Abstract
Introduction
Chronic pain affects about 20%–40% of the population and is linked to mental health outcomes and impaired daily functioning. Pharmacological interventions are commonly insufficient for producing relief and recovery of functioning. Behavioural health treatment is key to generate lasting benefits across outcome domains. However, most people with chronic pain cannot easily access evidence-based behavioural interventions. The overall aim of the DAHLIA project is to develop, evaluate and implement a widely accessible digital behavioural health treatment to improve well-being in individuals with chronic pain.
Methods and analysis
The project follows the four phases of the mHealth Agile Development and Evaluation Lifecycle: (1) development and pre-implementation surveillance using focus groups, stakeholder interviews and a business model; (2) iterative optimisation studies applying single case experimental design (SCED) method in 4–6 iterations with n=10 patients and their healthcare professionals per iteration; (3) a two-armed clinical randomised controlled trial enhanced with SCED (n=180 patients per arm) and (4) interview-based post-market surveillance. Data analyses include multilevel modelling, cost-utility and indicative analyses.
In October 2021, inter-sectorial partners are engaged and funding is secured for four years. The treatment content is compiled and the first treatment prototype is in preparation. Clinical sites in three Swedish regions are informed and recruitment for phase 1 will start in autumn 2021. To facilitate long-term impact and accessibility, the treatment will be integrated into a Swedish health platform (www.1177.se), which is used on a national level as a hub for advice, information, guidance and e-services for health and healthcare.
Ethics and dissemination
The study plan has been reviewed and approved by Swedish ethical review authorities. Findings will be actively disseminated through peer-reviewed journals, conference presentations, social media and outreach activities for the wider public.
Trial registration number
Keywords: PAIN MANAGEMENT, MENTAL HEALTH, PUBLIC HEALTH
Strengths and limitations of this study.
An agile, iterative and data-driven process is ideally suited to navigate the complex challenges faced during the development, evaluation and implementation of a digital behavioural treatment.
Executing the project with a multidisciplinary, inter-sectorial and international team brings expertise and insights from complementary views together.
Patients and different stakeholders, such as healthcare professionals, managers and digital developers, are involved in the project from the start, thus ensuring that individual needs to use and/or promote the treatment can be met.
The richness of methodologies combining traditional clinical trial evaluations on the population level, fine-graded momentary data collection on the individual level, explicit focus on cost-effectiveness and determinants of implementation allows for a treatment evaluation from all angles.
Due to the complexity and stepwise approach of this project, problems (eg, delays in recruitment) in earlier phases might negatively affect the execution of later phases, thus calling for mitigation strategies to address potential delays.
Introduction
Chronic pain (CP) affects 20%–40% of the adult population.1 Due to the COVID-19 pandemic, prevalence rates may increase further since CP can develop as a post-viral syndrome, from insufficient risk factor management during lockdown (eg, inactivity, stress), or from accumulated unmet rehabilitation needs in overburdened rehab services.2 3 CP impacts not only individuals’ daily activities and overall quality of life, but also social and working contexts.4 Thus, considerable direct and indirect health-related costs are associated with CP5 and it represents a major issue for healthcare services and society at large.
A consensus exists regarding the importance of a holistic perspective integrating social, psychological and biological factors of CP to accommodate this condition and its implications, and to guide interventions aimed at providing support.6 Considering the typical complexity of CP, pharmacological treatment alone is usually insufficient in producing sustained relief and recovery of functioning.7 Instead, management plans should target key behavioural, emotional, cognitive and social factors in everyday functioning and quality of life.8
To generate general and lasting benefits across outcome domains, person-centred, behavioural health interventions are critical. The necessity to match the pain treatment with specific needs of each patient has been the focus of discussion for the past decades.9 Existing evidence supports methods that stem from cognitive behavioural therapy (CBT) frameworks,10 including the fear-avoidance model of pain and disability11 and the psychological flexibility model, the model underlying acceptance and commitment therapy (ACT).12 13 In this type of treatment, the objective is to optimise effects by individualising treatment through evidence-based therapeutic procedures.14 In clinical practice, face-to-face therapy dominates in effectively promoting well-being in patients with CP.7 15 Modes of treatment delivery are evolving, however, as new models of care emerge.
Until now and despite the empirical support, interdisciplinary treatment, including behavioural interventions, is commonly not available or difficult to access for most individuals with CP.16 17 Digital solutions aiming at promoting health, also known as eHealth, appear promising to bridge this gap as they appear cost-effective, can be tailored to individual needs, applied in everyday life and used at the patients’ convenience.18 Particularly in light of the COVID-19 pandemic, distance approaches are gaining more attention in the management of CP.19 However, the development and implementation of evidence-based digital interventions face challenges.
Innovative digital treatments require an accurate scientific evaluation to ensure clinical effectiveness. As it is still seen as the ‘gold standard’, digital interventions for CP are often assessed through research-led randomised controlled trials (RCTs).18 20 21 However, a call for real-world and n-of-1 evaluations of efficacy and safety of individual assessment and treatment approaches is also being heard.22 Compared with RCTs, n-of-1 study designs use repeated measurements to provide a more fine-graded, time-sensitive and context-sensitive picture of individual trajectories and pattern, thus allowing to evaluate effects at the within-person level.23
Moreover, it has been shown that eHealth innovations purely originated from an academic context are rarely sustainably implemented into healthcare practice due to a lack of infrastructure, funding and time.24 To avoid research waste when creating new eHealth solutions, a strong user-centred design and focus on implementation is suggested.25 26 A framework that combines the scientific rigour of traditional research methods with a rapid and iterative digital product development approach is needed. Then, the development of an evidence-based and user-friendly digital behavioural treatment is facilitated that is implementation-ready for applied healthcare.
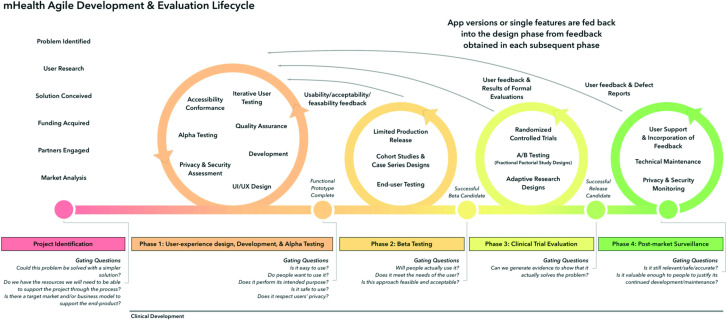
The ‘mHealth agile development and evaluation lifecycle’ (figure 1) is a framework created to promote the development of evidence-based, effective and sustainable digital solutions.27 This framework emphasises practicality, flexibility, rapid evaluation and the possibility to adjust protocols to meet technological changes and insights that emerge as part of the process. Therefore, Wilson et al’s27 framework will guide the present project. Additionally, the framework commissioned by the Medical Research Council and National Institute for Health Research (MRC/NHIR framework) for developing and evaluating complex interventions will inform the processes.26 28 By applying these perspectives, the ultimate goal to develop, evaluate and implement an effective and accessible behavioural treatment will be reached, thus improving health in individuals with CP across Sweden.
Figure 1.
mHealth agile development and evaluation lifecycle (Wilson et al, 2018).
Research objectives
The overall aim of this project is to develop, evaluate and implement a digital behavioural health treatment to improve well-being in individuals with CP. The treatment will be integrated into a nationally available healthcare web-platform, which facilitates large scale evaluations, further development, dissemination and long-term use in clinical practice across Sweden. Within the project, we will (1) develop a prototype of the digital treatment matching the needs of individuals with CP, using focus groups to assess user demands, and discuss possible treatment structures and content; (2) pilot the treatment in several iterations to evaluate its feasibility and acceptability, efficacy and individual change processes by combining intensive (single case experimental design (SCED)) and extensive methods; (3) conduct a two-armed RCT enhanced with SCED to assess the clinical effectiveness, cost-effectiveness and long-term effects compared with treatment as usual (TAU) on a between-person and within-person level and (4) identify barriers and facilitators, and monitor the implementation process of the treatment, through a business model and stakeholder interviews.
Methods and analysis
Following the mHealth agile lifecycle,27 the DAHLIA (Digital behaviourAl HeaLth for chronIc pAin) project consists of an identification phase 0 and four main phases: development, optimisation, clinical trial evaluation and post-market surveillance (see overview of the DAHLIA project in figure 2). Phase 1 includes two studies: focus groups with patients and healthcare professionals (HCPs) to develop the treatment prototype (study 1), and stakeholder interviews to prepare for the implementation process by creating a business model and identifying barriers and facilitators (study 2). Phase 2 (optimisation) aims at optimising the treatment and entails 4–6 iterations to test and gradually improve the prototype in a data-driven manner (study 3). Phase 3 consists of a large-scale clinical trial to evaluate the digital treatment in comparison to TAU in a two-armed RCT enhanced with SCED (study 4). Finally in phase 4, a post-market surveillance is conducted using interviews with stakeholders from different Swedish regions, also presenting lessons-learnt (study 5). Each phase may inform and alter subsequent phases, in line with the agile approach. Project planning started in January 2020, data collection takes place since end of 2021 and the anticipated completion of the project is 2025. Details of the studies are described in the following paragraphs.
Figure 2.
DAHLIA project overview including highlights of each study and time plan. FU, follow-up; HCP, healthcare professional; RCT, randomised controlled trial; SCED, single case experimental design; TAU, treatment as usual.
Project identification
Involvement of inter-sectorial partners and international collaborators
This project is a collaboration between academia, healthcare and industry. The academic partners come from seven universities in four countries (Sweden, Belgium, the Netherlands and the USA). The researchers contribute to the project with their scientific and clinical experience in developing and evaluating digital treatments, implementation sciences, cost-utilisation analysis, CP and related health issues and the SCED method. The DAHLIA treatment will be designed within the www.1177.se platform in collaboration with healthcare developers and digital designers in Region Kalmar and supported by the industry partner Inera, who is responsible for the maintenance of the platform. The healthcare partners currently represent 3 of the 21 regions in Sweden, and include primary care centres in Region Kalmar, the Pain Clinic at Capio St. Göran Hospital, Region Stockholm and the Rehabilitation centre in Region Örebro.
Personas as early user research
Personas are typical patient-profiles or user-profiles illustrating the target group of a treatment or product and can be useful in the development of digital interventions to communicate user needs to the development team.29 30 By giving a narrative and name, personas facilitate a more concrete discussion of patient needs, and to what extent the treatment might match those needs.31 In the DAHLIA project, three distinct patient personas evolved in an online workshop and were edited over several months until the project partners were developed in a stepwise manner. The personas originated from patient interviews in a previous study,29 and discussed in an online workshop to assess the relevance for the DAHLIA project. The personas were then adjusted based on factors identified in research,32–34 other personas used in digital development projects region Kalmar, and input from the clinical researchers (RW, IFI, KB, LMM, SP). The personas were continuously edited over several months until the project partners agreed on the final versions. The categories for each persona are: (1) personal information, including employment, education, family, background and social context, social support and living area; (2) patient pain profile, including pain problem, consequences, pain behaviour and attitude to treatment; (3) healthcare and treatment, including contact with healthcare, comorbidities and medicine and (4) personal needs and goals, specifically related to the treatment. Figure 3 illustrates one of the personas used in the DAHLIA project.
Figure 3.
Example of a DAHLIA Persona with chronic pain.
During the early development of the DAHLIA treatment prototype (version 1.0), and prior to patient involvement, personas were used to ensure that relevant characteristics and contextual factors were considered.35 The personas were presented at the start of treatment workshops to discuss, for instance, if and how the treatment content and structure fit the personas’ characteristics and met their needs. Potential problems for a persona in relation to treatment elements were identified, resulting in further discussions and consensus-based adjustments.
Guiding principles in the development process of the DAHLIA treatment
When developing and evaluating complex interventions, one might either rely on already existing treatments or adapt these to the context, or chose to build a new treatment based on research evidence and theory of the problem.26 In the present project, the latter was chosen for the following reasons. First, the initiative for this project originated from the Swedish Region Kalmar identifying the need for a digital treatment for patients with CP, which resulted in a collaboration with the research team. Furthermore, contextual factors such as organisational aspects, technical systems and licencing agreements define the conditions for in this project. Finally, by creating a new treatment together with stakeholders (ie, managers, regional developers, therapists, patients) and building on an existing digital structure (www.1177.se), the digital treatment can accommodate all identified requirements.
The following process was therefore followed to create the new treatment: four 3-hour online workshops took place between June 2020 and June 2021 to discuss the theoretical framework, conceptual model and treatment components. Project partners presented their previous work related to behavioural treatment approaches and conferred on the guiding principles for the prototype development. The group reached consensus on using learning theory36 as the theoretical framework for assessment and treatment. Furthermore, it was agreed that the fear-avoidance model11 and psychological flexibility model10 14 37 should be used as conceptual models for the DAHLIA treatment. Conclusively, the primary objective of the treatment is to increase resilience to pain and distress by promoting and training behavioural skills of relevance to the individual’s functioning and well-being. Furthermore, a self-guided microlearning format38 was chosen, including brief and frequent sessions (microsessions), delivered digitally and accessible via a smartphone or desktop computer (www.1177.se; for details, see ‘Stakeholder interviews’ (study 2)).
Based on the theoretical framework and conceptual models, values-oriented exposure is considered to be the core procedure. Exposure implies the use of systematic contact with negative experience such as pain and feelings of emotional distress that promotes avoidance, in a way that reduces their adverse influence and produces more flexible, varied and engaged patterns of behaviour. Essentially, the function of exposure is to reduce negatively reinforced behaviour focused on alleviating unwanted experiences, in favour of positively reinforced behaviour focused on approaching goals in daily life. Exposure is enabled by several behavioural processes, such as identifying life values and noticing own thoughts and emotions, known as defusion (OPEN), flexible attention to the present (AWARE) and the building of extended habits of engagement (ACTIVE).10
At the end of Phase 0, the following is envisioned: The DAHLIA treatment will run over 6 weeks and includes four self-guided microsessions per week. Each session will include a set of key elements (see figure 4). The extent to which each of these elements will be included in the session can vary. It should be noted that due to the agile process, data-driven decisions might result in changes to this suggested structure.
Figure 4.
DAHLIA treatment micro-session elements. Note: the name ‘DAHLIA treatment’ is mainly for academic settings; in the www.1177.se web-platform, a more intuitive treatment name will be chosen. HCP, healthcare professional.
A chat function will enable patients to connect with their HCPs (see details in the section Participants and recruitment) for additional guidance, asynchronous feedback and further instructions. The role of the HCP is to encourage and motivate patients to remain in the programme and intervene in case the individual situation worsens. At the start of the treatment, a specific weekday will be agreed on, during which the HCP replies to the patient’s message. Potentially, the reply could also be a chat message, a phone call or a video call. The contact with the HCP will take place once a week, with a minimum of six individual interactions between the HCP and patient. HCPs will receive training, a manual and supervision to provide the treatment.
Furthermore, patients will be prompted to fill in a pre-scheduled digital diary two times a day. The digital diary has the purpose to enable self-monitoring for increased self-awareness of own behaviours, emotions and routines, and thus enhanced orientation towards values and goals,39 and data collection to gain insight into the individual change processes and effects of the treatment in the context of the SCED. The full list of the daily diary items can be found in the Individual change processes section.
After the main 6-week intervention period, the treatment also entails booster-sessions delivered through the www.1177.se web-platform after 2 and 4 months. The participants get invited via SMS or emails to revisit the web-platform where they can engage in short behavioural exercises. Booster sessions are suggested in other contexts to support long-term behavioural changes40 and reinforce patients learnt coping strategies. Figure 5 summarises the DAHLIA treatment components.
Figure 5.
The DAHLIA treatment components.
Participants and recruitment
In the DAHLIA project, participants will be people who either use or deliver the digital treatment, or who facilitate the treatment implementation. Thus, study participants are (1) patients with CP, (2) HCPs treating patients with CP, (3) healthcare managers, (4) developers of the www.1177.se web platform, (5) other stakeholders identified in the process (eg, policy makers, representatives from patient organisations). HCPs will be licensed psychologists or psychotherapists trained in cognitive behavioural therapy. Healthcare managers, developers and other stakeholders need to be directly or indirectly connected with the treatment (eg, decision-making on an organisational level, technical support and so on), but no other requirements apply.
Patients are eligible for inclusion if they: are older than 18 years of age; report a pain duration of ≥3 months; are able to communicate in Swedish; and have access to a computer, smartphone and internet in their home environment. The exclusion criteria are: injury or illness that require immediate assessment and treatment, or is expected to progress significantly during the next 6 months; unstable medication (based on self-report: changes in medication during the past 3 months or expected within the next 3 months that could influence well-being and functioning substantially, such as opioids, anti-epileptic drugs, antidepressants); previous CBT treatment (including ACT) during the past 6 months; severe psychiatric comorbidity (eg, high risk of suicide). For study 1 (focus groups), only the exclusion criteria ‘severe psychiatric comorbidity’ (eg, high risk of suicide) will be applied as long-term health aspects are not expected to cause practical or ethical issues.
Information regarding the DAHLIA project and specific substudies will be provided to the clinics, including detailed instructions for eligibility. Regions recruiting patients are Kalmar, Stockholm and Örebro. Additional regions have expressed interest in participating and recruitment might be extended. Patients will be approached via their healthcare centres and once patients have expressed interest in study participation, a formal eligibility check will be conducted. Potential participants will be screened at their respective clinic via a face-to-face or online meeting by their treating care professionals, including psychologist and pain physicians. A short interview will be conducted to confirm eligibility and ensure that none of the exclusion criteria are met. Informed consent is then obtained from all participants prior to enrolment in the study. Sociodemographic and pain-descriptive information will be collected from all participants including age, sex, level of education, occupation, location, level and duration of pain, pain diagnosis (if applicable), and approaches to relief pain (eg, medication, heat, physiotherapy).
Phase 1: development
Focus groups (study 1)
The aim of this study is to (1) identify the needs of patients and HCPs and (2) match the treatment content to their needs. At least three focus groups will be conducted in autumn 2021, one with HCPs (ie, psychologists/psychotherapists trained in CBT) and two with patients. Per focus group, 6–8 participants will join.41 An attempt will be made to recruit a heterogeneous group of patients in terms of such characteristics as pain condition, sex and socioeconomic background. The focus groups will be held online and take 90–120 min. A semistructured guide inspired by Gruters et al42 will be followed. In addition to a general discussion around health and individual needs at the start, the focus group leader (ie, research assistant and clinical coordinator) will ask participants to reflect on the design, set-up, content and prospective feasibility of the DAHLIA treatment (for details, see online supplemental appendix 1). The group conversations will be audio-taped and video-taped. Field notes will provide further insight into relevant cues and observations.
bmjopen-2021-059152supp001.pdf (65.2KB, pdf)
The recordings will be transcribed verbatim and the data analysis will be performed by two independent researchers. The information for the patient groups and HCP group will be analysed separately. A combination of inductive and deductive content analysis will be used. First, the deductive approach will determine the themes emerging from the semistructured guide: (1) health needs and determinants to live well with CP, and (2) feedback on the DAHLIA treatment. Then, an indicative analysis will be performed to identify categories within the themes. The transcript will be read carefully and open coding will be used. A consensus meeting with a third researcher will be conducted as a final step. This approach has been described previously and appears valid to answer the research question.42 43 The results from the focus groups will be integrated into the treatment prototype (version 2.0).
Stakeholder interviews (study 2)
The aim of this study is to develop a preliminary business model for the digital behavioural treatment and identify barriers and facilitators of the prospective implementation process. An explicit focus on implementation and economic aspects early during treatment development has been recommended.44 45 Particularly, business modelling in the context of eHealth technologies can help to create a set of success factors that will influence uptake, sustainability and effectiveness.46 A business model is part of the implementation strategy and also presented a foundation for conversations with users and stakeholders regarding the value and purpose of an eHealth technology.46 Moreover, to build the knowledge base across the multiple studies and settings, the consolidated framework for implementation research (CFIR)47 will be used. The CFIR has five major domains: intervention characteristics, outer setting, inner setting, characteristics of the individuals involved and the process of implementation. It is used as part of the analysis, as explained below.
As a first step, a preliminary version of the business model canvas was filled in by the research team (SLB, SIJ, RW, HC). As suggested by Osterwalder and Pigneur48 ‘a business model describes the rationale of how an organisation creates, delivers and captures value’ (p. 14) and demonstrates the logic of how a company or organisation intends to generate profit for a service or product. The nine blocks of the business model cover four areas of a business: customers, offers, infrastructure and financial viability. Figure 6 presents the template of the business model canvas and short definitions for each segment, including example aspects relevant for the DAHLIA project.
Figure 6.
Template of business model canvas (based on Osterwald and Pigneur, 2010). Grey boxes: example aspects of the DAHLIA business model; the final model will be a result of the stakeholder interviews.
In the present study, the treatment will be integrated into the national public healthcare website (www.1177.se), using the digital platform for behavioural health (‘Stöd och Behandling’). This digital platform is free from commercial interests, maintained by Inera, which is owned by the county councils and regions. The general aim of this national website is to increase access to healthcare, strengthen the position of the patient and contribute to improved public health. The website (www.1177.se) contains healthcare information, inspiration and e-services. Each of the 21 regions in Sweden is responsible for coordinating activities and services provided on www.1177.se, which are conducted by own staff or contracted providers. Through a national network, providers and regions can cooperate and share licenses for services.
The business model will be discussed and refined as part of the stakeholder interviews. Currently identified stakeholders are software developers, HCPs and healthcare managers. A semistructured guide inspired by a previous study on eHealth implementation49 will structure the interviews and gather information on gatekeepers, barriers and facilitators for prospective dissemination and use. Questions are tailored to the different stakeholders and include, for example, ‘If/how is the interventions’ content updated?’, ‘Who is responsible/involved in the maintenance of the intervention?’, ‘What could facilitate/hinder the implementation process?’ and ‘Do you think this intervention has the potential to become successful in your care facility?’. The full guide can be seen in online supplemental appendix 2. As part of the agile process, the guide may be adjusted based on information collected during the interviews and tailored to additional stakeholders including policy makers or representatives from patient organisations.
bmjopen-2021-059152supp002.pdf (76.9KB, pdf)
A minimum of eight interviews will be conducted and snow-ball sampling will identify additional participants that can inform the process. Interviews will be conducted until data saturation is achieved and no new topics seem to emerge. The interviews will be executed online, take 60–90 min, and the conversation will be recorded. The qualitative data will be transcribed. Then, a qualitative thematic analysis will be performed50 with statements related to potential barriers and facilitators. An inductive approach to group the information will be applied in order to best scope the replies and map categories onto the CFIR domains47 as previously described.
Finally, implementation strategies matching the emerging topics will be formulated.51 Together with the business model, these two elements represent the implementation plan for the DAHLIA project. Findings from this study may furthermore influence the post-market surveillance (study 5, see details below).
Phase 2: optimisation (study 3)
The aim of the optimisation phase is to pilot the treatment and improve it through an iterative data-driven process using small patient cohorts. The primary objective is to determine the treatment feasibility and acceptability, and the secondary objectives are to examine individual change processes, and efficacy across iterations on a group-level. The general procedures include the eligibility check, and four assessment periods: baseline, main treatment period, post-intervention and 3 months and 6 months follow-ups. Results from each iteration will be integrated into the subsequent iteration, then tested again, until satisfaction is reached and no new major issues seem to emerge. In the optimisation studies, different methodologies will be combined namely momentary data collection using digital diaries, retrospective questionnaires and semistructured interviews. The latter will be conducted by a research assistant, while the diaries and questionnaires will be completed online. Figure 7 provides an overview of the procedure in relation to the research objectives.
Figure 7.
General overview of the optimisation studies and specific procedure in each iteration. FU, follow-up; HCP, healthcare professional; SCED, single case experimental design.
In total, 40–60 patients and their treating HCPs will be included, with n=10 patient–HCP dyads each iteration. Four iterations have been seen as sufficient in a previous study to optimise a digital treatment,52 therefore, a minimum of four iterations will be conducted in the DAHLIA project. In accordance with the agile approach, additional iterations may be performed if deemed necessary. The rationales for the approaches and methodological details are described below.
Feasibility and acceptability
The mixed-method procedure to evaluate the feasibility and acceptability of the treatment includes self-reports, interviews and technical data. Short self-reports will be collected after each microsession and booster-session. Specifically, patients will be asked to rate the microsession on its usefulness, enjoyment and comprehension (‘I experienced today’s session as helpful/enjoyable/understandable.’, rated on a 7-point numerical scale from 1=not at all, to 7=very much).
Furthermore, at the end of the main intervention period, interviews will be conducted following a semistructured guide to assess the participants’ general experience and different treatment components, specifically the diary, microsessions and chat function. Questions are first rated on a 7-point numeric scale and participants are then encouraged to elaborate on their response with further details, if possible. Examples of questions are ‘Did the intervention hinder your daily occupation?’, ‘Were the micro-sessions difficult or unclear?’, ‘Did you experience the digital diary as burdensome?’ or ‘Would you recommend the treatment to a friend?’ (for details, see online supplemental appendix 3). This guide is based on other feasibility studies52 53 and tailored to the DAHLIA treatment components. The HCPs will also be interviewed using a guide that follows the same structure (ie, numeric scale and open elaborations), but the specific questions will be informed by the focus groups (study 1).
bmjopen-2021-059152supp003.pdf (50.9KB, pdf)
Additionally, technical data generated from the www.1177.se website will be collected. These data include time and frequency of log-ins, duration of engagement with the treatment and use of components. Technical data will be used to describe the overall use and adherence, and allows mediation analyses to determine the influence of engagement rates on treatment outcomes.
Data from the feasibility assessments will be analysed using descriptive statistics and qualitative synthesis to identify trends. The results will be presented reflecting the two core variables from the Technology Acceptance Model: ‘Perceived Usefulness’ and ‘Perceived Ease of Use’.54 After each iteration, the insight gathered will be fed back to the developers and integrated to gradually improve the feasibility and acceptability through data-driven adjustments of the treatment. Next to the qualitative self-report, quantitative ratings of the treatment components and technical usage data, outcome measure to determine the feasibility and acceptability also include flow of participant recruitment and retention (ie, number of participants that were approached, signed informed consent and started/completed the treatment), treatment-fidelity rates (ie, post-treatment therapist self-report ‘Was the treatment delivered as planned?’), treatment compliance (ie, indicated through log-in data, self-report from patients and therapists) and (reasons for) dropouts in each iteration.
Individual change processes
The optimisation studies implement a sequential replicated and randomised SCED to gain detailed insight into within-person behavioural changes, and to develop and test the DAHLIA intervention, which has been recommended in the context of CP.55 In SCEDs, each case functions as their own control and changes are evaluated comparing levels of the outcome variables across different phases (eg, baseline phase ‘A’ and treatment phase ‘B’).56 The methodology aims to demonstrate cause–effect relationships between the treatment (independent variable) and the target behaviour (dependent variable).57
When planning an SCED study, the Risk of Bias in N-of-1 Trials (RoBiNT) Scale, a critical appraisal tool that evaluates the methodological quality of intervention studies using single-case methodology, can be followed as guidance.57 58 The design decision made in the present study was based on this appraisal tool to ensure a scientifically robust approach. Table 1 provides details on the design elements.
Table 1.
Methodological SCED approach of the DAHLIA study based on the RoBiNT Scale
| Item | RoBiNT Scale | SCED details, per optimisation iteration (anticipated points) |
| Internal validity subscale | ||
| 1 | Design | A replicated randomised AB-design with 10×A-B (total of 20 phases), providing the opportunity to observe the experimental effect 10 times. (2 points) |
| 2 | Randomisation | The start of the treatment phase and therefore length of baseline phase will be determined randomly for each participant, with the baseline phase lasting between 5 and 10 days. This means that the treatment phase will start on any day between the 6th and 11th assignment. (2 points) |
| 3 | Sampling behaviour during all phases | The baseline phase will last at least 5 days, with two times a day sampling, resulting in 10 data points or more (phase A) (assuming 100% compliance to diary). The treatment phase will run over 6 weeks, with two times a day sampling on at least 4 days per week (6 weeks×4 days×two times a day sampling), resulting in 48 data points or more (phase B) (assuming 100% compliance to diary). Even if the compliance rate should be lower, the amount of data points will lie >5 data points. (2 points) |
| 4 | Blinding of participants and HCP delivering the treatment | Blinding of the participant and practitioner is not feasible in the DAHLIA project. The behavioural treatment is delivered through a web-platform independently of the HCP; however, the HCP provides weekly, tailored support in addition to the online treatment. Neither the participant nor the HCP is blinded. (0 points) |
| 5 | Blinding (masking) of assessors | Patients complete self-report diaries and are not blinded to treatment phase, therefore, not independent of the therapy process. (0 point) |
| 6 | Inter-rater agreement | The measure of the target behaviour is a subject measure relying on self-reports from the digital diaries. (0 points) |
| 7 | Treatment adherence | The treatment is delivered through a web-platform following a standardised approach. Adherence to treatment (%) is calculated using digital log-in data. (2 points) |
| External validity and interpretation subscale | ||
| 8 | Baseline characteristics | A short interview by an HCP as part of the eligibility check will be conducted. Furthermore, a case formulation including information on age, sex, aetiology of CP and severity of CP will be presented when presenting the results; this information will be based on a baseline assessment (online self-report). (2 points) |
| 9 | Setting | Information on the general location (Swedish region, hospital/pain clinic) will be provided; however, the participant will engage with the online treatment in their everyday life, and therefore, it will not be possible to include details about the specific environment. (1 point) |
| 10 | Dependent variable (target behaviour) | Table 2 provides an overview of all diary items, which are scores on a 7-point Likert-Scale, except from the pain level item (0–100). Process outcome measures: 5 items on psychological (in)flexibility (see table 2), 2 items on pain self-efficacy, 1 item on pain avoidance. Primary outcome measures: 1 item on pain level, 1 item on pain interference, 1 item on pain catastrophising. Secondary outcome measures: 3 items on sleep, 2 items on affect, 1 item on stress, 1 item on fatigue. (2 points) |
| 11 | Independent variable (treatment) | A detailed description of the DAHLIA treatment is given above, including the treatment content, and number, duration and frequency of sessions. (2 points) |
| 12 | Raw data record | Ten cases will be recorded (4–6 iteration with n=10 participants per iteration). Raw data will be presented with a data point for each diary entry. (2 points) |
| 13 | Data analysis | Data will be analysed and reported for each participant individually. Structured visual analysis, effect size measures and a randomisation test wrapper for multilevel models will be applied. (2 points) |
| 14 | Replication | Ten participants will be included (per optimisation iteration). Across all iterations, data from n=40–60 participants will be available. (2 points) |
| 15 | Generalisation | Patients will be heterogeneous in their characteristics. Furthermore, retrospective self-reports will be completed by each participant pre–post treatment, including two FUs (for details, see table 3). (1 point) |
CP, chronic pain; DAHLIA, Digital behaviourAl HeaLth for chronIc pAin; FU, follow-up; HCP, healthcare professional; RoBiNT, Risk of Bias in N-of-1 Trials; SCED, single case experimental design.
Under the condition that all choices can be executed as intended, the internal validity of this SCED study will reach 8/14 points and the external validity will reach 14/16 points. The total interpretation score will be 22/30 points. This score indicates a moderate methodological rigour.59
Target behaviours will be assessed via self-reports collected through a digital diary. This diary will be prompted through the SMS function of REDCap, or a smartphone application (eg, www.mpath.io). Both data collection methods will be piloted with participants to ensure that the diary works reliably. Participants will be prompted to complete the diary two times a day (for details, see table 2). Proposed diary items are based on traditional questionnaires and diary studies,60 and were chosen as they assess relevant aspects in the context of CP. More specifically, sleep items are based on the Insomnia Severity Index,61 mood, stress and fatigue items are adapted from previous digital diaries studies,60 psychological (in-) flexibility items (experiential avoidance/acceptance; lack of contact with present moment/present moment awareness; self as context/context; (de-)fusion; (lack of contact with) values); inaction/committed action) are based on Multidimensional Psychological Flexibility Inventory,62 the pain level item is based on a Pain Rating Scale,63 pain catastrophising item are based on the Pain Catastrophising Scale,64 the pain avoidance item is based on the Psychological Inflexibility in Pain Scale,65 pain interference categories are based on the Brief Pain Inventory Scale66 and pain self-efficacy items are based on the Pain Self-Efficacy Questionnaire.67
Table 2.
Proposed daily diary items
| Lunch/evening diary | |||
| Instructions (Availability to fill out: lunch diary 12–14 hours, evening diary 18–20 hours) |
Lunch: Hello & welcome to your digital diary! Please reflect on last night and this morning, and rate the following statements. Self-reflections can help to understand your daily routines and needs better. Let’s get started. Evening: Welcome back to your daily diary. Please take 2–3 min to reflect on this afternoon. |
||
| Construct | Item | Answering scale | |
| Last night, … | |||
| 1 | Sleep* | … I had problems falling asleep. | 7-point numeric scale |
| 2 | Sleep* | … I had problems sleeping. | 7-point numeric scale |
| 3 | Sleep* | … I woke up too early. | 7-point numeric scale |
| During the morning/During the afternoon… | |||
| 4 | Positive affect | … I felt happy, energetic, at ease or enthusiastic. | 7-point numeric scale |
| 5 | Negative affect | … I felt down, irritated, depressed or hopeless. | 7-point numeric scale |
| 6 | Stress | … I felt stressed. | 7-point numeric scale |
| 7 | Fatigue | … I felt tired. | 7-point numeric scale |
| 8 | Experiential avoidance/acceptance† | … I tried to distract myself when I felt unpleasant emotions. … I opened myself to all my feelings, the good and the bad. |
7-point numeric scale |
| 9 | Lack of contact with present moment/present moment awareness† | … I did most things on ‘automatic’ with little awareness of what I was doing. … I was attentive and aware of my emotions. |
7-point numeric scale |
| 10 | Self as content/self as context† | … I criticised myself for having irrational or inappropriate emotions. … I tried to see the larger picture, even when I was down, depressed or hopeless. |
7-point numeric scale |
| 11 | Fusion/defusion† | … distressing thoughts tended to spin around in my mind like a broken record. … I was able to notice my thoughts and feelings without getting overwhelmed by them. |
7-point numeric scale |
| 12 | Lack of contact with values/values† | … I didn’t have time to focus on things that are important to me. … I tried to connect with what is truly important to me. |
7-point numeric scale |
| 13 | Inaction/committed action† | … negative feelings trapped me in inaction. … I didn’t quit working towards what is important even if it was though. |
7-point numeric scale |
| 14 | Pain level | … my overall pain level was: | 0 (no pain) to 10 (worst pain imaginable) |
| 15 | Pain interference | … my pain interfered with my… | 7-point numeric scale
|
| 16 | Pain catastrophising (rumination) | … I kept thinking about how much I hurt. | 7-point numeric scale |
| 17 | Pain catastrophising (magnification) | … I felt my pain overwhelmed me. | 7-point numeric scale |
| 18 | Pain catastrophising (Helplessness) | … I was afraid that my pain would get worse. | 7-point numeric scale |
| 19 | Pain avoidance | … I avoided planning activities because of my pain. | 7-point numeric scale |
| 20 | Pain self-efficacy | … I could do some form of housework/paid/unpaid work, despite the pain. | 7-point numeric scale |
| 21 | Pain self-efficacy | … I could live a normal lifestyle, despite the pain. | 7-point numeric scale |
| 22 | Open question | I would also like to share this about my morning/afternoon: | Free text |
| 23 | Treatment interaction‡ | Today, I completed a treatment module. |
|
| Instructions | Lunch: Thank you & have a nice afternoon! Evening: Thank you very much for taking the time to fill in your diary. Have a nice evening! |
||
7-point numerical scale ranges from 1: not at all, to 7: very much; alternatively, based on user input, a visual analogue slider scale from 0: not at all, to 100: very much might be used.
*Sleep items only as part of the morning questionnaire.
†Both psychological flexibility and inflexibility items will be tested to determine which are more feasible and suitable to use.
‡Treatment interaction item only as part of the evening questionnaire.
Generally, items should be short and easily to answer quickly.60 The order of the items will be the same in each prompt to allow participants to get used to the questions, minimise time to complete the diary and thus limit interference with their daily flow. The reliability, validity and sensitivity of the items will be explored through pilot studies and as part of the optimisation studies using suggested statistics (eg, P-technique factor analysis). Idiosyncratic items might also be discussed with patients, in line with the agile approach, to improve validity and potentially patient engagement and ownership. Based on user-input, scientific evidence and insight gained, diary items might be optimised and adjusted, and any adjustments made will be reported in prospective publications.
In addition to the information in table 1, the analysis will be executed as follows. Diary data have a multilevel structure because repeated measurements (level 1) are nested within individuals (level 2). First, structured visual analysis will be conducted for each individual separately following the four steps described in Kratochwill et al56 to examine the within-phase and between-phase patterns in respect to the effects on level, trend, variability, immediacy, overlap and consistency. Additionally, effect size measures will be calculated at the individual level using standardised mean difference and Tau-U, and at a group level using the between-case standardised mean difference.68 Finally, to avoid making distributional and random sampling assumptions, the randomisation test wrapper for multilevel models will be used to synthesise the data from the whole group of cases and evaluate treatment effects.69 Scientific advisors of this project will provide expertise and support in the SCED analyses. Results will be presented following the RoBiNT Scale and SCRIBE guideline.70
Efficacy across iterations
In the optimisation studies, efficacy will be determined using both intensive (SCED) as well as extensive methods (retrospective self-reports from baseline, post-intervention and follow-ups (FUs); see figure 7). The diary and questionnaire data will be aggregated across all iterations, thus include data from 40 to 60 participants. This approach allows to investigate the generalisability of results of the SCED and evaluate treatment effects in applied research.71 MultiSCED will be used for the SCED data.72
The proposed retrospective questionnaires used can be separated into process, primary and secondary outcome measures (see table 3). Additionally, negative treatment effects may occur in the context of internet interventions, and therefore, need to be acknowledged and systematically assessed.73 Negative treatment effects are here assessed post-treatment using the negative effects questionnaire (NEQ), a tool with reliable and valid psychometrics.74
Table 3.
Proposed outcome variables and tools used to assess efficacy using extensive methods
| Focus | Variables | Instrument | Supported psychometrics |
| Process outcome measures | Open/acceptance | Chronic Pain Acceptance Questionnaire (CPAQ) | Internal consistency and criterion validity (Swedish version)89 |
| Aware | 5 items on, ‘acting with awareness’ from the Five Facets Mindfulness Questionnaire (FFMQ) | Internal consistency, reliability and construct validity (Swedish version)90 | |
| Engaged/committed actions | (1) Valuing questionnaire; (2) Committed action questionnaire |
(1) Internal consistency and construct validity (Swedish version)91; (2) Proven validity and reliability (Swedish version)92 | |
| Psychological flexibility | Swedish translation of the Multidimensional Psychological Flexibility Inventory (MPFI) | Convergent and discriminant validities (English version)62 | |
| Self-efficacy | General Self-Efficacy Scale (S-GSE) | Reliable with high internal consistency (Swedish version)93 | |
| Pain self-efficacy | Pain Self-Efficacy Questionnaires (PSEQ-2) | Evidence for reliability and validity (English version),67 translated into Swedish94 | |
| Avoidance | Avoidance subscale of Psychological Inflexibility in Pain Scale (PIPS) | Internal validity and construct validity (Swedish version)65 | |
| Primary outcome measure | Catastrophising | 3-Item Daily Pain Catastrophising Scale (PCS) | Recommended instrument to understand mechanims64 |
| (Dis)ability/pain screening | Örebro Musculoskeletal Pain Screening Questionnaire (ÖMPSQ) | Clinically reliable and valid (Swedish version)95 | |
| Work ability | Work Ability Index (WAI) | Validated (Swedish version)96 | |
| Functioning | Brief Pain Inventory (BPI-SF) | Reliable and valid in multiple languages (including Swedish version)66 | |
| Secondary outcome measure | Well-being/depression | Patient Health Questionnaire (PHQ-9) | Satisfactory content validity and sufficient reliability (Swedish version)97 |
| Perceived stress | Perceived Stress Scale (PSS) | Internal reliability and construct validity (Swedish version)98 | |
| Sleep problems | Insomnia Severity Index (ISI) | Satisfactory factor structure, internal reliability and concurrent validity (Swedish version)61 | |
| Health-related quality of life | EQ-5D | Standardised measure of health-related quality of life develop by the EuroQol Group81 |
Descriptive statistics of the retrospective questionnaires will summarise demographics and pre-treatment clinical characteristics of the sample. To evaluate changes in treatment outcomes over time, linear multilevel modelling (MLM) will also be used. MLM accounts for repeated measures within subjects and can handle missing data, which will be addressed per variable. Using a random intercept model, time will be treated as a categorial variable and pre-treatment values will be specified as the reference point. Therefore, results will be interpreted as a change from pre-treatment to post-treatment and, from pre-treatment to follow-up assessments. Anchor-based methods will be applied to determine clinical significance of changes in outcome measures.75 Separate linear growth models76 will be computed for each variable, while controlling for multiple testing. Significance level is set at Alpha (α)=0.05.
Phase 3: clinical evaluation (study 4)
RCT enhanced by SCED
To determine the clinical effectiveness of the DAHLIA treatment, an RCT enhanced with SCED will be conducted. While RCTs provide estimates of between-subject treatment responses, differences in average scores between groups, they are unable to indicate specific within-subject responses. Simons et al77 apply a similar design and argue that SCED is a valuable addition to a traditional RCT design. One reason for this combined approach is that RCTs provide information on the population level, whereas SCEDs focus on the individual level. Furthermore, heterogeneity of treatment effects might remain undetected in a traditional RCT design.78 Additionally, the need for large cohorts of patients for adequate subgroup analysis,79 and a lack of feasibility to reach certain patient groups80 limits the insights from a traditional RCT. Applying SCED and multilevel modelling, even group results from small and distinct cohorts can be performed on a meta-analysis level.77
Outcome measures will be the same as in the optimisation studies, including the diary items for the SCED (see table 2), and retrospective questionnaires (see details table 3; including NEQ post-treatment.74 A priori computations based on a power of 0.95, four questionnaire assessment points and a medium effect size shows that 360 participants (180 in each arm) are sufficient to generate stable findings in the analyses of treatment effects. With an estimated attrition rate of 18%, this implies that 295 participants will provide post-treatment data, which is considered adequate also for moderator/predictor and cost-effectiveness evaluations. However, outcome measures and calculated sample size will be updated and might be modified based on iterations in the prior phase.
Treatment arm randomisation is conducted by a research assistant following the decision on study inclusion by the HCP and after the baseline assessment (sociodemographic information, questionnaires, A-phase of SCED) is completed. Participants are randomised to the treatment arm or TAU using a block randomisation strategy to ascertain equal distributions across the arms. Randomisation is conducted by a local project manager who is not involved in the screening or intervention. Next, participants undergo treatment; then all participants complete the post-intervention assessment (questionnaires and 5-day digital diary). Booster-sessions will be sent to the participants in the intervention group at 2 months and 4 months. Finally, at the 3-month and 6-month FUs, all participants complete the questionnaires and 5-day digital diary period. In case participants decide to discontinue the study at any point in time, they might choose to provide a reason.
To examine changes in process, primary and secondary outcome measures (table 3), linear mixed models will be conducted comparing the DAHLIA treatment to TAU. Analysis will be performed using group as a fixed between-person factor (two levels: DAHLIA treatment and TAU), and time as a fixed within-person variable (four levels: baseline, post-treatment, 3-month FU, 6-month FU). The linear mixed model will estimate fixed effects (regression slopes) for change in the intervals during (baseline to post-treatment assessment), and after (post-treatment to 3-month and 6-month FU) the treatment period. The intervals will be entered as a categorical dummy variable (three levels). Potential confounders will be added to the model as covariates (ie, age, gender, pain diagnosis, pain duration). Data will be analysed with the support of a statistician and using the latest version of SPSS. Mean change will be reported and test of significance will be two-sided with a set alpha level of 0.05.
Health economic evaluation
A short-term health economic evaluation will compare the DAHLIA treatment and the TAU at the primary endpoint (post-treatment). Additionally, an equivalent long-term evaluation will be performed at the end of the FU period using cumulative data collected up to that assessment point. Costs in both trial arms will be estimated from a societal perspective for each participant in the trial based on resource items and associated relevant unit costs. The use of societal resources comprises information on the use of resources related to healthcare contacts and medication (medical records and register data), and productivity losses related to absence from work (the LISA database). Costs to deliver the digital intervention will be estimated based on, for instance, HCPs’ time spent on treatment. Total costs will be aggregated by trial arm.
The self-report tool EQ-5D81 will be completed by the participants at pre-treatment, post-treatment and FUs and used to measure changes in health-related quality of life, to calculate quality adjusted life years (QALYs). Total QALY gains for participants over the trial will be estimated using the area under the curve method.82 Cost data and QALYs will be analysed using generalised linear models to account for non-normal distributions.83 Data will be analysed controlling for the influence of covariates, and by adjusting for baseline data. Cost-utility analysis will be conducted with QALYs gained as primary outcome, comparing incremental costs with incremental changes in QALYs for digital treatment and TAU. Results will be presented as an incremental cost-effectiveness ratio (ICER), representing the ratio between the difference in costs and the difference in QALY gained between the digital treatment and TAU. ICER will be expressed as cost per additional QALY, which is the most common approach in health economics.84 Uncertainty around the cost and outcome data will be explored and presented on cost-effectiveness plans, representing the distribution of the cost and outcome differences between both conditions. The probability of digital treatment being cost-effective compared with TAU will be presented across a range of price values a decision-maker would be willing to pay, represented by a cost-effectiveness acceptability curve.85
Phase 4: post-market surveillance (study 5)
Similar to the development phase (study 2), interviews with stakeholders will be conducted, recorded and transcribed. The stakeholders participating in study 2 will be approached, along with additional key stakeholders identified during the implementation process. Online supplemental appendix 4 provides the full overview of the interview questions. Questions reflect on the process so far (eg, ‘What kind and how many resources were needed to bring this intervention into practice?’), on the current status (eg, ‘What issues are you currently facing?’) and prospective adjustments (eg, ‘What will the prospective maintenance and upkeep look like?’). These questions are preliminary and may be adjusted based on findings of Phases 1–3. Even though the www.1177.se website is free for the end users (ie, patients and HCPs), special attention may also be paid to financing, as a lack thereof can be a barrier for long-term implementation of eHealth interventions.86
bmjopen-2021-059152supp004.pdf (68.7KB, pdf)
The qualitative data will be analysed following the same process as that used in Phase 1. Specifically, an inductive analysis to identify and summarise themes will be performed, and information will be mapped onto the domains of the CFIR.47 The implementation strategy and plan will be reviewed, and lessons-learnt will be presented to inform prospective implementation studies.
Patient and public involvement
This is a study protocol and due to ethical and practical reasons, no patients were directly involved in the project yet. However, the Personas originated from interviews with patients, as described above, and patients and other stakeholders will be involved in all planned phases of the DAHLIA project. Dissemination to patients and the public is described in more detail in the section Ethics and dissemination.
Discussion
CP is a huge public health problem, in suffering, disability and costs for individuals and society. Widely accessible and sustainable behavioural treatment options could help to address this problem. An agile and user-centred development integrating a data-driven decision-making process and scientific evaluation of effects is essential to produce an evidence-based intervention of this type for individuals with CP. To our knowledge, this is the first project using the mHealth agile development framework27 to systematically build a digital behavioural treatment within a nationally used healthcare hub. The purpose of this project is to improve the standard of care for individuals with CP by applying the innovative development framework, thus providing an accessible, user-friendly and empirically supported behavioural treatment to maintain or improve resilience, functioning and well-being in this population.
Strengths include (1) the execution of the project by a multidisciplinary, inter-sectorial and international research team, (2) the overall agile, iterative and data-driven process, and (3) the involvement of patients and different stakeholders early and throughout the development. Furthermore, (4) the richness of methodologies using mixed methods, combining a traditional clinical trial evaluation on the population level (RCT), fine-graded data collection (SCED) on the level of the individual, and (5) an explicit focus on cost-effectiveness and determinants of implementation will be highlighted. The project is (6) based on innovative strategies in the field of eHealth and digital treatments, and (7) key gatekeepers such as regional leaders support the initiative. The DAHLIA approach is also in line with the widely used MRC/NIHR framework by considering contextual and economical aspects, building on theory, involving stakeholders, and refining the intervention.26 45
Due to the ambitious and multifaceted nature of the project, several inherent challenges and risks should also be acknowledged. In case a substudy should be delayed, for example, due to recruitment difficulties or technical development issues, this delay could affect the whole project. Subsequently, adjustments following the agile approach could be discussed to balance the practical feasibility of executing the study and limiting the impact on its robustness.
Furthermore, the multidisciplinary, inter-sectorial approach is certainly a strength of the DAHLIA project, however, it might also have inherent challenges. For example, interests of stakeholders might differ, which needs to be considered and addressed. Here, communication is key, but compromises might be needed to ascertain satisfactory benefits for all parties involved.
Regarding the DAHLIA treatment itself, a high level of patient engagement (eg, four microsession per week combined with frequent diary assessments) will be required. These demands might be perceived as burdensome by some individual. However, contact with HCPs will support participants’ motivation and engagement. Furthermore, the focus groups and optimisation studies will provide insight into the perceived intensity, thus feasibility of the intervention set-up, and the agile process allows to adjust it accordingly. Specially, tailoring of the length of the microsessions and frequency of diary prompts will be explored.
Furthermore, the DAHLIA treatment may not be suitable for all people with CP and the question of ‘what fits for whom’ will be continuously discussed. The website (www.1177.se) is a national healthcare hub in Sweden, but research shows that older adults, people with cognitive problems or disabilities are less likely to use technologies,87 which could result in a bias in recruitment and usability. To improve inclusivity, the possibility to provide additional training for certain populations, such as older adults,88 will be explored. An additional issue is that the project is currently executed in Swedish, which excludes people with limited proficiency in Swedish. Therefore, translation into other languages and further cultural adaptations will be considered.
The DALHIA treatment may have the potential to become a widely implemented first line of treatment. However, some CP groups will likely benefit from an alternative treatment format (eg, face-to-face), or complementary interventions. Thus, additional studies may explore if and how physiotherapists, general practitioners or occupational therapists can deliver the DAHLIA treatment.
Finally, the treatment could prospectively be scaled and adjusted for other groups of patients with CP, for example, children and adolescents, people with disabilities and/or other medical conditions such as individuals with severe mental or physical comorbidities. In addition, support offered as part of the DAHLIA treatment can be extended to significant others and family members of people living with CP. Thus, by using an agile development approach, the DAHLIA project might grow to support the heterogeneous group of individuals with CP and their complex health needs.
Ethics and dissemination
The study received approval from Swedish ethical review authorities (Dnr 2021-02437). All participants will receive a detailed patient information sheet, have 1 week time to consider participation, and sign informed consent prior to participation. Each study participant will receive a unique study code to ensure anonymity and confidentiality. Data will be stored in accordance with Swedish regulations on secure servers at Karolinska Institutet.
The project is announced on the Karolinska Institutet website (Rikard Wicksell’s research group), and on social media, primarily twitter. The general outline of the project has been presented at online conferences. Next to the study protocol paper, the intention is to publish a number of peer-reviewed manuscripts, in which any protocol modifications will also be communicated. The results will be presented at (inter-)national conferences and networking events. Popular science articles, podcasts, radio interviews and animated videos are additionally planned to disseminate the results to the wider public.
Supplementary Material
Acknowledgments
We would like to thank Julia Ström and Anna Norrenge for a great collaboration. Furthermore, thanks go to Evalill Nilsson, Lauren Harrison, and Felicia Sundström for their input and feedback. All icons presented in figures 4, 5 and 7 are from www.freepik.com.
Footnotes
Twitter: @SaraLBartels
Contributors: SLB, SIJ, KB, LMC, IFl, SP, and RW were involved in the conception and design of this project. RW acquired and received the funding. HC provided specific input on the topic of implementation, IFe contributed with her expertise on health economy, and LS, PO, and JV added valuable knowledge on the single-case experimental design aspects of the project. SB drafted the manuscript, and all authors revised the manuscript and checked the intellectual content. All authors gave final approval and agree to be accountable for all aspects of the work.
Funding: This work was supported by AFA insurance grant number dnr 190 252.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Todd A, McNamara CL, Balaj M, et al. The European epidemic: pain prevalence and socioeconomic inequalities in pain across 19 European countries. Eur J Pain 2019;23:1425–36. 10.1002/ejp.1409 [DOI] [PubMed] [Google Scholar]
- 2.Clauw DJ, Häuser W, Cohen SP, et al. Considering the potential for an increase in chronic pain after the COVID-19 pandemic. Pain 2020;161:1694. 10.1097/j.pain.0000000000001950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kemp HI, Corner E, Colvin LA. Chronic pain after COVID-19: implications for rehabilitation. Br J Anaesth 2020;125:436–40. 10.1016/j.bja.2020.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Breivik H, Collett B, Ventafridda V, et al. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain 2006;10:287–333. 10.1016/j.ejpain.2005.06.009 [DOI] [PubMed] [Google Scholar]
- 5.Langley P, Müller-Schwefe G, Nicolaou A, et al. The societal impact of pain in the European Union: health-related quality of life and healthcare resource utilization. J Med Econ 2010;13:571–81. 10.3111/13696998.2010.516709 [DOI] [PubMed] [Google Scholar]
- 6.Miaskowski C, Blyth F, Nicosia F, et al. A biopsychosocial model of chronic pain for older adults. Pain Med 2020;21:1793–805. 10.1093/pm/pnz329 [DOI] [PubMed] [Google Scholar]
- 7.McCracken LM, Turk DC. Behavioral and cognitive-behavioral treatment for chronic pain: outcome, predictors of outcome, and treatment process. Spine 2002;27:2564–73. 10.1097/00007632-200211150-00033 [DOI] [PubMed] [Google Scholar]
- 8.Clauw DJ, Essex MN, Pitman V, et al. Reframing chronic pain as a disease, not a symptom: rationale and implications for pain management. Postgrad Med 2019;131:185–98. 10.1080/00325481.2019.1574403 [DOI] [PubMed] [Google Scholar]
- 9.Vlaeyen JWS, Morley S. Cognitive-Behavioral treatments for chronic pain: what works for whom? Clin J Pain 2005;21:1–8. 10.1097/00002508-200501000-00001 [DOI] [PubMed] [Google Scholar]
- 10.Hayes SC, Villatte M, Levin M, et al. Open, aware, and active: contextual approaches as an emerging trend in the behavioral and cognitive therapies. Annu Rev Clin Psychol 2011;7:141–68. 10.1146/annurev-clinpsy-032210-104449 [DOI] [PubMed] [Google Scholar]
- 11.Vlaeyen JWS, Crombez G, Linton SJ. The fear-avoidance model of pain. Pain 2016;157:1588–9. 10.1097/j.pain.0000000000000574 [DOI] [PubMed] [Google Scholar]
- 12.Hughes LS, Clark J, Colclough JA, et al. Acceptance and commitment therapy (act) for chronic pain. Clin J Pain 2017;33:552–68. 10.1097/AJP.0000000000000425 [DOI] [PubMed] [Google Scholar]
- 13.Feliu-Soler A, Montesinos F, Gutiérrez-Martínez O, et al. Current status of acceptance and commitment therapy for chronic pain: a narrative review. J Pain Res 2018;11:2145. 10.2147/JPR.S144631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayes SC, Hofmann SG, Stanton CE, et al. The role of the individual in the coming era of process-based therapy. Behav Res Ther 2019;117:40–53. 10.1016/j.brat.2018.10.005 [DOI] [PubMed] [Google Scholar]
- 15.McCracken L. Psychological approaches to chronic pain management: where are we coming from and where might we go? Revista de la Sociedad Española del Dolor 2018;25:57–63. [Google Scholar]
- 16.Breivik H, Eisenberg E, O’Brien T. The individual and societal burden of chronic pain in Europe: the case for strategic prioritisation and action to improve knowledge and availability of appropriate care. BMC Public Health 2013;13:1–14. 10.1186/1471-2458-13-1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fashler SR, Cooper LK, Oosenbrug ED, et al. Systematic review of multidisciplinary chronic pain treatment facilities. Pain Res Manag 2016;2016:5960987. 10.1155/2016/5960987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moman RN, Dvorkin J, Pollard EM, et al. A systematic review and meta-analysis of Unguided electronic and mobile health technologies for chronic Pain-Is it time to start prescribing electronic health applications? Pain Med 2019;20:2238–55. 10.1093/pm/pnz164 [DOI] [PubMed] [Google Scholar]
- 19.Puntillo F, Giglio M, Brienza N, et al. Impact of COVID-19 pandemic on chronic pain management: looking for the best way to deliver care. Best Pract Res Clin Anaesthesiol 2020;34:529-537. 10.1016/j.bpa.2020.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slattery BW, Haugh S, O'Connor L, et al. An evaluation of the effectiveness of the modalities used to deliver electronic health interventions for chronic pain: systematic review with network meta-analysis. J Med Internet Res 2019;21:e11086. 10.2196/11086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee J-A, Choi M, Lee SA, et al. Effective behavioral intervention strategies using mobile health applications for chronic disease management: a systematic review. BMC Med Inform Decis Mak 2018;18:1–18. 10.1186/s12911-018-0591-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eccleston C, Blyth FM, Dear BF, et al. Managing patients with chronic pain during the COVID-19 outbreak: considerations for the rapid introduction of remotely supported (eHealth) pain management services. Pain 2020;161:889. 10.1097/j.pain.0000000000001885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Onghena P, Edgington ES. Customization of pain treatments: single-case design and analysis. Clin J Pain 2005;21:56–68. 10.1097/00002508-200501000-00007 [DOI] [PubMed] [Google Scholar]
- 24.Schreiweis B, Pobiruchin M, Strotbaum V, et al. Barriers and facilitators to the implementation of eHealth services: systematic literature analysis. J Med Internet Res 2019;21:e14197. 10.2196/14197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higgins KS, Tutelman PR, Chambers CT, et al. Availability of researcher-led eHealth tools for pain assessment and management: barriers, facilitators, costs, and design. Pain Rep 2018;3:e686. 10.1097/PR9.0000000000000686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skivington K, Matthews L, Simpson SA, et al. A new framework for developing and evaluating complex interventions: update of medical Research Council guidance. BMJ 2021;374:n2061. 10.1136/bmj.n2061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson K, Bell C, Wilson L, et al. Agile research to complement agile development: a proposal for an mHealth research lifecycle. NPJ Digit Med 2018;1:1–6. 10.1038/s41746-018-0053-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Craig P, Dieppe P, Macintyre S, et al. Developing and evaluating complex interventions: the new medical Research Council guidance. BMJ 2008;337:a1655. 10.1136/bmj.a1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gentili C, Zetterqvist V, Rickardsson J, et al. ACTsmart – development and feasibility of digital acceptance and commitment therapy for adults with chronic pain. NPJ Digit Med 2020;3:1–12. 10.1038/s41746-020-0228-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silva S, Teixeira A. Design and development for individuals with ASD: fostering multidisciplinary approaches through personas. J Autism Dev Disord 2019;49:2156–72. 10.1007/s10803-019-03898-1 [DOI] [PubMed] [Google Scholar]
- 31.Miaskiewicz T, Kozar KA. Personas and user-centered design: how can personas benefit product design processes? Des Stud 2011;32:417–30. 10.1016/j.destud.2011.03.003 [DOI] [Google Scholar]
- 32.Gerdle B, Åkerblom S, Brodda Jansen G, Jansen GB, et al. Who benefits from multimodal rehabilitation - an exploration of pain, psychological distress, and life impacts in over 35,000 chronic pain patients identified in the Swedish Quality Registry for Pain Rehabilitation. J Pain Res 2019;12:891. 10.2147/JPR.S190003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gerdle B, Åkerblom S, Stålnacke B-M, et al. The importance of emotional distress, cognitive behavioural factors and pain for life impact at baseline and for outcomes after rehabilitation - a SQRP study of more than 20,000 chronic pain patients. Scand J Pain 2019;19:693–711. 10.1515/sjpain-2019-0016 [DOI] [PubMed] [Google Scholar]
- 34.Huijnen IPJ, Rusu AC, Scholich S, et al. Subgrouping of low back pain patients for targeting treatments: evidence from genetic, psychological, and activity-related behavioral approaches. Clin J Pain 2015;31:123–32. 10.1097/AJP.0000000000000100 [DOI] [PubMed] [Google Scholar]
- 35.Børøsund E, Mirkovic J, Clark MM, et al. A stress management APP intervention for cancer survivors: design, development, and usability testing. JMIR Form Res 2018;2:e19. 10.2196/formative.9954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cordier L, Diers M. Learning and Unlearning of pain. Biomedicines 2018;6:67. 10.3390/biomedicines6020067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharp TJ. Chronic pain: a reformulation of the cognitive-behavioural model. Behav Res Ther 2001;39:787–800. 10.1016/s0005-7967(00)00061-9 [DOI] [PubMed] [Google Scholar]
- 38.Rickardsson J, Zetterqvist V, Gentili C, et al. Internet-Delivered acceptance and commitment therapy (iACT) for chronic pain-feasibility and preliminary effects in clinical and self-referred patients. Mhealth 2020;6:27. 10.21037/mhealth.2020.02.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bandura A. Social cognitive theory of self-regulation. Organ Behav Hum Decis Process 1991;50:248–87. 10.1016/0749-5978(91)90022-L [DOI] [Google Scholar]
- 40.Bartels SL, van Knippenberg RJM, Köhler S, et al. The necessity for sustainable intervention effects: lessons-learned from an experience sampling intervention for spousal carers of people with dementia. Aging Ment Health 2020;24:2082–93. 10.1080/13607863.2019.1647130 [DOI] [PubMed] [Google Scholar]
- 41.Guest G, Namey E, McKenna K. How many focus groups are enough? building an evidence base for nonprobability sample sizes. Field methods 2017;29:3–22. 10.1177/1525822X16639015 [DOI] [Google Scholar]
- 42.Gruters AAA, Christie HL, Ramakers IHGB, et al. Neuropsychological assessment and diagnostic disclosure at a memory clinic: a qualitative study of the experiences of patients and their family members. Clin Neuropsychol 2021;35:1398–414. 10.1080/13854046.2020.1749936 [DOI] [PubMed] [Google Scholar]
- 43.Graneheim UH, Lundman B. Qualitative content analysis in nursing research: concepts, procedures and measures to achieve trustworthiness. Nurse Educ Today 2004;24:105–12. 10.1016/j.nedt.2003.10.001 [DOI] [PubMed] [Google Scholar]
- 44.Kirk MA, Kelley C, Yankey N, et al. A systematic review of the use of the consolidated framework for implementation research. Implementation Science 2015;11:1–13. 10.1186/s13012-016-0437-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Skivington K, Matthews L, Craig P, et al. Developing and evaluating complex interventions: updating medical Research Council guidance to take account of new methodological and theoretical approaches. The Lancet 2018;392:S2. 10.1016/S0140-6736(18)32865-4 [DOI] [Google Scholar]
- 46.van Limburg M, van Gemert-Pijnen JEWC, Nijland N, et al. Why business modeling is crucial in the development of eHealth technologies. J Med Internet Res 2011;13:e124. 10.2196/jmir.1674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Damschroder LJ, Aron DC, Keith RE, et al. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implementation Science 2009;4:1–15. 10.1186/1748-5908-4-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Osterwalder A, Pigneur Y. Business model generation: a Handbook for visionaries, game changers, and challengers. John Wiley & Sons, 2010. [Google Scholar]
- 49.Christie HL, Schichel MCP, Tange HJ, et al. Perspectives from Municipality Officials on the adoption, Dissemination, and implementation of electronic health interventions to support caregivers of people with dementia: inductive thematic analysis. JMIR Aging 2020;3:e17255. 10.2196/17255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Braun V, Clarke V. What can “thematic analysis” offer health and wellbeing researchers? Taylor & Francis, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Christie HL, Boots LMM, Peetoom K, et al. Developing a plan for the sustainable implementation of an electronic health intervention (partner in balance) to support caregivers of people with dementia: case study. JMIR Aging 2020;3:e18624. 10.2196/18624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dam AEH, van Boxtel MPJ, Rozendaal N, et al. Development and feasibility of Inlife: a pilot study of an online social support intervention for informal caregivers of people with dementia. PLoS One 2017;12:e0183386. 10.1371/journal.pone.0183386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bartels SL, van Knippenberg RJM, Malinowsky C, et al. Smartphone-Based experience sampling in people with mild cognitive impairment: feasibility and usability study. JMIR Aging 2020;3:e19852. 10.2196/19852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Davis FD. A technology acceptance model for empirically testing new end-user information systems: theory and results. Massachusetts Institute of Technology, 1985. [Google Scholar]
- 55.Lavefjord A, Sundström FTA, Buhrman M, et al. Assessment methods in single case design studies of psychological treatments for chronic pain: a scoping review. J Contextual Behav Sci 2021;21:121–35. 10.1016/j.jcbs.2021.05.005 [DOI] [Google Scholar]
- 56.Kratochwill TR, Hitchcock J, Horner R. Single-case designs technical documentation. What works clearinghouse, 2010. [Google Scholar]
- 57.Tate RL. The risk-of-bias in N-of-1 trials (RoBiNT) scale : an expanded manual for the critical appraisal of single-case reports / Robyn L Tate, Ulrike Rosenkoetter, Donna Wakim, Linda Sigmundsdottir, Janet Doubleday, Leanne Togher, Skye McDonald, Michael Perdices. John Walsh Centre for Rehabilitation Research: St Leonards NSW, 2015. [Google Scholar]
- 58.Tate RL, Perdices M, Rosenkoetter U, et al. Revision of a method quality rating scale for single-case experimental designs and N-of-1 trials: the 15-item risk of bias in N-of-1 trials (RoBiNT) scale. Neuropsychol Rehabil 2013;23:619–38. 10.1080/09602011.2013.824383 [DOI] [PubMed] [Google Scholar]
- 59.Perdices M, Tate RL, Rosenkoetter U. An algorithm to evaluate methodological rigor and risk of bias in single-case studies. Behav Modif 2019;0145445519863035:145445519863035. 10.1177/0145445519863035 [DOI] [PubMed] [Google Scholar]
- 60.Verhagen SJW, Hasmi L, Drukker M, et al. Use of the experience sampling method in the context of clinical trials. Evid Based Ment Health 2016;19:86–9. 10.1136/ebmental-2016-102418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dragioti E, Wiklund T, Alföldi P, et al. The Swedish version of the insomnia severity index: factor structure analysis and psychometric properties in chronic pain patients. Scand J Pain 2015;9:22–7. 10.1016/j.sjpain.2015.06.001 [DOI] [PubMed] [Google Scholar]
- 62.Rolffs JL, Rogge RD, Wilson KG. Disentangling components of flexibility via the Hexaflex model: development and validation of the multidimensional psychological flexibility inventory (MPFI). Assessment 2018;25:458–82. 10.1177/1073191116645905 [DOI] [PubMed] [Google Scholar]
- 63.McCaffery M, Beebe A. The numeric pain rating scale instructions. Pain 1989. [Google Scholar]
- 64.Darnall BD, Sturgeon JA, Cook KF, et al. Development and validation of a daily pain catastrophizing scale. J Pain 2017;18:1139–49. 10.1016/j.jpain.2017.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wicksell RK, Lekander M, Sorjonen K, et al. The Psychological Inflexibility in Pain Scale (PIPS)--statistical properties and model fit of an instrument to assess change processes in pain related disability. Eur J Pain 2010;14:771.e1-14. 10.1016/j.ejpain.2009.11.015 [DOI] [PubMed] [Google Scholar]
- 66.Cleeland CS, Ryan KM. Pain assessment: global use of the brief pain inventory. Ann Acad Med Singap 1994;23:129-38. [PubMed] [Google Scholar]
- 67.Nicholas MK, McGuire BE, Asghari A. A 2-item short form of the pain self-efficacy questionnaire: development and psychometric evaluation of PSEQ-2. J Pain 2015;16:153–63. 10.1016/j.jpain.2014.11.002 [DOI] [PubMed] [Google Scholar]
- 68.Valentine JC, Tanner‐Smith EE, Pustejovsky JE, et al. Between‐case standardized mean difference effect sizes for single‐case designs: a primer and tutorial using the scdhlm web application. Campbell Syst Rev 2016;12:1–31. 10.4073/cmdp.2016.1 [DOI] [Google Scholar]
- 69.Michiels B, Tanious R, De TK, et al. A randomization test wrapper for synthesizing single-case experiments using multilevel models: a Monte Carlo simulation study. Behav Res Methods 2020;52:654–66. 10.3758/s13428-019-01266-6 [DOI] [PubMed] [Google Scholar]
- 70.Tate RL, Perdices M, Rosenkoetter U, et al. The single-case reporting guideline in behavioural interventions (SCRIBE) 2016 statement. Phys Ther 2016;96:e1–10. 10.2522/ptj.2016.96.7.e1 [DOI] [PubMed] [Google Scholar]
- 71.Van den Noortgate W, Onghena P. The aggregation of single-case results using hierarchical linear models. Behav Anal Today 2007;8:196–209. 10.1037/h0100613 [DOI] [Google Scholar]
- 72.Declercq L, Cools W, Beretvas SN, et al. MultiSCED: A tool for (meta-)analyzing single-case experimental data with multilevel modeling. Behav Res Methods 2020;52:177–92. 10.3758/s13428-019-01216-2 [DOI] [PubMed] [Google Scholar]
- 73.Rozental A, Andersson G, Boettcher J, et al. Consensus statement on defining and measuring negative effects of internet interventions. Internet Interv 2014;1:12–19. 10.1016/j.invent.2014.02.001 [DOI] [Google Scholar]
- 74.Rozental A, Kottorp A, Forsström D, et al. The negative effects questionnaire: psychometric properties of an instrument for assessing negative effects in psychological treatments. Behav Cogn Psychother 2019;47:559–72. 10.1017/S1352465819000018 [DOI] [PubMed] [Google Scholar]
- 75.Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain 2008;9:105–21. 10.1016/j.jpain.2007.09.005 [DOI] [PubMed] [Google Scholar]
- 76.Shek DTL, Ma CMS, Ma C. Longitudinal data analyses using linear mixed models in SPSS: concepts, procedures and illustrations. ScientificWorldJournal 2011;11:42–76. 10.1100/tsw.2011.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Simons LE, Harrison LE, O'Brien SF, et al. Graded exposure treatment for adolescents with chronic pain (get living): protocol for a randomized controlled trial enhanced with single case experimental design. Contemp Clin Trials Commun 2019;16:100448. 10.1016/j.conctc.2019.100448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kent DM, Rothwell PM, Ioannidis JP. Assessing and reporting heterogeneity in treatment effects in clinical trials: a proposal. Trials 2010;11:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lillie EO, Patay B, Diamant J, et al. The N-of-1 clinical trial: the ultimate strategy for individualizing medicine? Per Med 2011;8:161–73. 10.2217/pme.11.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nikles J, Mitchell GK, Schluter P, et al. Aggregating single patient (N-of-1) trials in populations where recruitment and retention was difficult: the case of palliative care. J Clin Epidemiol 2011;64:471–80. 10.1016/j.jclinepi.2010.05.009 [DOI] [PubMed] [Google Scholar]
- 81.Björk S, Norinder A. The weighting exercise for the Swedish version of the EuroQol. Health Econ 1999;8:117–26. [DOI] [PubMed] [Google Scholar]
- 82.Matthews JN, Altman DG, Campbell MJ, et al. Analysis of serial measurements in medical research. BMJ 1990;300:230–5. 10.1136/bmj.300.6719.230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Barber J, Thompson S. Multiple regression of cost data: use of generalised linear models. J Health Serv Res Policy 2004;9:197–204. 10.1258/1355819042250249 [DOI] [PubMed] [Google Scholar]
- 84.Drummond MF, Sculpher MJ, Claxton K. Methods for the economic evaluation of health care programmes. Oxford university press, 2015. [Google Scholar]
- 85.Fenwick E, Claxton K, Sculpher M. Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Econ 2001;10:779–87. 10.1002/hec.635 [DOI] [PubMed] [Google Scholar]
- 86.Boots LM, de Vugt ME, Smeets CM, et al. Implementation of the blended care self-management program for caregivers of people with early-stage dementia (partner in balance): process evaluation of a randomized controlled trial. J Med Internet Res 2017;19:e423. 10.2196/jmir.7666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gell NM, Rosenberg DE, Demiris G, et al. Patterns of technology use among older adults with and without disabilities. Gerontologist 2015;55:412–21. 10.1093/geront/gnt166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kampmeijer R, Pavlova M, Tambor M, et al. The use of e-health and m-health tools in health promotion and primary prevention among older adults: a systematic literature review. BMC Health Serv Res 2016;16:467–79. 10.1186/s12913-016-1522-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wicksell RK, Olsson GL, Melin L. The Chronic Pain Acceptance Questionnaire (CPAQ)-further validation including a confirmatory factor analysis and a comparison with the Tampa Scale of Kinesiophobia. Eur J Pain 2009;13:760–8. 10.1016/j.ejpain.2008.09.003 [DOI] [PubMed] [Google Scholar]
- 90.Lilja JL, Frodi-Lundgren A, Hanse JJ, et al. Five Facets Mindfulness Questionnaire--reliability and factor structure: a Swedish version. Cogn Behav Ther 2011;40:291–303. 10.1080/16506073.2011.580367 [DOI] [PubMed] [Google Scholar]
- 91.Rickardsson J, Zetterqvist V, Kemani MK, et al. Assessing values – psychometric properties of the Swedish version of the Valuing questionnaire in adults with chronic pain. J Contextual Behav Sci 2019;14:40–9. 10.1016/j.jcbs.2019.08.009 [DOI] [Google Scholar]
- 92.Åkerblom S, Perrin S, Fischer MR, et al. A validation and generality study of the committed action questionnaire in a Swedish sample with chronic pain. Int J Behav Med 2016;23:260–70. 10.1007/s12529-016-9539-x [DOI] [PubMed] [Google Scholar]
- 93.Löve J, Moore CD, Hensing G. Validation of the Swedish translation of the general self-efficacy scale. Qual Life Res 2012;21:1249–53. 10.1007/s11136-011-0030-5 [DOI] [PubMed] [Google Scholar]
- 94.Larsson M, Nordeman L, Holmgren K, et al. Prevention of sickness absence through early identification and rehabilitation of at-risk patients with musculoskeletal pain (PREVSAM): a randomised controlled trial protocol. BMC Musculoskelet Disord 2020;21:1–13. 10.1186/s12891-020-03790-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Linton SJ, Boersma K. Early identification of patients at risk of developing a persistent back problem: the predictive validity of the Orebro musculoskeletal pain questionnaire. Clin J Pain 2003;19:80–6. 10.1097/00002508-200303000-00002 [DOI] [PubMed] [Google Scholar]
- 96.Ilmarinen J. Work ability--a comprehensive concept for occupational health research and prevention. Scand J Work Environ Health 2009;35:1–5. 10.5271/sjweh.1304 [DOI] [PubMed] [Google Scholar]
- 97.Mattsson M, Sandqvist G, Hesselstrand R, et al. Validity and reliability of the patient health Questionnaire-8 in Swedish for individuals with systemic sclerosis. Rheumatol Int 2020;40:1675–87. 10.1007/s00296-020-04641-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nordin M, Nordin S. Psychometric evaluation and normative data of the Swedish version of the 10-item perceived stress scale. Scand J Psychol 2013;54:502–7. 10.1111/sjop.12071 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-059152supp001.pdf (65.2KB, pdf)
bmjopen-2021-059152supp002.pdf (76.9KB, pdf)
bmjopen-2021-059152supp003.pdf (50.9KB, pdf)
bmjopen-2021-059152supp004.pdf (68.7KB, pdf)