Abstract
We report a case of a woman in her 50s with chronic teprotumumab-associated sensorineural hearing loss. The patient presented with chronic thyroid eye disease with proptosis and diplopia despite systemic thyroid control and orbital decompression. She was started on teprotumumab but developed tinnitus after the third dose, followed by frank hearing loss after the fifth dose. Her audiogram showed bilateral mild to moderate-severe hearing loss, which was significantly worse compared with her baseline audiogram obtained prior to treatment. Teprotumumab was immediately stopped, however repeat audiogram 6 weeks later showed no improvement. Given potentially irreversible sensorineural hearing loss, we recommend close monitoring with regular audiometric testing before, during and after teprotumumab therapy and propose potential treatment to reverse its effects in the ear.
Keywords: Ear, nose and throat/otolaryngology; Ophthalmology; Thyroid disease; Contraindications and precautions; Eye
Background
Thyroid eye disease, also known as Graves’ ophthalmopathy, is an orbital autoimmune condition associated with inflammation, tissue fibrosis, infiltration and expansion. Signs of thyroid eye disease include proptosis, lagophthalmos and strabismus, which can lead to debilitation, disfigurement, and in the worst cases permanent visual loss.
The pathogenesis of thyroid eye disease is not fully understood, however there is growing evidence that increased activity of insulin-like growth factor-1 receptor (IGF-1R) is involved. Patients with active disease demonstrate elevated IGF-1R expression in orbital fibroblasts as well as activating autoantibodies targeting these receptors.1 Teprotumumab (Horizon Therapeutics, Dublin, Ireland) is a human monoclonal antibody with activity against IGF-1R. It is the first drug approved by the US Food and Drug Administration for the treatment of thyroid eye disease. Clinical studies have found teprotumumab effective for improving proptosis, diplopia, clinical activity score and quality of life measures.2–4 Insulin-like growth factor-1 (IGF-1) is a ubiquitous molecule found throughout the body playing various roles in multiple systems. Consequently, side effects involving virtually every body system have been reported with teprotumumab use, including muscle spasms, hearing loss, hyperglycemia and inflammatory bowel disease.2 5–8
Notably, the Phase III Horizon clinical study reported subjective hearing loss in 10% of patients that was purportedly reversible without intervention; however this was not substantiated by audiometric testing.2 Subsequent literature has found the incidence of otological side effects to be as high as 30%.7 One clinical study reported borderline audiometry in two patients with tinnitus, however baseline pretreatment audiometry was not obtained for comparison.9 Multiple published cases of teprotumumab-associated hearing loss objectively demonstrated persistent and potentially permanent sensorineural hearing loss.10–12 IGF-1 plays a significant role in the development, maintenance and protection of hearing function within the inner ear.13 The inhibition of the IGF-1 signalling pathway by teprotumumab would explain its association with sensorineural hearing loss.
Here, we present a case of sensorineural hearing loss after partial teprotumumab therapy. We recommend practice guidelines for patient screening and suggest potential therapeutic modalities for teprotumumab-associated hearing loss.
Case presentation
A woman in her 50s with a history of chronic Graves’ disease presented with persistent diplopia and proptosis despite orbital decompression. Her exam was notable for extraocular muscle restriction in up gaze, right proptosis and right hypoglobus. Her thyroid stimulating immunoglobulin was stable but elevated at 431 (normal <140). Her thyroid stimulating hormone was 1.11 ulU/mL (normal 0.40–4.50 ulU/mL), free T4 was 1.00 ng/dL (normal 0.7–1.7 ng/dL) and free T3 was 2.89 pg/mL (normal 2.3–4.2 pg/mL). The patient was on methimazole 2.5 mg daily. MRI of the head and orbits with and without contrast showed marked right greater than left enlargement of the medial and inferior rectus muscles and proptosis consistent with thyroid ophthalmopathy. There was bony evidence of the prior orbital decompression of the medial wall and floor.
After considerable discussion, the patient was started on teprotumumab with the plan to receive an initial dose of 10 mg/kg, followed by 20 mg/kg every 3 weeks to complete a total of eight infusions.
Investigations
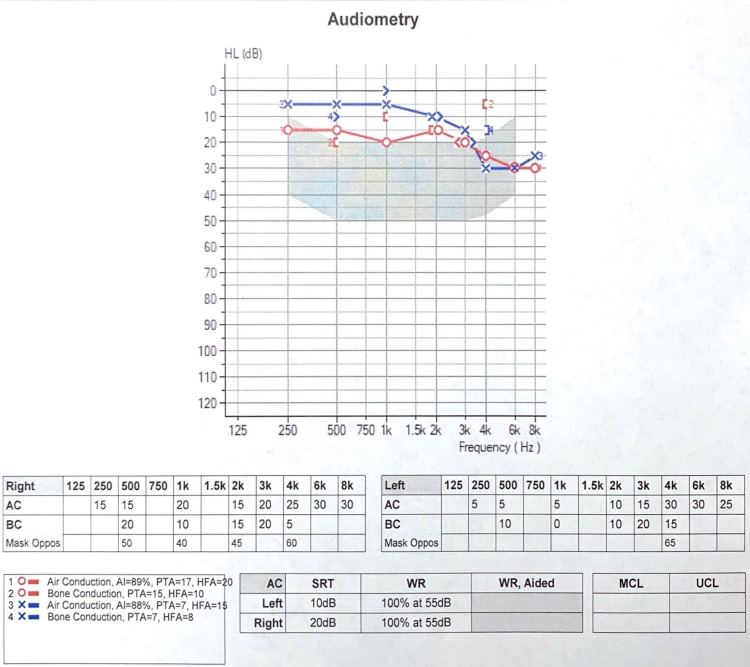
Prior to her first dose of teprotumumab, the patient underwent baseline otological and audiometric evaluation, which showed a normal otological examination and normal tympanogram. Baseline audiometry demonstrated mild hearing loss at 4000–8000 Hz bilaterally (normal hearing threshold - 250–3000 Hz) v consistent with normal age-related hearing loss (figure 1). The right ear was slightly worse than the left ear by 10 dB at 250–1000 Hz. Of note, the patient had a history of benign paroxysmal positional vertigo, she reported that her right ear had not felt the same since her first episode several years ago.
Figure 1.
Baseline audiogram prior to initiation of teprotumumab demonstrates mild hearing loss at 4000–8000 Hz, otherwise normal hearing threshold at 250–3000 Hz bilaterally. The right ear is slightly worse than the left ear by 10 dB at 250–1000 Hz.
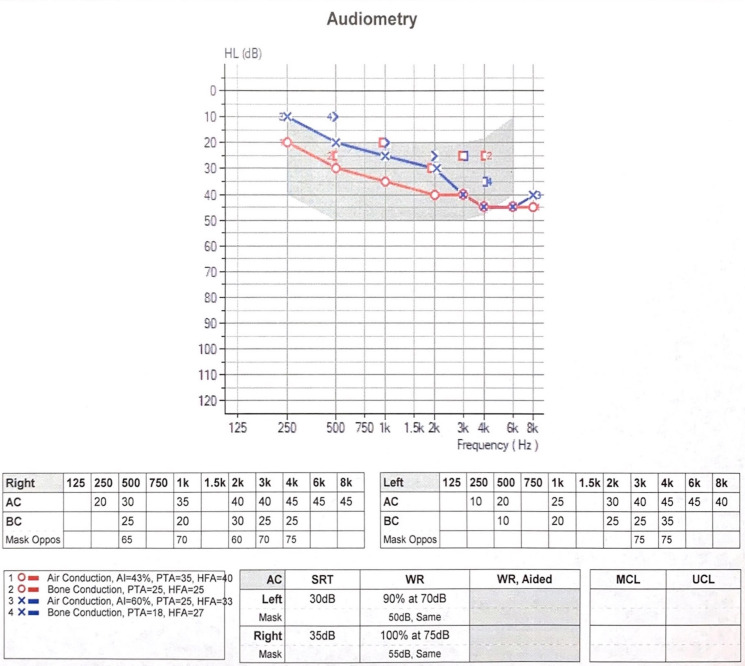
After the third infusion, the patient reported intermittent ringing in her ears. On the fifth dose, she had noticeable hearing loss. Repeat audiogram revealed bilateral mild to moderate-severe down sloping sensorineural hearing loss with a 15–30 dB decline at 500–8000 Hz compared with her baseline (figure 2).
Figure 2.
Audiogram after the fifth dose of teprotumumab demonstrates bilateral mild to moderate-severe down sloping sensorineural hearing loss with a 15–30 dB decline at 500–8000 Hz compared with baseline.
Outcome and follow-up
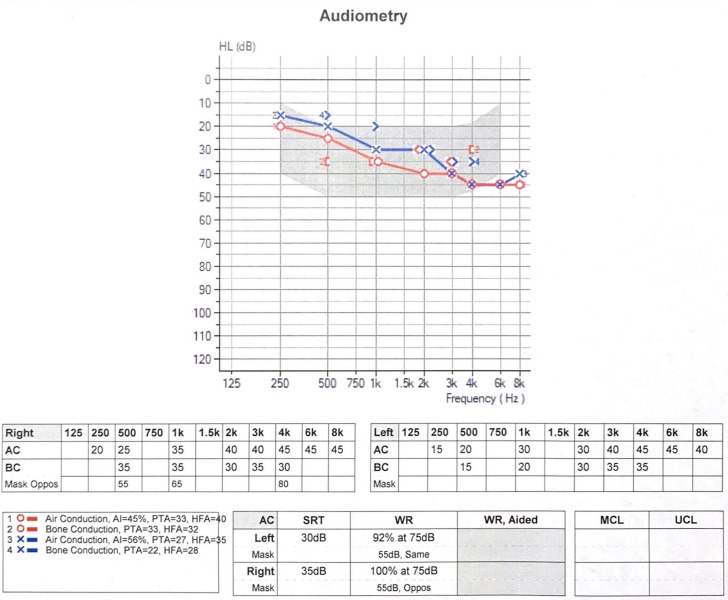
After discussion of these findings with the patient and her otolaryngologist, the decision was made to stop teprotumumab, forgoing the remaining doses to avoid potential dose-dependent ototoxicity. Repeat audiogram 6 weeks after cessation of teprotumumab showed no improvement (figure 3). The patient continues to be followed closely by her otolaryngologist with plans to consider hearing aids should there be no improvement after 3 months.
Figure 3.
Audiogram 6 weeks after cessation of teprotumumab shows no change from her audiogram after the fifth dose.
Discussion
This case describes a patient who objectively developed chronic sensorineural hearing loss after partial teprotumumab therapy for the treatment of thyroid eye disease.
Teprotumumab is a biological agent that inhibits the IGF-1 pathway by targeting the IGF-1R. IGF-1 is a neuroprotective agent that regulates the development and maintenance of the inner ear. It participates in cellular metabolism, growth, proliferation, differentiation and prevention of cell death of hair cells. IGF-1 deficiency is associated with profound sensorineural hearing loss in mice models and humans.13–15
Objective teprotumumab-associated chronic sensorineural hearing loss has been documented in the literature. In all cases, audiometry obtained following the initiation of treatment was performed only after the onset of subjective symptoms of hearing impairment, which varied from the third to the fifth dose. The timing of audiometric testing after the development of symptoms also varied widely from immediately to months later when symptoms persisted or worsened with subsequent infusions.11 12 The magnitude of hearing loss in these cases was typically mild to moderate-severe, with no improvement on audiometry several months after discontinuation of therapy.10 11
Patient screening and monitoring
Given the findings above, it is imperative to counsel patients on the risk of potentially irreversible sensorineural hearing loss prior to the initiation of teprotumumab. Risk factors should be considered such as history of otological insult, including age-related hearing loss, loud noise exposure and other conditions that degrade baseline otological function. Based on these factors, patients in collaboration with clinical providers may wish to defer or elect alternative treatments. A multidisciplinary team involving ophthalmology, otolaryngology, audiology and endocrinology is important for the care of patients with thyroid eye disease considering teprotumumab therapy.16 Baseline otological examination and audiometry should be established prior to treatment to identify susceptible patients and detect changes associated with drug therapy. This practice is standard with other medications with known ototoxicity potential such as cisplatin.17
The relationship between serum IGF-1 level and hyperthyroidism is unclear. Several studies suggest hyperthyroidism is associated with elevated serum IGF-1, while other studies found no difference in serum IGF-1 between hyperthyroid and euthyroid patients.18–21 In some animal models, low serum IGF-1 was associated with greater severity of hyperthyroid disease.22 Interestingly, one study found 18.4% of patients with newly diagnosed Graves’ disease had IGF-1 deficiency. Low IGF-1 was associated with higher thyroglobulin level and more severe free T3 hyperthyroidism at diagnosis.23 Serum IGF-1 testing may thus be useful to identify individuals predisposed to teprotumumab-associated hearing loss in the setting of a normal baseline audiogram. Interestingly, serum IGF-1 in our patient was elevated at 501 ng/mL (normal 37–208 ng/mL) 6 weeks after stopping teprotumumab, suggesting that elevated IGF-1 may not be protective either.
The published cases of teprotumumab-associated hearing loss performed audiograms after patients reported symptoms of hearing loss.10 11 Given that subjective hearing loss does not reliably reflect objective hearing loss, the onset of otological dysfunction in relation to drug dose and duration is unclear.12 In addition to baseline testing, we propose regular surveillance audiometric testing ideally after every infusion throughout the therapeutic course and after drug cessation. This degree of monitoring may not be practical but does provide the abililty to identify early, subclinical hearing impairment. It may also help characterize patterns of dose-dependent toxicity. We recommend at a minimum audiology testing pre, mid and post treatment.
Patients taking teprotumumab should be counselled and monitored closely for symptoms of early hearing dysfunction (ear fullness, muffled sounds, tinnitus, autophony, hypoacusis). Ototoxic medications and loud noise exposure should also be avoided.24
Potential treatment
Sensorineural hearing loss is primarily caused by damage or dysfunction of cochlear hair cells, which have little ability to proliferate or regenerate in postnatal mammalians. Systemic corticosteroids with or without local intratympanic corticosteroid injection has traditionally been used to treat idiopathic sudden onset sensorineural hearing loss, with variable success.25 26 Unfortunately, steroids would not be expected to help teprotumumab-associated hearing loss since its mechanism is not inflammatory. Oral steroids were used in one reported case of teprotumumab-associated hearing loss but were ineffective.10 Hearing aids and cochlear implantation are options to improve functional hearing but do not address the underlying pathophysiological cause. They do not prevent or reverse otological pathology and would be needed lifelong.
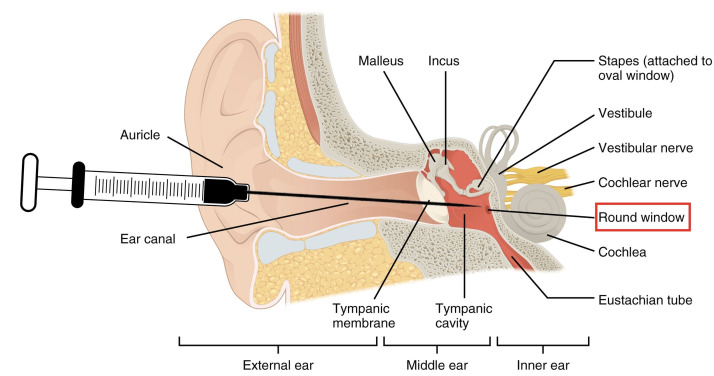
Local IGF-1 therapy has shown promising results in the treatment of refractory sensorineural hearing loss but is not yet currently widely used. It is given intratympanically and diffuses through the round window membrane to enter the inner ear (figure 4). Risks of any intratympanic injection include pain, headache, vertigo, nausea, infection and tympanic membrane perforation.27–31 This targeted therapy should be considered for treatment of teprotumumab-associated sensorineural hearing loss. Acting on the same signalling pathway in a timely manner could theoretically negate the local effects of teprotumumab in the ear. This could potentially prevent or reverse teprotumumab-associated sensorineural hearing loss. Prophylactic treatment in susceptible patients could also be considered.
Figure 4.
Schematic of intratympanic membrane injection to the round window. Modified and printed with permission from OpenStax Anatomy and Physiology. https://openstax.org/books/anatomy-and-physiology/pages/preface, https://creativecommons.org/licenses/by/4.0/deed.en
Stable drug delivery to the inner ear remains a challenge. Systemic IGF-1 is not well tolerated and is unlikely to pass through the blood-labyrinth barrier.32 External topical application such as ear drops would theoretically be the safest and least invasive route of delivery. However, this is significantly limited by the impermeable nature of the tympanic membrane which separates the outer and middle ear. Small peptide-linked particles have been shown to be actively transported across the intact tympanic membrane in in vivo rat models.33 Chemical permeation enhancers (bupivacaine, limonene, sodium dodecyl sulfate) may also aid increased drug permeability across the tympanic membrane, while hydrogel formulations maintain direct contact for maximal delivery.34 A chemical permeation enhanced hydrogel formulation of IGF-1 applied to tympanic membrane in the external ear could be a novel treatment strategy for teprotumumab-associated hearing loss. Drug delivery to the middle ear via the eustachian tube could also be considered.35 36 Currently, intratympanic injection remains the most widely used strategy for drug delivery to the inner ear.
Additionally, entry into the inner ear is limited by the permeability of the round window membrane. Several strategies have been employed to increase permeability including ultrasound microbubbles and drug carriers.37 Combined with these strategies to increase penetration into the inner ear, local application of topical IGF-1 could potentially be beneficial for patients with teprotumumab-associated hearing loss specifically.
Alternatives
Novel agents continue to be investigated as an alternative to teprotumumab for the treatment of thyroid eye disease. A low volume subcutaneous formulation of IGF-1R antagonist is currently in development, which could significantly improve convenience and cost of treatment. The longer half-life and greater stability of this agent would allow for fewer or less frequent dosing.38 39 Stable serum concentration would also minimize peaks and troughs, limiting drug exposure and dose-dependent toxicity to other organ systems. This is particularly important for areas with limited regenerative potential such as the inner ear.
In summary, teprotumumab has been associated with sensorineural hearing loss, through its antagonistic action on the IGF-1 signalling pathway, which engages in otological health. Further investigation is needed to better characterize teprotumumab-associated hearing loss, including its risk factors, frequency, magnitude and reversibility in relation to individual susceptibility, drug dosage and duration of treatment. This would potentially help establish safer, clearer guidelines and eligibility criteria for teprotumumab therapy.
Learning points.
Teprotumumab is associated with potentially irreversible sensorineural hearing loss.
Careful patient selection and counselling regarding these risks is imperative. Potential risk factors should be assessed including age, prior otological history and serum insulin-like growth factor-1 (IGF-1).
We recommend regular otological examination and audiometric testing before treatment, after each infusion, and after treatment, as well as close monitoring of hearing loss symptoms.
Topical IGF-1 therapy may be helpful in treating teprotumumab-associated hearing loss, however this is still undergoing evaluation.
Footnotes
Twitter: @rsilkiss
Contributors: AC drafted the manuscript. RS critically revised the manuscript and contributed to the discussion.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained directly from the patient(s).
References
- 1.Smith TJ, Hegedüs L, Douglas RS. Role of insulin-like growth factor-1 (IGF-1) pathway in the pathogenesis of Graves' orbitopathy. Best Pract Res Clin Endocrinol Metab 2012;26:291–302. 10.1016/j.beem.2011.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kahaly GJ, Douglas RS, Holt RJ, et al. Teprotumumab for patients with active thyroid eye disease: a pooled data analysis, subgroup analyses, and off-treatment follow-up results from two randomised, double-masked, placebo-controlled, multicentre trials. Lancet Diabetes Endocrinol 2021;9:360–72. 10.1016/S2213-8587(21)00056-5 [DOI] [PubMed] [Google Scholar]
- 3.Smith TJ, Kahaly GJ, Ezra DG, et al. Teprotumumab for thyroid-associated ophthalmopathy. N Engl J Med 2017;376:1748–61. 10.1056/NEJMoa1614949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Douglas RS, Kahaly GJ, Patel A, et al. Teprotumumab for the treatment of active thyroid eye disease. N Engl J Med 2020;382:341–52. 10.1056/NEJMoa1910434 [DOI] [PubMed] [Google Scholar]
- 5.Amarikwa L, Sears CM, Clauss K. Teprotumumab-Related adverse events in thyroid eye disease. Poster presented at: the American Society of ophthalmic plastic and reconstructive surgery 52nd annual fall scientific symposium; November 11-12, 2021; New Orleans, Louisiana, United States.
- 6.Chiou CA, Reshef ER, Freitag SK. Understanding the side effect profile of Teprotumumab. poster presented at: the American Society of ophthalmic plastic and reconstructive surgery 52nd annual fall scientific symposium; November 11-12, 2021; new Orleans, Louisiana, United States.
- 7.Sears CM, Pham B, Men C, et al. Teprotumumab for the treatment of recalcitrant TED. Poster presented at: The American Academy of Ophthalmology; 13 Nov 2021, New Orleans, Louisiana, United States, 2021. [Google Scholar]
- 8.Safo MB, Silkiss RZ. A case of ulcerative colitis associated with teprotumumab treatment for thyroid eye disease. Am J Ophthalmol Case Rep 2021;22:101069. 10.1016/j.ajoc.2021.101069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Douglas RS, Kahaly GJ, Ugradar S, et al. Teprotumumab efficacy, safety, and durability in Longer-Duration thyroid eye disease and Re-treatment: OPTIC-X study. Ophthalmology 2022;129:438–49. 10.1016/j.ophtha.2021.10.017 [DOI] [PubMed] [Google Scholar]
- 10.Highland J, Gordon S, Reddy D, et al. Ototoxicity and teprotumumab. Ann Otol Rhinol Laryngol 2021:000348942110427. 10.1177/00034894211042740 [DOI] [PubMed] [Google Scholar]
- 11.Belinsky I, Creighton FX, Mahoney N, et al. Teprotumumab and hearing loss: case series and proposal for audiologic monitoring. Ophthalmic Plast Reconstr Surg 2022;38:73–8. 10.1097/IOP.0000000000001995 [DOI] [PubMed] [Google Scholar]
- 12.Yu CY, Correa T, Simmons BA, et al. Audiology findings in patients with teprotumumab associated otologic symptoms. Am J Ophthalmol Case Rep 2021;24:101202. 10.1016/j.ajoc.2021.101202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodríguez-de la Rosa L, Lassaletta L, Calvino M, et al. The role of insulin-like growth factor 1 in the progression of age-related hearing loss. Front Aging Neurosci 2017;9:411. 10.3389/fnagi.2017.00411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Attias J, Zarchi O, Nageris BI, et al. Cochlear hearing loss in patients with Laron syndrome. Eur Arch Otorhinolaryngol 2012;269:461–6. 10.1007/s00405-011-1668-x [DOI] [PubMed] [Google Scholar]
- 15.Camarero G, Avendano C, Fernandez-Moreno C, et al. Delayed inner ear maturation and neuronal loss in postnatal Igf-1-deficient mice. J Neurosci 2001;21:7630–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chern A, Dagi Glass LR, Gudis DA. Thyroid eye disease, Teprotumumab, and hearing loss: an evolving role for otolaryngologists. Otolaryngol Head Neck Surg 2021;165:019459982110042. 10.1177/01945998211004240 [DOI] [PubMed] [Google Scholar]
- 17.Nagy JL, Adelstein DJ, Newman CW, et al. Cisplatin ototoxicity: the importance of baseline audiometry. Am J Clin Oncol 1999;22:305–8. 10.1097/00000421-199906000-00020 [DOI] [PubMed] [Google Scholar]
- 18.Völzke H, Friedrich N, Schipf S, et al. Association between serum insulin-like growth factor-I levels and thyroid disorders in a population-based study. J Clin Endocrinol Metab 2007;92:4039–45. 10.1210/jc.2007-0816 [DOI] [PubMed] [Google Scholar]
- 19.Tseng F-Y, Chen Y-T, Chi Y-C, et al. Serum levels of insulin-like growth factor 1 are negatively associated with log transformation of thyroid-stimulating hormone in Graves' disease patients with hyperthyroidism or subjects with euthyroidism: a prospective observational study. Medicine 2019;98:14862. 10.1097/MD.0000000000014862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lakatos P, Foldes J, Nagy Z, et al. Serum insulin-like growth factor-I, insulin-like growth factor binding proteins, and bone mineral content in hyperthyroidism. Thyroid 2000;10:417–23. 10.1089/thy.2000.10.417 [DOI] [PubMed] [Google Scholar]
- 21.Eke Koyuncu C, Turkmen Yildirmak S, Temizel M, et al. Serum resistin and insulin-like growth factor-1 levels in patients with hypothyroidism and hyperthyroidism. J Thyroid Res 2013;2013:306750. 10.1155/2013/306750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rochel D, Burger M, Nguyen P, et al. Insulin-like growth factor type 1 concentrations in hyperthyroid cats before and after treatment with thiamazole. J Feline Med Surg 2018;20:179–83. 10.1177/1098612X17694735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin S, Sirbu A, Betivoiu M, et al. IGF1 deficiency in newly diagnosed Graves' disease patients. Hormones 2015;14:651–9. 10.14310/horm.2002.1577 [DOI] [PubMed] [Google Scholar]
- 24.Reed DS, Kostosky N, Davies BW, et al. Rifle blast exacerbating hearing loss in a patient treated with teprotumumab for thyroid eye disease. Ophthalmic Plast Reconstr Surg 2022;38:e41–3. 10.1097/IOP.0000000000002078 [DOI] [PubMed] [Google Scholar]
- 25.Wei BP, Stathopoulos D, O'Leary S. Steroids for idiopathic sudden sensorineural hearing loss. Cochrane Database Syst Rev 2013;2013:CD003998. 10.1002/14651858.CD003998.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spear SA, Schwartz SR. Intratympanic steroids for sudden sensorineural hearing loss: a systematic review. Otolaryngol Head Neck Surg 2011;145:534–43. 10.1177/0194599811419466 [DOI] [PubMed] [Google Scholar]
- 27.Yamahara K, Yamamoto N, Nakagawa T, et al. Insulin-like growth factor 1: a novel treatment for the protection or regeneration of cochlear hair cells. Hear Res 2015;330:2–9. 10.1016/j.heares.2015.04.009 [DOI] [PubMed] [Google Scholar]
- 28.Nakagawa T, Sakamoto T, Hiraumi H, et al. Topical insulin-like growth factor 1 treatment using gelatin hydrogels for glucocorticoid-resistant sudden sensorineural hearing loss: a prospective clinical trial. BMC Med 2010;8:76. 10.1186/1741-7015-8-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee KY, Nakagawa T, Okano T, et al. Novel therapy for hearing loss: delivery of insulin-like growth factor 1 to the cochlea using gelatin hydrogel. Otol Neurotol 2007;28:976–81. 10.1097/MAO.0b013e31811f40db [DOI] [PubMed] [Google Scholar]
- 30.Dave VJ, Joshi A, Bradoo R, et al. Effects of insulin-like growth factor (IGF-1) in patients with sensorineural hearing loss. J Int Adv Otol 2021;17:207–14. 10.5152/iao.2021.8549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakagawa T, Kumakawa K, Usami S-ichi, et al. A randomized controlled clinical trial of topical insulin-like growth factor-1 therapy for sudden deafness refractory to systemic corticosteroid treatment. BMC Med 2014;12:219. 10.1186/s12916-014-0219-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu H, Hao J, Li KS. Current strategies for drug delivery to the inner ear. Acta Pharm Sin B 2013;3:86–96. 10.1016/j.apsb.2013.02.003 [DOI] [Google Scholar]
- 33.Kurabi A, Pak KK, Bernhardt M, et al. Discovery of a biological mechanism of active transport through the tympanic membrane to the middle ear. Sci Rep 2016;6:22663. 10.1038/srep22663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khoo X, Simons EJ, Chiang HH, et al. Formulations for trans-tympanic antibiotic delivery. Biomaterials 2013;34:1281–8. 10.1016/j.biomaterials.2012.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klug C, Fabinyi B, Tschabitscher M. Endoscopy of the middle ear through the eustachian tube: anatomic possibilities and limitations. Am J Otol 1999;20:299–303. [PubMed] [Google Scholar]
- 36.Todt I, Seidl R, Ernst A. A new minimally invasive method for the transtubal, microendoscopic application of fluids to the middle ear. Minim Invasive Ther Allied Technol 2008;17:300–2. 10.1080/13645700802274786 [DOI] [PubMed] [Google Scholar]
- 37.Lin Y-C, Lin Y-Y, Chen H-C, et al. Ultrasound microbubbles enhance the efficacy of insulin-like growth factor-1 therapy for the treatment of noise-induced hearing loss. Molecules 2021;26:3626. 10.3390/molecules26123626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Viridian Therapeutics, Inc . VRDN-002, a second-generation insulin like growth factor-1 receptor (IGF-1R) antagonist antibody for thyroid eye disease: preclinical pharmacokinetic profile and clinical promise. Presented at: the 90th annual meeting of the American thyroid association. Virtual event; September 30, 2021, 2021. [Google Scholar]
- 39.Viridian Therapeutics . Viridian corporate presentation, 2021. Available: https://investors.viridiantherapeutics.com/presentations/default.aspx [Accessed 8 Nov 2021].