Abstract
Mutation in SP7, encoding the osteoblast-specific transcription factor SP7 (also known as osterix), has been described to cause osteogenesis imperfecta (OI) type XII. However, the exact dental phenotype has not been well described. We report the detailed dental manifestation of a boy known to have OI type XII, presented with impacted dentition, necessitating combined oral and maxillofacial surgical and orthodontic treatment. This case also highlighted the need of multidisciplinary team assessment in this group of children.
Keywords: Dentistry and oral medicine, Paediatrics (drugs and medicines), Osteoporosis
Background
Osteogenesis imperfecta (OI) is a heterogeneous group of connective tissue disorders, characterised by bone fragility with repeated fractures and other extraskeletal features, including ligamentous laxity, blue sclerae, dentinogenesis imperfecta (DI) and other dental development disorders.1 The majority of individuals with OI have autosomal dominant mutations in either COL1A1 and COL1A21 2 which encode the α1(I) and the α2(I) chains of collagen type I—a crucial component in both bone and dentine. Other than COL1A1 and COL1A2, mutations in 20 other genes have been associated with OI phenotypes. Most of these genes are expressed in osteoblasts, and are directly involved in the metabolism of collagen type I.3
Mutation in SP7, encoding the osteoblast-specific transcription factor SP7 (also known as osterix), which is essential for bone formation, has been described to cause OI type XII, resulting in bone fragility with recurrent long bone fractures since early childhood, long bone bowing, scoliosis, short stature, early onset hearing impairment and delayed tooth eruption.4 However, the exact dental phenotype has not been well described. Here, we report a boy known to have OI type XII, presented with impacted permanent dentition.
Case presentation
The proband is a 17-year old boy known to have OI type XII due to a homozygous frameshift mutation in SP7 (p.Glu351Argfs*19). Parents were healthy, non-consanguineous Chinese couple. He had recurrent long bone fractures since the age of 2 years and was started on intravenous pamidronate infusions since the age of 5 years. With decreased frequency of fractures, bisphosphonate treatment was stopped at 10 years of age. He developed vertebral collapse and another episode of long bone fracture at the age of 13 years, and hence bisphosphonate was reinitiated again with 6-monthly zoledronic acid until 15 years, after the closure of growth plates. He also had repeated orthopaedic surgeries, including left tibia corrective osteotomy and modified Sofield procedures of bilateral tibiae. He was ambulatory with normal development, and he had no hearing issue at the moment. No obvious dental issue was noted until he reached his adolescence, when he reported to have delayed shedding of primary dentitions.
Investigations
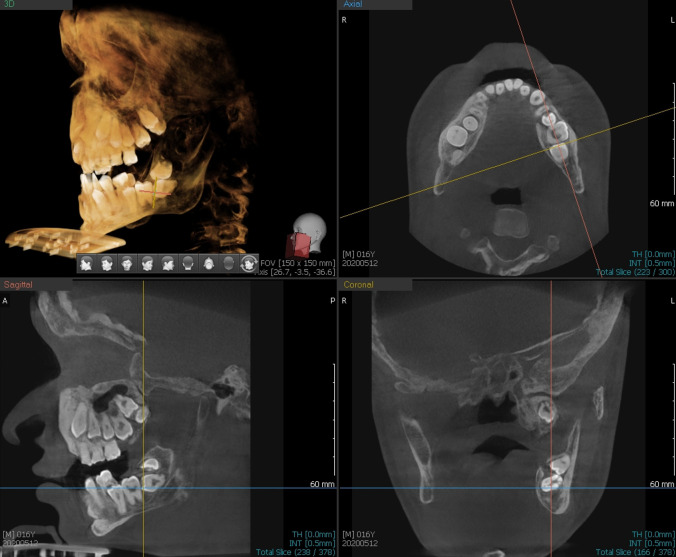
He was eventually assessed by dental surgeon in our multidisciplinary joint bone clinic at the age of 16 years. The patient had maxillary hypoplasia in both anteroposterior and vertical dimension, resulting in overclosure of the mandible and a pseudo-class III malocclusion, as well as a small face when compared with the cranium (figure 1). The perimeters of upper and lower dental arch were small, leading to relative macroglossia. Enamel hypoplasia was observed in the primary dentition, while the permanent teeth were not affected. He had retained primary dentition with only six permanent teeth (lower incisors, upper right and lower left first premolar) erupted. The primary dentition was yellowish-brown in colour and severely worn especially at the upper anterior teeth, which further leads to the loss of vertical dimension and overclosure of mandible (figure 2). Orthopantomogram and cone-beam CT showed poor development of the upper and lower dento-alveoli, which was probably related to multiple unerupted permanent teeth. The roots of the retained primary teeth were partially resorbed, and the pulp chambers of upper and lower right primary teeth were obliterated. The permanent teeth had bulbous crown and short roots. Most of the unerupted permanent teeth positioned cervically to their retained primary predecessors. However, the lower right first molar and all second and third permanent molars were impacted (figure 3). Lower right wisdom tooth was distally angulated. The lower right second molar was mesially angulated with the crown placed inferiorly to the root of the lower right wisdom tooth. The lower right first molar was further placed apically than the adjacent teeth. The upper right wisdom tooth was mesially angulated and upper right second molar was impacted apically (figures 4 and 5). The lower left wisdom tooth was inverted and horizontally impacted the lower left second molar, it also mesially impacted the upper left wisdom, while the upper left second molar was also impacted on contralateral side.
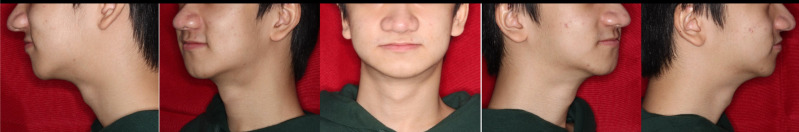
Figure 1.
Photos showed maxillary hypoplasia in both anteroposterior and vertical dimension, resulting in overclosure of the mandible and a pseudo-class III malocclusion as well as a small face as compared with the cranium.
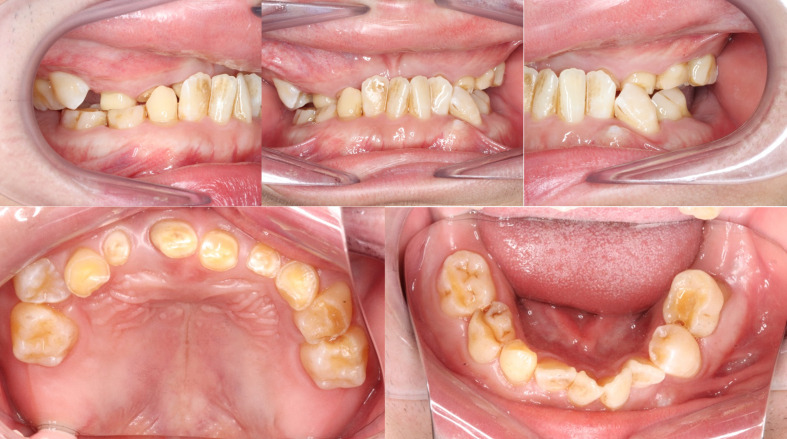
Figure 2.
The primary dentition was yellowish-brown in colour and severely worn especially at the upper anterior teeth, which leads to the loss of vertical dimension and overclosure of mandible.
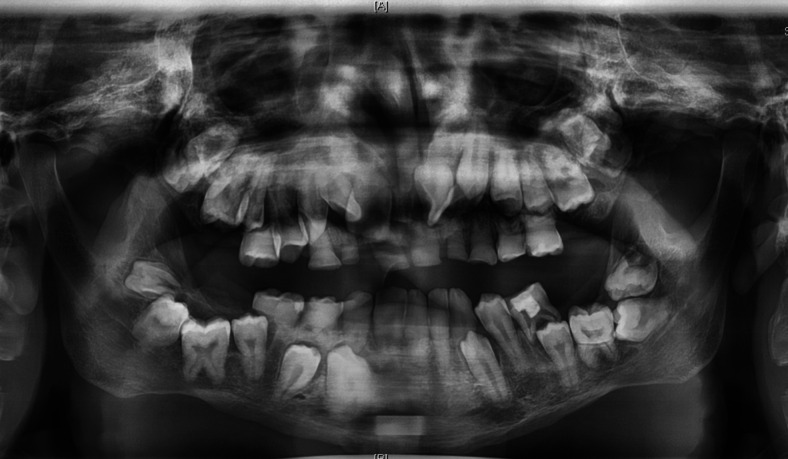
Figure 3.
Orthopantomogram showed poor development of the upper and lower dento-alveoli with multiple unerupted permanent teeth. The roots of the retained primary teeth were partially resorbed, and the pulp chambers of upper and lower right primary teeth were obliterated. The permanent teeth had bulbous crown and short roots. The lower right first molar and all second and third permanent molars were impacted. Others unerupted permanent teeth positioned cervically to their retained primary predecessors.
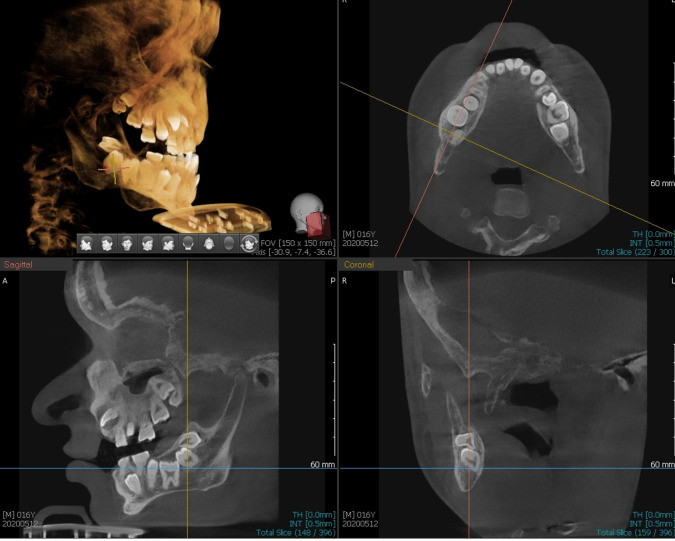
Figure 4.
Cone-beam CT showed that lower right wisdom tooth was distally angulated and lower right first and second molars were impacted. It also showed that upper right wisdom tooth was mesially angulated and upper right second molar was impacted.
Figure 5.
Cone-beam CT showed inverted lower left wisdom tooth, horizontal impacted lower left second molar, mesially impacted upper left wisdom and impacted upper left second molar.
Outcome and follow-up
A multidisciplinary therapeutic approach was adopted in view of his medical history and dental condition. In view of his unpredictable dental eruption pattern, different possible treatment modalities had been considered. One possible treatment option would be selective extraction of primary teeth to facilitate the eruption of the permanent successors. Another option would be surgical exposure of the unerupted permanent teeth with bonding of orthodontic attachments, followed by orthodontic traction to facilitate eruption. If permanent dentition fails to erupt, surgical removal of permanent teeth followed by prosthesis would be considered. He would be reviewed regularly for the treatment progress and the need of combined orthognathic and orthodontic surgery to correct the underlying dentofacial deformity in the future.
Discussion
While delayed teeth eruption has been described in OI type XII, impaction of permanent dentition necessitating combined treatment of oral maxillofacial and orthodontic surgery has not been described. This is the first case describing the detailed dental manifestation of patient with OI type XII.
For OI due to mutations in collagen 1, DI is predominantly observed in those with qualitative collagen defects with moderate–severe OI rather than those having quantitative defects with mild OI.5 Discolouration of teeth, cervical constriction and pulp obliteration were reported as frequent findings in patients with moderate–severe OI, yet these features vary between different OI populations.5–8 DI affects both dentitions, but the primary dentition is usually more severely affected than the permanent dentition,9 and this corresponded to our observation in our patient. Other than DI, tooth agenesis is a also common observation in OI and could contribute to mandibular and maxillary dysplasia, which could lead to dental malocclusion.10 11 Retained deciduous teeth past the normal range of exfoliation, retention of molar teeth or impaction of permanent teeth has been reported in various OI types, including classical OI due to mutations in collagen type 1, as well as OI type V.12 13 However, it has not been reported in OI type XII.
The dentition development involves an orchestrated process with multiple signalling pathways that are also important for skeletal development,14 therefore, it is not surprising that genetic defects causing OI would also affect tooth development. However, the exact mechanism by which the variants in SP7 leading to dental impaction remained unknown.
The Sp7/Osx gene is protein coding gene that encodes a zinc finger transcription factor—osterix, which is expressed primarily by osteoblasts. It promotes the differentiation and maturation of pre-osteoblasts into functional osteoblasts and is crucial for bone formation and bone homeostasis. The clinical phenotypes of individuals with SP7 mutations include recurrent fracture, short stature, early onset hearing loss (secondary to otospongiosis and poor mineralisation of ossicles and petrous temporal bone) and delayed teeth eruption.4 The role of Sp7 in tooth development remains unknown. Based on mice model, Sp7 is obligatory for the differentiation of both ameloblasts and odontoblasts but not for the initial tooth morphogenesis. Sp7-null mice exhibited features of craniofacial dysmorphogenesis, completely void of alveolar bone yet normal progression of initial tooth morphogenesis. With reduced proliferative capacity of Sp7-deficient ectomesenchyme, it resulted in small and misshapen teeth with randomly arranged cuboidal pre-odontoblasts and pre-ameloblasts. This implies the role of Sp7 in promoting the functional maturation and polarisation of odontoblasts.15
Eruption of permanent teeth involves resorption of the surrounding alveolar bone and the roots of primary teeth. Bisphosphonates, a well-established medical treatment for OI, works by inhibiting osteoclast function and hence it could theoretically cause delayed tooth eruption. This phenomenon has been observed in animals treated with bisphosphonates.16 17 However, there is only limited evidence for similar effects in humans.
In our patient, the tooth eruption was significantly delayed, and the permanent molars are severely impacted in four quadrants. Whether the dental phenotype is solely related to the SP7 mutation, or it was, at least, partially, contributed by prior bisphosphonates use, remained to be elucidated. Bisphosphonates treatment, being an anti-resorptive treatment, has been shown to be associated with delayed dental development and tooth eruption.18–20 However, most available evidence came from cross-sectional observational studies with various OI types, the association should be interpreted with caution. It could be possible that, patients with more severe OI types would be more likely to be treated with bisphosphonates when compared with the milder ones, and that these patients with more severe phenotype could be associated with more prominent dental manifestation with delayed dental development. In addition, the so-called ‘delay in tooth eruption’ reported was only around 1 year. This does not explain the clinical phenotype in our case.
As for the treatment, one possible option would be selective extraction of the primary teeth to facilitate the eruption of their permanent successors. However, it would be technically challenging in view of complex impactions and closed apices with low eruption potential. Surgical exposure of unerupted permanent teeth and bonding of orthodontic attachments might be necessary, followed by orthodontic traction to facilitate eruption. Forced eruption of impacted teeth would be needed and the teeth could be treated as abutments for future dental prosthesis. Similar successful orthodontic treatments in patients with different OI types have been reported.21 However, the treatment time could be considerably longer, and the goal of tooth movement might not be achieved with the history of bisphosphonates use.22 If the eruption of permanent dentition could not be facilitated, surgical removal of permanent teeth followed by prosthesis would be needed. The underlying jaw discrepancy would be ultimately corrected by combined orthognathic/orthodontic treatment approach. This combined therapy for patients with severe malocclusion has been reported to lead to satisfactory aesthetic and functional outcome.22
Our case highlighted the importance of multidisciplinary assessment in managing children with OI, and that the complaint of ‘delayed shedding of primary dentitions’ should be taken seriously with proper assessment by dental specialists. However, it could be challenging practically. Clark et al reported the real-life challenges in referring individuals with OI to a tertiary dental clinic for further assessment, with the main barrier being geographic factors and need of extra travel.23 In addition, individuals with OI usually have relatively short neck and some extent of immobility, which makes routine dental examination even more challenging.5 This underscored the advantage of joint assessment in a combined multidisciplinary clinic with orthopaedic surgeons, paediatric endocrinologist/paediatric bone specialist, geneticist and dental specialists of oral and maxillofacial surgeon and orthodontist. This does not just save patients from another visit to the hospital, but also enhance communication between various disciplines. This also allows concentration of expertise to identify abnormalities and recommend for timely interventions, which, in the long run, would help to optimise the overall health outcomes of these individuals.
In conclusion, we described the detailed dental manifestation of a boy with OI type XII. While delayed teeth eruption has been described in OI type XII, impaction of dentition necessitating combined oral and maxillofacial surgical and orthodontic treatment has not been described. The case highlighted the need of multidisciplinary team assessment in this group of children.
Learning points.
This case described the detailed dental manifestation in a patient with osteogenesis imperfecta due to mutation in SP7 gene.
Delayed dental eruption in a child with osteogenesis imperfecta would have functional implication in the long run.
In managing children with osteogenesis imperfecta presenting with delayed dental eruption, early multidisciplinary team assessment would be beneficial to decide on the most appropriate and personalised treatment plan.
Acknowledgments
This study was previously presented as an abstract at the International Conference on Children’s Bone Health (ICCBH) Virtual Forum Meeting on Bone Fragility Disorders in Children, 18–20 November 2020.
Footnotes
Contributors: JY-lT and JL-iH prepared the manuscript for publication. All authors contributed to clinical care of the patient and critically revised the manuscript and approved the final version of the manuscript for submission.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
References
- 1.Van Dijk FS, Sillence DO. Osteogenesis imperfecta: clinical diagnosis, nomenclature and severity assessment. Am J Med Genet A 2014;164:1470–81. 10.1002/ajmg.a.36545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forlino A, Marini JC. Osteogenesis imperfecta. Lancet 2016;387:1657–71. 10.1016/S0140-6736(15)00728-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tauer JT, Robinson M-E, Rauch F. Osteogenesis imperfecta: new perspectives from clinical and translational research. JBMR Plus 2019;3, :e10174. 10.1002/jbm4.10174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fiscaletti M, Biggin A, Bennetts B, et al. Novel variant in Sp7/Osx associated with recessive osteogenesis imperfecta with bone fragility and hearing impairment. Bone 2018;110:66–75. 10.1016/j.bone.2018.01.031 [DOI] [PubMed] [Google Scholar]
- 5.Thuesen KJ, Gjørup H, Hald JD, et al. The dental perspective on osteogenesis imperfecta in a Danish adult population. BMC Oral Health 2018;18:175. 10.1186/s12903-018-0639-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Retrouvey J-M, Taqi D, Tamimi F, et al. Oro-dental and cranio-facial characteristics of osteogenesis imperfecta type V. Eur J Med Genet 2019;62:103606. 10.1016/j.ejmg.2018.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scaramuzzo L, Raffaelli L, Spinelli MS, et al. Orthopaedic and dental abnormalities in osteogenesis imperfecta: a review of the literature. J Biol Regul Homeost Agents 2011;25:313–21. [PubMed] [Google Scholar]
- 8.Waltimo-Sirén J, Tuurala H, Säämäki E, et al. Dental and dentoalveolar dimensions in individuals with osteogenesis imperfecta. Acta Odontol Scand 2021;79:390–5. 10.1080/00016357.2021.1881160 [DOI] [PubMed] [Google Scholar]
- 9.O'Connell AC, Marini JC, A. C. O’Connell. Evaluation of oral problems in an osteogenesis imperfecta population. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1999;87:189–96. 10.1016/S1079-2104(99)70272-6 [DOI] [PubMed] [Google Scholar]
- 10.Malmgren B, Andersson K, Lindahl K, et al. Tooth agenesis in osteogenesis imperfecta related to mutations in the collagen type I genes. Oral Dis 2017;23:42–9. 10.1111/odi.12568 [DOI] [PubMed] [Google Scholar]
- 11.Rizkallah J, Schwartz S, Rauch F, et al. Evaluation of the severity of malocclusions in children affected by osteogenesis imperfecta with the peer assessment rating and discrepancy indexes. Am J Orthod Dentofacial Orthop 2013;143:336–41. 10.1016/j.ajodo.2012.10.016 [DOI] [PubMed] [Google Scholar]
- 12.Andersson K, Dahllöf G, Lindahl K, et al. Mutations in COL1A1 and COL1A2 and dental aberrations in children and adolescents with osteogenesis imperfecta - A retrospective cohort study. PLoS One 2017;12, :e0176466. 10.1371/journal.pone.0176466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Retrouvey J-M, Taqi D, Tamimi F, et al. Oro-dental and cranio-facial characteristics of osteogenesis imperfecta type V. Eur J Med Genet 2019;62, :103606. 10.1016/j.ejmg.2018.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Juuri E, Balic A. The biology underlying abnormalities of tooth number in humans. J Dent Res 2017;96:1248–56. 10.1177/0022034517720158 [DOI] [PubMed] [Google Scholar]
- 15.Bae J-M, Clarke JC, Rashid H, et al. Specificity protein 7 is required for proliferation and differentiation of ameloblasts and odontoblasts. J Bone Miner Res 2018;33:1126–40. 10.1002/jbmr.3401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hiraga T, Ninomiya T, Hosoya A, et al. Administration of the bisphosphonate zoledronic acid during tooth development inhibits tooth eruption and formation and induces dental abnormalities in rats. Calcif Tissue Int 2010;86:502–10. 10.1007/s00223-010-9366-z [DOI] [PubMed] [Google Scholar]
- 17.Bradaschia-Correa V, Massa LF, Arana-Chavez VE. Effects of alendronate on tooth eruption and molar root formation in young growing rats. Cell Tissue Res 2007;330:475–85. 10.1007/s00441-007-0499-y [DOI] [PubMed] [Google Scholar]
- 18.Malmgren B, Tsilingaridis G, Monsef-Johansson N, et al. Bisphosphonate therapy and tooth development in children and adolescents with osteogenesis imperfecta. Calcif Tissue Int 2020;107:143–50. 10.1007/s00223-020-00707-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marçal FF, Ribeiro EM, Costa FWG, et al. Dental alterations on panoramic radiographs of patients with osteogenesis imperfecta in relation to clinical diagnosis, severity, and bisphosphonate regimen aspects: a STROBE-compliant case-control study. Oral Surg Oral Med Oral Pathol Oral Radiol 2019;128:621–30. 10.1016/j.oooo.2019.07.001 [DOI] [PubMed] [Google Scholar]
- 20.Vuorimies I, Arponen H, Valta H, et al. Timing of dental development in osteogenesis imperfecta patients with and without bisphosphonate treatment. Bone 2017;94:29–33. 10.1016/j.bone.2016.10.004 [DOI] [PubMed] [Google Scholar]
- 21.Hartsfield JK, Hohlt WF, Roberts WE. Orthodontic treatment and Orthognathic surgery for patients with osteogenesis imperfecta. Semin Orthod 2006;12:254–71. 10.1053/j.sodo.2006.08.004 [DOI] [Google Scholar]
- 22.Friedrich RE, Scheuer HA, Höltje W. The effect of bisphosphonate medication on orthodontics and orthognathic surgery in patients with osteogenesis imperfecta. GMS Interdiscip Plast Reconstr Surg DGPW 2019;8:Doc06. 10.3205/iprs000132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clark R, Burren CP, John R. Challenges of delivery of dental care and dental pathologies in children and young people with osteogenesis imperfecta. Eur Arch Paediatr Dent 2019;20:473–80. 10.1007/s40368-019-00424-w [DOI] [PubMed] [Google Scholar]