Abstract
Introduction
The study aim was to determine if rapid enteric diagnostics followed by the provision of targeted antibiotic therapy (‘test-and-treat’) and/or Lactobacillus reuteri DSM 17938 would improve outcomes in children hospitalised in Botswana with acute gastroenteritis.
Methods
This was a multicentre, randomised, factorial, controlled, trial. Children aged 2–60 months admitted for acute non-bloody diarrhoea to four hospitals in southern Botswana were eligible. Participants were assigned to treatment groups by web-based block randomisation. Test-and-treat results were not blinded, but participants and research staff were blinded to L. reuteri/placebo assignment; this was dosed as 1×108 cfu/mL by mouth daily and continued for 60 days. The primary outcome was 60-day age-standardised height (HAZ) adjusted for baseline HAZ. All analyses were by intention to treat. The trial was registered at Clinicaltrials.gov.
Results
Recruitment began on 12 June 2016 and continued until 24 October 2018. There were 66 participants randomised to the test-and-treat plus L. reuteri group, 68 randomised to the test-and-treat plus placebo group, 69 to the standard care plus L. reuteri group and 69 to the standard care plus placebo group. There was no demonstrable impact of the test-and-treat intervention (mean increase of 0.01 SD, 95% CI −0.14 to 0.16 SD) or the L. reuteri intervention (mean decrease of 0.07 SD, 95% CI −0.22 to 0.08 SD) on adjusted HAZ at 60 days.
Conclusions
In children hospitalised for acute gastroenteritis in Botswana, neither a test-and-treat algorithm targeting enteropathogens, nor a 60-day course of L. reuteri DSM 17938, were found to markedly impact linear growth or other important outcomes. We cannot exclude the possibility that test-and-treat will improve the care of children with significant enteropathogens (such as Shigella) in their stool.
Trial registration number
Keywords: Child health, Medical microbiology, Individual randomized trial
WHAT IS ALREADY KNOWN ON THIS TOPIC?
Acute diarrhoeal disease remains a major paediatric health hazard in low-income and middle-income countries, causing half a million deaths yearly in children under the age of 5 years and significant growth restriction and cognitive delay in those who survive.
WHAT THIS STUDY ADDS
There was no apparent benefit of test-and-treat observed in all participants randomised to this intervention in an intention-to-treat analysis; however, the point estimate for the change in age-standardised height was greater than zero for children with Shigella, Campylobacter, enterotoxigenic Escherichia coli or Cryptosporidium detected in their stools who received specific treatment.
There was no apparent benefit of Lactobacillus reuteri treatment observed.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE AND/OR POLICY
We did not demonstrate that a test-and-treat approach for acute diarrhoeal disease prevents linear height deficits; however, we have not excluded the possibility that a small beneficial effect might result from specific treatment of certain enteropathogens.
Further studies enrolling children with acute gastroenteritis, especially those with shigellosis, might be useful to better define outcomes associated with targeted specific antimicrobial therapy.
Introduction
Diarrhoeal disease is the second leading cause of death of young children worldwide, with an estimated 499 000 deaths yearly in children under the age of 5 years.1 2 Diarrhoea is also a key driver of both severe acute malnutrition3 and stunting,4 mediated in part through losses of essential nutrients through malabsorption, increased catabolism, decreased intake and other mechanisms.5 Malnutrition and stunting have been associated with both cognitive maldevelopment6 7 and mortality through a number of different pathways8–10 and lead to sequelae in adulthood that have significant societal ramifications.11 Interestingly, gastrointestinal infection may have more pernicious impact than the physiological effect of diarrhoea itself; it has been demonstrated that both overt infectious diarrhoea and subclinical infections with selected enteropathogens have substantial negative associations with linear growth.12–14 These observations led to the hypothesis that mitigating exposures to Shigella, Campylobacter, Cryptosporidium and other enteropathogens might be an effective method to reduce stunting and optimise child development.12
The mainstay of treatment for acute diarrhoeal disease is the prompt treatment of fluid depletion using oral rehydration salts, once called ‘the most important medical advance of the 20th century’.15 16 The WHO also recommends providing zinc, as it has been shown to reduce the duration of infectious diarrhoea, even at low doses.17–19 Ensuring effective rehydration is critical to prevent early deaths resulting from dehydration and hypovolaemic shock; unfortunately, most diarrhoea-associated mortality occurs long after presentation to healthcare providers and fluid rehydration.20 Furthermore, stunting and cognitive delay are chronic processes and are unlikely to be mitigated by transient fluid rehydration. It is critical that we understand the precise mechanisms by which diarrhoeal disease causes mortality and morbidity, acute or delayed, in low-income and middle-income countries (LMICs), and discover new strategies to diminish the impact of these infections.21 Could timely antimicrobial use in children with treatable gastroenteritis improve symptoms and prevent subsequent growth deficits and/or mortality, as others have suggested?22 One major multicountry case–control study found that appropriate antibiotic therapy provided to young children with Shigella infection was associated with significantly less growth faltering than untreated children, although in this type of observational study confounding could not be excluded.14
It has been theorised that probiotic treatment could mitigate the impact of gastroenteritis in resource-limited settings. A previous Cochrane systematic review incorporating 56 trials in infants and young children found that probiotics reduced the duration of diarrhoea by 1 day23; a later systematic review and network meta-analysis found that there was moderate-to-high evidence that Saccharomyces boulardii +zinc was substantially superior to standard of care and reduced diarrhoea duration by 35–40 hours.24
The aim of this study was to determine if growth and other important outcomes could be improved in children hospitalised with severe acute diarrhoeal disease in Botswana through the use of two separate and distinct interventions:
Use of rapid stool diagnostics to identify those with potentially treatable bacterial or protozoan gastroenteritis, facilitating the provision of targeted therapy (‘test-and-treat’).
Lactobacillus reuteri DSM 17938 given daily × 60 days.
Methods
Study design
We conducted a multicentre, randomised, factorial (2×2), controlled, superiority trial with parallel assignment and a 1:1:1:1 allocation ratio. The study hospitals, all located in southern Botswana, were Princess Marina Hospital in Gaborone (the main tertiary referral hospital), Bamalete Lutheran Hospital in Ramotswa, Scottish Livingstone Hospital in Molepolole and Deborah Retief Hospital in Mochudi.
Participants
Eligible participants were children aged 2–60 months admitted to the paediatric medical wards of the study hospitals for acute diarrhoeal disease. Acute diarrhoeal disease was defined as at least three watery stools in a 24-hour period preceding admission to hospital. Children admitted to hospital for another reason who developed nosocomial diarrhoea>48 hours after admission were not included. Exclusion criteria included visibly bloody diarrhoea, diarrhoea for >14 days, known inflammatory bowel disease, cystic fibrosis, malignancy, household contact with another individual documented to have a bacterial or parasitic enteric infection of defined aetiology, no ready access to a telephone, no permanent address or an address outside the hospitals’ catchment areas. Children were not permitted to participate more than once. All potential participants were approached within 48 hours of hospitalisation by the study team. Written informed consent was obtained from all participants’ parents or legal guardians.
Randomisation and masking
A statistician independent of the study generated a randomisation list, using a random number generator, such that participants could be assigned at random to one of the four study groups in a 1:1:1:1 ratio (blocked randomly in groups of 4–8) using a web-based interface (Research Electronic Data Capture). Randomisation was stratified by the presence or absence of severe acute malnutrition. The randomisation process and treatment allocation were performed by individuals uninvolved with recruitment, enrolment and follow-up. The probiotics and matching placebo (oil vehicle only), provided by the manufacturer (BioGaia A.B., Stockholm, Sweden), were prepared by the study pharmacist and dispensed in identical containers. All were aware of treatment assignment to the test-and-treat (vs standard care) group, but participants, research assistants and outcome assessors were blinded to the probiotic vs placebo assignment. (The study pharmacist who prepared medications was not blinded but did not meet the participants and had no role in participant follow-up.)
Procedures
Consenting caregivers provided demographic information, details about the presenting illness and participant medical history. Physical examination findings were abstracted from the clinical team records. All study participants were weighed and measured in duplicate by the trained study team at enrolment using standardised protocols and dedicated, calibrated stadiometers and digital scales purchased specifically for study purposes. Middle upper-arm circumference was determined using specialised tape measures. The study was not involved with the management of dehydration or the establishment of rehydration practice by the clinical team. On recruitment, all participants had rectal flocked swab specimens taken (Copan Italia S.A.) that were immediately placed in Cary-Blair transport medium.
Rectal swabs from participants randomised to the test-and-treat arms were processed as soon as possible using molecular methods, that is, either the BioFire FilmArray GI Panel (bioMérieux, Lyon, France), which we had previously validated for flocked rectal swab specimens,25 or two previously described real-time laboratory-developed multiplex PCR assays.26 27 Testing results were communicated to the clinical team using standardised reporting forms. It was recommended explicitly to the clinical team that children with samples positive for Shigella, enterotoxigenic Escherichia coli (ETEC), enteropathogenic E. coli (EPEC), Campylobacter and/or Vibrio cholerae be treated with azithromycin (10 mg/kg/day by mouth once daily × 3 days) and those positive for Cryptosporidium be treated with nitazoxanide (7.5 mg/kg to maximum of 100 mg by mouth twice daily for ages 5–11 months, 100 mg by mouth twice daily for ages 1–3 years and 200 mg by mouth twice daily for ages 4+ years, all × 3 days).
Participants randomised to the standard care arms were treated as per the Botswana/WHO routine practice by the clinical team, namely fluid rehydration and zinc therapy. These participants had their rectal swabs batched and processed using the BioFire FilmArray GI Panel at the end of the study, so that these results were not available to treating clinicians.
Participants randomised to the probiotic arms were started on Lactobacillus reuteri DSM 17938 1×108 colony forming units (five drops) by mouth once daily within 24 hours of enrolment; participants’ caregivers were instructed to continue this for 60 days. This specific probiotic preparation was chosen because of previous studies suggesting efficacy28 29; the long duration of treatment was established in an effort to provide maximum benefit to study participants. Those randomised to the placebo arm were given the same instructions and managed in the same manner, except that they received placebo. All probiotic/placebo was kept refrigerated while the participant was in hospital, and all families were advised to maintain refrigeration after discharge, if possible.
All participants were re-evaluated 60 days after enrolment; at this time, probiotic/placebo adherence was evaluated, details of intercurrent illness were recorded, height and weight measurements were redone and probiotic/placebo containers were collected.
Outcomes
All outcomes were measured at 60 days, or within the 60-day follow-up period, unless otherwise specified. The primary outcome was height z-score (age-standardised height (HAZ)) adjusted for baseline HAZ. Secondary outcomes included the following: 7-day and 60-day mortality, weight z-score (age-standardised weight (WAZ)) adjusted for initial WAZ; presence of stunting (HAZ more than 2 SD below the mean); length of stay in hospital; development of sepsis; blood cultures positive for Lactobacillus species; admission to the intensive care unit in hospital; recurrence of acute diarrhoea; re-presentation to a medical professional because of acute diarrhoea or any other reason; readmission to a paediatric medical ward; retreatment with antimicrobials; and caregiver missed work due to participant illness. Adverse events discovered by research staff during daily visits to participants in-hospital or after discharge (either at the 60-day follow-up visit or through caregiver-initiated communication) were recorded and reported, as required. All adverse events were assessed for possible causality to study procedures or medications.
Statistical analysis
Based on results from the pilot study,30 we estimated an approximate difference of 0.2 SD in final HAZ adjusted for baseline height between rapid diagnostic and standard care arms, as well as between probiotic and placebo arms. We also assumed a SD of these means of approximately 0.5, thereby necessitating a total sample size of 400 to have 80% power to discern a statistical benefit of test-and-treat over standard care and probiotic over placebo.
Demographic and clinical characteristics were reported using descriptive statistics: means and SD for normally distributed data; medians and quartiles for non-normal distributed data; and counts and percentages for categorical data. All analyses were conducted separately to compare groups, first rapid test-and-treat versus standard care, and then L. reuteri probiotic versus placebo. Primary and secondary outcomes were also evaluated by groups. The primary analyses were intention-to-treat. To determine the treatment effect in each group, analysis of covariance (ANCOVA) was performed for continuous outcomes, logistic regression for binary outcomes and Poisson regression for count outcomes; all anthropometric outcome analysis was adjusted for initial age-standardised growth. The treatment effect sizes from these analyses were reported as mean differences for continuous outcomes, ORs for binary outcomes and incident rate ratios for count outcomes. All effect sizes as z-scores or functions of SD were accompanied by corresponding 95%CIs and p values; alpha was set at 0.05 (two sided). The ANCOVA and regression analyses were all adjusted for severe acute malnutrition. Observations with missing data were excluded from analysis. No plan was made to investigate the interaction between the test-and-treat intervention and the probiotic intervention.
Sensitivity analyses on outcomes HAZ at 60 days and recurrent diarrhoea were performed to further explore the impact of treatment effect within the following groups of patients:
All patients.
Patients with Shigella.
Patients with Shigella, ETEC or Campylobacter.
Patients with Shigella, ETEC, Campylobacter or Cryptosporidium.
Patients with Shigella, ETEC, Campylobacter, or EPEC.
All analyses were done with SAS (V.9.4) or STATA (V.11.2). There was no study data safety monitoring board. This study was registered at Clinicaltrials.gov.
Patient and public involvement
Patients and the public were not involved in the design of the trial.
Role of the funding source
The funders of the study had no role in the study design, data collection, data analysis, data interpretation or writing of the report. The main funder was Grand Challenges Canada; the probiotic and placebo were donated by BioGaia A.B.; the flocked swabs were donated by Copan Italia S.A.; and bioMérieux provided the FilmArray device and supplemental funding. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
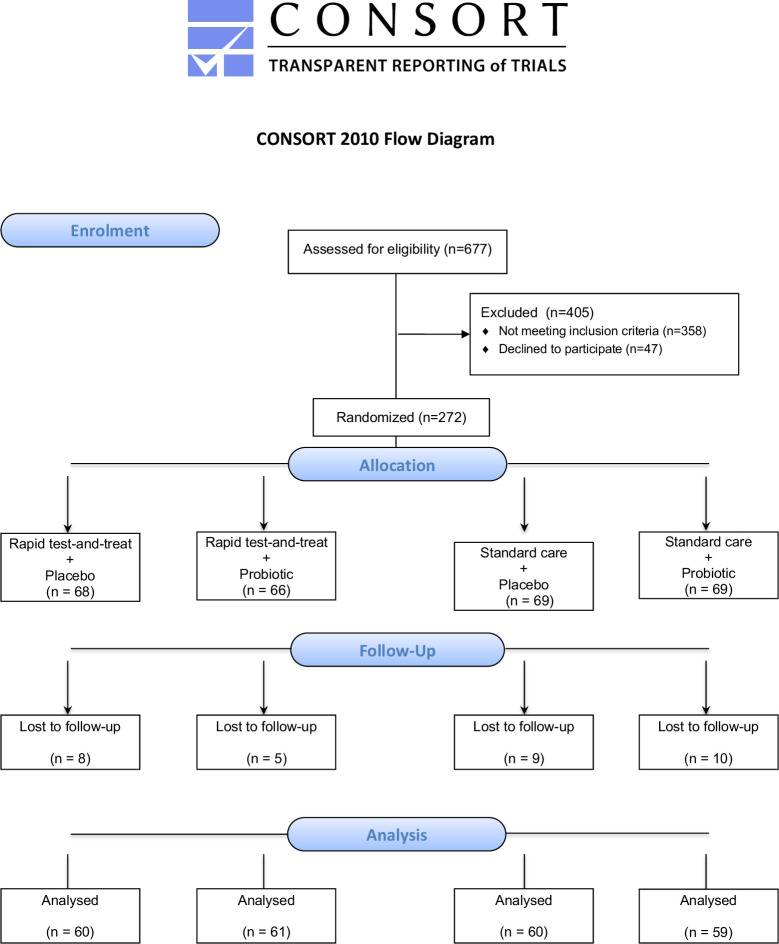
Recruitment began on 12 June 2016 and continued until 30 November 2017 at all four sites; recruitment then proceeded at Princess Marina Hospital only until October 2018. During the study period, at participating sites, there were 1038 admissions documented in hospital registers for children with severe acute gastroenteritis. Only 677 were screened for eligibility, due to many presenting at hours or dates (ie, weekends, holidays) when study staff were not present, or because the legal caregiver was not with the child to permit a discussion about informed consent. Of those screened, 272 were eligible and consented to participate in the study (see figure 1). We failed to reach our prespecified sample size, due to logistical and funding considerations stemming primarily from a drop in the incidence of severe acute diarrhoeal disease requiring hospitalisation as compared with previous years.31
Figure 1.
CONSORT flow diagram.
As can be seen from table 1, study participants were infants and young children who were predominantly previously healthy and who had received rotavirus vaccine. Sex ratios and baseline growth parameters were comparable between treatment groups, as was severity of illness and the type of rehydration the participants received in hospital.
Table 1.
Baseline characteristics of study participants
| Characteristic | Rapid test-and-treat n=134 | Standard care (no rapid test-and-treat) n=138 | ||
| Placebo n=68 | Lactobacillus reuteri n=66 | Placebo n=69 | L. reuteri n=69 | |
| Age in months: median (Q1, Q3) | 11.8 (8.4,18.6) | 10.6 (7.7,16.7) | 12.4 (7.4, 17.0) | 9.8 (8.0,15.2) |
| Male; n (%) | 37 (54.4) | 38 (57.6) | 39 (56.5) | 39 (56.5) |
| Medical comorbidities; n (%) | 2 (2.9) | 1 (1.5) | 4 (5.8) | 1 (1.5) |
| Receipt of at least one dose of rotavirus vaccine; n (%) | 67 (98.5) | 62 (93.9) | 65 (94.2) | 64 (94.1) |
| Child HIV status; n (%) | ||||
| Positive | 2 (3.0) | 0 | 2 (2.9) | 0 |
| Exposed, infection status unknown | 2 (3.0) | 3 (4.6) | 2 (2.9) | 5 (7.4) |
| Exposed but uninfected | 12 (17.9) | 14 (21.2) | 9 (13.0) | 19 (27.9) |
| Exposure unclear, infection status unknown | 10 (14.9) | 14 (21.2) | 16 (23.2) | 9 (13.2) |
| Exposure unclear, but known uninfected | 2 (3.0) | 3 (4.6) | 0 | 1 (1.5) |
| Unexposed | 39 (58.2) | 32 (48.5) | 40 (58.0) | 34 (50.0) |
| Severe acute malnutrition; n (%) | 3 (4.4) | 3 (4.5) | 3 (4.3) | 3 (4.3) |
| Weight-for-age z score; median (Q1, Q3) | −0.79 (-1.33, 0.24) | −0.82 (-1.72, 0.08) | −0.60 (-1.63, 0.50) | −0.46 (-1.22, 0.62) |
| Height-for-age z score; median (Q1, Q3) | −0.43 (-1.33, 0.34) | −0.49 (-1.50, 0.24) | −0.82 (-1.63, 0.29) | −0.60 (-1.19, 0.63) |
| Child middle upper arm circumference (cm); mean (SD) | 13.87 (1.88) | 13.69 (1.60) | 13.83 (1.84) | 14.03 (1.66) |
| Diarrhoea days before admission; median (Q1, Q3) | 2 (1,4) | 2 (1,3) | 2 (1, 4) | 2 (1,3) |
| Diarrhoea number/24 hours; mean (SD) | 6.66 (5.08) | 6.78 (2.78) | 6.72 (3.09) | 7.29 (3.71) |
| Vomit days before admission; median (Q1, Q3) | 2 (1, 3) | 2 (1,3) | 2 (1, 3) | 2 (1,2) |
| Missing | 6 | 9 | 6 | 4 |
| Vomit number/24 hours; median (Q1, Q3) | 5 (3,9.75) | 4 (2,6) | 4 (3,6) | 4 (2,6) |
| Missing | 6 | 9 | 8 | 4 |
| Oral rehydration solution prior to admission; n (%) | 50 (73.5) | 57 (86.4) | 55 (79.7) | 52 (75.4) |
| Traditional medicine given prior to admission?; n (%) | ||||
| Yes, ingested | 6 (8.8) | 3 (4.5) | 6 (8.7) | 4 (5.8) |
| Yes, inhaled | 1 (1.5) | 1 (1.5) | 1 (1.4) | 1 (1.4) |
| No | 61 (89.7) | 62 (93.9) | 62 (89.9) | 63 (91.3) |
| Don’t know | 0 | 0 | 0 | 1 (1.4) |
| Level of dehydration; n (%) | ||||
| None | 5 (7.4) | 8 (12.1) | 7 (10.1) | 9 (13.0) |
| Some | 43 (63.2) | 30 (45.5) | 43 (62.3) | 36 (52.1) |
| Severe | 14 (20.6) | 22 (33.3) | 16 (23.2) | 15 (21.7) |
| Unknown | 6 (8.8) | 6 (9.1) | 3 (4.3) | 9 (13.0) |
| Rehydration therapy at the hospital; n(%) | ||||
| Both IV and oral rehydration | 53 (77.9) | 49 (74.2) | 52 (75.4) | 56 (81.2) |
| IV only | 8 (11.8) | 10 (15.2) | 10 (14.5) | 9 (13.0) |
| Oral only | 7 (10.3) | 7 (10.6) | 6 (8.7) | 3 (4.3) |
| None | 0 | 0 | 1 (1.4) | 1 (1.4) |
| Zinc treatment; n (%) | 61 (89.7) | 56 (84.8) | 60 (87.0) | 62 (89.9) |
| Number of children under age of 5 years living in the same household; n (%) | ||||
| 1 | 41 (61.2) | 37 (56.1) | 42 (60.9) | 36 (52.2) |
| 2 | 13 (19.4) | 14 (21.2) | 17 (24.6) | 21 (30.4) |
| 3 | 9 (13.4) | 13 (19.7) | 6 (8.7) | 8 (11.6) |
| 4 | 3 (4.5) | 0 | 4 (5.8) | 4 (5.8) |
| 5 | 1 (1.5) | 2 (3.0) | 0 | 0 |
| Mother’s marital status; n (%) | ||||
| Married | 11 (16.2) | 7 (10.6) | 10 (14.5) | 12 (17.4) |
| Single | 56 (82.4) | 56 (84.8) | 58 (84.1) | 57 (82.6) |
| Divorced | 0 | 1 (1.5) | 0 | 0 |
| Cohabitation | 1 (1.5) | 2 (3.0) | 1 (1.4) | 0 |
| Mother’s education; n (%) | ||||
| Did not complete primary school | 3 (4.4) | 1 (1.5) | 3 (4.3) | 4 (5.8) |
| Completed primary school | 7 (10.3) | 13 (19.7) | 5 (7.2) | 6 (8.7) |
| Completed secondary school | 39 (57.4) | 36 (54.5) | 41 (59.4) | 38 (55.1) |
| Completed postsecondary training (vocational /technical) | 14 (20.6) | 11 (16.7) | 13 (18.8) | 12 (17.4) |
| Completed university degree | 5 (7.4) | 5 (7.6) | 7 (10.1) | 9 (13.0) |
| Socioeconomic parameters*; n(%) | ||||
| Electricity in home | 62 (91.2) | 47 (71.2) | 56 (81.2) | 52 (75.4) |
| Ownership of car | 38 (55.9) | 32 (48.5) | 33 (47.8) | 37 (53.6) |
| Ownership of mobile phone | 68 (100) | 65 (98.5) | 67 (97.2) | 69 (100) |
| Refrigerator in home | 57 (83.8) | 37 (56.1) | 49 (71.0) | 43 (62.3) |
| Water source; n (%) | ||||
| Tap to house | 41 (60.3) | 37 (56.9) | 31 (44.9) | 37 (53.6) |
| Shared community tap | 27 (39.7) | 28 (43.1) | 38 (55.1) | 31 (44.9) |
| Bore hole | 0 | 0 | 0 | 1 (1.4) |
| Method of feeding (for only those aged <6 months); n (%) | ||||
| Exclusively breast fed | 31 (45.6) | 26 (40.6) | 27 (39.7) | 19 (27.5) |
| Exclusively formula fed | 17 (25.0) | 16 (25.0) | 13 (19.1) | 19 (27.5) |
| Mixed feeding | 19 (27.9) | 22 (34.4) | 28 (41.2) | 31 (44.9) |
| Don’t know | 1 | 0 | 0 | 0 |
| Missing | 0 | 2 | 1 | 0 |
All variables with missing data are listed as such.
*Multiple responses possible, percentages may add to more than 100%.
Enteropathogens were commonly detected in study participants (table 2), and the frequency of detection of enteropathogens targeted for treatment (Shigella, Campylobacter, ETEC, Cryptosporidium and EPEC) was comparable between treatment arms (table 3).
Table 2.
Whole-cohort microbiology
| Pathogen | Frequency (%) |
| Bacterial | |
| Enteroaggregative Escherichia coli (EAEC) | 126 (46) |
| Enteropathogenic E. coli (EPEC) | 115 (42) |
| Enterotoxigenic E. coli (ETEC) | 51 (19) |
| Campylobacter | 43 (16) |
| Shigella/enteroinvasive E. coli (EIEC) | 31 (11) |
| Salmonella | 4 (1.5) |
| Shiga-toxin producing E. coli (STEC) | 3 (1.1) |
| Plesiomonas shigelloides | 1 (0.37) |
| Protozoan | |
| Giardia | 20 (7.3) |
| Cryptosporidium | 12 (4.4) |
| Viral | |
| Rotavirus | 151 (55) |
| Norovirus | 39 (14) |
| Adenovirus | 33 (12) |
| Sapovirus | 10 (3.6) |
| Astrovirus | 8 (2.9) |
| Negative | |
| Negative for all pathogens assayed | 9 (3.3) |
| Negative for all viral pathogens | 64 (23) |
| Negative for all bacterial and protozoan pathogens | 63 (23) |
No Vibrio cholerae, Yersinia, E. coli O157, Entamoeba histolytica or Cyclospora cayetensis detected. Clostridioides difficile was omitted from this table as its role as a pathogen in infants is unclear.
Table 3.
Microbiology and antimicrobial therapy by treatment group
| Standard care n=138 | Rapid test-and-treat n=134 | Placebo n=137 | Lactobacillus reuteri probiotic n=135 | |
| Participants with treatable bacterial pathogens (Campylobacter, Shigella, enterotoxigenic Escherichia coli, enteropathogenic E. coli); n (%) | 82 (59.4) | 84 (62.7) | 84 (61.3) | 82 (60.7) |
| Participants with Cryptosporidium; n (%) | 4 (2.9) | 8 (6.0) | 5 (3.6) | 7 (5.2) |
| Participants documented as treated with cefotaxime; n (%) | 21 (15.6) | 21 (16.0) | 21 (15.7) | 21 (15.9) |
| Missing | 3 | 3 | 3 | 3 |
| Participants documented as treated with amoxicillin/clavulanic acid; n (%) | 7 (5.3) | 7 (5.3) | 9 (6.8) | 5 (3.8) |
| Missing | 6 | 3 | 5 | 4 |
| Participants documented as treated with nalidixic acid; n (%) | 8 (6.0) | 8 (6.1) | 11 (8.2) | 5 (3.8) |
| Missing | 4 | 3 | 3 | 4 |
| Participants documented as treated with gentamicin; n (%) | 28 (20.7) | 30 (22.7) | 31 (23.0) | 27 (20.5) |
| Missing | 3 | 2 | 2 | 3 |
| Participants documented as treated with azithromycin; n (%) | 0 | 69 (51.5) | 36 (26.3) | 33 (24.4) |
| Participants documented as treated with nitazoxanide; n (%) | 0 | 5 (3.7) | 2 (1.5) | 3 (2.2) |
There were 86 of 134 participants (64%) randomised to test-and-treat who had a targeted bacterial or protozoan pathogen detected and so could have received an intervention (ie, antimicrobials) that could plausibly impact outcome; only 71/86 (83%) were documented as having received this targeted therapy. There was no demonstrable impact of the test-and-treat intervention (mean difference 0.01 SD increase, 95% CI −0.14 to 0.16 SD) on the primary outcome, HAZ at 60 days, in an intention-to-treat analysis (see table 4). There was no significant decrease in recurrent diarrhoea within the 60-day follow-up period observed in the test-and-treat arm (19% vs 28%, OR 0.60 95% CI 0.33 to 1.08). Stunting at 60 days was detected in 12% of the test-and-treat arm as compared with 17% of the standard care arm (OR 0.71, 95% CI 0.33 to 1.51). There was no demonstrable impact of the intervention on 60-day WAZ adjusted for initial WAZ, length of stay in hospital, development of sepsis in the 60-day follow-up period or 7-day mortality. There was also no evident difference in parent absenteeism or healthcare provider visits for non-diarrhoea causes in the follow-up period; 10% of those in the test-and-treat arm and 14% of those in the standard care arm sought care for diarrhoeal illnesses (OR 0.69, 95% CI 0.33 to 1.43).
Table 4.
Outcomes
| Outcomes | Treatment group | Regression analysis | Treatment group | Regression analysis | ||
| Delayed testing n=138 |
Rapid testing n=134 |
Effect estimate (95% CI) p value | Placebo n=137 |
Probiotic n=135 |
Effect estimate (95% CI) p value | |
| Height-for-age z-score at 60 days*; median (Q1, Q3) | −0.77 (−1.64,0.21) | −0.74 (−1.32,0.14) | Mean difference: 0.01 (−0.14 to 0.16) 0.923 |
−0.70 (−1.52, 0.20) |
−0.81 (−1.47,0.16) |
Mean difference: −0.07 (−0.22 to 0.08) 0.340 |
| Missing | 19 | 13 | 32 | 17 | 15 | 32 |
| Recurrent diarrhoea: n (%) | 36 (28.35) | 24 (19.05) |
OR: 0.60 (0.33 to 1.08) 0.089 |
27 (21.09) | 33 (26.40) |
OR: 1.29 (0.72 to 2.33) 0.395 |
| Missing | 11 | 8 | 19 | 9 | 10 | 19 |
| 60-day mortality | 2 (1.47) | 5 (3.76) | 2.86 (0.50 to 16.24) 0.237 | 6 (4.41) | 1 (0.75) | 0.14 (0.02 to 1.25) 0.078 |
| Missing | 2 | 1 | 3 | 1 | 2 | 3 |
| Presence of stunting at 60-day follow-up (Y/N) | 20 (16.81) | 15 (12.40) |
OR: 0.71 (0.33 to 1.51) 0.369 |
14 (11.67) | 21 (17.50) |
OR: 1.49 (0.70 to 3.18) 0.304 |
| Missing | 19 | 13 | 32 | 17 | 15 | 32 |
| Weight z-score at 60-day follow-up adjusted for initial weight*; median (Q1, Q3) | −0.56 (−1.30,0.68) | −0.54 (−1.19,0.15) | Mean difference: −0.05 (−0.21 to 0.11) 0.564 |
−0.43 (−1.19,0.45) | −0.71 (−1.32,0.24) | Mean difference: −0.08 (−0.24 to 0.08) 0.319 |
| Missing | 35 | 40 | 75 | 34 | 41 | 76 |
| Length of stay in hospital (days); median (Q1, Q3) | 3 (2,4) | 3 (2,4) |
Incident rate ratio: 0.99 (0.88 to 1.12) 0.932 |
3 (2,4) | 3 (2,4) | Incident rate ratio: 1.06 (0.94 to 1.19) 0.361 |
| Development of sepsis in the 60-day follow-up period (Y/N) | 1 (0.72) | 0 | NA | 0 | 1 (0.74) | NA |
| Blood cultures positive for Lactobacillus species in the 60-day follow-up period | 0 | 0 | NA | 0 | 0 | NA |
| 7-day mortality (Y/N) | 0 | 1 (0.76) | NA | 1 (0.74) | 0 | NA |
| Missing | 2 | 2 | 2 | 2 | ||
| Re-presentation to a healthcare provider because of acute diarrhoea or any other reason during the 60-day follow-up period | ||||||
| (a) Acute diarrhoea | 20 (14.49) | 14 (10.45) | OR: 0.69 (0.33 to 1.43) 0.314 |
15 (10.95) | 19 (14.07) | OR: 1.33 (0.65 to 2.75) 0.437 |
| (b) Other reason (unrelated to diarrhoea) | 55 (39.86) | 49 (36.57) | OR: 0.87 (0.53 to 1.42) 0.576 |
52 (37.96) | 52 (38.52) | OR: 1.02 (0.63 to 1.67) 0.924 |
| Readmission to a paediatric medical ward during the 60-day follow-up period (Y/N) | 7 (5.07) | 4 (2.99) | Not enough events | 5 (3.65) | 6 (4.44) | Not enough events |
| Caregiver missed work in the 60-day follow-up period due to participant illness (Y/N) | 17 (12.32) | 14 (10.45) | OR: 0.83 (0.39 to 1.76) 0.628 |
13 (9.49) | 18 (13.33) | OR: 1.47 (0.69 to 3.13) 0.320 |
All analyses in table 4 adjusted for severe acute malnutrition.
*Adjusted for baseline score and severe acute malnutrition.
In the sensitivity analyses (see table 5), looking at only those participants who had Shigella, Campylobacter, ETEC or Cryptosporidium (the pathogens of greatest interest) detected, there was still no significant benefit of the test-and-treat intervention observed, though the point estimate was greater (mean difference 0.09 SD increase in HAZ at 60 days, 95% CI −0.11 to 0.29 SD). This was not the case when participants positive for EPEC were included, whether or not those with rotavirus-positive stools were excluded.
Table 5.
Sensitivity analysis for height-for-age z-score at 60 days
| Outcome: HAZ at 60 days adjusted for baseline HAZ | Treatment group | Regression analysis | |
| Delayed testing N; median (Q1, Q3) |
Rapid testing N; median (Q1, Q3) |
Mean difference (95% CI) p value | |
| 1. All patients | 119; −0.77 (−1.64 to 0.21) |
121; −0.74 (−1.32 to 0.14) |
240; 0.01 (−0.14 to 0.16) 0.923 |
2. Patients with at least one of:
|
52; −0.99 (−1.87 to 0.20) |
43; −0.74 (−1.26 to 0.26) |
95; 0.09 (−0.11 to 0.29) 0.379 |
3. Patients with at least one of:
|
74; −0.90 (−1.75 to 0.21) |
74; −0.75 (−1.44 to 0.02) |
148; −0.06 (−0.27 to 0.15) 0.565 |
4. Patients with at least one of:
|
39; −0.48 (−1.64 to 0.57) |
35; −0.96 (−1.70 to 0.30) |
74; −0.08 (−0.31 to 0.15) 0.485 |
Comment: adjusted for baseline height-for-age z score and severe acute malnutrition.
EPEC, enteropathogenic Escherichia coli; ETEC, enterotoxigenic E. coli; HAZ, age-standardised height.
There was no demonstrable impact of the probiotic intervention (mean difference −0.07 SD, 95% CI −0.22 to 0.08 SD) on the primary outcome, HAZ at 60 days, in an intention-to-treat analysis. There was also no demonstrable benefit of the probiotic intervention on recurrent diarrhoea within the 60-day follow-up period, stunting at 60 days, 60-day WAZ adjusted for initial WAZ, length of stay in hospital, development of sepsis in the 60-day follow-up period, 7-day mortality, 60-day mortality, parent absenteeism or healthcare provider visits for non-diarrhoea causes in the follow-up period.
Discussion
In children hospitalised for acute diarrhoeal disease in Botswana, neither a test-and-treat enteric diagnostic strategy nor the provision of L. reuteri DSM 17938 led to a significant increase in age-standardised height, in an intention-to-treat analysis. To our knowledge, this is the first time such a rapid test-and-treat strategy has been prospectively evaluated in a resource-limited context.
As enteropathogens are known to be associated with the onset of enteropathy and growth failure,32 substantial effort has been put into observing putative routes of transmission of diarrhoea-causing microbes and designing multifaceted multisite cluster randomised trials evaluating water, sanitation and hygiene interventions. Unfortunately, three recent large trials evaluating comprehensive interventions found them to have no impact on the incidence of childhood diarrhoea or non-viral enteropathogen infection rates.33–37 This does not disprove the hypothesis that clean water and sanitation are salubrious processes but instead reinforces the notion that novel approaches to ameliorating enteric infections in childhood are required.36
In 1469 children followed prospectively in a seminal multicohort study,38 acute diarrhoeal episodes caused by specific pathogens were associated with negative length decrements at 90 days, most notably Shigella (0.03 SD loss in HAZ, 95% CI 0 to −0.05), ETEC (0.04 SD loss in HAZ, 95% CI −0.01 to −0.07), Cryptosporidium (0.06 SD loss in HAZ, 95% CI 0 to −0.13), norovirus and adenovirus; unsurprisingly, the cumulative impact of these infections on height measured at age 2 years was substantially larger.12 A secondary analysis of 3408 episodes of moderate-to-severe diarrhoea in infants from a large multicountry study found that infections with Cryptosporidium (0.09 SD loss in HAZ, 95% CI 0.04 to 0.14), untreated Shigella (0.17 SD loss in HAZ, 95% CI 0.04 to 0.31), and typical EPEC (0.08 SD loss in HAZ, 95% CI 0.02 to 0.15) were all associated with significant height decrements at 60 days.14 Other studies have also found that diarrhoeal episodes caused by Shigella, Cryptosporidium and Campylobacter are associated with significant height deficits at 12 months.13 However, there is little evidence as to whether prospective antibiotic treatment will mitigate this growth failure, even were it feasible to do so at large scale. A recent secondary analysis of the Global Enteric Multicenter Study demonstrated that toddlers with Shigella-positive stools that were treated with WHO-recommended antibiotics for dysentery actually had increased standardised length at 60 days postenrolment, an obvious and clinically relevant difference compared with those who did not receive treatment.14 In contrast, a recently published large multicountry clinical trial randomising young children with acute watery diarrhoea to azithromycin or placebo found only a small (0.03 SD) benefit in age-standardised length at 90 days postenrolment39; of note, this trial did not attempt to distinguish prospectively between those with shigellosis and those with alternate diarrhoeal aetiologies. In our study, the point estimates for the effectiveness of the test-and-treat intervention to prevent loss of linear growth and recurrent diarrhoea within the 60-day follow-up period in participants harbouring treatable pathogens previously associated with growth delays12–14 (ie, Shigella, Campylobacter and ETEC) were favourable, so we cannot exclude the possibility that there might be a benefit to test-and-treat for those children with acute severe diarrhoeal disease caused by selected enteropathogens. Unfortunately, given that our sample size calculations incorporated an overly optimistic prediction of the benefit attributable to test-and-treat given the results of our pilot trial,30 coupled with the fact that only one-third of the patients randomised to test-and-treat had one of these high-impact pathogens, diluted this trial’s ability to discern a significant benefit for this subgroup. Our study found that test-and-treat was associated with a 0.10 SD (95% CI −0.11 to 0.31) increase in HAZ at 60 days in children with Shigella, ETEC or Campylobacter in their stool; given the expected HAZ loss at 90 days observed in the previously mentioned observational studies,12–14 it would seem that an increase in linear growth of this magnitude might be reasonable.
Our study is the only randomised controlled trial to assess the potential impact of providing antimicrobial treatment for EPEC in children presenting with acute diarrhoeal illness in a resource-limited setting. A relatively large proportion of children had EPEC detected (42%), and there was no evidence of improvement in growth outcomes for children treated with azithromycin. The assay used in the study, unfortunately, which detects all EPEC strains using the eaeA gene target, did not differentiate typical (bfp positive) from atypical (bfp negative) EPEC.
Although we assessed the impact of the ‘test-and-treat’ approach on several outcomes, there are other potential benefits of using such an approach that were not assessed in this study; these include improved antimicrobial stewardship and reduced secondary transmission of treatable pathogens. One study recently found that the median duration of Shigella shedding in children after an incident infection was 14 days (95% CI 10 to 18 days), which may be abbreviated with appropriate antibiotic treatment.40 In a cohort of adults with shigellosis treated with azithromycin, those with azithromycin-resistant Shigella infection had culture-positive stools far longer than those with azithromycin-sensitive infection.41
Many studies have now shown that it is common to detect multiple pathogens in the stool of children presenting with diarrhoea in LMICs.20 31 42 43 Of these pathogens, some are more likely than others to be the causative agent of a given episode of diarrhoeal illness.12 42–44 Generally, only Shigella and rotavirus detection are more likely than not to reflect the cause of a given episode of diarrhoea,42 45 suggesting that the detection of other pathogens can also represent either colonisation or past infection, as shedding can be prolonged.40 In our study, we could not be certain whether the enteropathogens detected truly caused the diarrhoea that prompted their hospitalisation. However, the aim of this study was to investigate the impact of a potential test-and-treat diagnostic pathway in an LMIC hospital setting, and qualitative molecular detection should be very sensitive, if less specific, for the detection of diarrhoea caused by Campylobacter, Cryptosporidium and ETEC.
One limitation of our study is that we did not perform stool culture and do not have antimicrobial susceptibility data for the detected bacterial isolates. Azithromycin resistance has been associated with worse clinical outcomes for shigellosis in adults.41 Thankfully, a contemporaneous study in Gaborone (the primary study site) found no macrolide resistance in Campylobacter isolates drawn from both paediatric clinical specimens and zoonotic reservoirs.46 We note that azithromycin is not available in the public formulary in Botswana, and its use has been infrequent.47
It is difficult to identify the precise reason that this trial’s results differ from those of our previous pilot study.30 In an effort to optimise recruitment, we did not exclude children with suspected concomitant bacterial infection outside the gastrointestinal tract from the current study; consequently, approximately 15% of study participants also received treatment with cefotaxime, which would attenuate the observed benefit of test-and-treat for those children with Shigella or ETEC infections. There has been much interest in the ability of probiotics to improve human health, including in sub-Saharan Africa.48 Unfortunately, our study found no benefit associated with the use of Lactobacillus reuteri DSM 17938 for severe gastroenteritis in southern Africa; these results are consistent with those of an updated Cochrane systematic review.49 Although underpowered, the point estimates for almost all outcomes favoured placebo over probiotic, making a type II error less likely. It is possible that treatment with either gentamicin or amoxicillin/clavulanate (much more common in this study than in the previous pilot) had direct antimicrobial effects on L. reuteri, explaining the lack of benefit observed in this, larger, trial. We note that the probiotic preparation may not have been maintained at optimal temperatures in many households after discharge. It is also probable that adherence to the 60-day treatment course was suboptimal; however, if a 60-day treatment course did not result in any obvious beneficial effects, it seems unlikely that shorter, more implementable, treatment courses of L. reuteri would yield a clinically significant benefit either.
In summary, we found that neither a rapid test-and-teat strategy targeting bacterial and/or protozoan enteropathogens, nor L. reuteri DSM 17938, was associated with a statistically significant improvement in important outcomes. Given that the point estimates for the benefit of the test-and-treat strategy were favourable, but our study was underpowered, further studies of rapid diagnostics to mitigate the sequelae of paediatric acute diarrhoeal disease in specific settings may be useful.
Footnotes
Handling editor: Seye Abimbola
Twitter: @JeffPernica, @Norah Mawoko
Contributors: JMP conceptualised the study, acquired funding, designed the study, supervised data collection, wrote the initial draft, revised the manuscript critically, verified the underlying data, and is responsible for overall content as guarantor. DG conceptualised the study, revised study design, acquired funding, revised the manuscript critically and verified the underlying data. TA-M was responsible for study administration, revised study design, supervised data collection, revised the manuscript critically and verified the underlying data. APS revised study design and revised the manuscript critically. MM revised study design, supervised investigation and revised the manuscript critically. BM, MB, OM, BB, AL and JB provided input on study design, collected study data and revised the manuscript critically. NM provided study resources and revised the manuscript critically. KL and CM contributed to study investigation and revised the manuscript critically. LT, MS and MSK revised study design and revised the manuscript critically. TV and JE conducted data analysis and revised the manuscript critically. AMG and LM provided study resources and revised the manuscript critically. KS revised study design and revised the manuscript critically.
Funding: The study was funded by Grand Challenges Canada and the Government of Canada (Transition to Scale 0768–05) and bioMérieux. Copan Italia provided swabs and BioGaia provided probiotic and placebo medications. JMP was supported by Hamilton Health Sciences (Early Career Award). This publication was made possible through core services and support from the Penn Centre for AIDS Research, an NIH-funded programme (P30 AI 045008). MSK was supported by a NIH Career Development Award (K23-AI135090).
Competing interests: JMP’s institution has received grant funding from MedImmune for a study outside the submitted work.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available on reasonable request. Individual participant data that underlie the results reported in this article, after deidentification, will be made available, 12 months after publication until 36 months postpublication, to researchers whose proposed use of the data has been approved by an independent review committee. The study protocol has been attached. Requests for data sharing can be directed to the primary author.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by the Hamilton Integrated Research Ethics Board, the Botswana Ministry of Health and Wellness Health Research Development Committee Institutional Research Board, the Princess Marina Hospital Research Ethics Committee, the Scottish Livingstone Hospital Ethics Committee and the Bamalete Lutheran Hospital Ethics Committee. Participants gave informed consent to participate in the study before taking part.
References
- 1.GBD 2015 Mortality and Causes of Death Collaborators . Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the global burden of disease study 2015. Lancet 2016;388:1459–544. 10.1016/S0140-6736(16)31012-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD Diarrhoeal Diseases Collaborators . Estimates of global, regional, and national morbidity, mortality, and aetiologies of diarrhoeal diseases: a systematic analysis for the global burden of disease study 2015. Lancet Infect Dis 2017;17:909–48. 10.1016/S1473-3099(17)30276-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mata LJ, Urrutia JJ, Albertazzi C, et al. Influence of recurrent infections on nutrition and growth of children in Guatemala. Am J Clin Nutr 1972;25:1267–75. 10.1093/ajcn/25.11.1267 [DOI] [PubMed] [Google Scholar]
- 4.Checkley W, Buckley G, Gilman RH, et al. Multi-country analysis of the effects of diarrhoea on childhood stunting. Int J Epidemiol 2008;37:816–30. 10.1093/ije/dyn099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown KH. Diarrhea and malnutrition. J Nutr 2003;133:328S–32. 10.1093/jn/133.1.328S [DOI] [PubMed] [Google Scholar]
- 6.Oriá RB, Murray-Kolb LE, Scharf RJ, et al. Early-Life enteric infections: relation between chronic systemic inflammation and poor cognition in children. Nutr Rev 2016;74:374–86. 10.1093/nutrit/nuw008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grantham-McGregor S, Cheung YB, Cueto S, et al. Developmental potential in the first 5 years for children in developing countries. Lancet 2007;369:60–70. 10.1016/S0140-6736(07)60032-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walker CLF, Perin J, Katz J, et al. Diarrhea as a risk factor for acute lower respiratory tract infections among young children in low income settings. J Glob Health 2013;3:010402. 10.7189/jogh.03.010402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scrimshaw NS. Historical concepts of interactions, synergism and antagonism between nutrition and infection. J Nutr 2003;133:316S–21. 10.1093/jn/133.1.316S [DOI] [PubMed] [Google Scholar]
- 10.Collins S, Dent N, Binns P, et al. Management of severe acute malnutrition in children. Lancet 2006;368:1992–2000. 10.1016/S0140-6736(06)69443-9 [DOI] [PubMed] [Google Scholar]
- 11.Victora CG, Adair L, Fall C, et al. Maternal and child undernutrition: consequences for adult health and human capital. Lancet 2008;371:340–57. 10.1016/S0140-6736(07)61692-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rogawski ET, Liu J, Platts-Mills JA, et al. Use of quantitative molecular diagnostic methods to investigate the effect of enteropathogen infections on linear growth in children in low-resource settings: longitudinal analysis of results from the MAL-ED cohort study. Lancet Glob Health 2018;6:e1319–28. 10.1016/S2214-109X(18)30351-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schnee AE, Haque R, Taniuchi M, et al. Identification of Etiology-Specific diarrhea associated with linear growth faltering in Bangladeshi infants. Am J Epidemiol 2018;187:2210–8. 10.1093/aje/kwy106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nasrin D, Blackwelder WC, Sommerfelt H, et al. Pathogens associated with linear growth faltering in children with diarrhea and impact of antibiotic treatment: the global enteric multicenter study. J Infect Dis 2021;224:S848–55. 10.1093/infdis/jiab434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Santosham M, Chandran A, Fitzwater S, et al. Progress and barriers for the control of diarrhoeal disease. Lancet 2010;376:63–7. 10.1016/S0140-6736(10)60356-X [DOI] [PubMed] [Google Scholar]
- 16.Water with sugar and salt. Lancet 1978;2:300–1. [PubMed] [Google Scholar]
- 17.The treatment of diarrhoea; a manual for physicians and other senior health workers, 2005. Available: http://apps.who.int/iris/bitstream/10665/43209/1/9241593180.pdf [Accessed 13 Apr 2020].
- 18.Lazzerini M, Wanzira H. Oral zinc for treating diarrhoea in children. Cochrane Database Syst Rev 2016;12:CD005436. 10.1002/14651858.CD005436.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dhingra U, Kisenge R, Sudfeld CR, et al. Lower-Dose Zinc for Childhood Diarrhea - A Randomized, Multicenter Trial. N Engl J Med 2020;383:1231–41. 10.1056/NEJMoa1915905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kotloff KL, Nataro JP, Blackwelder WC, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the global enteric multicenter study, GEMs): a prospective, case-control study. Lancet 2013;382:209–22. 10.1016/S0140-6736(13)60844-2 [DOI] [PubMed] [Google Scholar]
- 21.Bhutta ZA, Black RE. Global maternal, newborn, and child health--so near and yet so far. N Engl J Med 2013;369:2226–35. 10.1056/NEJMra1111853 [DOI] [PubMed] [Google Scholar]
- 22.Salam RA, Das JK, Bhutta ZA. Current issues and priorities in childhood nutrition, growth, and infections. J Nutr 2015;145:1116S–22. 10.3945/jn.114.194720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allen SJ, Martinez EG, Gregorio GV, et al. Probiotics for treating acute infectious diarrhoea. Cochrane Database Syst Rev 2010;11:CD003048. 10.1002/14651858.CD003048.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Florez ID, Veroniki A-A, Al Khalifah R, et al. Comparative effectiveness and safety of interventions for acute diarrhea and gastroenteritis in children: a systematic review and network meta-analysis. PLoS One 2018;13:e0207701. 10.1371/journal.pone.0207701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walker CR, Lechiile K, Mokomane M, et al. Evaluation of anatomically designed flocked rectal swabs for use with the BioFire FilmArray gastrointestinal panel for detection of enteric pathogens in children admitted to hospital with severe gastroenteritis. J Clin Microbiol 2019;57. 10.1128/JCM.00962-19. [Epub ahead of print: 22 11 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldfarb DM, Steenhoff AP, Pernica JM, et al. Evaluation of anatomically designed flocked rectal swabs for molecular detection of enteric pathogens in children admitted to hospital with severe gastroenteritis in Botswana. J Clin Microbiol 2014;52:3922–7. 10.1128/JCM.01894-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mokomane M, Kasvosve I, Gaseitsiwe S, et al. A comparison of flocked swabs and traditional swabs, using multiplex real-time PCR for detection of common gastroenteritis pathogens in Botswana. Diagn Microbiol Infect Dis 2016;86:141–3. 10.1016/j.diagmicrobio.2016.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Francavilla R, Lionetti E, Castellaneta S, et al. Randomised clinical trial: Lactobacillus reuteri DSM 17938 vs. placebo in children with acute diarrhoea--a double-blind study. Aliment Pharmacol Ther 2012;36:363–9. 10.1111/j.1365-2036.2012.05180.x [DOI] [PubMed] [Google Scholar]
- 29.Szajewska H, Urbańska M, Chmielewska A, et al. Meta-Analysis: Lactobacillus reuteri strain DSM 17938 (and the original strain ATCC 55730) for treating acute gastroenteritis in children. Benef Microbes 2014;5:285–93. 10.3920/BM2013.0056 [DOI] [PubMed] [Google Scholar]
- 30.Pernica JM, Steenhoff AP, Mokomane M, et al. Rapid enteric testing to permit targeted antimicrobial therapy, with and without Lactobacillus reuteri probiotics, for paediatric acute diarrhoeal disease in Botswana: a pilot, randomized, factorial, controlled trial. PLoS One 2017;12:e0185177. 10.1371/journal.pone.0185177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pernica JM, Steenhoff AP, Welch H, et al. Correlation of clinical outcomes with multiplex molecular testing of stool from children admitted to hospital with gastroenteritis in Botswana. J Pediatric Infect Dis Soc 2016;5:312–8. 10.1093/jpids/piv028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scharf RJ, Deboer MD, Guerrant RL. Recent advances in understanding the long-term sequelae of childhood infectious diarrhea. Curr Infect Dis Rep 2014;16:408. 10.1007/s11908-014-0408-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Humphrey JH, Mbuya MNN, Ntozini R, et al. Independent and combined effects of improved water, sanitation, and hygiene, and improved complementary feeding, on child stunting and anaemia in rural Zimbabwe: a cluster-randomised trial. Lancet Glob Health 2019;7:e132–47. 10.1016/S2214-109X(18)30374-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luby SP, Rahman M, Arnold BF, et al. Effects of water quality, sanitation, handwashing, and nutritional interventions on diarrhoea and child growth in rural Bangladesh: a cluster randomised controlled trial. Lancet Glob Health 2018;6:e302–15. 10.1016/S2214-109X(17)30490-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Null C, Stewart CP, Pickering AJ, et al. Effects of water quality, sanitation, handwashing, and nutritional interventions on diarrhoea and child growth in rural Kenya: a cluster-randomised controlled trial. Lancet Glob Health 2018;6:e316–29. 10.1016/S2214-109X(18)30005-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cumming O, Arnold BF, Ban R, et al. The implications of three major new trials for the effect of water, sanitation and hygiene on childhood diarrhea and stunting: a consensus statement. BMC Med 2019;17:173. 10.1186/s12916-019-1410-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grembi JA, Lin A, Karim MA, et al. Effect of water, sanitation, handwashing and nutrition interventions on enteropathogens in children 14 months old: a cluster-randomized controlled trial in rural Bangladesh. J Infect Dis 2020. 10.1093/infdis/jiaa549. [Epub ahead of print: 29 Aug 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.MAL-ED Network Investigators . The MAL-ED study: a multinational and multidisciplinary approach to understand the relationship between enteric pathogens, malnutrition, gut physiology, physical growth, cognitive development, and immune responses in infants and children up to 2 years of age in resource-poor environments. Clin Infect Dis 2014;59 Suppl 4:S193–206. 10.1093/cid/ciu653 [DOI] [PubMed] [Google Scholar]
- 39.Antibiotics for Children With Diarrhea (ABCD) Study Group, Ahmed T, Chisti MJ, et al. Effect of 3 days of oral azithromycin on young children with acute diarrhea in low-resource settings: a randomized clinical trial. JAMA Netw Open 2021;4:e2136726. 10.1001/jamanetworkopen.2021.36726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McMurry TL, McQuade ETR, Liu J, et al. Duration of Postdiarrheal enteric pathogen carriage in young children in low-resource settings. Clin Infect Dis 2021;72:e806–14. 10.1093/cid/ciaa1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Houpt ER, Ferdous T, Ara R, et al. Clinical outcomes of drug-resistant shigellosis treated with azithromycin in Bangladesh. Clin Infect Dis 2021;72:1793–8. 10.1093/cid/ciaa363 [DOI] [PubMed] [Google Scholar]
- 42.Liu J, Platts-Mills JA, Juma J, et al. Use of quantitative molecular diagnostic methods to identify causes of diarrhoea in children: a reanalysis of the gems case-control study. Lancet 2016;388:1291–301. 10.1016/S0140-6736(16)31529-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taniuchi M, Sobuz SU, Begum S, et al. Etiology of diarrhea in Bangladeshi infants in the first year of life analyzed using molecular methods. J Infect Dis 2013;208:1794–802. 10.1093/infdis/jit507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Platts-Mills JA, Gratz J, Mduma E, et al. Association between stool enteropathogen quantity and disease in Tanzanian children using TaqMan array cards: a nested case-control study. Am J Trop Med Hyg 2014;90:133–8. 10.4269/ajtmh.13-0439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Platts-Mills JA, Liu J, Rogawski ET, et al. Use of quantitative molecular diagnostic methods to assess the aetiology, burden, and clinical characteristics of diarrhoea in children in low-resource settings: a reanalysis of the MAL-ED cohort study. Lancet Glob Health 2018;6:e1309–18. 10.1016/S2214-109X(18)30349-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Vries SPW, Vurayai M, Holmes M, et al. Phylogenetic analyses and antimicrobial resistance profiles of Campylobacter spp. from diarrhoeal patients and chickens in Botswana. PLoS One 2018;13:e0194481. 10.1371/journal.pone.0194481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mashalla Y, Setlhare V, Massele A, et al. Assessment of prescribing practices at the primary healthcare facilities in Botswana with an emphasis on antibiotics: findings and implications. Int J Clin Pract 2017;71. 10.1111/ijcp.13042. [Epub ahead of print: 27 11 2017]. [DOI] [PubMed] [Google Scholar]
- 48.Reid G, Nduti N, Sybesma W, et al. Harnessing microbiome and probiotic research in sub-Saharan Africa: recommendations from an African workshop. Microbiome 2014;2:12. 10.1186/2049-2618-2-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Collinson S, Deans A, Padua-Zamora A, et al. Probiotics for treating acute infectious diarrhoea. Cochrane Database Syst Rev 2020;12:CD003048. 10.1002/14651858.CD003048.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on reasonable request. Individual participant data that underlie the results reported in this article, after deidentification, will be made available, 12 months after publication until 36 months postpublication, to researchers whose proposed use of the data has been approved by an independent review committee. The study protocol has been attached. Requests for data sharing can be directed to the primary author.