Abstract
Introduction
Physical rehabilitation delivered early following admission to the intensive care unit (ICU) has the potential to improve short-term and long-term outcomes. The use of supine cycling together with other rehabilitation techniques has potential as a method of introducing rehabilitation earlier in the patient journey. The aim of the study is to determine the feasibility of delivering the designed protocol of a randomised clinical trial comparing a protocolised early rehabilitation programme including cycling with usual care. This feasibility study will inform a larger multicentre study.
Methods and analysis
90 acute care medical patients from two mixed medical–surgical ICUs will be recruited. We will include ventilated patients within 72 hours of initiation of mechanical ventilation and expected to be ventilated a further 48 hours or more. Patients will receive usual care or usual care plus two 30 min rehabilitation sessions 5 days/week.
Feasibility outcomes are (1) recruitment of one to two patients per month per site; (2) protocol fidelity with >75% of patients commencing interventions within 72 hours of mechanical ventilation, with >70% interventions delivered; and (3) blinded outcome measures recorded at three time points in >80% of patients. Secondary outcomes are (1) strength and function, the Physical Function ICU Test–scored measured on ICU discharge; (2) hospital length of stay; and (3) mental health and physical ability at 3 months using the WHO Disability Assessment Schedule 2. An economic analysis using hospital health services data reported with an embedded health economic study will collect and assess economic and quality of life data including the Hospital Anxiety and Depression Scales core, the Euroqol-5 Dimension-5 Level and the Impact of Event Score.
Ethics and dissemination
The study has ethical approval from the South Central Hampshire A Research Ethics Committee (19/SC/0016). All amendments will be approved by this committee. An independent trial monitoring committee is overseeing the study. Results will be made available to critical care survivors, their caregivers, the critical care societies and other researchers.
Trial registration number
Keywords: Critical care, physical therapy, rehabilitation, cycling
Strengths and limitations of this study.
Will investigate the implementation of a protocolised early rehabilitation intervention that is usual care in one NHS/university teaching institution, into other NHS institutions with different organisational structures.
The defined cohort has been demonstrated to benefit from this type of rehabilitation in alternative healthcare systems.
Results will inform the design of a multicentre randomised controlled trial.
This study is not designed to assess the effectiveness of the intervention.
Inability to blind the intervention to patients, physiotherapist and clinicians involved in the delivery of the intervention.
Introduction
In 2018/2019, there were over 290 000 admissions to adult intensive care units (ICUs) in the UK.1 Treatment advances have reduced mortality associated with critical illness2 3; however, survival does not represent the end of the story.4 A complex interplay between baseline health status, acute disease and the traumatic effects of intensive care treatment is associated with long-term physical, psychological and social hardship.5–10 Patients discharged from the ICU have higher mortality, higher health service costs and a reduction in employment status compared with hospitalised patients not requiring ICU.8 11
ICU-acquired weakness is characterised by rapid muscle wasting, polyneuropathy and bone demineralisation, causing pain, weakness and impaired physical function.12–14 Contributing factors are multifactorial, although immobility due to the sedation required for tolerance of ventilation plays an important role.15 16 Early mobilisation may mitigate these effects.17–19 In 2009, Schweickert et al reported that patients who underwent early physical therapy (within 1.5 days of mechanical ventilation) had greater functional independence at hospital discharge than patients who had usual care physical therapy.20 A recent randomised controlled trial (RCT) on the impact of a progressive ICU mobility programme reported improved functional status at ICU discharge.21 Meta-analyses and systematic reviews report that early mobilisation of ICU patients may reduce duration of mechanical ventilation and improve short-term physical outcomes,22–24 but mobilisation can be difficult to implement during a patient’s stay in the ICU. Moreover, studies which used delayed rehabilitation, often more than a week after ICU admission,25–27 have not replicated these outcomes.28–34 Barriers to early mobilisation include heavy sedation, patient’s illness, lack of resources and/or clinician buy-in.35–38In-bed cycle ergometry can provide passive activity in heavily sedated patients who are receiving vasopressors39 40 with minimal physiological demand40 41 and can be transitioned to active cycling as the patient’s condition improves.23 42–44
We implemented cycle ergometry as part of an early protocolised rehabilitation quality improvement programme with physiotherapy technicians supporting the additional workload.45 Like other investigators, we reported reduced number of ventilator days and ICU length of stay.21 46–49
The primary aim of this study was to evaluate the feasibility of an RCT investigating the effect of early protocolised rehabilitation versus usual physiotherapy care in ICU patients. Results will inform a prospective fully powered multicentre RCT. This protocol is reported according to Standard protocol items for clinical trials (SPIRIT 2013 Statement)50 and Template for Intervention Description and Replication51 guidelines.
Aim
The aim of this study was to determine the feasibility of delivering study procedures comparing an early protocolised mobilisation programme that includes cycling with usual care.
Objectives
Feasibility will be determined by measures of the recruitment process, intervention fidelity and outcome measurement completeness, specifically, (1) study accrual rates: a minimum of 30% of eligible patients or one to two patients per site per month are enrolled; (2) protocol adherence: 75% of patients commencing intervention within 72 hours of ICU admission, with a minimum of 70% of planned interventions delivered; and (3) blinded outcome assessment: functional assessment performed at three time points in 80% of survivors. The results will inform a larger fully powered RCT.
Methods and analysis
Study design
This is a two-centre feasibility study using a two-arm RCT, randomised 1:1, with blinded outcome assessments at ICU discharge, hospital discharge and 3-month follow-up. Patients will be recruited from two general ICUs, located in the south of the UK. They will not be recruited from our ICU on account that the intervention is now standard practice at this site. Prior to each site opening to recruitment, an audit of current physiotherapy practice will be undertaken over a 4-week period to evaluate what constitutes ‘usual care’ in each institution.
Participants
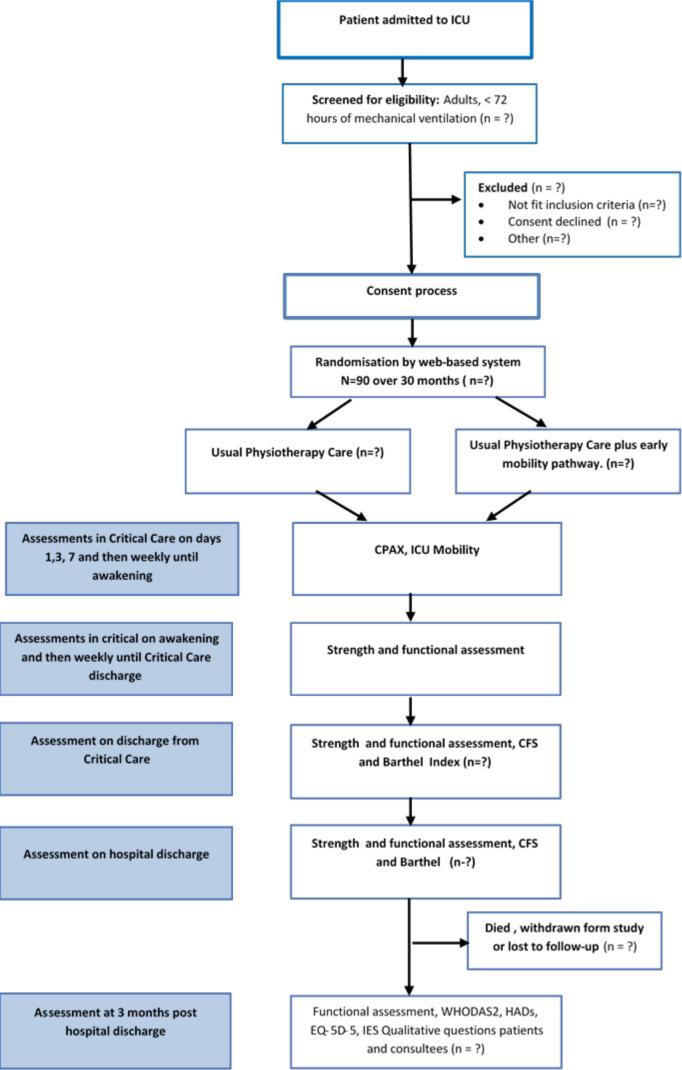
Ninety patients will be recruited. Eligible patients will be over 42 years old and will have an acute/unplanned medical admission to the ICU. They will be functionally independent prior to ICU admission (Barthel Index>80), in the hospital for <5 days prior to intubation and ventilation, intubated and ventilated for <72 hours, and expected to remain ventilated for a further 48 hours. Patients will be excluded if they are in the hospital for 5 days or more prior to ICU admission, have acute brain or spinal cord injury, known or suspected neurological/muscular impairment, condition limiting use of cycle ergometry (eg, lower limb fracture/amputation), not expected to survive >48 hours decided by consulting an intensivist and persistent therapy exemptions in the first 3 days of mechanical ventilation. Figure 1 presents the planned flow of patients through the study.
Figure 1.
Study design. CFS, Clinical Frailty Score; CPAx, Chelsea Critical Care Physical Assessment Tool; EQ-5D-5L, Euroqol-5 Dimension-5 Level; HAD, ICU, intensive care unit; IES, Impact of Event Score; WHODAS 2.0, WHO Disability Assessment Schedule 2.
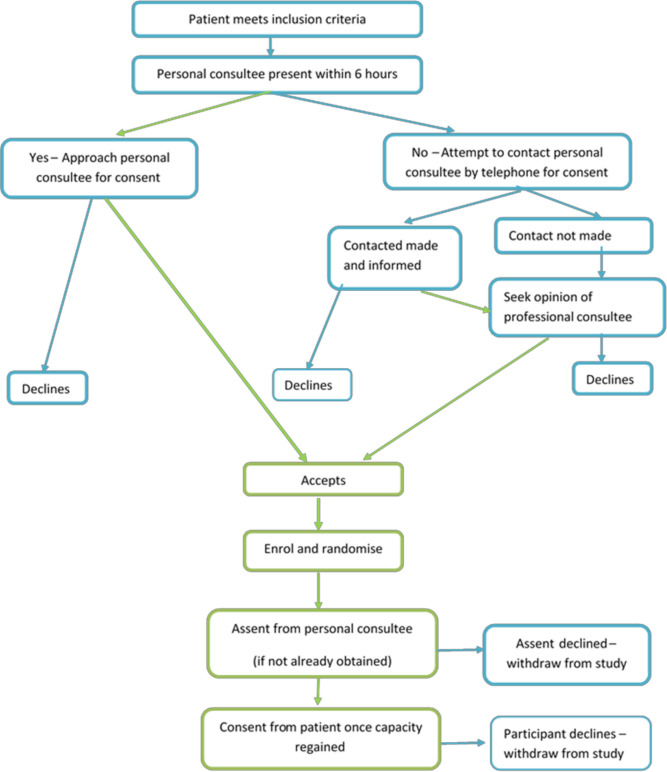
Recruitment, consent and randomisation
The study team will screen all patients for eligibility. Recruitment began in June 2019 (and was temporarily suspended in March 2020 due to the COVID-19 pandemic). It is anticipated recruitment will continue until mid 2022. The majority of patients will have diminished capacity when first eligible; therefore, the consent process is multilayered and designed in accordance with the Mental Capacity Act (MCA) 200552 (figure 2):
Figure 2.
Consent pathway.
Patient informed consent: wherever possible, informed consent will be directly sought from the patient (see online supplemental files 1 and 2).
Personal consultee informed assent: if the patient is unable to provide consent, informed assent will be sought from the patient’s personal consultee, within 6 hours of confirmation of eligibility. If the personal consultee is not available in person, attempts will be made to contact them by telephone. They will be asked to provide written assent, at the earliest possible convenience (see online supplemental files 3 and 4).
Professional consultee informed assent: where both patient and personal consultee are not available to approve enrolment within 6 hours of confirmation of eligibility, assent will be sought from a professional consultee in accordance with the MCA. The professional consultee will be a consultant medical practitioner, independent from the study. The patient’s personal consultee will be consulted at the earliest possible opportunity and assent will be requested to continue in the study.
In all cases, once the patient has regained capacity, they will be informed of the study and consent continuation will be sought. Following consent or assent, patients will be registered on a bespoke electronic data collection tool (ALEA) and randomly assigned to the protocolised early rehabilitation or usual care.
Staff training/site set-up
Participating sites will employ the equivalent of a full-time therapy technician to deliver the study intervention, under the supervision of a senior critical care therapist. Both senior critical care therapists and therapy technicians will complete a training package delivered by the primary institution (University Hospital Southampton NHS Foundation Trust), where early rehabilitation with cycling is well established and embedded in usual care. This package includes seminars on the delivery of the protocolised early rehabilitation, use of the bespoke electronic database and 5 days of clinical shadowing.
Interventions
All patients will receive usual medical, nursing and physiotherapy care while in intensive care. Each bedside nurse will be asked at the start of the shift if they have been involved caring for a patient in the intervention arm of the study. The ICU physiotherapy team, who are not involved with the delivery of the study delivery, will deliver all usual physiotherapy interventions in both groups. The physiotherapist delivering usual care will be asked to verify if they have delivered any of the study interventions. In the intervention arm, the protocolised physiotherapy programme will commence within 72 hours of ICU admission or as soon as possible thereafter and will continue for 28 days or until ICU discharge, whichever occurs first. Patients’ respiratory support can range from full mandatory ventilation through to oxygen supplementation with no mechanical support following extubation. Sedation is targeted throughout the time that the patient is intubated, and the ventilation mode is adjusted to patients’ needs, compliance and comfort at discretion at the start of each physiotherapy intervention, the participants’ level of sedation will be assessed using the Richmond Agitation–Sedation Scale (RASS)53 54 and the Confusion Assessment Method for ICU55will be undertaken. RASS will be targeted to a RASS between −1 and +1 by the bedside nurse. After 28 days of ICU admission, all patients will receive usual care physiotherapy interventions.
Group 1: usual care control group
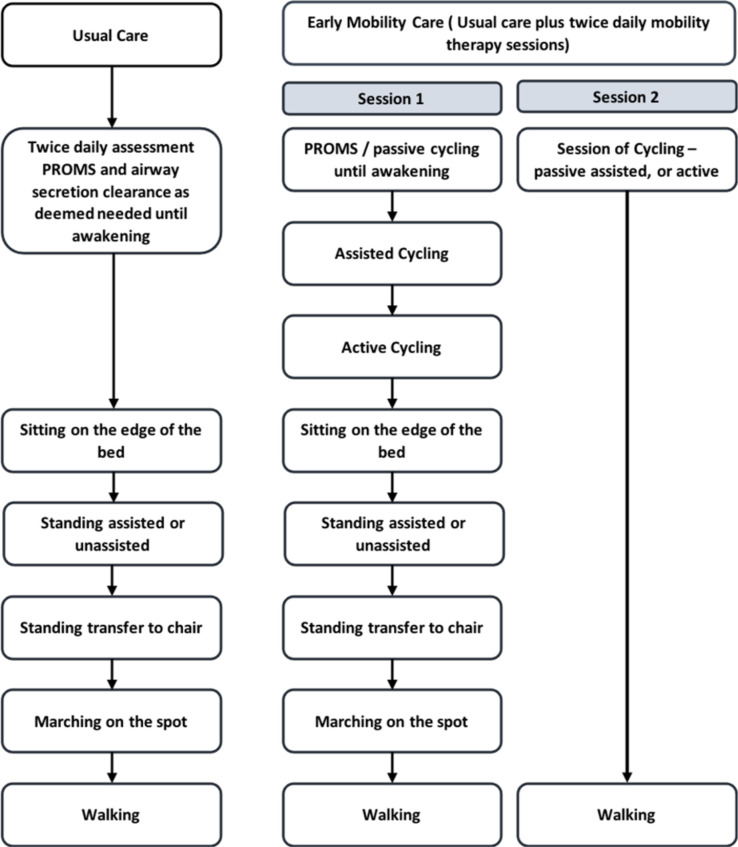
In this pragmatic study, physiotherapy interventions will be guided by individual assessment and start in accordance with the usual care pathway within each institution. The focus of each session may be respiratory support, mobilisation or a combination of both. Interventions delivered will be determined by the physiotherapist in conjunction with the attending physician. Interventions include, where appropriate, passive or active range of movement, positioning and respiratory physiotherapy, and when able, sitting on the edge of the bed, standing (assisted or unassisted), standing to transfer to chair, marching on the spot and walking (figure 3). Usual interventions may occur at any time of day.
Figure 3.
Study intervention pathway. (PROM = Passive Range of Movement)
Group 2: Protocolised rehabilitation pathway
Patients will have usual care physiotherapy, in addition to the two protocolised intervention within 72 hours of ICU admission or as soon as possible thereafter. Patients will be screened for safety criteria to withhold the intervention prior to each planned intervention session (table 1).
Table 1.
Safety criteria for delivery of physical therapy interventions
| Criteria to commence physiotherapy | Criteria to stop/withhold physiotherapy intervention | |
| Blood pressure | Mean Arterial Blood Pressure (MAP) 60–100 mm Hg, no change in vasopressor dose requirement for preceding 2 hours | Catecholamine-resistant hypotension with MAP <60 mm Hg |
| Heart rate | Between 40 and 140 beats/min | <50 or >140 beats/min |
| Respiratory rate | Sustained <40 breaths/min | Sustained >40 breaths/min |
| Temperature | >40°C | |
| Oxygen requirement | If Fraction inspired oxygen (FiO2)>0.8 for passive exercise only | |
| FiO2 <0.8 and (Positive End Expiratory Pressure) PEEP <15 cmH2O | ||
| Desaturation | Sats fall <85% for>1 min | |
| Other |
|
Those meeting criteria to withhold interventions will have issues addressed and reassessed for interventions 2 hours later. The two additional rehabilitation sessions will be delivered by the research physiotherapy staff including a therapy technician. This will comprise two mobility sessions the modality of the first, chosen at the discretion of the physiotherapist. The second session will be 30 min of supine cycling delivered in the afternoon.
The first rehabilitation intervention each day will be delivered in the morning. Planned interventions include passive or active range of movements, passive cycling, active cycling, in-bed exercises, sitting, mobilisation out of bed and walking. Daily assessment of the patient will be made to ensure the highest level of activity possible is provided for each individual patient given safety considerations and capability of the patient.
The second session will be cycling based. An in-bed supine cycle ergometer (MotoMed Letto 2) will be used to engage the participant in passive, assisted or active cycling, or a combination, depending on the degree of patient cooperation (figure 3). The aim was for the patient to have 30 min of cycling per day, following a standardised cycling programme. If cycling is in passive mode, patients will commence cycling at 5 revolutions per minute (RPM), building up to 20 RPM over 5 min and continue this for 20 min before 5 min 5 RPM cool down. In the assisted or active mode, after the 5 min warm-up, cycling will continue for 20 min at patient-selected RPM followed by a 5 min cool-down at 5 RPM. In-bed cycling sessions will stop when the patient is deemed to be able to stand and transfer from bed to chair for both mobility sessions for two consecutive days. If patients are considered unable to have concurrent mobility therapy and respiratory weaning, mobility therapy will take priority, in agreement with the senior clinical team. Individual participants will receive the trial intervention on 5 days/week (Monday–Friday) for the duration of their ICU stay or a maximum of 28 days, whichever comes first. Patients will be monitored for cardiovascular and respiratory stability and safety of indwelling lines, tubes and catheters with predetermined criteria for termination of any session (table 1). Deviations from the planned protocol will be reported to determine potential barriers to implementation. Patients will be able to decline any intervention or outcome assessment at any time without compromise to their care.
Primary outcome: feasibility to deliver the protocol as designed
Feasibility will be determined by measures of the recruitment process, intervention fidelity and outcome measurement completeness, specifically,
Study accrual rates: a minimum of 30% of eligible patients or one to two patients per site per month are enrolled.
Protocol adherence: 75% of patients commencing intervention within 72 hours of ICU admission, minimum of 70% of planned interventions delivered.
Blinded outcome assessment: functional assessment performed at three time points in 80% of survivors by physiotherapists working within the hospital but not within the ICU.
Secondary outcomes
The schedule of outcome assessments is detailed in table 2.
Table 2.
Schedule of assessments
| Randomisation | Day 1 | Day 3 | Day 7 | Awakening | Weekly | ICU discharge | Hospital discharge | 3 months posthospital discharge | |
| Demographic data | X | ||||||||
| Muscle assessment | |||||||||
| Medicial Research Council sum-score (MRCss)60 61 | X | X | X | X | |||||
| Grip strength62 | X | X | X | X | |||||
| Physical function | |||||||||
| CPAx63 | X | X | X | X | X | X | |||
| ICU mobility64 | X | X | X | X | X | X | |||
| PFITs59 | X | X | X | ||||||
| Timed-Up and Go | X | X | X | ||||||
| Clinical Frailty Score69 | (X) | X | X | X | |||||
| Barthel Index | (X) | X | X | X | |||||
| 6 min Walk Test70 | X | X | |||||||
| Health Related Quality of LIfe (HRQL) | |||||||||
| WHODAS 2.071 | X | ||||||||
| HADS72 73 | X | ||||||||
| EQ-5D-5L74 | X | ||||||||
| Impact of Event Scale75 | X | ||||||||
| Health Economic Evaluation (CSRI)* | X | ||||||||
CPAx, Chelsea Critical Care Physical Assessment Tool; CSRI, Client Service Receipt Inventory; EQ-5D-5L, Euroqol-5 Dimension-5 Level; HADS, Hospital Anxiety and Depression Scale; ICU, intensive care unit; PFITs, Physical Function ICU Test–scored; WHODAS 2.0, WHO Disability Assessment Schedule 2.
Strength and function
We will measure the Physical Function ICU Test–scored (PFITs) at awakening as described by De Jonghe et al56 then weekly within ICU and on ICU discharge.57 PFITs is a reliable and valid four-item scale (arm strength, leg strength, ability to stand and step cadence), with a score range of 0–10 and is responsive to change and predictive of key outcomes.58 Medical Research Council Manual Muscle Test Sum Score (MRC-ss)59 60 and handheld dynamometry61 will be measured on awakening, weekly, on ICU discharge and hospital discharge. Chelsea Critical Care Physical Assessment Tool (CPAx)62 and ICU Mobility Scale63 will be assessed three times during the first week within ICU, on awakening, weekly thereafter within the ICU and at ICU discharge. Timed Up and Go,64 65 Clinical Frailty Score (CFS)66–68 and Barthel Index will be assessed at ICU discharge, hospital discharge and 3 months posthospital discharge. Preadmission Barthel Index and CFS will be assessed by proxy on admission from family member or next of kin. Six-minute walk test69 will be performed, in accordance with American Thoracic Society guidelines, at hospital discharge and 3 months posthospital discharge.
Health-related quality of life (QOL) outcomes
The following will be measured at 3 months posthospital discharge : WHODAS 2.0,70 Hospital Anxiety and Depression Scale score,71 72 Euroqol-5 Dimension-5 Level (EQ-5D-5L),73 Impact of Event Score74 and Client Service Receipt Inventory questionnaire, designed for this study to evaluate costs that fall on patients and their carers. Resource use and costs including direct intervention costs of therapists and equipment and general hospital costs (per bed day) will be recorded for each patient.
Health economic substudy
We will also conduct an embedded health economic study to identify and define data collection for a future RCT where a full cost-effectiveness analysis (CEA) can be conducted. Within the feasibility study, we aim to address the following:
What the quality of the data and what potential problems are there for reporting QoL (EQ-5D-5L), resource use and costs.
The cost implications of the proposed intervention in terms of impact for the NHS (inpatient stay bed days) and identifying the main cost drivers.
Is the EQ-5D-5L appropriate for use in the future RCT?
The economic outcomes will include secondary care resource use within hospitals during inpatient stay, primary care resource use following discharge up to 3 months and resource use related to providing the intervention. The results will be reported in the form of descriptive statistics and will be used to inform a future CEA within a definitive RCT.
Additional data collection
We will collect baseline data including demographic information, Functional Comorbidity Index, ICU diagnosis, APACHE II score, ventilation duration, ventilator-free days, ICU and hospital length of stay, within ICU drug history and duration and type of usual care physiotherapy.
Implementation evaluation
We aim to investigate whether the protocolised early rehabilitation programme used in one NHS institution is transferable, as an RCT, into other similar NHS institutions. The design of a future multicentre study will be informed by identified facilitators and barriers to implementation. Implementation assessment will be based on the measures described by Proctor.75 A cross section of ICU staff and patients will be interviewed and complete questionnaires at trial completion to identify barriers impacting delivery of the study. Understanding of the integration and sustainability of the intervention are necessary to inform the design of a powered RCT. Acceptability will be measured at the beginning and end of the study from investigators and clinical staff by direct discussions and questionnaire. Our experience informs us that the introduction of this intervention is dependent on a cultural change within any unit for a proactive focus on early mobilisation. We aim to explore measures to help optimise implementation. Adoption, feasibility and fidelity measures will be monitored during the study by regular meetings with the investigators. Patient screening logs will identify the number of patients eligible for the study and barriers to enrolment. We will assess the degree to which it is possible to separate the staff caring for the intervention group from those caring for the patients in the control group.
We will report whether trial participation has influenced usual care within the participating units by prestudy and poststudy audits. Participating sites will collect data regarding number and seniority of therapy staff with dedicated time to work within the ICU; delirium and sedation protocols used; time, type and frequency of rehabilitation interventions delivered, who delivers the interventions and reasons why usual care may not be delivered.
The feasibility outcomes described earlier will be used to power a larger RCT.
Data entry and checks
Data will be entered into the secure electronic case report form (ALEA) and data validation will take place according to the procedures set out in the data management plan and data validation plan, both developed apriori. Missing data will be assessed to identify any specific challenges with any items of data collected. Missing data level is expected to be less than 20%. Data loss and mortality will inform number of participants needed to design a larger RCT. As this is a feasibility study data imputation will not be undertaken. Prior to statistical analysis, variables will be checked for missing and impossible and improbable values as defined by clinical opinion. Questions regarding the data will be directed to the data manager.
Sample size calculation
This is a feasibility study, the results of which will be used to power a definitive study if appropriate; as such, no formal sample size calculation for effectiveness of the intervention has been undertaken. Ninety patients will be recruited, aiming for 30–45 participants at each site. We anticipate a 30% in hospital mortality /loss to follow-up with an estimate of 60 patients completing the study. This sample size of 90 will allow the estimate of recruitment rate to be made with a 95% CI of ±5.2% if the rate is observed to be around 30%, and with a CI of ±7.3% if the recruitment rate is observed to be around 50%. In addition, the sample of 90 recruited patients will allow the estimate of the mortality rate to be made with a 95% CI of ±9.5%, assuming the mortality rate was around 30%. Finally, assuming the recruitment rate was around 30%, a sample of 300 patients approached to take part in the study, leading to 90 enrolled patients would allow for the recruitment rate to be estimated with a 95% CI of ±5.2%. If the recruitment rate was nearer 50%, with 180 patients approached to recruit the 90 enrolled patients, the recruitment rate would be estimated with a 95% CI of ±7.3%.
Statistical analysis
The analysis will be reported in line with the feasibility studies extension to the Consolidated Standards of Reporting Trials statement.76 The aims of the study were to estimate the recruitment, compliance and retention rates to inform the design of a future study and is not powered for hypothesis testing regarding the effectiveness of the intervention. Feasibility outcomes (recruitment, compliance and retention rates) will be presented with 95% CIs across the whole study population. Compliance and retention rates will also be presented by treatment arm to ensure balanced recruitment, but no formal statistical comparison tests will be made. Mortality and participant dropout rates will be presented with 95% CIs across the whole study population and within treatment arm. Clinical outcome data (secondary outcomes) will be presented as summary statistics using means and SDs or medians and ranges/IQRs, as applicable, across the whole study population and by treatment arm. These data will be used to inform the future trial but will not be used to draw conclusions about the effectiveness of the protocolised early rehabilitation intervention within this study.
Trial management
The chief investigator will ensure all study personnel are appropriately orientated and trained, oversee recruitment and report to the trial safety monitoring committee. Training will occur across sites using competency-based training developed at the primary site (University Hospital Southampton NHS Foundation Trust). A study steering group, consisting of an independent chair, expert members and two lay advisors will meet every 3 months. Fortnightly teleconferences with trial sites will be held to monitor conduct and progress. Timing and intervals of visits and teleconferences will be reviewed at 3 months to ensure optimal time use.
The chief investigator and principal investigators will facilitate local monitoring by the research and development quality manager, research ethics committee (REC) review and provide access to source data as required. A monitoring report will be produced, summarising the visit, documents and findings. The chief investigator will ensure that all findings are addressed appropriately. The steering group will review all events in a timely manner. Additional monitoring will be scheduled where there is evidence or suspicion of non-compliance with the study protocol.
A data management and safety committee will be chaired by an independent expert. Quarterly reports will be given to the committee once recruitment has commenced.
Patient and public involvement
The study has been supported by patient advisory representatives. These representatives are members of the trial steering committee. Patient advisors partnered with us for the design of the study, the informational material to support the intervention, the burden of the intervention from the patient’s perspective and contributed to the dissemination plan
Ethics and dissemination
Ethical approval has been granted by South Central Hampshire A Research Ethics Committee (REC reference 19/SC/0016). This study entitled: A feasibility study of Early Mobilisation Programmes in Critical Care (EMPRESS) was registered with Clinical Ttrials.gov on 10 December 2018.
Results of this proposed feasibility study will be disseminated for four key audiences: (1) patients and public; (2) intensive care staff, healthcare workers and potential future research delivery partners; (3) service delivery organisations; and (4) academic and potential future research collaborators. Dissemination activities will include feedback to patients and public involvement study focus group, feedback to study participants, presentations to local clinical teams and managers and commissioners and presentation at conferences attended by appropriate healthcare professionals. Where appropriate, results will be published in peer reviewed journals.
Safety and adverse events
Early mobility within ICUs is safe. In a review of physiotherapy in a critical care rehabilitation programme, 1110 patients underwent 5267 rehabilitation sessions; physiological abnormalities or potential adverse events occurred in only 6 per 1000 interventions.77 Mobilisation interventions will only be delivered if patients fit the safety criteria defined in table 1. Similar safety criteria have been used in other ICU rehabilitation studies.78 79
All adverse events will be documented. Any intervention will cease according to stopping criteria detailed in table 1. Any such event will be recorded as an adverse event. The chief investigator will provide a monthly update to the safety monitoring committee. Serious adverse events are events that result in death, are life-threatening or require prolonged hospitalisation. Any such event will be reported in accordance with the NHS Health Research Authority guidance.
Discussion
EMPRESS is a feasibility study to assess if an RCT of protocolised rehabilitation with supine cycling can be delivered in ventilated patients in ICUs with differing organisational structures with blinded follow-up assessments. A recent meta-analysis indicated that protocolised rehabilitation significantly reduces duration of mechanical ventilation and ICU length of stay.23 This is consistent with our findings when we introduced the early rehabilitation programme outlined here in our ICU.45 Passive cycling commenced on ventilated patients may assist the recovery muscle strength in ICU patients,43 although the overall benefits of leg cycle ergometry in the critically ill is inconclusive.44 We describe a protocolised rehabilitation programme with supine cycling delivered as close to intubation as possible, at an intensity according to the patients’ highest performance capability.
Both patient and organisational issues are recognised to the delivery of early rehabilitation of the critically ill patients.35 A frequently reported challenge is the lack of appropriately qualified staff.80 This study evaluates the safety, feasibility, effectiveness of delivery and cost efficiency of using therapy technicians to deliver protocolised rehabilitation interventions. In addition to the clinical benefits, early physical rehabilitation can also be cost saving.49 Even with the cost of employment of additional therapy technicians specifically to assist in the delivery of we have found this early rehabilitation programme cost effective.81
This study will collect data on the dose of intervention delivered to all patients, reasons for non-delivery of protocol interventions, and the level of experience of therapists delivering the interventions. A qualitative process evaluation is designed to identify both patient and organisational challenges that have potential to be addressed in a potential future study. Findings will inform refinement of trial design and evaluation of the intervention, clarifying causal mechanisms behind study outcomes and providing additional context not adequately captured by the quantitative data. The process evaluation will be consistent with Medical Research Council guidance for conducting process evaluations of complex healthcare interventions.82
Targeted sedation is embedded within this protocol as oversedation is one of the more commonly cited barriers to mobilisation of the ventilated patient.35 This study opened to recruitment prior to the publication of the recommended core outcome set for critical care ventilation trials83; however, three of the six outcomes listed (duration of mechanical ventilation, duration of stay and health-related QOL) are secondary outcomes in this study and the other three outcomes are included in the data collected. This will be addressed should we proceed to a full RCT. Due to the nature of the intervention, it is not possible for this to be blinded; however, the follow-up assessments will be carried out by a blinded.
Results from EMPRESS will inform the design of a multi-centred RCT, both identifying barriers to the implementation of the designed protocol and exploring how these may be addressed from feedback from the therapy and nursing teams in addition to the feedback from patients and their next of kin.
bmjopen-2021-055285supp001.pdf (138.8KB, pdf)
bmjopen-2021-055285supp002.pdf (112.5KB, pdf)
bmjopen-2021-055285supp003.pdf (139.2KB, pdf)
bmjopen-2021-055285supp004.pdf (86.1KB, pdf)
Supplementary Material
Footnotes
Twitter: @AndyCBates, @MariaChorozo
Contributors: RC and AB contributed equally to the preparation of the paper. RC and ZvW had the original idea for the study. RC, LD, IR, NH, AD, GS, ID and MG developed the trial protocol. IR devised the statistical analysis plan. MC developed the economic analysis. AB, GS, ID and RC prepared and submitted documents for research and development and ethical approval. RC, KM and AB wrote the manuscript. All authors reviewed the final version.
Funding: This work is supported by the National Institute for Health Research (NIHR) under its Research for Patient Benefit (RfPB) Programme. Grant Reference Number PB-PG-0317-20045. KM is an NIHR Senior Nurse and Midwife Research Leader. Sponsor: University Hospital Southampton NHS Foundation Trust.
Disclaimer: The views expressed are those of the author(s) and not necessarily those of the National Institute for Health Research, NHS or the Department of Health and Social Care.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, conduct, reporting or dissemination plans of this research. Refer to the Methods and analysis section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Hospital Admitted Patient Care Activity 2018-19 . NHS England; 2019 [National stastics]. Available: https://digital.nhs.uk/data-and-information/publications/statistical/hospital-admitted-patient-care-activity/2018-19 [Accessed Acessed 21st Aug 2021].
- 2.Esteban A, Frutos-Vivar F, Muriel A, et al. Evolution of mortality over time in patients receiving mechanical ventilation. Am J Respir Crit Care Med 2013;188:220–30. 10.1164/rccm.201212-2169OC [DOI] [PubMed] [Google Scholar]
- 3.Kaukonen K-M, Bailey M, Suzuki S, et al. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000-2012. JAMA 2014;311:1308–16. 10.1001/jama.2014.2637 [DOI] [PubMed] [Google Scholar]
- 4.Iwashyna TJ, Netzer G. The burdens of survivorship: an approach to thinking about long-term outcomes after critical illness. Semin Respir Crit Care Med 2012;33:327–38. 10.1055/s-0032-1321982 [DOI] [PubMed] [Google Scholar]
- 5.Cheung AM, Tansey CM, Tomlinson G, et al. Two-Year outcomes, health care use, and costs of survivors of acute respiratory distress syndrome. Am J Respir Crit Care Med 2006;174:538–44. 10.1164/rccm.200505-693OC [DOI] [PubMed] [Google Scholar]
- 6.Cuthbertson BH, Roughton S, Jenkinson D, et al. Quality of life in the five years after intensive care: a cohort study. Critical Care 2010;14:R6. 10.1186/cc8848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Desai SV, Law TJ, Needham DM. Long-Term complications of critical care. Crit Care Med 2011;39:371–9. 10.1097/CCM.0b013e3181fd66e5 [DOI] [PubMed] [Google Scholar]
- 8.Griffiths J, Hatch RA, Bishop J, et al. An exploration of social and economic outcome and associated health-related quality of life after critical illness in general intensive care unit survivors: a 12-month follow-up study. Critical Care 2013;17:R100. 10.1186/cc12745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herridge MS, Tansey CM, Matté A, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med 2011;364:1293–304. 10.1056/NEJMoa1011802 [DOI] [PubMed] [Google Scholar]
- 10.Iwashyna TJ, Ely EW, Smith DM, et al. Long-Term cognitive impairment and functional disability among survivors of severe sepsis. JAMA 2010;304:1787–94. 10.1001/jama.2010.1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lone NI, Gillies MA, Haddow C, et al. Five-Year mortality and hospital costs associated with surviving intensive care. Am J Respir Crit Care Med 2016;194:198–208. 10.1164/rccm.201511-2234OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allen C, Glasziou P, Del Mar C. Bed rest: a potentially harmful treatment needing more careful evaluation. Lancet 1999;354:1229–33. 10.1016/S0140-6736(98)10063-6 [DOI] [PubMed] [Google Scholar]
- 13.Jolley SE, Bunnell AE, Hough CL. ICU-Acquired weakness. Chest 2016;150:1129–40. 10.1016/j.chest.2016.03.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parry SM, Puthucheary ZA. The impact of extended bed rest on the musculoskeletal system in the critical care environment. Extrem Physiol Med 2015;4:16. 10.1186/s13728-015-0036-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Puthucheary ZA, Rawal J, McPhail M, et al. Acute skeletal muscle wasting in critical illness. JAMA 2013;310:1591–600. 10.1001/jama.2013.278481 [DOI] [PubMed] [Google Scholar]
- 16.Levine S, Nguyen T, Taylor N, et al. Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N Engl J Med 2008;358:1327–35. 10.1056/NEJMoa070447 [DOI] [PubMed] [Google Scholar]
- 17.Needham DM. Mobilizing patients in the intensive care unit: improving neuromuscular weakness and physical function. JAMA 2008;300:1685–90. 10.1001/jama.300.14.1685 [DOI] [PubMed] [Google Scholar]
- 18.Kress JP, Hall JB. Critical care medicine ICU-Acquired weakness and recovery from critical illness. NEJM 2014;370:1626–35. [DOI] [PubMed] [Google Scholar]
- 19.Hodgson CL, Berney S, Harrold M, et al. Clinical review: early patient mobilization in the ICU. Crit Care 2012;17:207. 10.1186/cc11820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. The Lancet 2009;373:1874–82. 10.1016/S0140-6736(09)60658-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schujmann DS, Teixeira Gomes T, Lunardi AC, et al. Impact of a progressive mobility program on the functional status, respiratory, and muscular systems of ICU patients: a randomized and controlled trial. Crit Care Med 2020;48:491–7. 10.1097/CCM.0000000000004181 [DOI] [PubMed] [Google Scholar]
- 22.Calvo-Ayala E, Khan BA, Farber MO, et al. Interventions to improve the physical function of ICU survivors: a systematic review. Chest 2013;144:1469–80. 10.1378/chest.13-0779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Waldauf P, Jiroutková K, Krajčová A, et al. Effects of rehabilitation interventions on clinical outcomes in critically ill patients: systematic review and meta-analysis of randomized controlled trials. Crit Care Med 2020;48:1055–65. 10.1097/CCM.0000000000004382 [DOI] [PubMed] [Google Scholar]
- 24.Kayambu G, Boots R, Paratz J. Physical therapy for the critically ill in the ICU: a systematic review and meta-analysis. Crit Care Med 2013;41:1543–54. 10.1097/CCM.0b013e31827ca637 [DOI] [PubMed] [Google Scholar]
- 25.Berney SC, Harrold M, Webb SA, et al. Intensive care unit mobility practices in Australia and New Zealand: a point prevalence study. Crit Care Resusc 2013;15:260–5. [PubMed] [Google Scholar]
- 26.Harrold ME, Salisbury LG, Webb SA, et al. Early mobilisation in intensive care units in Australia and Scotland: a prospective, observational cohort study examining mobilisation practises and barriers. Crit Care 2015;19:336. 10.1186/s13054-015-1033-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nydahl P, Ruhl AP, Bartoszek G, et al. Early mobilization of mechanically ventilated patients: a 1-day point-prevalence study in Germany. Crit Care Med 2014;42:1178–86. 10.1097/CCM.0000000000000149 [DOI] [PubMed] [Google Scholar]
- 28.McWilliams D, Jones C, Atkins G, et al. Earlier and enhanced rehabilitation of mechanically ventilated patients in critical care: a feasibility randomised controlled trial. J Crit Care 2018;44:407–12. 10.1016/j.jcrc.2018.01.001 [DOI] [PubMed] [Google Scholar]
- 29.Wright SE, Thomas K, Watson G, et al. Intensive versus standard physical rehabilitation therapy in the critically ill (EPICC): a multicentre, parallel-group, randomised controlled trial. Thorax 2018;73:213–21. 10.1136/thoraxjnl-2016-209858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Denehy L, Skinner EH, Edbrooke L, et al. Exercise rehabilitation for patients with critical illness: a randomized controlled trial with 12 months of follow-up. Critical Care 2013;17:R156. 10.1186/cc12835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moss M, Nordon-Craft A, Malone D, et al. A randomized trial of an intensive physical therapy program for patients with acute respiratory failure. Am J Respir Crit Care Med 2016;193:1101–10. 10.1164/rccm.201505-1039OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walsh TS, Salisbury LG, Boyd J, et al. A randomised controlled trial evaluating a rehabilitation complex intervention for patients following intensive care discharge: the recover study. BMJ Open 2012;2:e001475. 10.1136/bmjopen-2012-001475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cuthbertson BH, Rattray J, Campbell MK, et al. The practical study of nurse led, intensive care follow-up programmes for improving long term outcomes from critical illness: a pragmatic randomised controlled trial. BMJ 2009;339:b3723–b23. 10.1136/bmj.b3723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elliott D, McKinley S, Alison J, et al. Health-Related quality of life and physical recovery after a critical illness: a multi-centre randomised controlled trial of a home-based physical rehabilitation program. Crit Care 2011;15:R142. 10.1186/cc10265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dubb R, Nydahl P, Hermes C, et al. Barriers and strategies for early mobilization of patients in intensive care units. Ann Am Thorac Soc 2016;13:724–30. 10.1513/AnnalsATS.201509-586CME [DOI] [PubMed] [Google Scholar]
- 36.Nydahl P, Sricharoenchai T, Chandra S, et al. Safety of patient mobilization and rehabilitation in the intensive care unit. systematic review with meta-analysis. Ann Am Thorac Soc 2017;14:766–77. 10.1513/AnnalsATS.201611-843SR [DOI] [PubMed] [Google Scholar]
- 37.Kho ME, Martin RA, Toonstra AL, et al. Feasibility and safety of in-bed cycling for physical rehabilitation in the intensive care unit. J Crit Care 2015;30:1419.e1–1419.e5. 10.1016/j.jcrc.2015.07.025 [DOI] [PubMed] [Google Scholar]
- 38.Sricharoenchai T, Parker AM, Zanni JM, et al. Safety of physical therapy interventions in critically ill patients: a single-center prospective evaluation of 1110 intensive care unit admissions. J Crit Care 2014;29:395–400. 10.1016/j.jcrc.2013.12.012 [DOI] [PubMed] [Google Scholar]
- 39.Kho ME, Molloy AJ, Clarke FJ, et al. TryCYCLE: a prospective study of the safety and feasibility of early In-Bed cycling in mechanically ventilated patients. PLoS One 2016;11:e0167561. 10.1371/journal.pone.0167561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Camargo Pires-Neto R, Fogaça Kawaguchi YM, Sayuri Hirota A, et al. Very early passive cycling exercise in mechanically ventilated critically ill patients: physiological and safety aspects--a case series. PLoS One 2013;8:e74182. 10.1371/journal.pone.0074182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilkinson OM, Bates A, Cusack R. An observational feasibility study - does early limb ergometry affect oxygen delivery and uptake in intubated critically ill patients – a comparison of two assessment methods. BMC Anesthesiol 2021;21:27. 10.1186/s12871-020-01227-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hickmann CE, Castanares-Zapatero D, Deldicque L, et al. Impact of very early physical therapy during septic shock on skeletal muscle: a randomized controlled trial. Crit Care Med 2018;46:1436–43. 10.1097/CCM.0000000000003263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Machado ADS, Pires-Neto RC, Carvalho MTX, et al. Effects that passive cycling exercise have on muscle strength, duration of mechanical ventilation, and length of hospital stay in critically ill patients: a randomized clinical trial. J Bras Pneumol 2017;43:134–9. 10.1590/s1806-37562016000000170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takaoka A, Utgikar R, Rochwerg B, et al. The efficacy and safety of In-Intensive care unit Leg-Cycle Ergometry in critically ill adults. A systematic review and meta-analysis. Ann Am Thorac Soc 2020;17:1289–307. 10.1513/AnnalsATS.202001-059OC [DOI] [PubMed] [Google Scholar]
- 45.van Willigen Z, Collings N, Richardson D, et al. Quality improvement: the delivery of true early mobilisation in an intensive care unit. BMJ Qual Improv Rep 2016;5:u211734.w4726. 10.1136/bmjquality.u211734.w4726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Needham DM, Korupolu R, Zanni JM, et al. Early physical medicine and rehabilitation for patients with acute respiratory failure: a quality improvement project. Arch Phys Med Rehabil 2010;91:536–42. 10.1016/j.apmr.2010.01.002 [DOI] [PubMed] [Google Scholar]
- 47.Morris PE, Goad A, Thompson C, et al. Early intensive care unit mobility therapy in the treatment of acute respiratory failure. Crit Care Med 2008;36:2238–43. 10.1097/CCM.0b013e318180b90e [DOI] [PubMed] [Google Scholar]
- 48.McWilliams D, Weblin J, Atkins G, et al. Enhancing rehabilitation of mechanically ventilated patients in the intensive care unit: a quality improvement project. J Crit Care 2015;30:13–18. 10.1016/j.jcrc.2014.09.018 [DOI] [PubMed] [Google Scholar]
- 49.Lord RK, Mayhew CR, Korupolu R, et al. Icu early physical rehabilitation programs: financial modeling of cost savings. Crit Care Med 2013;41:717–24. 10.1097/CCM.0b013e3182711de2 [DOI] [PubMed] [Google Scholar]
- 50.Chan A-W, Tetzlaff JM, Altman DG, et al. Spirit 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med 2013;158:200-+. 10.7326/0003-4819-158-3-201302050-00583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hoffmann TC, Glasziou PP, Boutron I, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348:g1687. 10.1136/bmj.g1687 [DOI] [PubMed] [Google Scholar]
- 52.Act MC. Availble at 2005., 2005. Available: http://www.legislation.gov.uk/ukpga/2005/9/contents [Accessed 7 Dec 2021].
- 53.Ely EW, Truman B, Shintani A, et al. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation scale (RASS). JAMA 2003;289:2983–91. 10.1001/jama.289.22.2983 [DOI] [PubMed] [Google Scholar]
- 54.Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation–Sedation scale. Am J Respir Crit Care Med 2002;166:1338–44. 10.1164/rccm.2107138 [DOI] [PubMed] [Google Scholar]
- 55.Ely EW, Inouye SK, Bernard GR. Delirium in mechanically ventilated patients - Validity and reliability of the Confusion Assessment Method for the intensive care unit (CAM-ICU). Jama-J Am Med Assoc 2001;286:2703–10. [DOI] [PubMed] [Google Scholar]
- 56.De Jonghe B, Sharshar T, Lefaucheur J-P, et al. Paresis acquired in the intensive care unit: a prospective multicenter study. JAMA 2002;288:2859–67. 10.1001/jama.288.22.2859 [DOI] [PubMed] [Google Scholar]
- 57.Denehy L, de Morton NA, Skinner EH, et al. A physical function test for use in the intensive care unit: validity, responsiveness, and predictive utility of the physical function ICU test (scored). Phys Ther 2013;93:1636–45. 10.2522/ptj.20120310 [DOI] [PubMed] [Google Scholar]
- 58.Skinner EH, Berney S, Warrillow S, et al. Development of a physical function outcome measure (PFIT) and a pilot exercise training protocol for use in intensive care. Crit Care Resusc 2009;11:110–5. [PubMed] [Google Scholar]
- 59.Fan E, Ciesla ND, Truong AD, et al. Inter-Rater reliability of manual muscle strength testing in ICU survivors and simulated patients. Intensive Care Med 2010;36:1038–43. 10.1007/s00134-010-1796-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hermans G, Clerckx B, Vanhullebusch T, et al. Interobserver agreement of medical Research Council sum-score and handgrip strength in the intensive care unit. Muscle Nerve 2012;45:18–25. 10.1002/mus.22219 [DOI] [PubMed] [Google Scholar]
- 61.Vanpee G, Segers J, Van Mechelen H, et al. The interobserver agreement of handheld dynamometry for muscle strength assessment in critically ill patients. Crit Care Med 2011;39:1929–34. 10.1097/CCM.0b013e31821f050b [DOI] [PubMed] [Google Scholar]
- 62.Corner EJ, Wood H, Englebretsen C, et al. The chelsea critical care physical assessment tool (CPAx): validation of an innovative new tool to measure physical morbidity in the general adult critical care population; an observational proof-of-concept pilot study. Physiotherapy 2013;99:33–41. 10.1016/j.physio.2012.01.003 [DOI] [PubMed] [Google Scholar]
- 63.Hodgson C, Needham D, Haines K, et al. Feasibility and inter-rater reliability of the ICU mobility scale. Heart Lung 2014;43:19–24. 10.1016/j.hrtlng.2013.11.003 [DOI] [PubMed] [Google Scholar]
- 64.Bischoff HA, Stähelin HB, Monsch AU, et al. Identifying a cut-off point for normal mobility: a comparison of the timed 'up and go' test in community-dwelling and institutionalised elderly women. Age Ageing 2003;32:315–20. 10.1093/ageing/32.3.315 [DOI] [PubMed] [Google Scholar]
- 65.Ng SS, Hui-Chan CW. The timed up & go test: its reliability and association with lower-limb impairments and locomotor capacities in people with chronic stroke. Arch Phys Med Rehabil 2005;86:1641–7. 10.1016/j.apmr.2005.01.011 [DOI] [PubMed] [Google Scholar]
- 66.Bagshaw M, Majumdar SR, Rolfson DB, et al. A prospective multicenter cohort study of frailty in younger critically ill patients. Crit Care 2016;20:175. 10.1186/s13054-016-1338-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bagshaw SM, Stelfox HT, Johnson JA, et al. Long-Term association between frailty and health-related quality of life among survivors of critical illness: a prospective multicenter cohort study. Crit Care Med 2015;43:973–82. 10.1097/CCM.0000000000000860 [DOI] [PubMed] [Google Scholar]
- 68.Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005;173:489–95. 10.1503/cmaj.050051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.statement ATS, ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories . Ats statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002;166:111–7. 10.1164/ajrccm.166.1.at1102 [DOI] [PubMed] [Google Scholar]
- 70.Ustun TB, Kostanjesek N, Chatterji S. Measuring health and disability : manual for WHO Disability Assessment Schedule (WHODAS 2.0) / edited by T.B. Üstün, N. Kostanjsek, S. Chatterji, J.Rehm. Geneva: World Health Organization, 2010. [Google Scholar]
- 71.Bjelland I, Dahl AA, Haug TT, et al. The validity of the hospital anxiety and depression scale. An updated literature review. J Psychosom Res 2002;52:69–77. 10.1016/s0022-3999(01)00296-3 [DOI] [PubMed] [Google Scholar]
- 72.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361–70. 10.1111/j.1600-0447.1983.tb09716.x [DOI] [PubMed] [Google Scholar]
- 73.Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727–36. 10.1007/s11136-011-9903-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schwarzwald J, Solomon Z, Weisenberg M, et al. Validation of the impact of event scale for psychological sequelae of combat. J Consult Clin Psychol 1987;55:251–6. 10.1037/0022-006X.55.2.251 [DOI] [PubMed] [Google Scholar]
- 75.Proctor E, Silmere H, Raghavan R, et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health 2011;38:65–76. 10.1007/s10488-010-0319-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Eldridge SM, Chan CL, Campbell MJ, et al. Consort 2010 statement: extension to randomised pilot and feasibility trials. BMJ 2016;355:i5239. 10.1136/bmj.i5239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sricharoenchai T, Parker AM, Zanni JM, et al. Safety of physical therapy interventions in critically ill patients: a single-center prospective evaluation of 1110 intensive care unit admissions. J Crit Care 2014;29:395–400. 10.1016/j.jcrc.2013.12.012 [DOI] [PubMed] [Google Scholar]
- 78.Kho ME, Molloy AJ, Clarke F, et al. Cycle pilot: a protocol for a pilot randomised study of early cycle ergometry versus routine physiotherapy in mechanically ventilated patients. BMJ Open 2016;6:e011659. 10.1136/bmjopen-2016-011659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kayambu G, Boots RJ, Paratz JD. Early rehabilitation in sepsis: a prospective randomised controlled trial investigating functional and physiological outcomes The i-PERFORM Trial(Protocol Article). BMC Anesthesiol 2011;11:21. 10.1186/1471-2253-11-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Parry SM, Knight LD, Connolly B, et al. Factors influencing physical activity and rehabilitation in survivors of critical illness: a systematic review of quantitative and qualitative studies. Intensive Care Med 2017;43:531–42. 10.1007/s00134-017-4685-4 [DOI] [PubMed] [Google Scholar]
- 81.van Willigen Z, Gallagher L, Cusack R. New roles on ICU: the cost effectiveness of employing therapy technicians on intensive care to reduce length of stay and improve functional outcomes. Intens Care Med 2013;39:S488–S88. [Google Scholar]
- 82.Skivington K, Matthews L, Simpson SA, et al. A new framework for developing and evaluating complex interventions: update of medical Research Council guidance. BMJ;2021:n2061. 10.1136/bmj.n2061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Blackwood B, Ringrow S, Clarke M, et al. A core outcome set for critical care ventilation trials. Crit Care Med 2019;47:1324–31. 10.1097/CCM.0000000000003904 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-055285supp001.pdf (138.8KB, pdf)
bmjopen-2021-055285supp002.pdf (112.5KB, pdf)
bmjopen-2021-055285supp003.pdf (139.2KB, pdf)
bmjopen-2021-055285supp004.pdf (86.1KB, pdf)