Abstract
Introduction
Arthropod-borne viruses (arboviruses) are of notable public health importance worldwide, owing to their potential to cause explosive outbreaks and induce debilitating and potentially life-threatening disease manifestations. This systematic review and meta-analysis aims to assess the relationship between markers of socioeconomic position (SEP) and infection due to arboviruses with mosquito vectors.
Methods
We conducted a systematic search on PubMed, Embase, and LILACS databases to identify studies published between 1980 and 2020 that measured the association of SEP markers with arbovirus infection. We included observational studies without geographic location or age restrictions. We excluded studies from grey literature, reviews and ecological studies. Study findings were extracted and summarised, and pooled estimates were obtained using random-effects meta-analyses.
Results
We identified 36 observational studies using data pertaining to 106 524 study participants in 23 geographic locations that empirically examined the relationship between socioeconomic factors and infections caused by seven arboviruses (dengue, chikungunya, Japanese encephalitis, Rift Valley fever, Sindbis, West Nile and Zika viruses). While results were varied, descriptive synthesis pointed to a higher risk of arbovirus infection associated with markers of lower SEP, including lower education, income poverty, low healthcare coverage, poor housing materials, interrupted water supply, marital status (married, divorced or widowed), non-white ethnicities and migration status. Pooled crude estimates indicated an increased risk of arboviral infection associated with lower education (risk ratio, RR 1.5 95% CI 1.3 to 1.9); I2=83.1%), interruption of water supply (RR 1.2; 95% CI 1.1 to 1.3; I2=0.0%) and having been married (RR 1.5 95% CI 1.1 to 2.1; I2=85.2%).
Conclusion
Evidence from this systematic review suggests that lower SEP increases the risk of acquiring arboviral infection; however, there was large heterogeneity across studies. Further studies are required to delineate the relationship between specific individual, household and community-level SEP indicators and arbovirus infection risks to help inform targeted public health interventions.
PROSPERO registration number
CRD42019158572.
Keywords: systematic review, arboviruses, public health, epidemiology
Key questions.
What is already known?
Arboviruses with mosquito vectors are of notable global public health importance owing to their potential to cause explosive outbreaks and induce debilitating and potentially life-threatening disease manifestations.
In regions with established arboviral circulation, factors indicative of socioeconomic position, such as increased population density, inadequate water management and poor housing conditions, may exacerbate vector proliferation and elevate infection risks.
What are the new findings?
Descriptive synthesis pointed to a higher risk of arboviral infection associated with markers of lower socioeconomic position, including lower education, income poverty, low healthcare coverage, poor housing materials, interruptions of water supply, marital status (married, divorced or widowed) and non-white ethnicity.
Pooled crude estimates from meta-analyses indicated an increased risk of arboviral infection associated with having lower education, interruption of water supply and having ever been married.
What do the new findings imply?
This review underscores the importance of evaluating the arbovirus-related impacts of social protection policies that aim to reduce the consequences of poverty (eg, conditional cash transfer, housing and public works programmes) alongside continuing research on more conventional vector control interventions.
Introduction
Arthropod-borne viruses (arboviruses) are transmitted between vertebrate hosts by haematophagous (blood-feeding) arthropod vectors, including mosquitoes and ticks.1 Arboviruses with mosquito vectors, such as dengue virus (DENV) and chikungunya virus (CHIKV), are of notable public health importance worldwide owing to their potential to cause explosive outbreaks and induce debilitating and potentially life-threatening disease manifestations.2 In addition, congenital arboviral infections, such as with Zika virus (ZIKV), may result in severe congenital malformations with the potential to incur lifelong health and social costs for affected individuals and their families.1–4
Infection due to arboviruses with mosquito vectors is becoming increasingly prevalent. The burden of DENV has grown dramatically in recent decades, with substantial impact on morbidity and mortality worldwide, and ZIKV, CHIKV and Yellow Fever virus (YFV) have re-emerged.5 Environmental factors, such as climate change (eg, rising temperatures) and habitat modification (eg, deforestation) along with social factors, such as increased international mobility, contribute to the global spread of competent vectors and arboviruses.6 7 In regions with established arboviral circulation, community-level factors, such as increased population density, inadequate water management, and poor housing, may exacerbate vector proliferation and elevate infection risks.8 This has been reported by several ecological studies, which have shown increased levels of arboviral infections in economically deprived areas at the population-level.9–11 Furthermore, a recent systematic review employing descriptive synthesis reported a greater presence of Aedes mosquito vectors and associated arboviral diseases in regions with lower socioeconomic conditions in 50%–60% of evaluated studies.12 As described in the early social epidemiology literature, steep inverse associations between social class and mortality from a wide range of diseases exist.13 To better understand individual- and household-level risk factors for arboviral infections, we conducted a systematic review and meta-analysis synthesising published evidence on the relationship between markers of socioeconomic position (SEP) and infection due to arboviruses with mosquito vectors.
Methods
Search strategy and eligibility criteria
The protocol for this systematic literature review was registered in the International Prospective Register of Systematic Reviews (PROSPERO) as CRD42019158572 and was conducted in line with the 2009 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.14 We searched for studies measuring the association between SEP and arboviral infection published between 1 January 1980 and 30 June 2020 in MEDLINE (PubMed), Embase (Ovid) and LILACS (see online supplemental material 1), hypothesising that studies published more than 40 years prior to this work would lack relevance to current research. The search and full-text review were restricted to articles published in English, Portuguese, Spanish and French. Studies were eligible from any geographic location and with individuals from any age group, and included peer-reviewed observational case reports, case series or studies that had a cross-sectional, case–control or cohort study design. Studies assessing the association between SEP and/or proxy measures of SEP (eg, individual social class, living conditions, education, employment, household income, race/ethnicity and asset ownership) at the individual-level or household-level and the occurrence of acute, recent or past arboviral infection, indicated by laboratory confirmation, were included. Laboratory confirmation of arbovirus infection was based on the presence of viral RNA, antigen and/or serological evidence (eg, IgM or IgG); the quality of assays used in the individual studies was not appraised. Studies from grey literature, using an ecological design, evaluating the economic burden of arboviral infections, or only describing the natural history of disease were excluded (online supplemental material 2).
bmjgh-2021-007735supp001.pdf (21.2KB, pdf)
bmjgh-2021-007735supp002.pdf (20.7KB, pdf)
Data extraction and meta-analysis
Data on the author, year of publication, study period, study type, source of population, data source, duration of follow-up (if applicable), geographic location, age, sex, individual-level and household-level socioeconomic characteristics, arbovirus infection type, comparison groups, confounders, frequency (number and percentage) and effect estimates (risk ratio (RR) or odds ratio (OR)) were extracted from studies and consolidated. Data screening was conducted in duplicate by four investigators (GMP, LQ, JMP and NSC) and extraction in duplicate by two investigators (GMP and AV). Discrepancies were resolved by consensus. Two reviewers (GMP and LQ) evaluated study quality by conducting a bias assessment using the Newcastle-Ottawa scale (NOS) for individual-level studies (NOS ranges from zero to nine). The NOS form for cohort studies was also used to evaluate data quality for cross-sectional studies; however, the maximum score is limited to six as it was not possible to demonstrate absence of infection at the start of these studies due to the lack of follow-up (online supplemental table 1). Evaluation was performed in duplicate, and discrepancies were resolved by consensus.
bmjgh-2021-007735supp003.pdf (112.9KB, pdf)
When effect estimates were provided for an indicator with comparable parameters in at least three cohort and/or cross-sectional studies, pooled effect sizes and the 95% CIs were calculated using random-effects meta-analyses. Since studies were highly heterogeneous, a random-effects model was preferred.15 Heterogeneity in RR estimates were assessed using I2 statistics and Cochran’s Q test p values. Case–control studies were not included in the meta-analyses since ORs with 95% CIs were calculated from these study data and, given the high frequency of infections in study populations, were considered to be not directly comparable with cohort and/or cross-sectional relative risk (RR) effect estimates. Further subgroup analyses were conducted for each virus within each of the meta-analyses. Analyses were performed using STATA (V.14.0). A map indicating locations where studies were based was created using Tableau software.
Patient and public involvement
The patients and the public were not involved in the design, conduct or reporting of our research.
Results
Our search generated 3928 published records. After screening titles and abstracts, 110 manuscripts were assessed for eligibility. Of these, 36 articles were deemed eligible for inclusion in this systematic review (figure 1).
Figure 1.
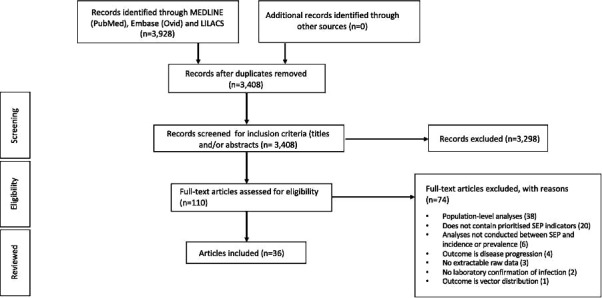
PRISMA flow chart illustrating selection of studies. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; SEP, socioeconomic position.
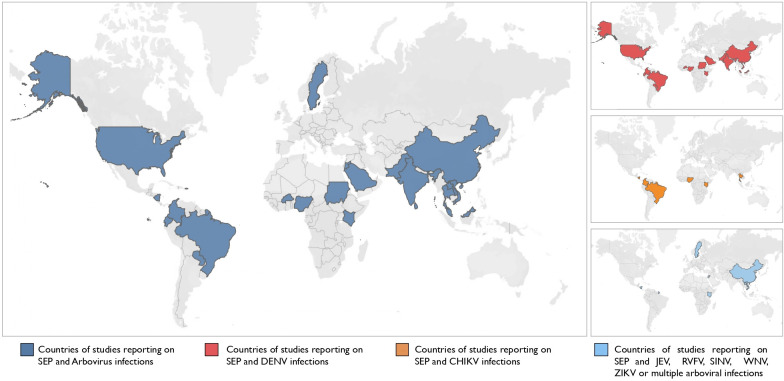
All studies included in this review were published between 1995 and 2020, the majority of which were published between 2015 and 2020 (n=28) and focused on DENV (n=21), CHIKV (n=6), Japanese encephalitis (JEV) (n=1), Sindbis virus (SINV) (n=1), West Nile virus (WNV) (n=1), ZIKV (n=1), DENV and JEV (n=2), DENV, CHIKV and Rift Valley fever virus (RVFV) (n=1) and flaviviruses in general with other arboviruses (n=2) (table 1, online supplemental table 2). There were no studies examining YFV. Included studies consisted of 2 cohort studies,16 17 4 case–control studies,18–21 27 cross-sectional studies,22–48 1 nested cross-sectional study within a cohort,49 1 combined cross-sectional and cohort study50 and 1 longitudinal serosurvey.51 Studies were conducted in 23 countries: 4 in low-income countries (Burkina Faso,42 Laos35 and Sudan26 43), 14 in lower-middle-income countries (Ecuador,41 India,19 Jordan,33 37 Kenya,17 36 Nicaragua,16 50 Nigeria,27 31 40 Pakistan,39 Sri Lanka18 and Vietnam34), 13 in upper-middle income (Brazil,23 30 45–47 China,20 24 38 Colombia,49 51 Malaysia,25 Paraguay44 and Thailand29) and 5 in high-income countries/territories (Mayotte (France),28 French Guiana,21 Saudi Arabia,22 Sweden32 and USA48) according to the Development Assistance Committee List of Official Development Assistance Recipients (figure 2).
Table 1.
Characteristics of included studies
| Author (year) | Country/ territory | Study period | Type of study | Population | Type of infection | Diagnostic test | Age range | Total size | Frequency measure | Cumulative incidence | NOS |
| DENV | |||||||||||
| Brunkard et al (2007)48 | USA | October 2004–November 2004 | Cross-sectional | Probability-based, household selection stratified, multistage, cluster-sampling design | DENV | DENV IgM+; DENV IgG+ | All ages | 600 | P | 2%–7.3%; 40%–78% |
5 |
| da Silva-Nunes et al (2008)47 | Brazil | 2004–2006 | Cross-sectional | Households in Ramal do Granada, were visited between March and April 2004. 466 dwellers <1–90 years of age (98.5% of the 473 areas permanent residents) were enrolled. |
DENV | DENV IgG+ | All ages | 405 | P | 18.3% | 6 |
| Pessanha et al (2010)46 | Brazil | June 2006–March 2007 | Cross- sectional | All residents aged over 1 year in the three Belo Horizonte districts (Venda Nova, DS Leste and DS Centro-Sul) | DENV | Not specified | All ages | 709 | P | 11.9% (95% CI 9.7% to 14.6%) | 5 |
| Kikuti et al (2015)45 | Brazil | 2009–2010 | Cross-sectional | Individuals seeking medical care for acute febrile illness at the only public emergency health unit | DENV | DENV IgM+ and/or RT-PCR+ | >5 years | 2962 | I | 22.0% | 5 |
| Pereira et al (2015)44 | Paraguay | 2014 | Cross-sectional | Inhabitants of three villages | DENV | DENV IgG+ | All ages | 418 | P | 24.2% (95% CI 20.2% to 28.6%) | 5 |
| Soghaier et al (2015)43 | Sudan | 2011 | Cross-sectional | Randomly selected community population through multi-stage cluster sampling | DENV | DENV IgG+ | All ages | 540 | P | 9.4% | 6 |
| Fournet et al (2016)42 | Burkina Faso | May 2004–September 2004 | Cross-sectional | Children from Ouagadougou districts with different types and degrees of urbanisation | DENV | DENV IgG+ | 0–12 years | 3015 | P | 22.7% | 6 |
| Kenneson et al (2017)41 | Ecuador | 2014–2015 | Cross-sectional | Individuals with DENV infections from sentinel clinics - as well as members of the same household and four neighbouring households located within 200 meters | DENV | DENV NS1 RDT+, RT-PCR+ and/or IgM+ | All ages | 219 | P | 36.5% | 5 |
| Nasir et al (2017)40 | Nigeria | May 2016–August 2016 | Cross-sectional | Patients with febrile illnesses seeking medical assistance at hospital | DENV | DENV NS1 RDT+; DENV IgG+ | 1–49 years | 171 | P | 8.8%; 43.3% |
3 |
| Khan et al (2018)39 | Pakistan | 2013–2015 | Cross-sectional | DENV patient samples | DENV | DENV RT-PCR+ | All ages | 59 765 | I | 9.2% | 4 |
| Liu et al (2018)38 | China | 2013–2015 | Cross-sectional | Samples selected from a 200,000-sample database holding serum collected from community residents living in Liwan and Yuexiu districts of Guangzhou | DENV | DENV IgM+; DENV IgG+ | All ages | 2085 | P | 3.98%; 11.8% |
3 |
| Obaidat and Roess (2018)37 | Jordan | 2015–2016 | Cross-sectional | Healthy relatives of patients at governmental human health centres at 11 governorates | DENV | DENV IgG+ | 0–80 years | 892 | P | 24.6% | 6 |
| Piedrahita et al (2018)51 | Colombia | 2010–2012 | Longitudinal serosurvey | School children | DENV | DENV IgG+ | 5–19 years | 4385 | I | 53.8% (2010) to 64.6% (2012) | 5 |
| Udayanga et al (2018)18 | Sri Lanka | February 2017– April 2017 | Case–control | Random selection of 200 households reporting past dengue incidence and 200 non-dengue reported households | DENV | N/A | All ages | 4000 | N/A | N/A | 4 |
| Al-Raddadi et al (2019)22 | Saudi Arabia | 2017 | Cross-sectional | Residents of the four cities of all genders, age groups, and socioeconomic classes | DENV | DENV IgG+ | All ages | 6397 | P | 26.7% | 6 |
| Chiaravalloti-Neto et al (2019)23 | Brazil | October 2015–March 2016 | Cross-sectional | Residents of Vila Toninho neighbourhood | DENV | DENV IgG+ | >10 y | 1322 | P | 74.6% | 8 |
| Jing et al (2019)24 | China | 2015 | Cross-sectional | 850 participants from seven selected communities in Guangzhou with no reported dengue cases before 2014 | DENV | DENV IgG+ | 1-84y | 850 | P | 6.6% | 6 |
| Abd-Jamil et al (2020)25 | Malaysia | 2007–2010 | Cross-sectional | Orange Asli populations residing in eight different villages in the forest or forest fringe areas of Peninsular Malaysia | DENV | DENV IgG+ | All ages | 491 | P | 17.0% | 6 |
| Eldigail et al (2020)26 | Sudan | August 2017–May 2018 | Cross-sectional | Eleven localities of Kassala state | DENV | DENV IgG+ | All ages | 600 | P | 11.4% | 6 |
| Omatola et al (2020)31 | Nigeria | 2019 | Cross-sectional | Visiting outpatients from the four hospitals in Anyigba | DENV | DENV IgG+ | All ages | 200 | P | 20.5% | 3 |
| Swain et al (2020)19 | India | 2017 | Case–control | Confirmed dengue patients within 1 year in six districts of the state | DENV | DENV IgM+ | All ages | 767 | N/A | N/A | 8 |
| CHIKV | |||||||||||
| Sissoko et al (2008)28 | Mayotte | 2005–2006 | Cross-sectional | Household-based; complex multistage cluster sampling of population of Mayotte | CHIKV | CHIKV IgG+ | ≥2 years | 1154 | P | 37.2% | 6 |
| Nakkhara et al (2013)29 | Thailand | 2008 | Cross-sectional | Residents aged 18 years or more from three villages | CHIKV | CHIKV IgG+ | >18 years | 507 | P | 61.9% | 5 |
| Kuan et al (2016)50 | Nicaragua | March 2015–April 2016 | Cross-sectional; Cohort |
Children aged 2–14 years enrolled in the Paediatric Dengue Cohort Study; Household recruitment |
CHIKV | CHIKV total antibody+ | 2–14 years; >15 years | 3362; 848 |
P | 6.1% (2-14 years); 13.1% (>15 years) |
9; 5 |
| Rueda et al (2019)49 | Colombia | 2014 | Cross-sectional nested in community cohort | 548 suspected CHIKV patients from the COPCORD cohort | CHIKV | CHIKV IgG+ | >18 years | 548 | P | 53.8% | 4 |
| Anjos et al (2020)30 | Brazil | 2016–2017 | Cross-sectional | All households of 3 contiguous valleys in Pau da Lima who are ≥5 years of age | CHIKV | CHIKV IgM+, CHIKV IgG+ | All ages | 1772 | P | 11.8% | 4 |
| Omatola et al (2020)27 | Nigeria | 2018 | Cross-sectional | Febrile participants at five hospitals in Anyigba who test negative for typhoid and malaria | CHIKV | CHIKV IgM+, CHIKV IgG+ | All ages | 243 | P | 34.2% | 3 |
| JEV | |||||||||||
| Luo et al (1995)20 | China | June 1991–September 1991 | Case–control | Active case finding in hospitals in Gusi County, Henan, China | JEV | JEV IgG+ | >6 months - 10 years | 150 | N/A | N/A | 8 |
| SINV | |||||||||||
| Ahlm et al (2014)32 | Sweden | 2009 | Cross-sectional | Randomly selected from population registers | SINV | SINV IgG+ | 25–75 years | 1729 | P | 2.9% | 6 |
| WNV | |||||||||||
| Obaidat et al (2019)33 | Jordan | November 2015–May 2016 | Cross-sectional | Healthy relatives of patients seeking healthcare at health centres throughout Jordan. | WNV | WNV IgG+ | 15–50 years | 801 | P | 8.6% | 6 |
| ZIKV | |||||||||||
| Burger-Calderon et al (2018)16 | Nicaragua | August 2016–October 2016 | Cohort | Laboratory-confirmed Zika index cases and their household members | ZIKV | ZIKV RT-PCR+ | All ages | 142 | I | 31.0% | 8 |
| Multiple arboviruses | |||||||||||
| Bartley et al (2002)34 | Viet Nam | April 1996–August 1997 | Cross-sectional | Community and hospital-based subjects | DENV; JEV | DENV or JEV IgG+ | All ages | 308 | P | 66.0% | 5 |
| Conlan et al (2015)35 | Laos | January 2009–March 2009 | Cross-sectional | Random selection of 14 households per village and all household members over 6 years age asked to participate | JEV; DENV | NC; JEV HI+; DENV1 HI+; DENV2 HI+; DENV3 HI+; DENV4 HI+ | ≥6 years | 1136 | P | 67.3% (Any flavivirus); 39.4% (JEV); 2.2% (DENV 1); 0.8% (DENV2); 0.8% (DENV3); 13.6% (DENV4) |
4 |
| Ochieng et al (2015)36 | Kenya | 2007 | Cross-sectional | HIV-negative blood specimens from the 2007 Kenya AIDS Indicator Survey | CHIKV; DENV; RVFV | CHIKV IgG+; DENV IgG+; RVFV IgG+ | 15–64 years | 1091 | P | 0.97%; 12.5%; 4.5% |
3 |
| Bonifay et al (2017)21 | French Guiana | March 2013–June 2014 | Case–controlE | Group of patients infected with CHIKV in 2014 with a group infected with DENV | CHIKV; DENV | CHIKV RT-PCR+; DENV IgM+ | >15 years and 3 months | 336 | N/A | N/A | 6 |
| Hortion et al (2019)17 | Kenya | December 2014–December 2015 | Cohort | Acutely ill children presenting at one of four healthcare centres | Flavivirus, CHIKV; DENV | CHIKV IgG+; DENV IgG+ | All ages | 1604 | P | 3.7% | 6 |
*The authors report it was not possible to distinguish between DENV and JEV IgG due to cross-reactivity.
CHIKV, Chikungunya virus; DENV, Dengue virus; HI, Hemagglutination inhibition; I, Incidence; Ig, Immunoglobulin; JEV, Japanese Encephalitis virus; N/A, not applicable; NC, not clear; NOS, Newcastle-Ottawa scale; NS1, Non-structural protein 1; P, Prevalence; RDT, Rapid diagnostic test; SINV, Sindbis virus; WNV, West Nile virus; ZIKV, Zika virus.
Figure 2.
Geographic distribution of studies included in the systematic review. (A) All countries reporting SEP and arboviral infections, (B) Countries reporting SEP and Dengue virus (DENV) infections, (C) Countries reporting SEP and Chikungunya virus infections, (D) Countries reporting on SEP and Japanese encephalitis virus (JEV), Rift Valley fever virus (RVFV), Sindbis virus (SINV), West Nile virus (WNV), Zika virus (ZIKV) or multiple arboviral infections. SEP, socioeconomic position.
bmjgh-2021-007735supp004.pdf (163.4KB, pdf)
Age and sex
Age and sex were investigated and/or adjusted for in 32 of the 36 studies on seven arboviruses (CHIKV, DENV, JEV, RVFV, SINV, WNV and ZIKV). These studies included three case–control, two cohort, 25 cross-sectional studies, one study comprising a cross-sectional and cohort investigation50 and 1 cross-sectional nested in a cohort study, spanning 21 countries.
Of the 20 studies that evaluated the relationship between age and arboviral infection, 18 (90%) reported evidence of an association between increasing age and seropositivity for arboviruses, while four studies (20%) found statistical evidence of an association between age and past arboviral infection (DENV23 36 37 and CHIKV50) in adjusted models.
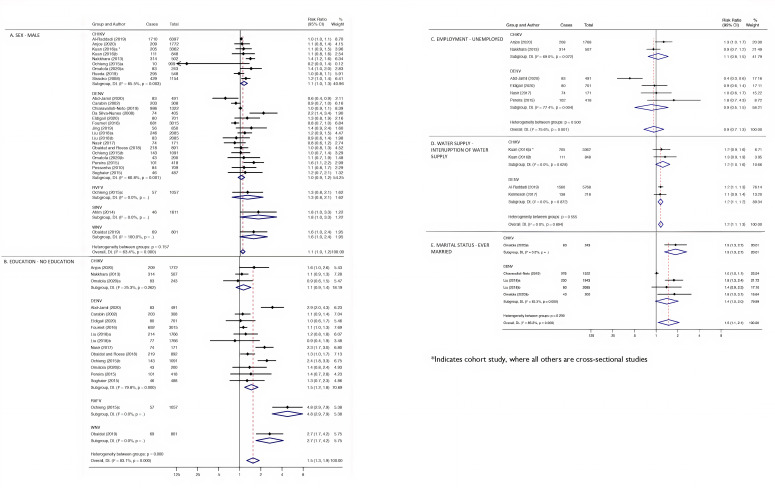
All 36 studies considered the direct relationship between sex and arboviral infection or adjusted for sex in the model. Five (13.9%) of these studies reported evidence of higher prevalence of arboviruses among males in crude analyses.28 32 39 45 47 However, statistical analyses were not provided for every study, and just eight provided an adjusted point estimate.16 23 34 36 37 47 50 51 A study conducted in Sweden32 found a crude statistical association between being male and seropositivity for SINV; however, on adjusting for age and smoking in multivariate analyses, neither sex nor age were significant predictors of seropositivity for SINV. Twenty-four studies with 28 crude estimates comprising a total of 34 373 individuals were included in the random-effects meta-analysis of the association of sex and arboviral infection. The crude combined RR for males was 1.1 (95% CI 1.0 to 1.2), with substantial heterogeneity between studies (I2=63.4%) (figure 3A). Disease-specific pooled estimates indicated a RR of 1.1 (95% CI 1.0 to 1.3) and 1.0 (95% CI 0.9 to 1.2) in CHIKV and DENV subgroups, respectively.
Figure 3.
Meta-analysis for the association between socioeconomic risk markers and arboviral infections. Pooled estimates using random-effects meta-analyses are calculated by subgroups of socioeconomic markers, sex (A), education (B), employment (C), water supply (D) and marital status (E). Subgroups of arboviruses are additionally presented per risk marker. Error bars show the point RR with their 95% CIs on the log scale for each study. Diamonds show the combined point estimate. I2 statistics and Q-test p values are reported. *Indicates cohort study, whereas all others are cross-sectional studies.
Education and occupation
The association between education and arboviral infection was analysed in 1 cross-sectional study nested in a cohort, 2 case–control and 22 cross-sectional studies, spanning 18 countries and 6 arboviruses (CHIKV, DENV, JEV, RVFV, SINV and WNV). In these studies, education was classified in distinct ways depending on context, and included level of education,19 24 26 27 29 31–34 36 38–41 43 44 49 schooling age,23 parental education,20 the attainment of any formal education,25 37 42 length of education in years28 and illiteracy.30 45
Overall, there tended to be a higher risk of infection among less educated individuals in crude analyses. However, studies that developed multivariate models indicated weak or no statistical evidence of an association between education and arboviral infection after accounting for confounding factors.19 20 23 32 36 37 In addition, a cross-sectional study conducted in China presented evidence that fewer years of parental schooling was associated with increased risk of JEV infection;20 however, on adjusting for JEV vaccination, there was very little evidence remaining. In the 17 investigations (n=15 760) included in the random-effects meta-analysis for education, the crude combined RR for lack of education was 1.5 (95% CI 1.3 to 1.9); however, there was considerable heterogeneity between studies (I2=83.1%) (figure 3B).
Random-effects meta-analysis for disease-specific pooled estimates revealed that individuals with no education had a crude combined RR of 1.5 (95% CI 1.2 to 1.8) for DENV infections and 1.1 (95% CI 0.9 to 1.4) for CHIKV infections.
Occupation was assessed in 11 cross-sectional studies and 1 case–control study. Eleven of the 12 studies presented frequencies, 6 presented crude effect estimates and 2 presented adjusted effect estimates. The occupation-related variables analysed were employment status,25 26 30 location of work (inside or outside),23 earnings (above the country’s minimum wage or not),41 employment stability and occupation types.19 27–29 31 40 44 In a study conducted by Chiaravalloti-Neto et al in Brazil, there was a crude association between working outside and seropositivity for DENV, which was lost on adjusting for other socioeconomic and demographic covariates.23 Swain et al indicated evidence to suggest that DENV infection was associated with occupations that required travel into certain parts of India.19 Collectively, in the six studies (n=4056) that were included in the random-effects meta-analysis for occupation, there was little evidence of an association between lack of employment and arboviral infection (pooled RR 0.9; CI 95% 0.7 to 1.3), with considerable heterogeneity between studies (I2=75.6%) (figure 3C).
Income poverty and social vulnerability
Variables indicating income poverty and social vulnerability varied considerably and thus were challenging to standardise; however, descriptive analyses indicate that lower income was a risk factor for arboviral infection, with limited empirical evidence.
The relationship between poverty or social vulnerability and arboviral infection was assessed in 1 cohort, 4 case–controls and 15 cross-sectional studies, across 16 countries and 4 arboviruses (CHIKV, DENV, JEV and WNV). Assessments were based on weekly or monthly household income,18 20 23 25 26 33 39 44–46 48 49 SEP categorised into groups,42 49 50 per capita income quartiles or quintiles.35 36 47 Health vulnerability was also assessed in two studies.21 46 This comprised estimating a health vulnerability index and health vulnerability through state or free care compared with social security and complimentary health insurance. Frequencies and/or effect estimates were extracted for 14. Four studies investigating DENV found evidence of a relationship between lower household income and increased arboviral infection.25 45 47 48 One case–control study, conducted in French Guiana, that specifically examined healthcare coverage status in relation to CHIKV and DENV infection, found that a lack of private health insurance was associated with higher CHIKV infection both in the crude and adjusted analyses. In contrast, however, DENV appears to affect a wealthier population.21 Since poverty indicators were not measured consistently between studies and study contexts, a meta-analysis was not possible for income or social vulnerability factors in this study.
Household conditions
Four case–control, three cohort, one longitudinal serosurvey and 18 cross-sectional studies investigated the association between household characteristics and arboviral infections. These studies examined the type or size of residence,19 22–24 30 32 34 44 46 house appearance or quality,20 28 42 number of rooms,22 41 building density,42 household crowding,17 18 22 23 28 30 31 41 43 44 48 50 type or presence of walls,47 wall gaps,47 presence of screens,41 48 residential area,17 21 32 37 waste management42 45 and asset ownership (air conditioning,48 refrigerator,16 television,34 land tenure and home ownership23 41 47 and asset ownership index (presence of electricity, flush toilet, piped water and possession of a television set, radio or refrigerator).28
Of the four studies that evaluated the association between type of residential area (urban vs rural) and arboviral infections,17 32 34 37 one reported higher risk of SINV infection in small, rural residential areas in Northern Sweden,32 one study showed that the risk of flavivirus infection was higher in urban residential areas or cities compared with surrounding rural areas and Southern Vietnam,34 while a study in Kenya observed no difference in flavivirus infection between rural and urban areas but did note a higher seroprevalence among coastal compared with western study participants.17 In Jordan, a higher risk of WNV infection was reported for those living in Badia and the Jordan Valley regions (arid and hot climates) compared with those living in the Highlands and Plains regions (colder and higher precipitation areas).37
The relationship between house or land ownership and arboviral infection was evaluated in three studies.23 41 47 A cross-sectional study conducted in Brazil showed little evidence of an association between home ownership and seropositivity in DENV, although living in a house compared with an apartment was positively associated with DENV seropositivity, after adjusting for socioeconomic and demographic covariates.23 Crude analyses indicated evidence of a negative association between land tenure in rural Amazonia, Brazil, and DENV seropositivity; however, this association was weak in the adjusted analysis.47
Of the seven studies that analysed building materials, three studies found an association between poor building materials or structures and arboviral infection.20 28 30 In addition, unstructured low building density households had higher prevalences of CHIKV and DENV.19 20 28 42
Crowding, categorised by number of individuals per household,17 22 23 28 30 43 44 48 50 residents per room41 or residents per bed27 was analysed in 11 studies, of which four found an association between crowding and arboviral infection.23 28 43 50 In a study conducted in Paraguay, DENV prevalence was higher for those who lived alone compared with those who lived with others.44
Water supply and sanitation
Water supply or service consumption was investigated in eight studies16 22 37 41–44 50 and waste collection or sanitation in three studies.22 42 48 Having adequate water supply (ie, tap or piped water) was associated with lower DENV infection in Ecuador41 and Paraguay.44 In addition, water supplied by water wells, onsite water storage and frequent/longer interruptions of water supply was associated with higher flavivirus seroprevalence in Burkina Faso,42 higher seropositivity for ZIKV in contacts of ZIKV index cases in Nicaragua,16 higher DENV infection in Ecuador41 and Saudi Arabia,22 and higher CHIKV infection in children in Nicaragua.50
Improper waste management practices were also significantly associated with flavivirus IgG in different building density strata in Burkina Faso,42 while an association was found between lack of street drainage and higher DENV infection on the US/Mexico border.48 The absence of sanitation was strongly associated with DENV infection in crude analysis in Saudi Arabia; however, this was not included in the multivariable analysis.22 The random-effects meta-analysis from three studies (one of which contained a cohort (A) and cross-sectional (B) study design) (n=10 196) revealed evidence of an association between interruption of water supply and arboviral infection (RR 1.2; 95% CI 1.1 to 1.3; I2=0.0%) (figure 3D).
Other (marital status, ethnicity and migration status)
A range of other sociodemographic factors that act as proxies for SEP were investigated by several articles identified in this review. Having been born overseas was associated with greater risk of past arboviral infection, evidenced by one study,21 and crude analyses indicated individuals who identified as non-white or of a schedule caste in India, had a higher risk of arboviral infection.19 23 45 49 The evidence was limited, concentrated in six countries and largely focused on DENV or CHIKV.
Having been married, including currently or previously (ie, divorced or widowed), was associated with an overall increase in risk of arbovirus infection.23 31 38 Marital status and its association with DENV and CHIKV IgG and/or IgM antibody levels was investigated in four cross-sectional studies, conducted in Guangzhou, China,38 São Paulo, Brazil,23 Guinea Savannah, Nigeria,31 and Kogi state, Nigeria.27 In São Paulo,23 adjusted analyses showed that being single was a risk factor for DENV compared with being married, while in Guangzhou, China,38 crude analyses showed that widowed or divorced individuals were at higher risk of infection compared with both their married and single counterparts. Adjusted analyses from these two studies, however, revealed no statistical evidence of an association. All four studies were included in the random-effects meta-analysis, which revealed statistical evidence that individuals who had ever been married, including currently married, divorced or widowed, had higher overall crude risks of arboviral infection (RR 1.5 95% CI 1.1 to 2.1; I2=85.2%) than those who were single (figure 3E).
Four studies examined race/caste as a correlate of arboviral infection, of which two were conducted in Brazil,23 45 one in Colombia49 and one in India.19 The two Brazilian studies found that Black and non-white individuals were at increased risk of DENV23 45 and a case–control study conducted in Odisha, India, revealed higher odds of DENV infection in those considered a schedule caste or schedule tribe (official term given in India to those who have historically faced deprivation, oppression and marginalisation) compared with those considered non-schedule caste or non-schedule tribe.19 The crude analyses showed evidence of this association; however, this was lost on adjusting for unmentioned confounders. A meta-analysis was not performed due to the heterogeneity of study contexts and the countries’ specific social constructions of race/caste.
Migration status, defined on the basis of the country of birth: French-born and Foreign-born, was investigated as a potential risk factor for arboviral infection in a case–control study conducted in French Guiana.21 This study found strong statistical evidence in crude analysis that individuals born abroad had over four times the odds of testing positive for DENV IgG than those born in French West Indies, French Guiana or Mainland France. One study additionally indicated that changing city within Brazil was not associated with an increase in DENV infection risk.46
Quality evaluation
The quality scores of the 36 individual studies varied across study designs. For cross-sectional studies, scores ranged from 3 to 6, with weaknesses related to selection bias of exposed cohorts and lack of adjustment for confounders. For the cohort studies, scores ranged from 6 to 9, with weaknesses related to no indication of absence of disease at the start of the study and to lack of adjustment for confounders (online supplemental table 1A). For case–control studies, scores ranged from 4 to 8, with weaknesses related to lack of adjustment for confounders (online supplemental table 1B).
Discussion
In this systematic review and meta-analysis, we summarised published evidence linking markers of SEP and infection due to arboviruses with mosquito vectors. Descriptive results indicated lower education, income poverty, low healthcare coverage, poor housing materials, interrupted water supply, marital status (married, single, divorced or widowed), non-white ethnicities and migration status as potential risk factors for arboviral infection. Meta-analyses provided statistical evidence of an increased risk of infection due to arboviruses with mosquito vectors associated with lack of education, interruption of water and having ever been married.
Overall, the seroprevalence of arboviral-specific antibodies (in particular, to DENV) was shown to be highest in older age groups. This finding corroborates a number of studies that found a positive association between age and seropositivity for DENV and is assumed to be related to the longer period of exposure to DENV over time.52–58 No clear association between arboviral infection and sex was observed.
In addition, individuals with lower education were at greater risk of arboviral infection in both the descriptive summary and meta-analysis. Education is commonly used as a generic indicator for SEP, highlighting the accumulation of advantage and disadvantage over the lifecourse.59 60 It is associated with permanent income status, whereas income itself, for example, captures the level of income at the time of data collection and is thus, in general, volatile. These findings, therefore, might suggest that structural poverty is a relatively more important factor than transient poverty. Education is also argued to capture the knowledge and skill-related assets of an individual, which may contribute to the receptivity of health messaging and thus permitting more informed use of vector control activities to reduce risk of infection.61
The descriptive analysis for employment assessed several occupations and occupational exposure types, while the meta-analysis looked at unemployment compared with being employed. No overall statistical evidence for unemployment as a risk factor for arboviral infection was apparent. The unobserved effect is likely because the degree of vulnerability linked to unemployment is highly dependent on both the type of employment (indoor or outdoor occupations) as well as the country’s overall economic circumstances.59 Thus, this indicator is limited when comparing across studies as well as geographic areas.
Poverty has long been considered a determinant of arboviral infections such as DENV and CHIKV; however, the scarcity of studies with consistent measures of income poverty and social vulnerability has meant that such a relationship has yet to be substantiated. Indeed, in this systematic review, a meta-analysis was not possible for the variables that indicated income poverty and social vulnerability, since contexts within which the data were collected for these were not standardised. Descriptive analyses, nonetheless, indicated that lower income appeared to be a risk factor, although with limited empirical evidence. This is additionally supported by the vast literature on social determinants of health.62 Income can influence a variety of material circumstances with direct implications for health and arbovirus exposure.63 The conversion of money and assets into health-enhancing commodities or behaviours may be more relevant to understanding how this variable affects arboviral infection directly.59
While a meta-analysis was not completed for the variables related to the constructs of race or caste, the descriptive analysis revealed that individuals who identified as non-white23 45 or of a schedule caste19 were at greater risk of arboviral infection. While there is no biological basis for an association between these constructs and health,64 ethnicity, caste and race are proxies for the embodiment of xenophobia, casteism and racism in their structural, cultural and interpersonal forms.65 Data from the US context, for example, observed that in areas where mortality rates are highest, the fraction of black residents is larger.66 These findings may be extrapolated to the Brazilian context, where racial inequality and segregation are reflected in social disadvantage65 and health inequities.
Substandard housing conditions are likely to lead to greater exposure to mosquitoes and thus increased risk of infection.67 The association between poor quality housing conditions and arboviral infection was a common finding in many of the studies assessed. However, due to the diversity of indicators relating to household conditions, it was not possible to evaluate this in a meta-analysis. Poor living conditions are often also characterised by overcrowding. Indeed, household crowding appeared to be an additional risk factor for DENV infection. While the reasons behind this are unknown, it is likely due to the association between household crowing and income poverty as well as to the higher concentration of carbon dioxide and other chemicals in crowded houses which attracts a greater number of mosquitoes.68 Furthermore, the meta-analysis conducted on water supply in this study provided evidence that interruption in water supply, likely resulting in storage of water in containers and creation of prime breeding spots for mosquitoes,69 may increase risk of CHIKV and DENV infection.
The meta-analysis provided evidence that having been married, including currently or previously (ie, divorced or widowed), was associated with an increase in arboviral infection risk; however, the descriptive analysis indicated that most of these associations diminish after adjusting for confounding. Age may be a particularly important confounder in this context. Migration was assessed in one study and presented descriptively in this analysis. Those classified as migrants were considered to be in a precarious social situation, since they did not have regular social security and health insurance and therefore were more at risk of arboviral infection.21
This review has strengths and limitations. First, it is among the first to conduct a systematic review and meta-analysis using diverse populations to assess SEP indicators that identify individuals at the highest risk of arboviral infection. Further research is required to understand the specific mechanisms by which these factors impact infection. The findings of this review should be interpreted with caution, since there were high levels of heterogeneity between studies, which is likely a result of differences in study design, study population and contexts within which these data were collected as well as differences inherent to the individual arboviruses and their mosquito vectors. While this review addressed several arboviruses that circulate in different ecological cycles and involve differences in vector-host preferences, local host abundances and herd immunity, assessing the social determinants of these arboviruses together allows for the analysis of distal risk factors, such as socioeconomic indicators, that have an overarching effect on all arboviral infections.7 However, we acknowledge that grouping findings from multiple arboviruses may obscure observations and the heterogeneity of the measures used to capture the range of socioeconomic factors analysed in these studies make it more difficult to delineate associations of interest. Furthermore, this review did not differentiate past infections from current infections and therefore changes in SEP, civil status and even location may have introduced misclassification bias.
Conclusion
Evidence from this systematic review suggests that indicators of lower SEP at the individual and household-levels are associated with increased risks of acquiring arboviral infection across a wide range of geographic and cultural contexts. Although not a sufficient determinant of arbovirus risk in itself, poverty is closely correlated with the risk factors for arbovirus infection identified in this review. Within settings experiencing a high burden of arbovirus infections, further work is required to delineate the roles of specific socioeconomic risk factors to inform locally relevant preventive activities. More broadly, the findings of this review underscore the importance of evaluating the arbovirus-related impacts of social protection policies that aim to reduce the consequences of poverty (eg, conditional cash transfer, housing and public works programmes) alongside continuing research on more conventional vector control interventions. To conclude, the findings of this review add to relatively sparse data on the socioeconomic determinants of infection due to arboviruses with mosquito vectors and emphasise the need for further research to disrupt the cycle of poverty, vulnerability and arbovirus-related illness.
bmjgh-2021-007735supp005.pdf (158.8KB, pdf)
bmjgh-2021-007735supp006.pdf (89.1KB, pdf)
Footnotes
Handling editor: Seema Biswas
Twitter: @grace_m_power, @aisling_vaughan, @Enny04762857, @ebbrickley
Contributors: GMP developed the search strategy and inclusion and exclusion criteria, conducted screening and data extraction, evaluated study quality by conducting a bias assessment, drafted and completed the manuscript. AV conducted data extraction and drafted the manuscript. LQ developed the inclusion and exclusion criteria, conducted screening and evaluated study quality by conducting a bias assessment, NSC conducted screening, resolved discrepancies and contributed to the drafting of the manuscript, JMP conducted screening and contributed to the drafting of the manuscript, EP reviewed the manuscript and offered insightful critique, LL built the map for the manuscript, AIR reviewed the manuscript and offered insightful critique, APS, MLB and EBB reviewed the manuscript, obtained funding and supervised the project. EBB is responsible for the overall content and serves as the guarantor. All authors helped refine the final version of the manuscript and approve with its submission.
Funding: This work was supported by the British Council Newton Fund (527418645); Wellcome Trust & the UK Department for International Development (205377/Z/16/Z, https://wellcome.ac.uk/); Wellcome Trust (202912/Z/16/Z).
Map disclaimer: The inclusion of any map (including the depiction of any boundaries therein), or of any geographic or locational reference, does not imply the expression of any opinion whatsoever on the part of BMJ concerning the legal status of any country, territory, jurisdiction or area or of its authorities. Any such expression remains solely that of the relevant source and is not endorsed by BMJ. Maps are provided without any warranty of any kind, either express or implied.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study is a systematic review of available literature and did not involve direct access to participants of the primary research studies included. Research ethics approval was therefore not required.
References
- 1.Tsai TF. Congenital arboviral infections: something new, something old. Pediatrics 2006;117:936–9. 10.1542/peds.2005-2729 [DOI] [PubMed] [Google Scholar]
- 2.Brasil P, Pereira JP, Moreira ME, et al. Zika virus infection in pregnant women in Rio de Janeiro. N Engl J Med Overseas Ed 2016;375:2321–34. 10.1056/NEJMoa1602412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuper H, Lyra TM, Moreira MEL, et al. Social and economic impacts of congenital Zika syndrome in Brazil: study protocol and rationale for a mixed-methods study. Wellcome Open Res 2018;3:127. 10.12688/wellcomeopenres.14838.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ximenes RAdeA, Miranda-Filho DdeB, Montarroyos UR, et al. Zika-related adverse outcomes in a cohort of pregnant women with rash in Pernambuco, Brazil. PLoS Negl Trop Dis 2021;15:e0009216. 10.1371/journal.pntd.0009216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gould E, Pettersson J, Higgs S, et al. Emerging arboviruses: why today? One Health 2017;4:1–13. 10.1016/j.onehlt.2017.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodrigues NCP, Daumas RP, de Almeida AS, et al. Risk factors for arbovirus infections in a low-income community of Rio de Janeiro, Brazil, 2015-2016. PLoS One 2018;13:e0198357. 10.1371/journal.pone.0198357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Esser HJ, Mögling R, Cleton NB, et al. Risk factors associated with sustained circulation of six zoonotic arboviruses: a systematic review for selection of surveillance sites in non-endemic areas. Parasit Vectors 2019;12:265. 10.1186/s13071-019-3515-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whiteman A, Gomez C, Rovira J, et al. Aedes mosquito infestation in socioeconomically contrasting neighborhoods of Panama City. Ecohealth 2019;16:210–21. 10.1007/s10393-019-01417-3 [DOI] [PubMed] [Google Scholar]
- 9.Souza WVde, Albuquerque MdeFPMde, Vazquez E, et al. Microcephaly epidemic related to the Zika virus and living conditions in Recife, Northeast Brazil. BMC Public Health 2018;18:130. 10.1186/s12889-018-5039-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Netto EM, Moreira-Soto A, Pedroso C, et al. High Zika virus seroprevalence in Salvador, northeastern Brazil limits the potential for further outbreaks. mBio 2017;8:01390-17. 10.1128/mBio.01390-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lobkowicz L, Power GM, De Souza WV, et al. Neighbourhood-level income and Zika virus infection during pregnancy in Recife, Pernambuco, Brazil: an ecological perspective, 2015-2017. BMJ Glob Health 2021;6. 10.1136/bmjgh-2021-006811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whiteman A, Loaiza JR, Yee DA, et al. Do socioeconomic factors drive Aedes mosquito vectors and their arboviral diseases? A systematic review of dengue, chikungunya, yellow fever, and Zika Virus. One Health 2020;11:100188. 10.1016/j.onehlt.2020.100188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marmot MG, Davey Smith G, Stansfeld S, et al. Health inequalities among British civil servants: the Whitehall II study. Lancet 1991;337:1387–93. 10.1016/0140-6736(91)93068-k [DOI] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barili F, Parolari A, Kappetein PA, et al. Statistical primer: heterogeneity, random- or fixed-effects model analyses? Interact Cardiovasc Thorac Surg 2018;27:317–21. 10.1093/icvts/ivy163 [DOI] [PubMed] [Google Scholar]
- 16.Burger-Calderon R, Gonzalez K, Ojeda S, et al. Zika virus infection in Nicaraguan households. PLoS Negl Trop Dis 2018;12:e0006518. 10.1371/journal.pntd.0006518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hortion J, Mutuku FM, Eyherabide AL, et al. Acute flavivirus and alphavirus infections among children in two different areas of Kenya, 2015. Am J Trop Med Hyg 2019;100:170–3. 10.4269/ajtmh.18-0297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Udayanga L, Gunathilaka N, Iqbal MCM, et al. Comprehensive evaluation of demographic, socio-economic and other associated risk factors affecting the occurrence of dengue incidence among Colombo and Kandy Districts of Sri Lanka: a cross-sectional study. Parasit Vectors 2018;11:478. 10.1186/s13071-018-3060-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swain S, Bhatt M, Biswal D, et al. Risk factors for dengue outbreaks in Odisha, India: a case-control study. J Infect Public Health 2020;13:625–31. 10.1016/j.jiph.2019.08.015 [DOI] [PubMed] [Google Scholar]
- 20.Luo D, Ying H, Yao R, et al. Socio-economic status and micro-environmental factors in relation to the risk of Japanese encephalitis: a case-control study. Southeast Asian J Trop Med Public Health 1995;26:276–9. [PubMed] [Google Scholar]
- 21.Bonifay T, Douine M, Bonnefoy C, et al. Poverty and arbovirus outbreaks: when Chikungunya virus hits more precarious populations than dengue virus in French Guiana. Open Forum Infect Dis 2017;4:ofx247. 10.1093/ofid/ofx247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al-Raddadi R, Alwafi O, Shabouni O, et al. Seroprevalence of dengue fever and the associated sociodemographic, clinical, and environmental factors in Makkah, Madinah, Jeddah, and Jizan, Kingdom of Saudi Arabia. Acta Trop 2019;189:54–64. 10.1016/j.actatropica.2018.09.009 [DOI] [PubMed] [Google Scholar]
- 23.Chiaravalloti-Neto F, da Silva RA, Zini N, et al. Seroprevalence for dengue virus in a hyperendemic area and associated socioeconomic and demographic factors using a cross-sectional design and a geostatistical approach, state of São Paulo, Brazil. BMC Infect Dis 2019;19:441. 10.1186/s12879-019-4074-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jing Q, Li Y, Liu J, et al. Dengue underestimation in Guangzhou, China: evidence of seroprevalence in communities with no reported cases before a large outbreak in 2014. Open Forum Infect Dis 2019;6:ofz256. 10.1093/ofid/ofz256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abd-Jamil J, Ngui R, Nellis S, et al. Possible factors influencing the seroprevalence of dengue among residents of the forest fringe areas of Peninsular Malaysia. J Trop Med 2020;2020:1019238. 10.1155/2020/1019238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eldigail MH, Abubaker HA, Khalid FA, et al. Recent transmission of dengue virus and associated risk Facors among residents of Kassala state, eastern Sudan. BMC Public Health 2020;20:530. 10.1186/s12889-020-08656-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Omatola CA, Onoja BA, Fassan PK, et al. Seroprevalence of Chikungunya virus infection in five hospitals within Anyigba, Kogi State of Nigeria. Braz J Infect Dis 2020;24:1–6. 10.1016/j.bjid.2020.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sissoko D, Moendandze A, Malvy D, et al. Seroprevalence and risk factors of Chikungunya virus infection in Mayotte, Indian Ocean, 2005-2006: a population-based survey. PLoS One 2008;3:e3066. 10.1371/journal.pone.0003066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakkhara P, Chongsuvivatwong V, Thammapalo S. Risk factors for symptomatic and asymptomatic Chikungunya infection. Trans R Soc Trop Med Hyg 2013;107:789–96. 10.1093/trstmh/trt083 [DOI] [PubMed] [Google Scholar]
- 30.Anjos RO, Mugabe VA, Moreira PSS, et al. Transmission of Chikungunya virus in an urban slum, Brazil. Emerg Infect Dis 2020;26:1364–73. 10.3201/eid2607.190846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Omatola CA, Onoja AB, Moses E. Dengue in parts of the guinea savannah region of Nigeria and the risk of increased transmission. International Health 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahlm C, Eliasson M, Vapalahti O, et al. Seroprevalence of Sindbis virus and associated risk factors in northern Sweden. Epidemiol Infect 2014;142:1559–65. 10.1017/S0950268813002239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Obaidat MM, Stringer AP, Roess AA. Seroprevalence, risk factors and spatial distribution of West Nile virus in Jordan. Trans R Soc Trop Med Hyg 2019;113:24–30. 10.1093/trstmh/try111 [DOI] [PubMed] [Google Scholar]
- 34.Bartley LM, Carabin H, Vinh Chau N, et al. Assessment of the factors associated with flavivirus seroprevalence in a population in southern Vietnam. Epidemiol Infect 2002;128:213–20. 10.1017/s0950268801006495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Conlan JV, Vongxay K, Khamlome B, et al. Patterns of flavivirus seroprevalence in the human population of northern Laos. Am J Trop Med Hyg 2015;93:1010–3. 10.4269/ajtmh.15-0072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ochieng C, Ahenda P, Vittor AY, et al. Seroprevalence of infections with dengue, Rift Valley fever and Chikungunya viruses in Kenya, 2007. PLoS One 2015;10:e0132645. 10.1371/journal.pone.0132645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Obaidat MM, Roess AA. First report on seroprevalence and risk factors of dengue virus in Jordan. Trans R Soc Trop Med Hyg 2018;112:279–84. 10.1093/trstmh/try055 [DOI] [PubMed] [Google Scholar]
- 38.Liu J, Deng Y, Jing Q, et al. Dengue infection spectrum in Guangzhou: a cross-sectional seroepidemiology study among community residents between 2013 and 2015. Int J Environ Res Public Health 2018;15:15061227. 10.3390/ijerph15061227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khan J, Ghaffar A, Khan SA. The changing epidemiological pattern of dengue in Swat, Khyber Pakhtunkhwa. PLoS One 2018;13:e0195706. 10.1371/journal.pone.0195706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nasir IA, Agbede OO, Dangana A, et al. Dengue virus non-structural protein-1 expression and associated risk factors among febrile patients attending University of Abuja teaching Hospital, Nigeria. Virus Res 2017;230:7–12. 10.1016/j.virusres.2016.12.011 [DOI] [PubMed] [Google Scholar]
- 41.Kenneson A, Beltrán-Ayala E, Borbor-Cordova MJ, et al. Social-ecological factors and preventive actions decrease the risk of dengue infection at the household-level: results from a prospective dengue surveillance study in Machala, Ecuador. PLoS Negl Trop Dis 2017;11:e0006150. 10.1371/journal.pntd.0006150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fournet F, Rican S, Vaillant Z, et al. The influence of urbanization modes on the spatial circulation of flaviviruses within Ouagadougou (Burkina Faso). Int J Environ Res Public Health 2016;13:13121226. 10.3390/ijerph13121226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soghaier MA, Himatt S, Osman KE, et al. Cross-sectional community-based study of the socio-demographic factors associated with the prevalence of dengue in the eastern part of Sudan in 2011. BMC Public Health 2015;15:558. 10.1186/s12889-015-1913-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pereira Y, Samudio M, Ojeda A, et al. Seroprevalencia de la infección POR dengue en un distrito del Chaco Paraguayo: estudio poblacional. Revista chilena de infectología 2015;32:618–27. 10.4067/S0716-10182015000700002 [DOI] [PubMed] [Google Scholar]
- 45.Kikuti M, Cunha GM, Paploski IAD, et al. Spatial distribution of dengue in a Brazilian urban slum setting: role of socioeconomic gradient in disease risk. PLoS Negl Trop Dis 2015;9:e0003937. 10.1371/journal.pntd.0003937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pessanha JEM, Caiaffa WT, Kroon EG, et al. [Dengue fever in three sanitary districts in the city of Belo Horizonte, Brazil: a population-based seroepidemiological survey, 2006 to 2007]. Rev Panam Salud Publica 2010;27:252–8. 10.1590/s1020-49892010000400003 [DOI] [PubMed] [Google Scholar]
- 47.da Silva-Nunes M, de Souza VAF, Pannuti CS, et al. Risk factors for dengue virus infection in rural Amazonia: population-based cross-sectional surveys. Am J Trop Med Hyg 2008;79:485–94. [PubMed] [Google Scholar]
- 48.Brunkard JM, Robles López JL, Ramirez J, et al. Dengue fever seroprevalence and risk factors, Texas-Mexico border, 2004. Emerg Infect Dis 2007;13:1477–83. 10.3201/eid1310.061586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rueda JC, Santos AM, Angarita J-I, et al. Demographic and clinical characteristics of Chikungunya patients from six Colombian cities, 2014-2015. Emerg Microbes Infect 2019;8:1490–500. 10.1080/22221751.2019.1678366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kuan G, Ramirez S, Gresh L, et al. Seroprevalence of anti-Chikungunya virus antibodies in children and adults in Managua, Nicaragua, after the first Chikungunya epidemic, 2014-2015. PLoS Negl Trop Dis 2016;10:e0004773. 10.1371/journal.pntd.0004773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Piedrahita LD, Agudelo Salas IY, Marin K, et al. Risk factors associated with dengue transmission and spatial distribution of high seroprevalence in schoolchildren from the urban area of medellin, Colombia. Can J Infect Dis Med Microbiol 2018;2018:2308095:1–11. 10.1155/2018/2308095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carabali M, Lim JK, Velez DC, et al. Dengue virus serological prevalence and seroconversion rates in children and adults in medellin, Colombia: implications for vaccine introduction. Int J Infect Dis 2017;58:27–36. 10.1016/j.ijid.2017.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Larrieu S, Michault A, Polycarpe D, et al. Dengue outbreaks: a constant risk for reunion island. results from a seroprevalence study among blood donors. Trans R Soc Trop Med Hyg 2014;108:57–9. 10.1093/trstmh/trt110 [DOI] [PubMed] [Google Scholar]
- 54.Mazaba-Liwewe ML, Siziya S, Monze M, et al. First sero-prevalence of dengue fever specific immunoglobulin G antibodies in Western and north-western provinces of Zambia: a population based cross sectional study. Virol J 2014;11:135. 10.1186/1743-422X-11-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Low S-L, Lam S, Wong W-Y, et al. Dengue seroprevalence of healthy adults in Singapore: serosurvey among blood donors, 2009. Am J Trop Med Hyg 2015;93:40–5. 10.4269/ajtmh.14-0671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Amaya-Larios IY, Martínez-Vega RA, Mayer SV, et al. Seroprevalence of neutralizing antibodies against dengue virus in two localities in the state of Morelos, Mexico. Am J Trop Med Hyg 2014;91:1057–65. 10.4269/ajtmh.14-0145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Al-Azraqi TA, El Mekki AA, Mahfouz AA. Seroprevalence of dengue virus infection in Aseer and Jizan regions, southwestern Saudi Arabia. Trans R Soc Trop Med Hyg 2013;107:368–71. 10.1093/trstmh/trt022 [DOI] [PubMed] [Google Scholar]
- 58.Suleman M, Faryal R, Alam MM, et al. Dengue virus serotypes circulating in Khyber Pakhtunkhwa Province, Pakistan, 2013-2015. Ann Lab Med 2017;37:151–4. 10.3343/alm.2017.37.2.151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shaw M, Galobardes B, Lawlor DA. The handbook of inequality and socioeconomic position concepts and measures. 1st edn. Bristol University Press, 2007. [Google Scholar]
- 60.Bartley M, Carpenter L, Dunnell K, et al. Measuring inequalities in health: an analysis of mortality patterns using two social classifications. Sociol Health & Illness 1996;18:455–74. 10.1111/1467-9566.ep10939068 [DOI] [Google Scholar]
- 61.Wadsworthx ME. Changing social factors and their long-term implications for health. Br Med Bull 1997;53:198–209. 10.1093/oxfordjournals.bmb.a011600 [DOI] [PubMed] [Google Scholar]
- 62.Braveman P, Gottlieb L. The social determinants of health: it's time to consider the causes of the causes. Public Health Rep 2014;129:19–31. 10.1177/00333549141291S206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pickett KE, Wilkinson RG. Income inequality and health: a causal review. Soc Sci Med 2015;128:316–26. 10.1016/j.socscimed.2014.12.031 [DOI] [PubMed] [Google Scholar]
- 64.Smolen JR, Araújo EMde. Race/skin color and mental health disorders in Brazil: a systematic review of the literature. Cien Saude Colet 2017;22:4021–30. 10.1590/1413-812320172212.19782016 [DOI] [PubMed] [Google Scholar]
- 65.Hicken MT. Measurement and modeling of race and health in Brazil: continuing the discussion. Cad Saude Publica 2017;33:e00084216. 10.1590/0102-311X00084216 [DOI] [PubMed] [Google Scholar]
- 66.Berkman LF, Kawachi I, Glymour MM. Social epidemiology. Oxford, UK: Oxford University Press, 2015. [Google Scholar]
- 67.Lindsay SW, Jawara M, Paine K, et al. Changes in house design reduce exposure to malaria mosquitoes. Trop Med Int Health 2003;8:512–7. 10.1046/j.1365-3156.2003.01059.x [DOI] [PubMed] [Google Scholar]
- 68.Alton C, Rattanavong H. Service delivery and resettlement: options for development planning. final report Livelihoods study, Lao/03 a 2004;1. [Google Scholar]
- 69.Ferdousi F, Yoshimatsu S, Ma E, et al. Identification of essential containers for Aedes larval breeding to control dengue in Dhaka, Bangladesh. Trop Med Health 2015;43:253–64. 10.2149/tmh.2015-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgh-2021-007735supp001.pdf (21.2KB, pdf)
bmjgh-2021-007735supp002.pdf (20.7KB, pdf)
bmjgh-2021-007735supp003.pdf (112.9KB, pdf)
bmjgh-2021-007735supp004.pdf (163.4KB, pdf)
bmjgh-2021-007735supp005.pdf (158.8KB, pdf)
bmjgh-2021-007735supp006.pdf (89.1KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as online supplemental information.