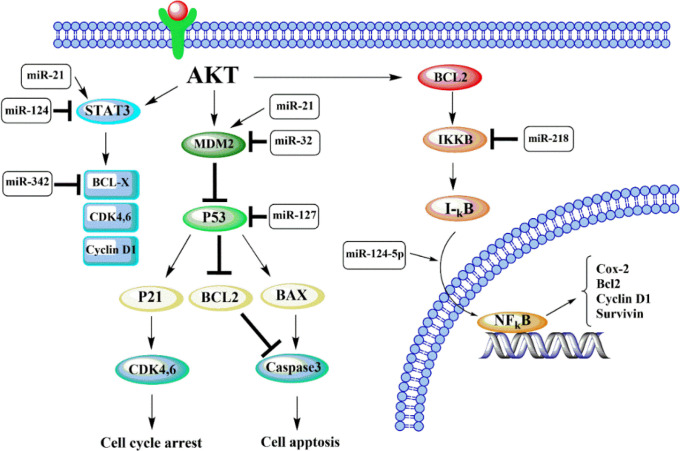
Fig. 4.
Schematic representation of apoptosis inhibition by AKT signaling pathway and its downstream effectors. Akt activates MDM2, which inhibits p53 via binding to this protein. P53 is a tumor suppressor that induces apoptosis by activating some downstream as p21 and BAX. Akt activation can trigger the IKKB, which decreases IκB as an inhibitor of NF-κB. IκB reduction allows NF-κB to move to the nucleus and activate the transcription of cell survival and apoptosis inhibitors, including the surviving and Bcl2 family
Furthermore, AKT activates anti-apoptotic components that play key roles in cellular functions (Figure 4) (77). As a result, STAT3 and β-catenin act as an oncogene in glioblastoma that can be modulated by various miRNAs (84, 85); miR-124 is suggested to suppress the tumor by inhibition STAT3 signaling pathway (86). In contrast, miR-21 can induce proliferation and invasion of gliomas through the STAT3/β-catenin pathway by targeting RECK (87).
1.5. AKT/NFkB/Bcl2 Signaling Pathway
One of the mechanisms of NFκB (Nuclear factor-kappa B) activation is the AKT phosphorylation of IκB mediated by IKKβ, a key regulator of NFκB (Figure 4) (88). Activation of such a pathway has an essential role in the capacity of tumor invasion, which is due to the up-regulation of matrix metalloproteinases (MMPs) (89). In contrast, miR-124-5p can target NRP-1 to promote tumorigenicity through PI3K/Akt/ NFkB pathway (90). Interestingly, some miRNAs regulate cellular functions and apoptosis through targeting the MMPs; miR-218 directly targets IKKβ that in turn reduces NFκB activity and MMP-9 expression in gliomas (91). Also, overexpression of miR-16 and miR-211 can suppress the MMP-9 and induce mitoch-ondrial apoptotic pathway by mediating caspase 3 and 9 (92, 93). However, miR-23a and miR-10b directly inhibit HOXD10 expression and induce gliomas invasion through modulating MMP-14 (44, 94).
The activated pathway of NFkB in glioblastoma can exert cell proliferation via inducing anti-apoptotic factors, including the Bcl2 family, Cyclin D1, and surviving (Figure 4) (95). Bcl2 family regulates apoptosis, including both pro- and anti-apoptotic components such as BCL-x and MCL1 (96). Both of these anti-apoptotic proteins can bind to BAK and BAX through the BH3 domain resulting in restraint of the apoptosis (97). In glioma cells, miR-342 targets directly BCL-x and MCL1, which significantly dec-reases their expression and induces apoptosis (98). In addition, miR-181 level in gliomas is inversely related to level of tumor, and Bcl2 is suggested as a target of this tumor suppressor (78).
1.6. AKT/MDM2 Signaling and p53 Regulation
According to findings, in glioblastoma, phosphor-ylated AKT can enhance the expression of MDM2; a pro-oncogene downstream of AKT pathway (99). MDM2 is considered a key regulator of p53 protein levels via binding to p53 and promoting its degradation (100). The p53 is identified as a significant tumor suppressor that induces growth arrest and apoptosis via overexpression of its target gene as p21 and blockage of anti-apoptotic effectors as Bcl2 family (101). Increasing the level of p53 leads to upregulating downstream p21 that results in G0/G1 cell cycle arrest (102). It can also activate BAX/caspase 3 pathways leading to apoptosis in tumor cells (Figure 4) (103). Some miRNAs affect the components of such pathways to up- or down-regulate glioma functions; for instance, the expression level of p53 protein is reported to increase after the silencing of miR-127 (104) (Table 1). In addition, miR-32 can suppress tumor growth by direct targeting MDM2 and TSC1, two main p53 inhibitors (76). miR-21 targets various components of this pathway; it can increase the level of MDM2 by activating the AKT pathway leading to downregulation of p53 and apoptosis inhibition (105).
Table 1.
Down- and up- regulated miRNAs and their functional pathways in glioblastoma
| Tumor suppresser miRNAs | |||
|---|---|---|---|
| miR-125b | TNFAIP3,NKIRAS2 | TRAIL/NFkB Caspase signaling cascade | (1 20 ) |
| Micro RNA | Target | Functional pathway | Reference |
| miR-34a | CDK6, CCND1, NOTCH | Notch signaling pathway | ( 130 , 13 1) |
| miR-145 | BNIP3 | Notch signaling pathway | ( 133 ) |
| miRNA146-a | Notch1 | Notch signaling pathway | ( 129 ) |
| miR-7 | PI3K, Raf-1, FAK | Raf/MEK/ERK pathway | ( 29 , 30 ) |
| miR-449b-5p miR-410 miR-206 miR-562 |
c-MET | c-MET/AKT pathway | ( 34 , 35 , 37 , 38 ) |
| miR-554 | PARK7 | PI3K/AKT pathway | ( 46 ) |
| miR-579 miR-548 miR-4698 |
- | PI3K/AKT pathway | ( 60 , 61 ) |
| miR-6071 | ULBP2 | PI3K/AKT/mTOR | ( 62 ) |
| miR-489 | SPIN1 | PI3K/AKT/mTOR | ( 63 ) |
| miR-326 | PKM2 | PI3K/AKT/mTOR | ( 64 ) |
| miR-652 | FOXK1 | AKT/mTOR pathway | ( 65 ) |
| miR-153 | RICTOR | AKT/mTOR pathway | ( 66 ) |
| miR-128 | P70S6K1 | AKT/mTOR pathway | ( 67 ) |
| miR-145 | Oct4 and Sox2 | AKT/mTOR pathway | ( 68 ) |
| miR-32 | TSC1 | AKT/mTOR pathway | ( 76 ) |
| miR-124 | - | AKT/ STAT3 pathway | ( 86 ) |
| miR-16 miR-211 |
MMP-9 | PI3K/Akt/ NFkB pathway | ( 92 , 93 ) |
| miR-342 | BCL-x , MCL1 | AKT/NFkB/Bcl2 | ( 98 ) |
| miR-181 | Bcl2 | AKT/NFkB/Bcl2 | ( 78 ) |
| miR-32 | MDM2, TSC1 | AKT/ MDM2/p53 | ( 76 ) |
| miR-218 | IKKβ | PI3K/Akt/ NFkB pathway TRAIL/NFkB/ Caspase signaling cascade |
( 91 ) |
| Onco-miRNA | |||
| Micro RNA | Target | Functional pathway | Reference |
| miR-26a miR-1908 |
- | PI3K/AKT pathway | ( 43 , 44 ) |
| miR-23a | PTEN | PI3K/AKT pathway |
(
45
)
( 44 ) |
| AKT/NFkB pathway | |||
| miR-21 | Sox2, S6K, 4E-BP1 PDCD4 RECK |
AKT/mTOR pathway STAT3/β-catenin pathway AKT/ MDM2 pathway |
(
69
,
70
)
( 87 ) ( 105 ) |
| Tap63 LRRFIP1 |
Caspase signaling cascade TRAIL/ NFkB Caspase signaling cascade |
( 116 , 119 ) | |
| miR-503 miR-96 |
PDCD4 | AKT/mTOR | ( 74 , 75 ) |
| miR-30 | Caspase3 | Caspase signaling cascade | ( 116 ) |
| miR-184 | - | AKT/FOXO3 | ( 79 ) |
| miR-221 miR-222 |
P27kip-1 | AKT/FOXO3 | ( 80 ) |
| miR-10b | HomeoboxD10, Bim | AKT/FOXO3 | ( 82 , 83 ) |
| AKT/NFkB | ( 119 ) | ||
| miR-124-5p | NRP-1 | PI3K/Akt/ NFkB pathway | ( 90 ) |
| miR-127 | AKT/ NFkB/p53 | ( 104 ) | |
The table presents tumor suppressor and onco-miRNAs with observed effects upon their regulation in glioblastoma and the functional pathway impressing their direct targets.
Signaling Pathway Related to Caspase Family
Caspase proteins, a family of proteolytic enzymes, play different roles in the cell; particularly, they are vital components of the apoptotic pathway (106). These proteins are classified into two categories; primary types, including caspase8 and caspase9, that activate the secondary caspases by cleaving, and conse-quently, activate caspases that cooperate in the apop-tosis process (107). According to the previous studies, the expression of caspase proteins has notably decre-ased in gliomas compared with normal cells associated with the reduction of apoptosis in glioblastoma (108).
Activation of some caspase proteins is effectively impressed with the receptors that are considered to initiate different cellular pathways; two of them, also known as the death receptors, are TNF-related apop-tosis inducing ligand receptor (TRAILR) and tumor necrosis factor receptor (TNFR) (109). The TRAIL receptor is involved in the activation of the caspase cascade in both extrinsic and intrinsic apoptosis pathways (110). It straightly activates the primary caspase8 via the extrinsic apoptotic path, which in subsequent, the downstream components, including caspase3 and caspase7, carry out the apoptosis process (111, 112). While intrinsic or mitochondrial pathway is tightly related to Bcl2 family including both anti- and pro-apoptotic proteins (113). Upon this pathway, a pro-apoptotic member of the Bcl2 family, protein Bid, is cleaved by activated caspase8 and then is translocated to mitochondria (112, 114). Consequently, some proteins such as cytochrome complex are released into the cytosol and combined with Apaf-1 resulting in Apaf-1/caspase 9 axis, which induces programmed cell death through activating caspases 3 and 7 (115). In glioma cells, some miRNAs can damage the signaling cascade of TRAIL and inhibit apoptosis; for instance, miR-21 can change TRAIL sensitivity by targeting the TAp63 (116). Likewise miR-30 targets the caspase3 and inhibits TRAIL-dependent apoptosis (116).
Furthermore, the TNF receptor triggers NFκB signaling cascade, which involves biological processes like cell survival and inflammation (117). Previous research has shown that the NFκB pathway intensifies because of the increased expression of the death rece-ptors in glioblastoma (118). According to evidence, the inappropriate activity of NFκB leads to inhibiting caspase8 and results in the resistance to apoptosis and induction of immortality in glioma cells (118).
Expression patterns of several miRNAs can alter the gliomas' functions by affecting the NFκB pathway; for example, the level of mir-218 expression is decreased in gliomas compared to normal tissues (91). Aberrant expression of this miRNA changes the transcriptional activity of NFκB by affecting the 3'UTR length of IKKβ (89). Another miRNA that is upreg-ulated in glioma cells is miR-21, which enhances the level of NFκB by targeting the LRRFIP1 gene (119). In addition, over-expression of mir-125b promotes the activity of NFκB by targeting both TNFAIP3 and NKIRAS2, leading to alter molecular mechanisms of main elements in the pathway (120).
Notch Signaling Pathway
Many core functions in the cell are induced by notch signaling, such as differentiation leading to cell development, proliferation, and maintenance of stem cells, and it seems mutation of notch proteins have been related to developmental diseases; for example, schizo-phrenia may associate with mutation of notch4 (121, 122). Notch signaling prevents neural stem cells (NSC) from differentiation and provides the maintenance of NSCs in immature glia (123). Of note such function, it is considered that notch signaling may play a similar role in maintaining glioma stem cells, and it may interfere with tumorigenesis (121, 124).
In glioblastoma, notch1 protein amplifies the transcription leading overexpress of epidermal growth factor receptor (EGFR) gene via TP53 signaling pathway that its mechanism is not clear enough (125). Following the block of Notch1 in gliomas, cell proli-feration decreases, and apoptosis increases. As the latter event, phosphorylation of AKT and STAT3 inhi-bits, while the pro-apoptotic form of caspase3 increases (126). Such pieces of evidence point out that the Notch1 pathway interferes in the growth and survival of glioblastoma cells (127).
Several miRNAs have an effective role in up- or down-regulation of proliferation and apoptosis through notch signaling pathways in glioblastoma (128). The Notch1 3′-UTR sequence is involved in the luciferase receptor gene, which is significantly targeted by miR-146a, reducing its activity (129). It results in blockage of Notch1 expression and flows signaling pathway that reduces the proliferation of gliomas and induces apoptosis (8).
It has been reported that miR-34a regulates cell proliferation through various targets such as CCND1, CDK6, and the notch protein. In glioblastoma, TP53 is targeted by miR-34a, leading to its downregulation compared to normal brain cells. According to in vivo findings, increased expression of miR-34a can inten-sely inhibit glioma growth via targeting the c-MET and Notch signaling pathway (130). Thereby, miR-34a reduces glioma's proliferation and invasion, making it a tumor suppresser agent (131). Also, miR-145 can suppress the gliomas and induce apoptosis via a notch pathway but with a different target known as BNIP3 (132). In glioma cells, BNIP3 is in the nucleus that inhibits the apoptosis as an oncogene through both paths: blockage of TRAL and up-regulation of the notch signaling pathway (133). miR-145 can block BNIP3 expression by binding to it, which results in the reduction of some proteins, including notch1, Hes1, and P2, thus, the apoptosis increases in glioma cells (132).
Conclusion
Apoptosis has been proved as a key function in all cells that occurs through various extrinsic and intrinsic cell pathways. Therefore, any dysfunction of up- or down-stream components can totally change the condition to benefit cell survival. Dysregulation in apoptotic signaling pathways is one of the most effective events confirmed as an indicator of cancer; as reported in the studies on glioblastoma, the signaling pathways alter to shut off apoptosis. Besides, there are numerous micro RNAs known as effective agents on the biological activity of tumor cells.
In this review, we investigated the PI3K/AKT sig-naling pathways and related miRNAs affecting such pathways in glioblastoma. According to the collected evidence, the changes in the expression level of various miRNAs can notably affect the proliferation, invasion, and apoptosis in glioma cells. Many onco-miRNAs can upregulate gliomas' functions and help the cell survival, while others suppress the tumor growth and promote apoptosis. Overview of such effectors and their influence on the PI3K/AKT pathway sub-com-ponent can help us find novel procedures for gliob-lastoma treatment. However, it required: 1) to improve our understanding of their regulatory functions and their corresponding targets in other apoptotic path-ways, 2) to find some ways to control therapeutic responses and better management of glioblastoma .
Conflict of Interest
The authors declared no conflict of interest.
Funding
None.
Acknowledgments
None.
References
- 1.Kleihues P, Sobin LH. World Health Organization classification of tumors. Cancer. 2000;88(12):2887. . doi: 10.1002/1097-0142(20000615)88:12<2887::aid-cncr32>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 2.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tan AC, Ashley DM, Lopez GY, Malinzak M, Friedman HS, Khasraw M. Management of glioblastoma: State of the art and future directions. CA Cancer J Clin. 2020;70(4):299–312. doi: 10.3322/caac.21613. [DOI] [PubMed] [Google Scholar]
- 4.Ohgaki H, Kleihues P. Epidemiology and etiology of gliomas. Acta Neuropathol. 2005;109(1):93–108. doi: 10.1007/s00401-005-0991-y. [DOI] [PubMed] [Google Scholar]
- 5.Burnet NG, Lynch AG, Jefferies SJ, Price SJ, Jones PH, Antoun NM, et al. High grade glioma: imaging combined with pathological grade defines management and predicts prognosis. Radiother Oncol. 2007;85(3):371–8. doi: 10.1016/j.radonc.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 6.Wesseling P, Capper D. WHO 2016 Classification of gliomas. Neuropathol Appl Neurobiol. 2018;44(2):139–50. doi: 10.1111/nan.12432. [DOI] [PubMed] [Google Scholar]
- 7.Lawler S, Chiocca EA. Emerging functions of microRNAs in glioblastoma. J Neurooncol. 2009;92(3):297–306. doi: 10.1007/s11060-009-9843-2. [DOI] [PubMed] [Google Scholar]
- 8.Ceccarelli M, Barthel FP, Malta TM, Sabedot TS, Salama SR, Murray BA, et al. Molecular Profiling Reveals Biologically Discrete Subsets and Pathways of Progression in Diffuse Glioma. Cell. 2016;164(3):550–63. doi: 10.1016/j.cell.2015.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lathia JD, Mack SC, Mulkearns-Hubert EE, Valentim CL, Rich JN. Cancer stem cells in glioblastoma. Genes Dev. 2015;29(12):1203–17. doi: 10.1101/gad.261982.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie H, Shi S, Chen Q, Chen Z. LncRNA TRG-AS1 promotes glioblastoma cell proliferation by competitively binding with miR-877-5p to regulate SUZ12 expression. Pathol Res Pract. 2019;215(8):152476. doi: 10.1016/j.prp.2019.152476. [DOI] [PubMed] [Google Scholar]
- 11.Novakova J, Slaby O, Vyzula R, Michalek J. MicroRNA involvement in glioblastoma pathogenesis. Biochem Biophys Res Commun. 2009;386(1):1–5. doi: 10.1016/j.bbrc.2009.06.034. [DOI] [PubMed] [Google Scholar]
- 12.Huang S-w, Zhong L, Shi J. MicroRNAs as biomarkers for human glioblastoma: progress and potential. Acta Pharmacol Sin. 2018;39(9):1405–13. doi: 10.1038/aps.2017.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–97. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 14.German MA, Pillay M, Jeong D-H, Hetawal A, Luo S, Janardhanan P, et al. Global identification of microRNA-target RNA pairs by parallel analysis of RNA ends. Nature Biotechnol. 2008;26(8):941–6. doi: 10.1038/nbt1417. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J, Lv J, Zhang F, Che H, Liao Q, Huang W, et al. MicroRNA-211 expression is down-regulated and associated with poor prognosis in human glioma. J Neuro-oncol. 2017;133(3):553–9. doi: 10.1007/s11060-017-2464-2. [DOI] [PubMed] [Google Scholar]
- 16.Wang N, Zhang Y, Liang H. MicroRNA-598 Inhibits Cell Proliferation and Invasion of Glioblastoma by Directly Targeting Metastasis Associated in Colon Cancer-1 (MACC1) Oncol Res. 2018;26(8):1275–83. doi: 10.3727/096504018X15185735627746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.García MA, Carrasco E, Ramírez A, Jiménez G, López-Ruiz E, Perán M, et al. Apoptosis as a therapeutic target in Cancer and Cancer stem cells: novel strategies and futures perspectives. Apoptosis and medicine: IntechOpen. 2012 [Google Scholar]
- 18.Zhang CZ, Zhang JX, Zhang AL, Shi ZD, Han L, Jia ZF, et al. MiR-221 and miR-222 target PUMA to induce cell survival in glioblastoma. Mol Cancer. 2010;9(1):229 . doi: 10.1186/1476-4598-9-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fu X, Tian J, Zhang L, Chen Y, Hao Q. Involvement of microRNA-93, a new regulator of PTEN/Akt signaling pathway, in regulation of chemotherapeutic drug cisplatin chemosensitivity in ovarian cancer cells. FEBS Lett. 2012;586(9):1279–86. doi: 10.1016/j.febslet.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 20.Yoshizawa A, Fukuoka J, Shimizu S, Shilo K, Franks TJ, Hewitt SM, et al. Overexpression of phospho-eIF4E is associated with survival through AKT pathway in non-small cell lung cancer. Clin Cancer Res. 2010;16(1):240–8. doi: 10.1158/1078-0432.CCR-09-0986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Slattery ML, Mullany LE, Sakoda LC, Wolff RK, Stevens JR, Samowitz WS, et al. The PI3K/AKT signaling pathway: Associations of miRNAs with dysregulated gene expression in colorectal cancer. Mol Carcinog. 2018;57(2):243–61. doi: 10.1002/mc.22752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gu JJ, Fan KC, Zhang JH, Chen HJ, Wang SS. Suppression of microRNA-130b inhibits glioma cell proliferation and invasion, and induces apoptosis by PTEN/AKT signaling. Int J Mol Med. 2018;41(1):284–92. doi: 10.3892/ijmm.2017.3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu X, Zhang Y, Qu D, Jiang T, Li S. Osthole induces G2/M arrest and apoptosis in lung cancer A549 cells by modulating PI3K/Akt pathway. J Exp Clin Cancer Res. 2011;30(1):33 . doi: 10.1186/1756-9966-30-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Møller HG, Rasmussen AP, Andersen HH, Johnsen KB, Henriksen M, Duroux M. A systematic review of microRNA in glioblastoma multiforme: micro-modulators in the mesenchymal mode of migration and invasion. Molecular Neurobiol. 2013;47(1):131–44. doi: 10.1007/s12035-012-8349-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schlegel J, Merdes A, Stumm G, Albert FK, Forsting M, Hynes N, et al. Amplification of the epidermal-growth-factor-receptor gene correlates with different growth behaviour in human glioblastoma. Int J Cancer. 1994;56(1):72–7. doi: 10.1002/ijc.2910560114. [DOI] [PubMed] [Google Scholar]
- 26.Dai Z, Wang L, Wang X, Zhao B, Zhao W, Bhardwaj SS, et al. Oxymatrine induces cell cycle arrest and apoptosis and suppresses the invasion of human glioblastoma cells through the EGFR/PI3K/Akt/mTOR signaling pathway and STAT3. Oncol Rep. 2018;40(2):867–76. doi: 10.3892/or.2018.6512. [DOI] [PubMed] [Google Scholar]
- 27.Padfield E, Ellis HP, Kurian KM. Current Therapeutic Advances Targeting EGFR and EGFRvIII in Glioblastoma. Front Oncol. 2015;5:5. doi: 10.3389/fonc.2015.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gallia GL, Tyler BM, Hann CL, Siu IM, Giranda VL, Vescovi AL, et al. Inhibition of Akt inhibits growth of glioblastoma and glioblastoma stem-like cells. Mol Cancer Ther. 2009;8(2):386–93. doi: 10.1158/1535-7163.MCT-08-0680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Z, Jiang Z, Huang J, Huang S, Li Y, Yu S, et al. miR-7 inhibits glioblastoma growth by simultaneously interfering with the PI3K/ATK and Raf/MEK/ERK pathways. Int J Oncol. 2014;44(5):1571–80. doi: 10.3892/ijo.2014.2322. [DOI] [PubMed] [Google Scholar]
- 30.Wu DG, Wang YY, Fan LG, Luo H, Han B, Sun LH, et al. MicroRNA-7 regulates glioblastoma cell invasion via targeting focal adhesion kinase expression. Chin Med J (Engl). 2011;124(17):2616–21. [PubMed] [Google Scholar]
- 31.Chu SH, Feng DF, Zhang H, Chen ET, Duan ZX, Li XY, et al. c-Met-targeted RNA interference inhibits growth and metastasis of glioma U251 cells in vitro. J Neurooncol. 2009;93(2):183–9. doi: 10.1007/s11060-008-9772-5. [DOI] [PubMed] [Google Scholar]
- 32.Giglio S, Vecchione A. c-Met and miRs in Cancer. Biomed. 2015;3(1):32–44. doi: 10.3390/biomedicines3010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yao Y, Dou C, Lu Z, Zheng X, Liu Q. MACC1 suppresses cell apoptosis in hepatocellular carcinoma by targeting the HGF/c-MET/AKT pathway. Cell Physiol Biochem. 2015;35(3):983–96. doi: 10.1159/000369754. [DOI] [PubMed] [Google Scholar]
- 34.Zhen L, Yun-Hui L, Hong-Yu D, Jun M, Yi-Long Y. Long noncoding RNA NEAT1 promotes glioma pathogenesis by regulating miR-449b-5p/c-Met axis. Tumour Biol. 2016;37(1):673–83. doi: 10.1007/s13277-015-3843-y. [DOI] [PubMed] [Google Scholar]
- 35.Chen L, Zhang J, Feng Y, Li R, Sun X, Du W, et al. MiR-410 regulates MET to influence the proliferation and invasion of glioma. Int J Biochem Cell Biol. 2012;44(11):1711–7. doi: 10.1016/j.biocel.2012.06.027. [DOI] [PubMed] [Google Scholar]
- 36.Salvi A, Sabelli C, Moncini S, Venturin M, Arici B, Riva P, et al. MicroRNA-23b mediates urokinase and c-met downmodulation and a decreased migration of human hepatocellular carcinoma cells. FEBS J. 2009;276(11):2966–82. doi: 10.1111/j.1742-4658.2009.07014.x. [DOI] [PubMed] [Google Scholar]
- 37.Nie X, Su Z, Yan R, Yan A, Qiu S, Zhou Y. MicroRNA-562 negatively regulated c-MET/AKT pathway in the growth of glioblastoma cells. Onco Targets Ther. 2019;12:41–9. doi: 10.2147/OTT.S186701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dai C, Xie Y, Zhuang X, Yuan Z. MiR-206 inhibits epithelial ovarian cancer cells growth and invasion via blocking c-Met/AKT/mTOR signaling pathway. Biomed Pharmacother. 2018;104:763–70. doi: 10.1016/j.biopha.2018.05.077. [DOI] [PubMed] [Google Scholar]
- 39.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296(5573):1655–7. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 40.Endersby R, Baker SJ. PTEN signaling in brain: neuropathology and tumorigenesis. Oncogene. 2008;27(41):5416–30. doi: 10.1038/onc.2008.239. [DOI] [PubMed] [Google Scholar]
- 41.Koul D. PTEN signaling pathways in glioblastoma. Cancer Biol Ther. 2008;7(9):1321–5. doi: 10.4161/cbt.7.9.6954. [DOI] [PubMed] [Google Scholar]
- 42.Lee J, Kim B, Park M, Lee Y, Kim Y, Lee B, et al. PTEN status switches cell fate between premature senescence and apoptosis in glioma exposed to ionizing radiation. Cell Death Differ. 2011;18(4):666–77. doi: 10.1038/cdd.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huse JT, Brennan C, Hambardzumyan D, Wee B, Pena J, Rouhanifard SH, et al. The PTEN-regulating microRNA miR-26a is amplified in high-grade glioma and facilitates gliomagenesis in vivo. Genes Dev. 2009;23(11):1327–37. doi: 10.1101/gad.1777409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hu X, Chen D, Cui Y, Li Z, Huang J. Targeting microRNA-23a to inhibit glioma cell invasion via HOXD10. Sci Rep. 2013;3(1):3423. doi: 10.1038/srep03423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xia X, Li Y, Wang W, Tang F, Tan J, Sun L, et al. MicroRNA-1908 functions as a glioblastoma oncogene by suppressing PTEN tumor suppressor pathway. Mol Cancer. 2015;14(1):1–14. doi: 10.1186/s12943-015-0423-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jin S, Dai Y, Li C, Fang X, Han H, Wang D. MicroRNA-544 inhibits glioma proliferation, invasion and migration but induces cell apoptosis by targeting PARK7. Am J Transl Res. 2016;8(4):1826–37. [PMC free article] [PubMed] [Google Scholar]
- 47.Shinbo Y, Taira T, Niki T, Iguchi-Ariga SM, Ariga H. DJ-1 restores p53 transcription activity inhibited by Topors/p53BP3. Int J Oncol. 2005;26(3):641–8. [PubMed] [Google Scholar]
- 48.Kim RH, Peters M, Jang Y, Shi W, Pintilie M, Fletcher GC, et al. DJ-1, a novel regulator of the tumor suppressor PTEN. Cancer Cell. 2005;7(3):263–73. doi: 10.1016/j.ccr.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 49.Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17(1):98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mao H, Lebrun DG, Yang J, Zhu VF, Li M. Deregulated signaling pathways in glioblastoma multiforme: molecular mechanisms and therapeutic targets. Cancer Invest. 2012;30(1):48–56. doi: 10.3109/07357907.2011.630050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ghobrial IM, Witzig TE, Adjei AA. Targeting apoptosis pathways in cancer therapy. CA Cancer J Clin. 2005;55(3):178–94. doi: 10.3322/canjclin.55.3.178. [DOI] [PubMed] [Google Scholar]
- 52.Franke TF, Hornik CP, Segev L, Shostak GA, Sugimoto C. PI3K/Akt and apoptosis: size matters. Oncogene. 2003;22(56):8983–98. doi: 10.1038/sj.onc.1207115. [DOI] [PubMed] [Google Scholar]
- 53.Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13(22):2905–27. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 54.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149(2):274–93. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fan Q-W, Weiss WA. Inhibition of PI3K-Akt-mTOR signaling in glioblastoma by mTORC1/2 inhibitors. mTOR: Springer. 2012: 349–59.. doi: 10.1007/978-1-61779-430-8_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang LW, Berry-Kravis E, Hagerman RJ. Fragile X: leading the way for targeted treatments in autism. Neurotherapeutics. 2010;7(3):264–74. doi: 10.1016/j.nurt.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sun S-Y. mTOR kinase inhibitors as potential cancer therapeutic drugs. Cancer Lett. 2013;340(1):1–8. doi: 10.1016/j.canlet.2013.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jacinto E, Facchinetti V, Liu D, Soto N, Wei S, Jung SY, et al. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell. 2006;127(1):125–37. doi: 10.1016/j.cell.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 59.Lisi L, Laudati E, Navarra P, Dello Russo C. The mTOR kinase inhibitors polarize glioma-activated microglia to express a M1 phenotype. J Neuroinflammation. 2014;11(1):125. doi: 10.1186/1742-2094-11-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kalhori MR, Irani S, Soleimani M, Arefian E, Kouhkan F. The effect of miR-579 on the PI3K/AKT pathway in human glioblastoma PTEN mutant cell lines. J Cell Biochem. 2019;120(10):16760–74. doi: 10.1002/jcb.28935. [DOI] [PubMed] [Google Scholar]
- 61.Ehsan A, Fereshteh FA, Kaveh K, Masoud S. miR-548x and miR-4698 controlled cell proliferation by affecting the PI3K/AKT signaling pathway in Glioblastoma cell lines. Sci Rep. 2020;10:1. doi: 10.1038/s41598-020-57588-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou Y, An H, Wu G. MicroRNA-6071 Suppresses Glioblastoma Progression Through the Inhibition of PI3K/AKT/mTOR Pathway by Binding to ULBP2. OncoTargets Ther. 2020;13:9429. doi: 10.2147/OTT.S265791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li Y, Ma X, Wang Y, Li G. miR-489 inhibits proliferation, cell cycle progression and induces apoptosis of glioma cells via targeting SPIN1-mediated PI3K/AKT pathway. Biomed Pharmacother. 2017;93:435–43. doi: 10.1016/j.biopha.2017.06.058. [DOI] [PubMed] [Google Scholar]
- 64.Kefas B, Comeau L, Erdle N, Montgomery E, Amos S, Purow B. Pyruvate kinase M2 is a target of the tumor-suppressive microRNA-326 and regulates the survival of glioma cells. Neuro-oncology. 2010;12(11):1102–12. doi: 10.1093/neuonc/noq080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang H, Song Z, Wu X, Wu Y, Liu C. MicroRNA-652 suppresses malignant phenotypes in glioblastoma multiforme via FOXK1-mediated AKT/mTOR signaling pathway. Onco Targets Ther. 2019;12:5563–75. doi: 10.2147/OTT.S204715. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 66.Cui Y, Zhao J, Yi L, Jiang Y. microRNA-153 Targets mTORC2 Component Rictor to Inhibit Glioma Cells. PloS one. 2016;11(6):e0156915. doi: 10.1371/journal.pone.0156915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen PH, Cheng CH, Shih CM, Ho KH, Lin CW, Lee CC, et al. The Inhibition of microRNA-128 on IGF-1-Activating mTOR Signaling Involves in Temozolomide-Induced Glioma Cell Apoptotic Death. PloS one. 2016;11(11):e0167096. doi: 10.1371/journal.pone.0167096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li X, Wu C, Chen N, Gu H, Yen A, Cao L, et al. PI3K/Akt/mTOR signaling pathway and targeted therapy for glioblastoma. Oncotarget. 2016;7(22):33440–50. doi: 10.18632/oncotarget.7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Luo G, Luo W, Sun X, Lin J, Wang M, Zhang Y, et al. MicroRNA21 promotes migration and invasion of glioma cells via activation of Sox2 and βcatenin signaling. Mol Med Rep. 2017;15(1):187–93. doi: 10.3892/mmr.2016.5971. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 70.Young MR, Santhanam AN, Yoshikawa N, Colburn NH. Have tumor suppressor PDCD4 and its counteragent oncogenic miR-21 gone rogue? Mol Interv. 2010;10(2):76. doi: 10.1124/mi.10.2.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gaur AB, Holbeck SL, Colburn NH, Israel MA. Downregulation of Pdcd4 by mir-21 facilitates glioblastoma proliferation in vivo. Neuro Oncol. 2011;13(6):580–90. doi: 10.1093/neuonc/nor033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rajesh Y, Pal I, Banik P, Chakraborty S, Borkar SA, Dey G, et al. Insights into molecular therapy of glioma: current challenges and next generation blueprint. Acta Pharmacol Sin. 2017;38(5):591–613. doi: 10.1038/aps.2016.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nasr Z, Pelletier J. Tumor progression and metastasis: role of translational deregulation. Anticancer Res. 2012;32(8):3077–84. [PubMed] [Google Scholar]
- 74.Guo P, Yu Y, Li H, Zhang D, Gong A, Li S, et al. TGF-β1-induced miR-503 controls cell growth and apoptosis by targeting PDCD4 in glioblastoma cells. Sci Rep. 2017;7(1):1–10. doi: 10.1038/s41598-017-11885-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ma QQ, Huang JT, Xiong YG, Yang XY, Han R, Zhu WW. MicroRNA-96 Regulates Apoptosis by Targeting PDCD4 in Human Glioma Cells. Technol Cancer Res Treat. 2017;16(1):92–8. doi: 10.1177/1533034616629260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Suh SS, Yoo JY, Nuovo GJ, Jeon YJ, Kim S, Lee TJ, et al. MicroRNAs/TP53 feedback circuitry in glioblastoma multiforme. Proc Natl Acad Sci U S A. 2012;109(14):5316–21. doi: 10.1073/pnas.1202465109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dobson M, Ramakrishnan G, Ma S, Kaplun L, Balan V, Fridman R, et al. Bimodal regulation of FoxO3 by AKT and 14-3-3. Biochim Biophys Acta. 2011;1813(8):1453–64. doi: 10.1016/j.bbamcr.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu N, Tu Y. Systematic review of microRNAs and its therapeutic potential in glioma. Cancer Transll Med. 2015;1(2):50 . [Google Scholar]
- 79.Tivnan A, Foley NH, Tracey L, Davidoff AM, Stallings RL. MicroRNA-184-mediated inhibition of tumour growth in an orthotopic murine model of neuroblastoma. Anticancer Res. 2010;30(11):4391–5. [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang C, Wang G, Kang C, Du Y, Pu P. [Up-regulation of p27(kip1) by miR-221/222 antisense oligonucleotides enhances the radiosensitivity of U251 glioblastoma] Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2009;26(6):634–8. doi: 10.3760/cma.j.issn.1003-9406.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 81.Gillies JK, Lorimer IA. Regulation of p27Kip1 by miRNA 221/222 in glioblastoma. Cell Cycle. 2007;6(16):2005–9. doi: 10.4161/cc.6.16.4526. [DOI] [PubMed] [Google Scholar]
- 82.Guan H, Song L, Cai J, Huang Y, Wu J, Yuan J, et al. Sphingosine kinase 1 regulates the Akt/FOXO3a/Bim pathway and contributes to apoptosis resistance in glioma cells. PloS one. 2011;6(5):e19946. doi: 10.1371/journal.pone.0019946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gabriely G, Teplyuk NM, Krichevsky AM. Context effect: microRNA-10b in cancer cell proliferation, spread and death. Autophagy. 2011;7(11):1384–6. doi: 10.4161/auto.7.11.17371. [DOI] [PubMed] [Google Scholar]
- 84.Woodward WA, Chen MS, Behbod F, Alfaro MP, Buchholz TA, Rosen JM. WNT/β-catenin mediates radiation resistance of mouse mammary progenitor cells. Proc Natl Acad Sci. 2007;104(2):618–23. doi: 10.1073/pnas.0606599104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cao Y, Lathia JD, Eyler CE, Wu Q, Li Z, Wang H, et al. Erythropoietin receptor signaling through STAT3 is required for glioma stem cell maintenance. Genes Cancer. 2010;1(1):50–61. doi: 10.1177/1947601909356352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wei J, Wang F, Kong L-Y, Xu S, Doucette T, Ferguson SD, et al. MiR-124 inhibits STAT3 signaling to enhance T cell-mediated immune clearance of glioma. Cancer Res. 2013;73(13):3913–26. doi: 10.1158/0008-5472.CAN-12-4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Han L, Yue X, Zhou X, Lan FM, You G, Zhang W, et al. MicroRNA‐21 expression is regulated by β‐catenin/STAT3 pathway and promotes glioma cell invasion by direct targeting RECK. CNS Neurosci Ther. 2012;18(7):573–83. doi: 10.1111/j.1755-5949.2012.00344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Johnson SM, Grosshans H, Shingara J, Byrom M, Jarvis R, Cheng A, et al. RAS is regulated by the let-7 microRNA family. Cell. 2005;120(5):635–47. doi: 10.1016/j.cell.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 89.Ghosh S, Karin M. Missing pieces in the NF-κB puzzle. Cell. 2002;109(2):S81–S96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 90.Zhang G, Chen L, Khan AA, Li B, Gu B, Lin F, et al. miRNA-124-3p/neuropilin-1(NRP-1) axis plays an important role in mediating glioblastoma growth and angiogenesis. Int J Cancer. 2018;143(3):635–44. doi: 10.1002/ijc.31329. [DOI] [PubMed] [Google Scholar]
- 91.Song L, Huang Q, Chen K, Liu L, Lin C, Dai T, et al. miR-218 inhibits the invasive ability of glioma cells by direct downregulation of IKK-beta. Biochem Biophys Res Commun. 2010;402(1):135–40. doi: 10.1016/j.bbrc.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 92.Asuthkar S, Velpula KK, Chetty C, Gorantla B, Rao JS. Epigenetic regulation of miRNA-211 by MMP-9 governs glioma cell apoptosis, chemosensitivity and radiosensitivity. Oncotarget. 2012;3(11):1439–54. doi: 10.18632/oncotarget.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yang TQ, Lu XJ, Wu TF, Ding DD, Zhao ZH, Chen GL, et al. Micro RNA‐16 inhibits glioma cell growth and invasion through suppression of BCL 2 and the nuclear factor‐κB1/MMP 9 signaling pathway. Cancer Sci. 2014;105(3):265–71. doi: 10.1111/cas.12351. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 94.Sun L, Yan W, Wang Y, Sun G, Luo H, Zhang J, et al. MicroRNA-10b induces glioma cell invasion by modulating MMP-14 and uPAR expression via HOXD10. Brain Res. 2011;1389:9–18. doi: 10.1016/j.brainres.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 95.Xu X, Yang H, Wang X, Tu Y. The significance of nuclear factor-kappa B signaling pathway in glioma: A review. Cancer Transll Med. 2017;3(5) [Google Scholar]
- 96.Wong RS. Apoptosis in cancer: from pathogenesis to treatment. J Exp Clin Cancer Res. 2011;30(1):87 . doi: 10.1186/1756-9966-30-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the BCL2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol. 2014;15(1):49–63. doi: 10.1038/nrm3722. [DOI] [PubMed] [Google Scholar]
- 98.Ghaemi S, Arefian E, Rezazadeh Valojerdi R, Soleimani M, Moradimotlagh A, Jamshidi Adegani F. Inhibiting the expression of anti-apoptotic genes BCL2L1 and MCL1, and apoptosis induction in glioblastoma cells by microRNA-342. Biomed Pharmacother. 2020;121:109641. doi: 10.1016/j.biopha.2019.109641. [DOI] [PubMed] [Google Scholar]
- 99.Ogawara Y, Kishishita S, Obata T, Isazawa Y, Suzuki T, Tanaka K, et al. Akt enhances Mdm2-mediated ubiquitination and degradation of p53. J Biol Chem. 2002;277(24):21843–50. doi: 10.1074/jbc.M109745200. [DOI] [PubMed] [Google Scholar]
- 100.Zhang J, Jiang H, Shao J, Mao R, Liu J, Ma Y, et al. SOX4 inhibits GBM cell growth and induces G0/G1 cell cycle arrest through Akt-p53 axis. BMC Neurol. 2014;14(1):207 . doi: 10.1186/s12883-014-0207-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vousden KH, Lu X. Live or let die: the cell's response to p53. Nat Rev Cancer. 2002;2(8):594–604. doi: 10.1038/nrc864. [DOI] [PubMed] [Google Scholar]
- 102.Deng C, Zhang P, Harper JW, Elledge SJ, Leder P. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell. 1995;82(4):675–84. doi: 10.1016/0092-8674(95)90039-x. [DOI] [PubMed] [Google Scholar]
- 103.Yuan L, Zhang Y, Xia J, Liu B, Zhang Q, Liu J, et al. Resveratrol induces cell cycle arrest via a p53-independent pathway in A549 cells. Mol Med Rep. 2015;11(4):2459–64. doi: 10.3892/mmr.2014.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Feng R, Dong L. Knockdown of microRNA-127 reverses adriamycin resistance via cell cycle arrest and apoptosis sensitization in adriamycin-resistant human glioma cells. Int J Clin Exp Pathol. 2015;8(6):6107–16. [PMC free article] [PubMed] [Google Scholar]
- 105.Papagiannakopoulos T, Shapiro A, Kosik KS. MicroRNA-21 targets a network of key tumor-suppressive pathways in glioblastoma cells. Cancer Res. 2008;68(19):8164–72. doi: 10.1158/0008-5472.CAN-08-1305. [DOI] [PubMed] [Google Scholar]
- 106.Li J, Yuan J. Caspases in apoptosis and beyond. Oncogene. 2008;27(48):6194–206. doi: 10.1038/onc.2008.297. [DOI] [PubMed] [Google Scholar]
- 107.Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli KJ, et al. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17(6):1675–87. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gdynia G, Grund K, Eckert A, Bock BC, Funke B, Macher-Goeppinger S, et al. Basal caspase activity promotes migration and invasiveness in glioblastoma cells. Mol Cancer Res. 2007;5(12):1232–40. doi: 10.1158/1541-7786.MCR-07-0343. [DOI] [PubMed] [Google Scholar]
- 109.Lavrik I, Golks A, Krammer PH. Death receptor signaling. J Cell Sci. 2005;118(Pt 2):265–7. doi: 10.1242/jcs.01610. [DOI] [PubMed] [Google Scholar]
- 110.Garofalo M, Condorelli G, Croce C, Condorelli G. MicroRNAs as regulators of death receptors signaling. Cell Death Differ. 2010;17(2):200–8. doi: 10.1038/cdd.2009.105. [DOI] [PubMed] [Google Scholar]
- 111.Crowder RN, El-Deiry WS. Caspase-8 regulation of TRAIL-mediated cell death. Exp Oncol. 2012;34(3):160–4. [PubMed] [Google Scholar]
- 112.Fulda S. Cell death-based treatment of glioblastoma. Cell Death Dis. 2018;9(2):121 . doi: 10.1038/s41419-017-0021-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ola MS, Nawaz M, Ahsan H. Role of Bcl2 family proteins and caspases in the regulation of apoptosis. Mol Cell Biochem. 2011;351(1-2):41–58. doi: 10.1007/s11010-010-0709-x. [DOI] [PubMed] [Google Scholar]
- 114.Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94(4):491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- 115.Slee EA, Harte MT, Kluck RM, Wolf BB, Casiano CA, Newmeyer DD, et al. Ordering the cytochrome c-initiated caspase cascade: hierarchical activation of caspases-2,-3,-6,-7,-8, and-10 in a caspase-9-dependent manner. J Cell Biol. 1999;144(2):281–92. doi: 10.1083/jcb.144.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Quintavalle C, Donnarumma E, Iaboni M, Roscigno G, Garofalo M, Romano G, et al. Effect of miR-21 and miR-30b/c on TRAIL-induced apoptosis in glioma cells. Oncogene. 2013;32(34):4001–8. doi: 10.1038/onc.2012.410. [DOI] [PubMed] [Google Scholar]
- 117.Atkinson GP, Nozell SE, Benveniste EN. NF-κB and STAT3 signaling in glioma: targets for future therapies. Expert Rev Neurother. 2010;10(4):575–86. doi: 10.1586/ern.10.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Valdés-Rives SA, Casique-Aguirre D, Germán-Castelán L, Velasco-Velázquez MA, González-Arenas A. Apoptotic signaling pathways in glioblastoma and therapeutic implications. BioMed Res Int. 2017:2017. doi: 10.1155/2017/7403747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sana J, Hajduch M, Michalek J, Vyzula R, Slaby O. MicroRNAs and glioblastoma: roles in core signalling pathways and potential clinical implications. J Cell Mol Med. 2011;15(8):1636–44. doi: 10.1111/j.1582-4934.2011.01317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Haemmig S, Baumgartner U, Gluck A, Zbinden S, Tschan MP, Kappeler A, et al. miR-125b controls apoptosis and temozolomide resistance by targeting TNFAIP3 and NKIRAS2 in glioblastomas. Cell Death Dis. 2014;5(6):e1279. doi: 10.1038/cddis.2014.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7(9):678–89. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- 122.Wei J, Hemmings GP. The NOTCH4 locus is associated with susceptibility to schizophrenia. Nature Genetics. 2000;25(4):376–7. doi: 10.1038/78044. [DOI] [PubMed] [Google Scholar]
- 123.Morrison SJ, Perez SE, Qiao Z, Verdi JM, Hicks C, Weinmaster G, et al. Transient Notch activation initiates an irreversible switch from neurogenesis to gliogenesis by neural crest stem cells. Cell. 2000;101(5):499–510. doi: 10.1016/s0092-8674(00)80860-0. [DOI] [PubMed] [Google Scholar]
- 124.Shimizu T, Kagawa T, Inoue T, Nonaka A, Takada S, Aburatani H, et al. Stabilized β-catenin functions through TCF/LEF proteins and the Notch/RBP-Jκ complex to promote proliferation and suppress differentiation of neural precursor cells. Mol Cell Biol. 2008;28(24):7427–41. doi: 10.1128/MCB.01962-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Purow BW, Sundaresan TK, Burdick MJ, Kefas BA, Comeau LD, Hawkinson MP, et al. Notch-1 regulates transcription of the epidermal growth factor receptor through p53. Carcinogenesis. 2008;29(5):918–25. doi: 10.1093/carcin/bgn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fan X, Khaki L, Zhu TS, Soules ME, Talsma CE, Gul N, et al. NOTCH pathway blockade depletes CD133-positive glioblastoma cells and inhibits growth of tumor neurospheres and xenografts. Stem Cells. 2010;28(1):5–16. doi: 10.1002/stem.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kanamori M, Kawaguchi T, Nigro JM, Feuerstein BG, Berger MS, Miele L, et al. Contribution of Notch signaling activation to human glioblastoma multiforme. J Neurosurg. 2007;106(3):417–27. doi: 10.3171/jns.2007.106.3.417. [DOI] [PubMed] [Google Scholar]
- 128.Banelli B, Forlani A, Allemanni G, Morabito A, Pistillo MP, Romani M. MicroRNA in Glioblastoma: An Overview. Int J Genomics. 2017;2017:7639084. doi: 10.1155/2017/7639084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bhaumik D, Scott G, Schokrpur S, Patil C, Campisi J, Benz C. Expression of microRNA-146 suppresses NF-κB activity with reduction of metastatic potential in breast cancer cells. Oncogene. 2008;27(42):5643–7. doi: 10.1038/onc.2008.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Guessous F, Zhang Y, Kofman A, Catania A, Li Y, Schiff D, et al. microRNA-34a is tumor suppressive in brain tumors and glioma stem cells. Cell Cycle. 2010;9(6):1031–6. doi: 10.4161/cc.9.6.10987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sun F, Fu H, Liu Q, Tie Y, Zhu J, Xing R, et al. Downregulation of CCND1 and CDK6 by miR-34a induces cell cycle arrest. FEBS Lett. 2008;582(10):1564–8. doi: 10.1016/j.febslet.2008.03.057. [DOI] [PubMed] [Google Scholar]
- 132.Shi L, Wang Z, Sun G, Wan Y, Guo J, Fu X. miR-145 inhibits migration and invasion of glioma stem cells by targeting ABCG2. Neuromolecular Med. 2014;16(2):517–28. doi: 10.1007/s12017-014-8305-y. [DOI] [PubMed] [Google Scholar]
- 133.Burton T, Henson E, Azad M, Brown M, Eisenstat D, Gibson S. BNIP3 acts as transcriptional repressor of death receptor-5 expression and prevents TRAIL-induced cell death in gliomas. Cell Death Dis. 2013;4(4):e587–e. doi: 10.1038/cddis.2013.100. [DOI] [PMC free article] [PubMed] [Google Scholar]