Abstract
Amoxicillin-clavulanate resistance (MIC >16 μg/ml) and the corresponding molecular mechanisms were prospectively studied in Escherichia coli over a 3-year period (1996 to 1998) in 14 French hospitals. The overall frequency of resistant E. coli isolates remained stable at about 5% over this period. The highest frequency of resistant isolates (10 to 15%) was observed, independently of the year, among E. coli isolated from lower respiratory tract samples, and the isolation rate of resistant strains was significantly higher in surgical wards than in medical wards in 1998 (7.8 versus 2.8%). The two most frequent mechanisms of resistance for the 3 years were the hyperproduction of the chromosomal class C β-lactamase (48, 38.4, and 39.7%) and the production of inhibitor-resistant TEM (IRT) enzymes (30.4, 37.2, and 41.2%). By using the single-strand conformational polymorphism–PCR technique and sequencing methods, we determined that 59 IRT enzymes corresponded to previously described IRT enzymes whereas 8 were new. Three of these new enzymes derived from TEM-1 by only one amino acid substitution (Ser130Gly, Arg244Gly, and Asn276Asp), whereas three others derived by two amino acid substitutions (Met69Leu and Arg244Ser, Met69Leu and Ile127Val, and Met69Val and Arg275Gln). The two remaining new IRTs showed three amino acid substitutions (Met69Val, Trp165Arg, and Asn276Asp and Met69Ile, Trp165Cys, and Arg275Gln). New genetic features were also found in blaTEM genes, namely, blaTEM-1B with either the promoters Pa and Pb, P4, or a promoter displaying a C→G transversion at position 3 of the −35 consensus sequence and new blaTEM genes, notably one encoding TEM-1 but possessing the silent mutations originally described in blaTEM-2 and then in some blaTEM-encoding IRT enzymes.
Throughout the world, microbiologists are encouraged to survey the antibiotic resistances of major pathogens in order to provide the people in charge of public health with epidemiological data helpful in making recommendations on the best use of antibiotics.
We surveyed amoxicillin-clavulanate resistance in Escherichia coli over a 3-year period (1996 to 1998) because amoxicillin-clavulanate is one of the most used antibiotics in France and also because E. coli is the major enterobacterial pathogen. In addition to the evolution of amoxicillin-clavulanate-resistant E. coli isolates, which was studied both globally and according to different parameters (patient status, wards, and biological specimens), we also analyzed the evolution of the principal mechanisms involved in amoxicillin-clavulanate resistance in E. coli. These mechanisms include hyperproduction of the chromosomal class C β-lactamase of E. coli, hyperproduction of plasmid-mediated TEM enzymes, production of oxacillinases, and the production of TEM-derived enzymes whose β-lactamase activities are no longer inhibited by clavulanate (inhibitor-resistant TEM [IRT] enzymes) (11–13, 25, 26, 28, 33). However, all these mechanisms which are able to generate amoxicillin-clavulanate resistance in E. coli do not generate the same β-lactam cross-resistance. Thus, a knowledge of the evolution of these different mechanisms might lead us to give different recommendations for the choice of antibiotics to treat an infection presumed to be caused by E. coli.
(Part of this study was presented at the 39th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, Calif., 1999.)
MATERIALS AND METHODS
Clinical isolates.
During the same 3 months of the years 1996, 1997, and 1998, the microbiological laboratories of 14 (13 in 1997) French hospitals distributed around the country (seven teaching and seven nonteaching hospitals) collected consecutive and nonrepetitive E. coli isolates (only one isolate with a given antibiogram per patient) and filled out a form for each isolate in which the patient status (out-or inpatient), the ward (pediatrics, geriatrics, medicine, surgery, or intensive-care unit) in which each inpatient was hospitalized, and the biological specimen from which each isolate was obtained were specified. The isolates considered resistant to amoxicillin-clavulanate on the basis of the agar disk diffusion method and in accordance with the recommendations of the French Antibiogram Committee (inhibition zone diameter, <14 mm) (1) were sent with their corresponding antibiograms to our laboratory, where we confirmed the resistance and analyzed the underlying mechanisms by molecular methods.
Susceptibility testing.
Amoxicillin-clavulanate resistance was confirmed for each isolate by the E-test method (AB Biodisk, Dammartin sur Tigeaux, France) performed according to the manufacturer's recommendations. The amoxicillin-clavulanate-resistant isolates were classified in two groups on the basis of the standard antibiogram performed by each laboratory: group I, comprising the isolates displaying an additional resistance to cefpodoxime (inhibition zone diameter, <21 mm) and a reduced susceptibility to cefoxitin (inhibition zone diameter, <22 mm) (1), and group II, comprising the isolates fully susceptible to these two antibiotics. The isolates of group I were considered to be hyperproducing their chromosomal class C β-lactamase and not to be producing plasmid-mediated AmpC-like enzymes on the basis of the conserved susceptibility to broad-spectrum cephalosporins or aztreonam (3, 6, 15), and no further molecular analysis was made in this study to characterize this mechanism of resistance.
Molecular detection of TEM, IRT, and OXA-1 enzymes.
As previously described, two specific primers of blaTEM genes were used to amplify the entire gene and its promoter region and two other primers specific for the gene encoding OXA-1 were used to amplify an internal 609-bp fragment of this gene. When the PCR was positive for the blaTEM gene, pairs of primers designed to obtain three overlapping fragments were used to carry out the single-strand conformational polymorphism (SSCP)–PCR, as we have previously demonstrated (34). Briefly, each 32P-labeled amplified fragment was submitted to electrophoresis on a native polyacrylamide gel after heat and formamide denaturation. The electrophoretic mobility of each fragment was compared to that of the corresponding fragment of the reference blaTEM genes: blaTEM-1A, blaTEM-1B, and blaTEM-2. The blaTEM genes whose three fragments comigrated with the corresponding fragments of reference blaTEM genes were assessed as TEM-encoding genes, whereas those whose fragment 1, 2, or 3 did not comigrate with the corresponding fragments of the reference genes were assessed as blaTEM gene derivatives and subsequently sequenced.
Nucleotide sequence.
PCR products for sequencing were prepared under the standard conditions described above. The PCR products were purified using the QIAquick PCR purification kit (QIAGEN, Courtaboeuf, France) following the manufacturer's recommendations. The nucleotide sequences of purified PCR fragments were determined with an automated cycle-sequencing system on a Perkin-Elmer R377 sequencer and the same primers used for PCR (34).
Statistical analysis.
The differences between proportions were evaluated by a chi-square test at the 5% level of significance.
Nucleotide sequence accession numbers.
The nucleotide sequence data reported here for blaTEM-76, blaTEM-77, blaTEM-78, and blaTEM-79 have been submitted to GenBank and have been assigned accession no. AF 190694, AF 190695, AF 190693, and AF 190692, respectively.
RESULTS
Distribution of E. coli clinical isolates.
One thousand six hundred twenty and 1,606 E. coli isolates were collected in 1996 and 1998, respectively; only 1,483 were collected in 1997 because one of the 14 laboratories was not able to participate in the study that year. There were no statistical differences in the distributions of these isolates according to the different parameters except for the deep-pus specimens, whose frequency was significantly (P < 0.001) higher in 1998 (10.3%) than in 1996 (6.7%) and 1997 (6.8%). Each year, the teaching hospitals provided approximately one-half of the isolates and the nonteaching hospitals provided the other half. Each year, 10% of the isolates came from children and 90% came from adults, and 18 and 82% were from outpatients and inpatients, respectively. About 8% of the isolates were obtained from adult patients hospitalized in intensive-care units, 8% were from patients in geriatrics, 16% were from patients in surgery, and 38% were from patients in medical wards. The great majority of isolates (80% in 1996 and 1997 and 75.5% in 1998) were urinary tract isolates, while 9 and 3% were isolated from blood and lower respiratory tract secretions, respectively.
Epidemiology of E. coli isolates resistant to amoxicillin-clavulanate.
Amoxicillin-clavulanate resistance, first determined by the agar disk diffusion method (inhibition zone diameter, <14 mm) for 79 isolates in 1996, 78 in 1997, and 68 in 1998, was subsequently confirmed by the E-test method (MIC >16 μg/ml) (1). Thus, 4.8% of E. coli isolates collected in 1996, 5.2% of those collected in 1997, and 4.2% of those collected in 1998 were resistant, but these frequency differences were not statistically significant.
As indicated in Table 1, there was no significant evolution of the frequency of amoxicillin-clavulanate-resistant E. coli isolates in children, adults, out- and inpatients, or teaching and nonteaching hospitals. The comparison of the frequency of amoxicillin-clavulanate-resistant isolates which was made each year among all the different patient and hospital categories showed that the frequency of these isolates was significantly higher for inpatients than for outpatients only in 1996 (P = 0.01) and 1997 (P = 0.03).
TABLE 1.
Frequencies of amoxicillin-clavulanate (AMC)-resistant E. coli isolates by patient and hospital characteristics
| Parameter | No. (%) of AMC-resistant E. coli isolates
|
||
|---|---|---|---|
| 1996 | 1997 | 1998 | |
| Children | 6 (3.8) | 5 (3.0) | 9 (5.1) |
| Adults | 78 (5.5) | 75 (5.7) | 66 (4.6) |
| Outpatients | 7 (2.5)a | 7 (2.7)a | 10 (3.4) |
| Inpatients | 81 (6.1)a | 73 (6.0)a | 65 (5.0) |
| Teaching hospitals | 42 (5.6) | 43 (5.5) | 45 (6.0) |
| Nonteaching hospitals | 46 (5.3) | 37 (5.3) | 30 (3.5) |
Significant difference between the frequencies of AMC-resistant E. coli isolated in outpatients and inpatients in 1996 (P = 0.01) and 1997 (P = 0.03).
Regarding the rate of amoxicillin-clavulanate-resistant E. coli isolates studied in the different wards (Table 2), it was shown that there was a significant evolution of this rate only in medical wards and only for the years 1997 and 1998. In fact, in this type of ward, the frequency of amoxicillin-clavulanate-resistant E. coli isolates significantly decreased, from 7% in 1997 to 2.8% in 1998 (P = 0.008). The comparative analysis of frequencies between different wards for each year showed that there was no significant difference, except in 1998 between medical and surgical wards, in which the frequencies of amoxicillin-clavulanate-resistant E. coli isolates were 2.8 and 7.8%, respectively (P = 0.001).
TABLE 2.
Frequencies of amoxicillin-clavulanate (AMC)-resistant E. coli isolates by wards
| Ward | No. (%) of AMC-resistant E. coli isolates
|
||
|---|---|---|---|
| 1996 | 1997 | 1998 | |
| Intensive-care units | 8 (6.0) | 7 (6.5) | 8 (6.7) |
| Surgery | 24 (9.0) | 13 (5.5) | 19 (7.8)b |
| Medicine | 27 (4.5) | 40 (7.0)a | 16 (2.8)ab |
| Geriatrics | 9 (7.3) | 6 (5.0) | 10 (7.0) |
Significant decrease between the frequencies of AMC-resistant E. coli in 1998 and 1997 in medical wards (P = 0.008).
Frequency of AMC-resistant E. coli was significantly lower in medical wards than in surgical wards in 1998 (P = 0.001).
Irrespective of the year in question, the highest frequency of amoxicillin-clavulanate-resistant E. coli isolates was observed among the E. coli strains isolated from lower respiratory tract samples (Table 3). In 1997 and 1998, this prevalence was significantly higher than those determined for the other samples (urine, blood samples, and deep pus). For the same type of sample, the frequency of amoxicillin-clavulanate-resistant E. coli isolates varied over the 3 years, but not significantly, except for blood samples, for which the frequency was significantly higher in 1996 (7.3%) than in 1998 (1.4%) (P = 0.04).
TABLE 3.
Frequencies of amoxicillin-clavulanate (AMC)-resistant E. coli isolates by sample
| Sample | No. (%) of AMC-resistant E. coli isolates
|
||
|---|---|---|---|
| 1996 | 1997 | 1998 | |
| Lower respiratory tract | 5 (9.4) | 6 (14.6)b | 7 (15.2)b |
| Urine | 67 (5.2) | 67 (5.6)b | 52 (4.3)b |
| Blood | 8 (6.0)a | 4 (3.1)b | 2 (1.4)ab |
| Deep pus | 8 (7.3) | 3 (2.9)b | 8 (4.8)b |
Significantly lower frequencies of AMC-resistant E. coli isolates in blood in 1998 than in 1996 (P = 0.04).
Significantly higher frequencies of AMC-resistant E. coli isolates in lower respiratory tract samples than in each other type of sample in 1997 and 1998; urine (1997, P = 0.03; 1998, P = 0.04), blood (1997, P = 0.01; 1998, P = 0.0007), and deep pus (1997, P = 0.02; 1998, P = 0.02).
Mechanisms of resistance to amoxicillin-clavulanate.
Among the 225 amoxicillin-clavulanate-resistant E. coli isolates collected over the 3-year period, 98 isolates which displayed a coresistance to amoxicillin-clavulanate and cefpodoxime and a reduced susceptibility to cefoxitin were considered to be hyperproducing their chromosomal class C β-lactamase. However, for 47 (48%) of them, the blaTEM PCR was positive, and TEM enzyme production was demonstrated in 41 of the cases on the basis of SSCP-PCR experiments, as the three amplified blaTEM fragments of the corresponding genes comigrated with those of reference blaTEM genes. For six isolates which displayed SSCP-PCR profiles different from those of the reference blaTEM genes, the nucleotide sequences of the corresponding blaTEM genes showed the presence of either blaTEM-like genes (n = 3) or blaTEM genes encoding IRT enzymes (n = 3). The blaTEM gene of strain 602042 differed from blaTEM-1B by the silent mutation G162T, whereas that of strain 710020 resembled blaTEM-1A except for the replacement of cytosines 436 and 913 by thymidines (Table 4). The third blaTEM-like gene, found in strain 606160, was more closely related to blaTEM-2, although the nucleotide mutation leading to the amino acid substitution Glu39Lys was absent and the promoter region was different, showing thymidine 32 replaced by cytosine and guanine 162 replaced by thymidine (Table 4). The amino acid sequences deduced from the nucleotide sequences of the blaTEM genes of strains 610087, 710085, and 609046 showed that the two first strains produced IRT-2 (TEM-30) whereas the third produced a new TEM derivative in which there was only one amino acid substitution, namely, Asn276Asp (Table 4).
TABLE 4.
Nucleotide mutations and amino acid substitutions in blaTEM-like genes and new IRT-encoding genes in comparison with blaTEM-1A, blaTEM-1B, blaTEM-1C, and blaTEM-2 genes
| Strain | blaTEM | Mutation (substitution) at nucleotide position (amino acid codon)
|
Reference | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 32 | 141 (−35) | 162 (−10) | 175 | 226 (6) | 317 (39) | 346 (48) | 407 (69) | 436 (78) | 581 (127) | 590 (130) | 604 (134) | 682 (160) | 695 (165) | 697 (165) | 913 (237) | 925 (242) | 929 (244) | 1020 (275) | 1022 (276) | |||
| 1A | C | C | G | A | C | C (Gln) | A | A (Met) | C | A (Ile) | A (Ser) | G | T | T (Trp) | G (Trp) | C | G | C (Arg) | G (Arg) | A (Asn) | 36 | |
| 1B | G | T | T | T | 18a | |||||||||||||||||
| 1C | T | 18 | ||||||||||||||||||||
| 2 | T | A (Lys) | G | T | C | A | 18a | |||||||||||||||
| 602042 | 1B-like | T | G | T | T | T | ||||||||||||||||
| 714028 | 1B-like | T | G | T | T | T | ||||||||||||||||
| 805009 | 1B-like | G | G | T | T | T | ||||||||||||||||
| 710020 | 1D | T | T | |||||||||||||||||||
| 801148 | 1E | G | T | G | T | T | ||||||||||||||||
| 606160 | 1F | T | G | T | C | A | ||||||||||||||||
| 609046 | 84 | T | G | T | A | G (Asp) | ||||||||||||||||
| 614078 | 84 | T | G | T | A | G (Asp) | ||||||||||||||||
| 811020 | 76 | G | T | T | G (Gly) | T | ||||||||||||||||
| 803013 | 79 | G | T | T | G (Gly) | |||||||||||||||||
| 801009 | 77 | G | T | C (Leu) | T | T | A (Ser) | |||||||||||||||
| 601105 | 81 | T | C (Leu) | G (Val) | ||||||||||||||||||
| 614024 | 82 | T | G (Val) | A (Gln) | ||||||||||||||||||
| 801155 | 78 | T | G | G (Val) | T | C | C (Arg) | A | G (Asp) | |||||||||||||
| 813147 | 83 | T | G | T (Ile) | T | C | T (Cys) | A | A (Gln) | |||||||||||||
Among the 127 isolates resistant to amoxicillin-clavulanate but susceptible to cefpodoxime and cefoxitin, 9 displayed an OXA-1-positive PCR, 117 had a TEM-positive PCR, and 1 had both OXA-1- and TEM-positive PCRs.
For 32 out of the 118 blaTEM PCR products, the electrophoretic mobilities of the three amplified fragments were identical to those of the reference blaTEM genes.
A particular SSCP-PCR profile was observed for 17 out of the 118 blaTEM PCR products, meaning that the profile of each fragment was composed of four bands instead of two (Fig. 1). As two of these bands comigrated with those of reference blaTEM genes and two did not, this profile was thought to have resulted from the coamplification of blaTEM and blaTEM derivative genes.
FIG. 1.
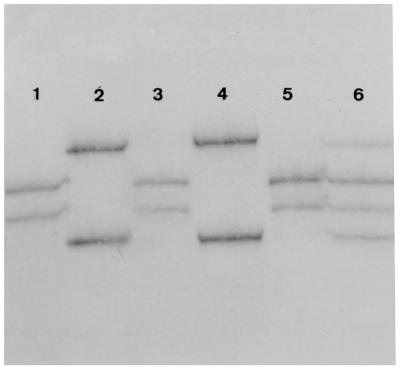
SSCP profiles of fragment 3 of blaTEM genes expressed by four clinical E. coli isolates and of reference genes blaTEM-1 and blaTEM-2. As the nucleotide sequences of blaTEM-1A and blaTEM-1B are identical for fragment 3, there is only one SSCP profile for the two genes, which is shown in lane 3. The SSCP profile of fragment 3 of the reference blaTEM-2 gene is shown in lane 4. Two clinical isolates (lanes 1 and 5) expressed a blaTEM gene whose fragment 3 comigrates with that of blaTEM-2. The migration profile of fragment 3 of the blaTEM gene expressed by a third clinical isolate (lane 2) differs slightly from that of blaTEM-2 (lane 4). In lane 6 is shown a migration profile consisting of four bands. As two of these bands comigrate with those of blaTEM-1 and the other two do not comigrate with those of any reference genes, this profile is thought to result from the coamplification of blaTEM-1 and a blaTEM derivative.
The nucleotide sequence determined for the remaining 69 blaTEM PCR products displaying SSCP-PCR profiles different from those of reference blaTEM genes showed that four of them were blaTEM-like genes (Table 4) and 65 were blaTEM gene derivatives. Three of the blaTEM-like genes were blaTEM-1B-like genes, each demonstrating a singular silent mutation to distinguish it from blaTEM-1B: C32T in strain 714028, A346G in strain 801148, and C141G in strain 805009 (Table 4). The fourth blaTEM-like gene was a blaTEM-1C gene which differed from blaTEM-1A by the mutation C436T.
The amino acid sequences deduced from the nucleotide sequences of the remaining 65 blaTEM genes showed that 57 isolates produced previously described IRT enzymes: 15 isolates produced IRT-2 (TEM-30; one of these also produced an OXA-1 enzyme), 14 produced IRT-11 (TEM-40), 9 produced IRT-4 (TEM-35), 6 produced IRT-8 (TEM-37), 4 produced IRT-7 (TEM-36), 3 produced IRT-6 (TEM-34), 3 produced IRT-10 (TEM-39), 1 produced IRT-5 (TEM-33), 1 produced IRT-1 (TEM-31), and 1 produced IRT-9 (TEM-38).
Eight isolates produced IRT enzymes which have not yet been described (Table 4). Three new IRT enzymes derived from TEM-1 by one amino acid substitution: Ser130Gly (strain 811020; TEM-76), Arg244Gly (strain 803013; TEM-79), and Asn276Asp (strains 614078 and 609046; TEM-84). Three other new IRT enzymes derived from TEM-1 by two amino acid substitutions: Met69Leu and Arg244Ser (strain 801009; TEM-77), Met69Leu and Ile127Val (strain 601105; TEM-81), and Met69Val and Arg275Gln (strain 614024; TEM-82). The last two new IRT enzymes derived from TEM-1 by three amino acid substitutions: Met69Val, Trp165Arg, and Asn276Asp (strain 805155; TEM-78) and Met69Ile, Trp165Cys, and Arg275Gln (strain 813147; TEM-83).
Evolution of the mechanisms involved in amoxicillin-clavulanate resistance.
As indicated in Table 5, the frequency of E. coli isolates whose amoxicillin-clavulanate resistances were considered to be related to the hyperproduction of their chromosomal class C β-lactamase decreased over the 3-year period (48% in 1996 and 39.7% in 1998), whereas the frequency of those whose amoxicillin-clavulanate resistances were presumed to be related to the production of IRT enzymes increased (30.4% in 1996 and 41.2% in 1998). However, this opposite evolution was not significant as was also the case for the decrease in TEM-producing isolates (15% in 1996 and 10.3% in 1998) and the increase in OXA-1-producing isolates (3.8% in 1996 and 7.3% in 1998). The association of two mechanisms responsible for amoxicillin-clavulanate resistance, such as OXA-1 and IRT enzymes or IRT enzymes and presumed hyperproduced class C β-lactamase, remained rare (Table 5).
TABLE 5.
Distribution of amoxicillin-clavulanate resistance mechanisms in 225 E. coli isolates by year
| Mechanisma | No. (%) of E. coli isolates
|
|||
|---|---|---|---|---|
| 1996 | 1997 | 1998 | Total | |
| CASE (± TEM) | 38 (48) | 30 (38.4) | 27 (39.7) | 95 (42.2) |
| TEM | 12 (15) | 17 (21.8) | 7 (10.3) | 36 (16) |
| OXA-1 | 3 (3.8) | 1 (1.3) | 5 (7.3) | 9 (4) |
| IRT (± TEM) | 24 (30.4) | 29 (37.2) | 28 (41.2) | 81 (36) |
| CASE + IRT | 2 (2.5) | 1 (1.3) | 0 | 3 (1.3) |
| OXA-1 + IRT | 0 | 0 | 1 (1.5) | 1 (0.4) |
| Total | 79 (100) | 78 (100) | 68 (100) | 225 (100) |
CASE, cephalosporinase; OXA-1, oxacillinase-1.
DISCUSSION
Amoxicillin-clavulanate, which was first produced commercially almost 20 years ago, continues to be a contributing antibiotic to overcome the problem of class A β-lactamase-producing bacteria, especially fastidious bacteria, such as Haemophilus influenzae and Moraxella catarrhalis. Such a feature can certainly explain why amoxicillin-clavulanate is so intensively prescribed, and sometimes abused, to empirically treat otitis, sinusitis, and respiratory tract infections (19). In the case of the normally amoxicillin-susceptible enterobacteria, particularly E. coli, the emergence of isolates less susceptible to amoxicillin-clavulanate was noted as early as 1987 among the amoxicillin-resistant isolates (25). Since then, several studies which evaluated the antibiotic susceptibilities of enterobacteria in different European countries have shown that the frequency of E. coli with reduced susceptibility to amoxicillin-clavulanate has not exceeded 25 to 30% in hospitals (8, 17, 24, 27, 29, 31; M. H. Nicolas-Chanoine, J. Sirot, and H. Chardon, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 2253, 1999) and 10% in the community (16) for the last 10 years. Nevertheless, some studies reported frequencies of nonsusceptible E. coli isolates of 40% (21, 27), whereas others reported frequencies as low as 5% (35). As recently pointed out by Simpson et al., differences in amoxicillin-clavulanate susceptibility among the E. coli isolates of different countries can result from real localized resistance problems but also from methodological differences in susceptibility testing and breakpoint criteria (32). Moreover, the group of nonsusceptible isolates includes intermediate as well as resistant isolates, and the classification of isolates into these two subcategories also depends on methodological criteria. Our study focused on E. coli strains for which the amoxicillin-clavulanate MIC was higher than 16 μg/ml, i.e., on E. coli considered to be resistant to amoxicillin-clavulanate irrespective of the methodology used (32).
To our knowledge, this is the first study which has observed the evolution of the amoxicillin-clavulanate susceptibility of E. coli over a 3-year period in a large number of hospitals by taking into account different criteria, such as patient characteristics, hospital status, medical specialties, and sample origins.
During the 3-year period of our study (1996 to 1998), the overall frequency of amoxicillin-clavulanate-resistant E. coli remained stable at about 5%. A similar observance of a stable frequency was also made by Stapleton, who conducted a survey from 1990 to 1994 but described much lower resistance rates (35). The percentage of 5% resistant isolates was found, in our study, for urinary tract isolates of E. coli, and this is quite a bit lower than that of Henquell et al., who reported about 25% resistant isolates obtained from a single hospital and 10% obtained from private laboratories in Clermont Ferrand, France, in 1993 (21). As their study included only one hospital, this difference could be attributed to the local spread of E. coli clones.
The highest frequencies of amoxicillin-clavulanate-resistant E. coli isolates were observed in samples from the lower respiratory tract. These frequencies, which increased from 9.4% in 1996 to 14.6% in 1997 and to 15.2% in 1998, were significantly higher than those in all other samples. Such an observation should be taken into account by clinicians in charge of patients with chronic lung diseases who are hospitalized for acute lower respiratory tract infections because amoxicillin-clavulanate is one of the primary drugs recommended for the treatment of such patients (2, 22). With regard to the different medical specialties, it is interesting to note that E. coli isolates obtained from patients in surgery wards, independent of the sample origin, were significantly more often resistant to amoxicillin-clavulanate than those from patients in medical wards in 1998. Those surgeons who are in charge of patients with abdominal problems should be aware of this result, because amoxicillin-clavulanate, which is indicated in France for the treatment of intra-abdominal infections, is one of the most prescribed antibiotics for its concomitant activity against enterobacteria and anaerobes.
In addition to the clinical epidemiology of amoxicillin-clavulanate-resistant E. coli, we also studied the corresponding mechanisms of resistance and their evolution. Although relevant for assessing the adaptation of bacteria to antibiotic pressure, studies of the molecular epidemiology of mechanisms of resistance remain rare. Henquell et al., who studied the mechanisms of resistance to amoxicillin-clavulanate of urinary tract E. coli isolates exclusively, found that TEM production (48%) was the first mechanism and IRT production (27.5%) the second mechanism for E. coli isolated from hospitalized patients and the converse (34% TEM and 45% IRT production) for E. coli isolates collected by private laboratories in Clermont Ferrand, France (21). In our study, we found quite a different distribution of mechanisms, showing presumed hyperproduction of the class C β-lactamase as the predominant mechanism (48%) in 1996, followed by IRT production (30.4%), while both mechanisms were found to be equivalent in 1997 (38.4 and 37.2%) and 1998 (39.7 and 41.2%). The fact that IRT production tended to increase could appear paradoxical, because this mechanism is linked to a narrower spectrum of β-lactam hydrolysis than that of class C β-lactamase production. However, it might reflect the antibiotic selective pressure by penicillin molecules, which have been widely used over the last 10 years. Amoxicillin-clavulanate resistance due to the pure production of TEM enzymes was, irrespective of the study year, always at a lower rate than that found by Henquell et al. (21). However, in both studies the frequencies of OXA-1-producing E. coli remained low. We confirm in this study the previously described relevance of the SSCP-PCR method for screening and differentiating genes encoding TEM- and TEM-derived β-lactamases. By using this method, we were able to detect new blaTEM-like genes and new IRT-encoding blaTEM genes. Regarding the new blaTEM-like genes, we found blaTEM-1B genes with either the strong promoters Pa and Pb (C32T), described by Chen (14) and initially present in blaTEM-2, or the strong promoter (G162T) recently designated P4 by Goussard et al. (18). Given that blaTEM-1B-derived genes, such as blaTEM-30 (10), blaTEM-33b (18), and blaTEM-38 (34), possess promoters Pa and Pb, we consider that we have discovered the ancestral blaTEM-1B gene of these derivatives. A third blaTEM1B-like gene also displays modifications in the promoter, namely, the mutation C141G located at position 3 in the −35 consensus of the Pribnow box, which is a new genetic feature in the promoters of blaTEM genes. A fourth blaTEM gene is similar to blaTEM-1C (18) except for the mutation C913T, whereas a fifth is similar to blaTEM-1B except for the mutation A346G, previously described in the blaTEM-2 gene. We suggest designating these two genes blaTEM-1D and blaTEM-1E, respectively. The last blaTEM-like gene we described is completely new. In fact, it possesses all the silent mutations observed in the blaTEM-2 gene in comparison with the blaTEM-1A gene but not the amino acid substitution Gln39Lys, and it displays the strong promoter P4 instead of Pa and Pb as in the blaTEM-2 gene. As these silent mutations and this promoter are shared by several blaTEM genes encoding IRT enzymes (TEM-33c, -34, -35, -36, -37, -39, and -45) (9, 10, 18, 34), we think we have identified the ancestor of these derivatives, and we therefore suggest calling this gene blaTEM-1F.
Our study has also contributed to the discovery of eight new IRT enzymes. One has a mutation only at position 276 which has been previously described only in an in vitro mutant (30). A second resembles TEM-59, as it possesses the amino acid substitution Ser130Gly, but derived from TEM-1 and not from TEM-2 (5, 23). A third differs from TEM-30, -31, -41, and -51 by a glycine at position 244 (7).
Three new enzymes show an association of two amino acid substitutions. The first combines two substitutions already described individually in TEM-33 and TEM-30 (4, 20). The second includes a known substitution and a new substitution in terms of its position. The third resembles TEM-45 (9).
The two remaining new IRT enzymes display three amino acid substitutions. One of them corresponds to a new combination of 3-amino-acid substitutions, whereas the other resembles TEM-39 (20).
In conclusion, this study, which permitted a large-scale evaluation of the frequencies of amoxicillin-clavulanate-resistant E. coli isolates in hospitals, also permitted the discovery of new blaTEM genes as well as new blaTEM derivatives.
ACKNOWLEDGMENTS
We thank all the hospitals which provided us with the clinical E. coli isolates: Hôpital d'Aix en Provence, Hôpital Hôtel Dieu de Clermont Ferrand, Hôpital Henri Mondor de Créteil, Hôpital du Mans, Hôpital A. Calmette de Lille, Hôpital L. Pradel de Lyon, Hôpital E. Muller de Mulhouse, Hôpital de l'Archet II de Nice, Hôpital de Perpignan, Hôpital Pontchaillou de Rennes, Hôpital Purpan de Toulouse, Hôpital de Troyes, Hôpital de Valenciennes, and Hôpital A. Mignot de Versailles.
We are grateful to Hoechst Marion Roussel for financial support of this study over the 3 years.
REFERENCES
- 1.Anonymous. Statement 1996 Ca-SFM. Zone sizes and MIC breakpoints for non-fastidious organisms. Clin Microbiol Infect. 1996;2:S46–S47. [PubMed] [Google Scholar]
- 2.Bartlett J G, Breiman R F, Mandell L A, File T M., Jr Community-acquired pneumonia in adults: guidelines for management. Clin Infect Dis. 1998;26:811–838. doi: 10.1086/513953. [DOI] [PubMed] [Google Scholar]
- 3.Bauernfeind A, Wagner S, Jungwirth R, Schneider I, Meyer D. A novel class C β-lactamase (FOX-2) in Escherichia coli conferring resistance to cephamycins. Antimicrob Agents Chemother. 1997;41:2041–2046. doi: 10.1128/aac.41.9.2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belaaouaj A, Lapoumeroulie C, Caniça M M, Vedel G, Névot P, Krishnamoorthy R, Paul G. Nucleotide sequences of the genes coding for the TEM-like β-lactamases IRT-1 and IRT-2 (formerly called TRI-1 and TRI-2) FEMS Microbiol Lett. 1994;120:75–80. doi: 10.1111/j.1574-6968.1994.tb07010.x. [DOI] [PubMed] [Google Scholar]
- 5.Bermudes H, Jude F, Arpin C, Quentin C, Morand A, Labia R. Characterization of an inhibitor-resistant TEM (IRT) beta-lactamase in a novel strain of Klebsiella pneumoniae. Antimicrob Agents Chemother. 1997;41:222. doi: 10.1128/aac.41.1.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradford P A, Urban C, Mariano N, Projan S J, Rahal J J, Bush K. Imipenem resistance in Klebsiella pneumoniae is associated with the combination of ACT-1, a plasmid-mediated AmpC β-lactamase, and the loss of an outer membrane protein. Antimicrob Agents Chemother. 1997;41:563–569. doi: 10.1128/aac.41.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bret L, Chaibi E B, Chanal-Claris C, Sirot D, Labia R, Sirot J. Inhibitor-resistant TEM (IRT) β-lactamases with different substitutions at position 244. Antimicrob Agents Chemother. 1997;41:2547–2549. doi: 10.1128/aac.41.11.2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buirma R J A, Horrevorts A M, Wagenvoort J H T. Incidence of multi-resistant Gram-negative isolates in eight Dutch hospitals. Scand J Infect Dis Suppl. 1991;78:35–44. [PubMed] [Google Scholar]
- 9.Caniça M M, Barthelemy M, Gilly L, Labia R, Krishnamoorthy R, Paul G. Properties of IRT-14 (TEM-45), a newly characterized mutant of TEM-type beta-lactamases. Antimicrob Agents Chemother. 1997;41:374–378. doi: 10.1128/aac.41.2.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caniça M M, Lu C Y, Krishnamoorthy R, Paul G C. Molecular diversity and evolution of blaTEM genes encoding β-lactamases resistant to clavulanic acid in clinical E. coli. J Mol Evol. 1997;44:57–65. doi: 10.1007/pl00006121. [DOI] [PubMed] [Google Scholar]
- 11.Caroff N, Espaze E, Berard I, Richet H, Reynaud A. Mutations in the ampC promoter of Escherichia coli isolates resistant to oxyiminocephalosporins without extended spectrum β-lactamase production. FEMS Microbiol Lett. 1999;173:459–465. doi: 10.1111/j.1574-6968.1999.tb13539.x. [DOI] [PubMed] [Google Scholar]
- 12.Chaibi E B, Sirot D, Paul G, Labia R. Inhibitor-resistant TEM β-lactamases: phenotypic, genetic and biochemical characteristics. J Antimicrob Chemother. 1999;43:447–458. doi: 10.1093/jac/43.4.447. [DOI] [PubMed] [Google Scholar]
- 13.Chardon H, Farzaneh S, Labia R, Jarlier V, Nicolas M H, Paul G, Poyart C, Sirot D, Sirot J. Analysis of β-lactamases produced by cephalothin-susceptible Escherichia coli clinical isolates resistant to co-amoxiclav and ticarcillin-clavulanic acid. J Antimicrob Chemother. 1995;36:267–269. doi: 10.1093/jac/36.1.267. [DOI] [PubMed] [Google Scholar]
- 14.Chen C. Two improved promoter sequences for the β-lactamase expression arising from a single base pair substitution. Nucleic Acids Res. 1984;12:3219–3234. doi: 10.1093/nar/12.7.3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gazouli M, Tzouvelekis L S, Prinarakis E, Miriagou V, Tzelepi E. Transferable cefoxitin resistance in enterobacteria from Greek hospitals and characterization of a plasmid-mediated group 1 β-lactamase (LAT-2) Antimicrob Agents Chemother. 1996;40:1736–1740. doi: 10.1128/aac.40.7.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldstein F W, Pean Y, Gertner J the Vigil'Roc Study Group. Resistance to ceftriaxone and other β-lactams in bacteria isolated in the community. Antimicrob Agents Chemother. 1995;39:2516–2519. doi: 10.1128/aac.39.11.2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldstein F W, Pean Y, Rosato A, Gertner J, Gutmann L. Characterization of ceftriaxone-resistant Enterobacteriaceae: a multicentre study in 26 French hospitals. Vigil'Roc Study Group. J Antimicrob Chemother. 1993;32:595–603. doi: 10.1093/jac/32.4.595. [DOI] [PubMed] [Google Scholar]
- 18.Goussard S, Courvalin P. Updated sequence information for TEM β-lactamase genes. Antimicrob Agents Chemother. 1999;43:367–370. doi: 10.1128/aac.43.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18a.Goussard S, Courvalin P. Sequence of the genes blaT-1B and blaT-2. Gene. 1991;102:71–73. doi: 10.1016/0378-1119(91)90540-r. [DOI] [PubMed] [Google Scholar]
- 19.Guillemot D, Maison P, Carbon C, Balkau B, Vauzelle-Kervroëdan F, Sermet C, Bouvenot G, Eschwege E. Trends in antimicrobial drug use in the community—France, 1981–1992. J Infect Dis. 1998;177:492–497. doi: 10.1086/517384. [DOI] [PubMed] [Google Scholar]
- 20.Henquell C, Chanal C, Sirot D, Labia R, Sirot J. Molecular characterization of nine different types of mutants among 107 inhibitor-resistant TEM beta-lactamases from clinical isolates of Escherichia coli. Antimicrob Agents Chemother. 1995;39:427–430. doi: 10.1128/aac.39.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henquell C, Sirot D, Chanal C, De C C, Chatron P, Lafeuille B, Texier P, Sirot J, Cluzel R. Frequency of inhibitor-resistant TEM beta-lactamases in Escherichia coli isolates from urinary tract infections in France. J Antimicrob Chemother. 1994;34:707–714. doi: 10.1093/jac/34.5.707. [DOI] [PubMed] [Google Scholar]
- 22.Huchon G. Guidelines for management of adult community-acquired lower respiratory tract infections. Eur Resp J. 1998;11:986–991. doi: 10.1183/09031936.98.11040986. [DOI] [PubMed] [Google Scholar]
- 23.Jacob F, Joris B, Lepage S, Dusart J, Frere J-M. Role of the conserved amino acids of the 'SDN' loop (Ser130, Asp131 and Asn132) in a class A β-lactamase studied by site-directed mutagenesis. Biochem J. 1990;271:399–406. doi: 10.1042/bj2710399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lepelletier D, Caroff N, Reynaud A, Richet H. Escherichia coli: epidemiology and analysis of risk factors for infections caused by resistant strains. Clin Infect Dis. 1999;29:548–552. doi: 10.1086/598632. [DOI] [PubMed] [Google Scholar]
- 25.Martinez J L, Cercenado E, Rodriguez-Creixems M, Vincente-Pérez M F, Delgado-Iribarren A, Baquero F. Resistance to β-lactam/clavulanate. Lancet. 1987;iii:1473. doi: 10.1016/s0140-6736(87)91180-9. [DOI] [PubMed] [Google Scholar]
- 26.Medeiros A A, Cohenford M, Jacoby G A. Five novel plasmid-determined β-lactamases. Antimicrob Agents Chemother. 1985;27:715–719. doi: 10.1128/aac.27.5.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Natsch S, Conrad C, Hartmeier C, Schmid R. Use of amoxicillin-clavulanate and resistance in Escherichia coli over a 4-year period. Infect Control Hosp Epidemiol. 1998;19:653–656. doi: 10.1086/647893. [DOI] [PubMed] [Google Scholar]
- 28.Nicolas-Chanoine M H. Inhibitor-resistant β-lactamases. J Antimicrob Chemother. 1997;40:1–3. doi: 10.1093/jac/40.1.1. [DOI] [PubMed] [Google Scholar]
- 29.Roy C, Segura C, Torrellas A, Reig R, Teruel D, Hermida M. Activity of amoxycillin/clavulanate against β-lactamase-producing Escherichia coli and Klebsiella spp. J Antimicrob Chemother. 1989;24(Suppl. B):41–47. doi: 10.1093/jac/24.suppl_b.41. [DOI] [PubMed] [Google Scholar]
- 30.Saves I, Burlet S O, Swaren P, Lefevre F, Masson J M, Prome J C, Samama J P. The asparagine to aspartic acid substitution at position 276 of TEM-35 and TEM-36 is involved in the β-lactamase resistance to clavulanic acid. J Biol Chem. 1995;270:18240–18245. doi: 10.1074/jbc.270.31.18240. [DOI] [PubMed] [Google Scholar]
- 31.Shah P M, Asanger R, Kahan F M. Incidence of multi-resistance in Gram-negative aerobes from intensive care units of 10 German hospitals. Scand J Infect Dis. 1991;78:22–34. [PubMed] [Google Scholar]
- 32.Simpson I, Durodie J, Knott S, Shea B, Wilson J, Machka K. Effects of following national committee for clinical laboratory standards and Deutsche Industrie Norm-Medizinische Mikrobiologie guidelines, country of isolate origin, and site of infection on susceptibility of Escherichia coli to amoxicillin-clavulanate (Augmentin) J Clin Microbiol. 1998;36:1361–1365. doi: 10.1128/jcm.36.5.1361-1365.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siu L K, Ho P L, Yuen K Y, Wong S S Y, Chau P Y. Transferable hyperproduction of TEM-1 β-lactamase in Shigella flexneri due to a point mutation in the Pribnow box. Antimicrob Agents Chemother. 1997;41:468–470. doi: 10.1128/aac.41.2.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Speldooren V, Heym B, Labia R, Nicolas-Chanoine M-H. Discriminatory detection of inhibitor-resistant β-lactamases in Escherichia coli by single-strand conformational polymorphism–PCR. Antimicrob Agents Chemother. 1998;42:879–884. doi: 10.1128/aac.42.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stapleton P, Wu P J, King A, Shannon K, French G, Phillips I. Incidence and mechanisms of resistance to the combination of amoxicillin and clavulanic acid in Escherichia coli. Antimicrob Agents Chemother. 1995;39:2478–2483. doi: 10.1128/aac.39.11.2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sutcliffe J G. Nucleotide sequence of the ampicillin resistance gene of Escherichia coli plasmid pBR322. Proc Natl Acad Sci USA. 1978;75:3737–3741. doi: 10.1073/pnas.75.8.3737. [DOI] [PMC free article] [PubMed] [Google Scholar]


