Abstract
Cepharanthin is a proprietary extract of Stephania cepharantha, widely used in Japan for the treatment of inflammatory diseases. Cephranthin, its component alkaloids, and the standard resistance modulator verapamil were tested against Plasmodium falciparum for capacity to modulate sensitivity to chloroquine. Cepharanthin enhanced the activity of chloroquine against resistant clones by a factor of 15 at a concentration of only 200 nM (1.2 ng/ml). It is 50 times more potent than verapamil and 3 times more potent than the sum of its individual alkaloids. Combinations of component alkaloids acted synergistically to sensitize the parasite to chloroquine, possibly explaining the enhanced potency of Cepharanthin. Cepharanthin differed from verapamil in that it further sensitized clones that are considered to be fully susceptible, improving the baseline activity of chloroquine. Potent sensitization of parasites to chloroquine in vitro coupled with low toxicity suggests that coadministration of Cepharanthin might extend the clinical utility of chloroquine.
Malaria is an immense public health problem, threatening almost half the world's population and killing nearly two million people each year (13). Until recently, chloroquine (CQ) has been a key weapon in the fight against this disease. Unfortunately, resistance to CQ is now widespread in Plasmodium falciparum, the most important human malaria pathogen.
New drugs are needed urgently but will take time to reach the market. One strategy that can be pursued in the meantime is to try to “reverse” CQ resistance chemically. It has been known for more than a decade that the CQ resistance of P. falciparum can be reversed in vitro by coadministration with compounds such as verapamil (VP) and desipramine (2, 7). Similar compounds have been used successfully to reverse CQ resistance in animal malaria models (6, 9), prompting studies in humans. Early clinical trials with desipramine and cyproheptadine (3, 12) failed to offer an advantage over CQ, but recent trials combining CQ with chlorpheniramine have been more successful. In an area of Nigeria with a high rate of CQ resistance, CQ-chlorpheniramine gave a cure rate equivalent to, or higher than, the standard antimalarials pyrimethamine-sulfadoxine or halofantrine (10, 11). These recent findings demonstrate that the strategy of using CQ in combination with resistance-modulating compounds is clinically feasible and suggest that the clinical utility of CQ can be extended.
The properties of an ideal CQ resistance modulator have not yet been elucidated. This is partly due to a poor understanding of the mechanism of CQ resistance itself. Nonetheless, it is clear that a potential resistance modulator must exhibit good activity at concentrations that are nontoxic and pharmacologically achievable.
Cepharanthin is an extract of the root tubers of Stephania cepharantha. It can be obtained both in liquid and in tablet form and is orally bioavailable. It is widely used in Japan for the treatment of chronic inflammatory diseases, radiation-induced leukopenia, asthma bronchiale, and alopecia aerata and is considered to have no serious side effects (8). The main constituents of the extract are the bisbenzylisoquinoline alkaloids isotetrandrine (IT), cepharanthine (CE), berbamine (BE), homoaromoline (HO), cepharanoline (CO), and cycleanine (CY). CE has been reported to reverse drug resistance in cultured cancer cells (1), and a related bisbenzylisoquinoline alkaloid has been shown to modulate CQ resistance in P. falciparum (14). Here we report that Cepharanthin exhibits a potent ability to sensitize P. falciparum to CQ. Cepharanthin improves the CQ responses of both CQ-resistant and CQ-susceptible strains, suggesting that the baseline activity of CQ is improved. The mixture is more active than the sum of the individual alkaloids and is far more active than the reference compound VP.
MATERIALS AND METHODS
Reagents and parasite culture.
Cepharanthin and pure samples of its six major alkaloids were obtained from M. Ono, Kaken Shoyaku Co., Ltd. Purity (>99%) was confirmed by high-performance liquid chromatography and quantitative analysis. All other reagents were obtained from Sigma UK. P. falciparum strains (K1, a CQ-resistant strain, and HB3, a CQ-sensitive strain) used in this study were obtained from D. Walliker, Edinburgh University, Edinburgh, United Kingdom. Strains were cloned twice by limiting dilution before use and were maintained in continuous culture by standard techniques (4).
Drug sensitivity assays.
Parasites were synchronized by using sorbitol 48 h before use, and ring stage parasites were used for the sensitivity assays. Firstly, the baseline antimalarial activity of each resistance modulator was determined by standard techniques (5). CQ sensitivity in the presence or absence of single fixed concentrations of potential resistance modulators was determined as described previously (5). Resistance modulators were used at concentrations lower than the measured 50% inhibitory concentrations (IC50). VP was used at fixed concentrations of 10, 50, 100, 200, and 500 nM and 1, 5, and 10 μM. Cepharanthin, IT, CE, BE, HO, CO, and CY were each used at fixed concentrations of 10, 20, 50, 100, 200, and 500 nM. The contribution of each individual alkaloid to the sensitization effect was assessed by using artificial mixtures of purified alkaloids that approximately reflected their relative abundances in Cepharanthin: IT, 35%; CE, 35%; BE, 10%; CY, 10%; CO, 5%; HO, 5%. Cepharanthin also contains low concentrations of 46 other compounds that together make up approximately 10% of its weight. Assessment of the contributions of these minor constituents is beyond the scope of the present study. The effect of removing each alkaloid from the six-alkaloid mixture on the CQ response was determined at a range of concentrations. The effects of alkaloid pairs on the CQ responses of parasites were tested by titration of the two drugs at fixed ratios of 100 nM stock solutions. Results were plotted as fold enhancement of CQ activity (IC50) at each ratio. Each sensitivity assay was performed in triplicate, and the IC50 were calculated for each assay by the four-parameter logistic method (Grafit program; Erithacus Software, Horley, Surrey, United Kingdom).
CQ accumulation assays.
Sensitization of CQ-resistant malaria parasites by VP and other compounds is attributed to an increase in the steady-state accumulation of CQ. This in turn reflects an increase in the amount of CQ bound to ferriprotoporphyrin IX (FPIX) in the infected cell (4, 5). We have measured the effect of fixed concentrations of VP and Cepharanthin on the steady-state accumulation of [3H]CQ in both CQ-susceptible and CQ-resistant clones. The concentrations of the modulators were the same as those used in the sensitivity assays, and the concentration of [3H]CQ was 5 nM. The assays were conducted over 1 h at 37°C. The other reagents used and assay conditions were as described previously (4).
RESULTS
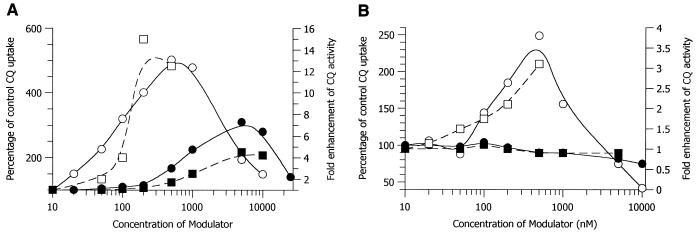
Cepharanthin and component alkaloids exhibited moderate antimalarial activity, with IC50 in the range of 350 to 3,500 nM (data not shown). Against the CQ-resistant K1 clone and at subinhibitory concentrations, Cepharanthin markedly enhanced both the accumulation of [3H]CQ and the antimalarial activity of CQ (Fig. 1A). There was a maximum 5-fold increase in CQ accumulation and a 15-fold enhancement of activity. Similar results were obtained with the CQ-susceptible clone HB3 (Fig. 1B), although the effect was less spectacular: a maximum 2.5-fold enhancement of accumulation and 3-fold enhancement of activity. Similar results were obtained with other CQ-susceptible and CQ-resistant clones (data not shown). VP was much less effective than Cepharanthin. In the K1 clone, VP increased CQ uptake by a maximum of threefold and enhanced antimalarial activity by a maximum of fivefold (Fig. 1A). This was achieved at a concentration of 5 μM. Similar effects were achieved with Cepharanthin at a concentration of only 100 nM. In the HB3 clone, VP did not enhance CQ accumulation or activity (Fig. 1B).
FIG. 1.
Comparative effects of Cepharanthin (open symbols) and VP (closed symbols) on the uptake of [3H]CQ (circles) and the antimalarial activity of CQ (squares). Shown are data obtained with the CQ-resistant clone K1 (A) and the CQ-susceptible clone HB3 (B).
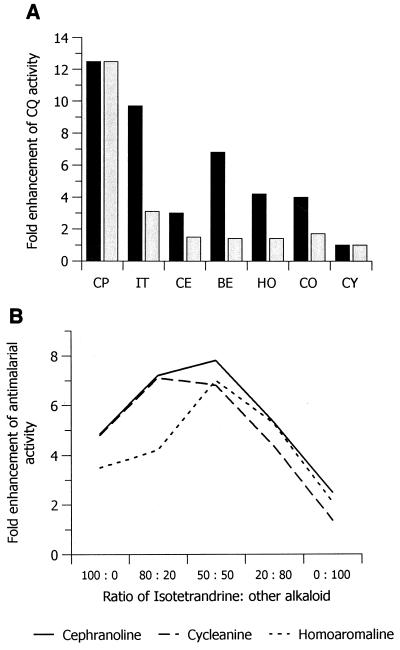
The major component alkaloids were tested for the ability to enhance the activity of CQ against the K1 clone (Fig. 2A). With the exception of CY, all of the alkaloids were effective. CE, CO, HO, BE, and IT enhanced the activity of CQ by maximum factors of 3, 4, 4.2, 6.8, and 9.7, respectively. Used at equivalent concentrations (500 nM), none of the individual alkaloids was as effective as Cepharanthin. The effects of the individual alkaloids at the proportional concentrations at which they occur in 500 nM Cepharanthin are also plotted. When summed, these individual effects account for only approximately one-third of the activity of Cepharanthin, suggesting synergy between individual alkaloids. This was confirmed by selectively omitting IT, CY, HO, and CO, which resulted in losses of activity of 95, 70, 50, and 43%, respectively. Omitting the other alkaloids (BE and CE) had little or no effect (data not shown). Pronounced synergy was found between IT and CY, IT and HO, and IT and CO (Fig. 2B). Other combinations were additive (data not shown).
FIG. 2.
(A) Comparative effects of Cepharanthin (CP) and component alkaloids on the activity of CQ against the K1 clone. Solid bars, all compounds at 500 nM; Shaded bars, all compounds at their relative concentrations in 500 nM Cepharanthin. (B) Synergy of the sensitizing effects of pairs of alkaloids on the activity of CQ against the K1 clone.
DISCUSSION
By definition, CQ resistance reversal agents specifically target the CQ resistance mechanism and do not enhance the baseline activity of CQ against susceptible strains. This group of compounds includes VP, desipramine, cyproheptadine, and chlorpheniramine (2, 7, 10). In contrast, Cepharanthin is able to potentiate the action of CQ both in susceptible and in resistant strains (Fig. 1). The potency of Cepharanthin is remarkable: by use of a concentration of Cepharanthin as low as 200 nM, the highly CQ resistant K1 clone becomes even more sensitive to CQ than the standard CQ-susceptible clone HB3. This effect is three times greater than the maximum available sensitization with VP, seen at a concentration of 5 μM (Fig. 1A). In fact, Cepharanthin is able to reproduce VP's maximum sensitivity enhancement at 1/50 of the concentration. Even the clone HB3, generally considered to be fully CQ susceptible, becomes threefold more sensitive to CQ in the presence of Cepharanthin (Fig. 1B). Thus, Cepharanthin may offer an advantage over current resistance reversal agents in that it may increase the baseline efficacy of CQ to a level over and above that achievable by simply overcoming the resistance mechanism.
The mechanism by which Cepharanthin increases the potency of CQ is not known at present. Our data indicate that increased potency may be related to enhanced accumulation of CQ (Fig. 1). CQ activity depends on the binding of CQ to FPIX in the parasite (4). We have shown recently that CQ-resistant parasites exhibit a reduced access of CQ to FPIX (5). We have preliminary data suggesting that Cepharanthin increases the access of CQ to FPIX, possibly by altering the parasite membrane potential (Haruki et al., unpublished data). Thus, it is likely that Cepharanthin increases the activity of CQ by increasing the intracellular binding of CQ to FPIX.
We have identified a novel method of sensitizing malarial parasites to CQ that is highly effective in both CQ-resistant and CQ-sensitive isolates. It is likely that the remarkable potency of Cepharanthin can be attributed largely to a pronounced synergy that exists between IT and either CO, CY, or HO (Fig. 2B). Because it is a safe therapeutic entity (8), the clinical use of Cepharanthin as a cheap and highly effective adjunct to CQ treatment holds great promise.
REFERENCES
- 1.Aogi K, Nishiyama M, Kim R, et al. Overcoming CPT-11 resistance by using a biscoclaurine alkaloid cepharanthine, to modulate plasma trans-membrane potential. Int J Cancer. 1997;72:295–300. doi: 10.1002/(sici)1097-0215(19970717)72:2<295::aid-ijc16>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 2.Bitonti A J, McCann P P. Desipramine and cyproheptadine for reversal of chloroquine resistance in Plasmodium falciparum. Lancet. 1989;ii:1282–1283. doi: 10.1016/s0140-6736(89)91895-3. [DOI] [PubMed] [Google Scholar]
- 3.Bjorkman A, Willcox M, Kihamia C M, et al. Field study of cyproheptadine/chloroquine synergism in falciparum malaria. Lancet. 1990;336:59–60. doi: 10.1016/0140-6736(90)91579-y. [DOI] [PubMed] [Google Scholar]
- 4.Bray P G, Janneh O, Raynes K J, et al. Cellular uptake of chloroquine is dependent on binding to ferriprotoporphyrin IX and is independent of NHE activity in Plasmodium falciparum. J Cell Biol. 1999;145:363–376. doi: 10.1083/jcb.145.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bray P G, Mungthin M, Ridley R G, Ward S A. Access to hematin: the basis of chloroquine resistance. Mol Pharmacol. 1998;54:170–179. doi: 10.1124/mol.54.1.170. [DOI] [PubMed] [Google Scholar]
- 6.Kyle D E, Milhous W K, Rossan R N. Reversal of Plasmodium falciparum resistance to chloroquine in Panamanian Aotus monkeys. Am J Trop Med Hyg. 1993;48:126–133. doi: 10.4269/ajtmh.1993.48.126. [DOI] [PubMed] [Google Scholar]
- 7.Martin S K, Oduola A M, Milhous W K. Reversal of chloroquine resistance in Plasmodium falciparum by verapamil. Science. 1987;235:899–901. doi: 10.1126/science.3544220. [DOI] [PubMed] [Google Scholar]
- 8.Okamoto M, Ono M, Baba M. Potent inhibition of HIV type 1 replication by an anti-inflammatory alkaloid, cepharanthine, in chronically infected monocytic cells. AIDS Res Hum Retrovir. 1998;14:1239–1245. doi: 10.1089/aid.1998.14.1239. [DOI] [PubMed] [Google Scholar]
- 9.Peters W, Ekong R, Robinson B L, Warhurst D C, Pan X Q. The chemotherapy of rodent malaria. XLV. Reversal of chloroquine resistance in rodent and human Plasmodium by antihistaminic agents. Ann Trop Med Parasitol. 1990;84:541–551. doi: 10.1080/00034983.1990.11812509. [DOI] [PubMed] [Google Scholar]
- 10.Sowunmi A, Oduola A M, Ogundahunsi O A, Salako L A. Comparative efficacy of chloroquine plus chlorpheniramine and pyrimethamine/sulfadoxine in acute uncomplicated falciparum malaria in Nigerian children. Trans R Soc Trop Med Hyg. 1998;92:77–81. doi: 10.1016/s0035-9203(98)90964-6. [DOI] [PubMed] [Google Scholar]
- 11.Sowunmi A, Fehintola F A, Ogundahunsi O A, Oduola A M. Comparative efficacy of chloroquine plus chlorpheniramine and halofantrine in acute uncomplicated falciparum malaria in Nigerian children. Trans R Soc Trop Med Hyg. 1998;92:441–445. doi: 10.1016/s0035-9203(98)91084-7. [DOI] [PubMed] [Google Scholar]
- 12.Warsame M, Wernsdorfer W H, Bjorkman A. Lack of effect of desipramine on the response to chloroquine of patients with chloroquine-resistant falciparum malaria. Trans R Soc Trop Med Hyg. 1992;86:235–236. doi: 10.1016/0035-9203(92)90288-n. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization. The World Health Report. Conquering suffering, enriching humanity. Geneva, Switzerland: World Health Organization; 1997. [Google Scholar]
- 14.Ye Z G, Van Dyke K, Castranova V. The potentiating action of tetrandrine in combination with chloroquine or qinghaosu against chloroquine-sensitive and resistant falciparum malaria. Biochem Biophys Res Commun. 1989;165:758–765. doi: 10.1016/s0006-291x(89)80031-2. [DOI] [PubMed] [Google Scholar]