Abstract
Introduction
Bladder cancer is a lethal disease with a rising incidence on a background of limited conventional imaging modalities for staging (either CT of the chest-abdomen-pelvis or 18F-fluorodeoxyglucose positron emitting tomography (FDG-PET/CT)). CT is known to have relatively low sensitivity for detecting low volume metastatic disease, an important goal when considering surgical interventions entailing significant potential morbidity. FDG is also limited, being predominantly renally excreted and, therefore, producing intense non-specific activity in the urinary tract, which limits its utility to detect bladder and upper tract lesions, or nodal metastases in close proximity to the urinary tract. 89Zirconium-labelled girentuximab (89Zr-TLX250) may have utility in the accurate staging of bladder and urothelial carcinomas, with less renal excretion as compared with FDG; however, this has not previously been investigated.
Methods and analysis
89Zirconium-labelled girentuximab PET in Urothelial Cancer Patients is a single-arm phase I trial examining the feasibility of using 89Zr-TLX250-PET/CT as a staging modality for urothelial and bladder carcinomas by examining isotope uptake by the cancer. This trial will also examine the safety and utility of 89Zr-TLX250-PET/CT in patients either undergoing preoperative staging of bladder or other urothelial carcinomas for curative intent, or with known metastatic urothelial carcinomas. All participants will undergo 89Zr-TLX250-PET/CT and will need to have undergone recent FDG-PET/CT for comparison. This trial aims to recruit 10 participants undergoing preoperative staging and 10 participants with known metastatic disease. The primary endpoint is feasibility defined by the ability to recruit to the target sample size within the study duration; secondary endpoints are safety, tolerability, sensitivity and specificity in detecting lymph node metastases compared with FDG-PET/CT.
Ethics and dissemination
Ethics approval has been obtained from the South Metropolitan Health Service Human Research Ethics Committee (RGS0000003940). Eligible patients will only be enrolled after providing written informed consent. Patients will be given a full explanation, in lay terms, of the aims of the study and potential risks including as a written patient information sheet.
Trial registration numbers
ACTRN12621000411842, NCT05046665.
Keywords: Urological tumours, Urological tumours, Nuclear radiology, NUCLEAR MEDICINE
STRENGTHS AND LIMITATIONS OF THIS STUDY
This will be the first study to generate data assessing the role of 89Zirconium-labelled girentuximab in the imaging of urothelial carcinoma patients.
As a high-volume quaternary centre, there is capacity to recruit suitable trial subjects within a realistic time frame.
As a small study, the ability to detect modest differences between the imaging modalities is limited.
The resolution quality of images that will be obtained and the optimum imaging timing have yet to be determined.
Introduction
Urothelial cancer
Bladder cancer is the most common malignancy involving the urinary system, and the 10th most common malignancy overall1 with a rising incidence worldwide.2 Transitional cell carcinoma is the predominant histological type, accounting for approximately 90% of all bladder cancers. Transitional cell carcinoma also affects the renal pelvis, ureter or urethra as all are lined with transitional cell urothelium. Diagnosis is usually made histologically with tissue obtained via transurethral resection, biopsy or from urine cytology. As with all malignancies, the prognosis and treatment of the disease is determined by the histopathology and staging investigations.
Current staging modalities
The following modalities are currently used to detect the distribution and extent of urothelial tumours:
CT of the chest, abdomen and pelvis including delayed-phase images are used to identify urothelial tumours, which may appear as filling defects on delayed-phase imaging or as enhancing soft tissue on the nephrographic phase. CT may demonstrate extravesical extension, tumour involvement or obstruction of the upper urinary tract nodal, involvement in the pelvis or retroperitoneum, and visceral or osseous metastasis. CT may miss tumours <1 cm in size, particularly those in the bladder trigone or dome, and it cannot accurately categorise depth of bladder wall invasion. The sensitivity of CT for identification of nodal involvement is relatively low (false-negative rate 68%, false-positive rate 16%) and may require biopsy for confirmation.3 Approximately 50% of patients with a filling defect in the renal pelvis or ureter will have associated hydronephrosis, hydroureter, or a delayed nephrogram secondary to obstruction.4
18F-fluorodeoxyglucose (FDG) positron emitting tomography (PET)/CT has limited value in the local staging of bladder cancer, largely due to urinary excretion of FDG affecting image interpretation of the bladder and any nodal disease in close proximity to the ureters.5 However, FDG PET/CT is often useful in the distant staging of urothelial cancer, especially in high-risk disease with sensitivity of 78% in detecting locoregional lymph node metastasis as compared with 44% with CT alone.6
Carbonic anhydrase IX
Carbonic anhydrase IX (CAIX) is an enzyme that functions as a regulator of intracellular pH, cell proliferation and cell adhesion in response to hypoxia.7 CAIX is expressed abundantly in response to hypoxia in a wide range of cancer cell lines including bladder, renal, head and neck, lung, and colon cancers.7
CAIX was distinctly expressed in >70% of urothelial carcinomas but was not expressed in normal urothelial tissue.8 Previous data have demonstrated sensitivity and specificity of urinary CAIX of 86.2% and 95.1%, respectively, for detection of urothelial bladder cancer (area under the curve 90.5%).9 A significant association between CAIX expression in paired urine and tumour specimens has been established. Notably, CAIX was shown to have significantly higher predictive accuracy for urothelial carcinomas compared with urinary cytology (90.5% vs 71.7%), especially in low-grade tumours (90% vs 61.8%).9
These findings provide strong rationale for investigating the potential use of CAIX as a targeted imaging agent for the identification and diagnosis of bladder cancer. By the same token, the utility of CAIX as a therapeutic target also merits future investigation.
PET/CT and 89Zirconium-girentuximab
Theranostic PET is a novel modality, combining the potential for both imaging and treatment as it enables the tracking of targeted vehicles and carriers using, for instance, isotope-labelled monoclonal antibodies. A notable example in the field of theranostics with an established therapeutic role is Lutetium-177 prostate-specific membrane antigen, a radionuclide agent that has garnered success in the treatment of castrate-resistant prostate cancer.10
Due to the intrinsic chemical properties of the relatively low energy positrons which provide high resolution PET images,11 89Zr has been identified as a suitable ligand candidate for this approach.12 TLX-250 is an antibody directed against CAIX that has been widely studied in the setting of renal cell carcinoma. In this context, 89Zirconium-labelled girentuximab (89Zr-TLX250) PET/CT has been shown to have a significant impact on clinical decision making in patients with an indeterminate renal mass.13 Studies have also explored the use of 89Zr-immuno-PET with other conjugated antibodies, demonstrating utility for monitoring treatment in animal models as well as potential as a tool in the clinical staging of breast cancer.14 Collectively these studies highlight the potential diagnostic and therapeutic applications of 89Zr-TLX250.
Urinary excretion of FDG PET/CT is intrinsically problematic when imaging urinary tract malignancies as outlined above with low sensitivity for low volume or urinary tract associated disease. The hepatic clearance of 89Zr-TLX250 with low urinary excretion is therefore anticipated to be advantageous for the local and regional staging of bladder and other urothelial carcinomas. This Phase I study aims to investigate 89Zr-TLX250 utilisation in the staging of urothelial carcinoma or bladder cancer exploiting the low urinary excretion.
Methods and analysis
Protocol overview
This is a non-randomised, non-blinded, single-centre, phase I trial comparing 89Zr-TLX250 PET/CT with FDG PET/CT in patients with urothelial carcinoma or bladder cancer. Study duration will be 18 months, having commenced in May 2021 with the anticipated date of last data collection being 31 December 2022. It is being conducted at a single centre in Western Australia and will include two cohorts of adult patients; ten patients undergoing preoperative primary staging for recently diagnosed bladder cancer or urothelial carcinoma for consideration of treatment with curative intent, and ten patients with known metastatic urothelial carcinoma or bladder cancer.
The primary objective of this study is to evaluate the feasibility of using 89Zr-TLX250 PET/CT as a staging modality for urothelial carcinoma or bladder cancer. The secondary objectives are to evaluate the safety and tolerability of 89Zr-TLX250 PET/CT, as well as its effectiveness as compared with FDG PET/CT.
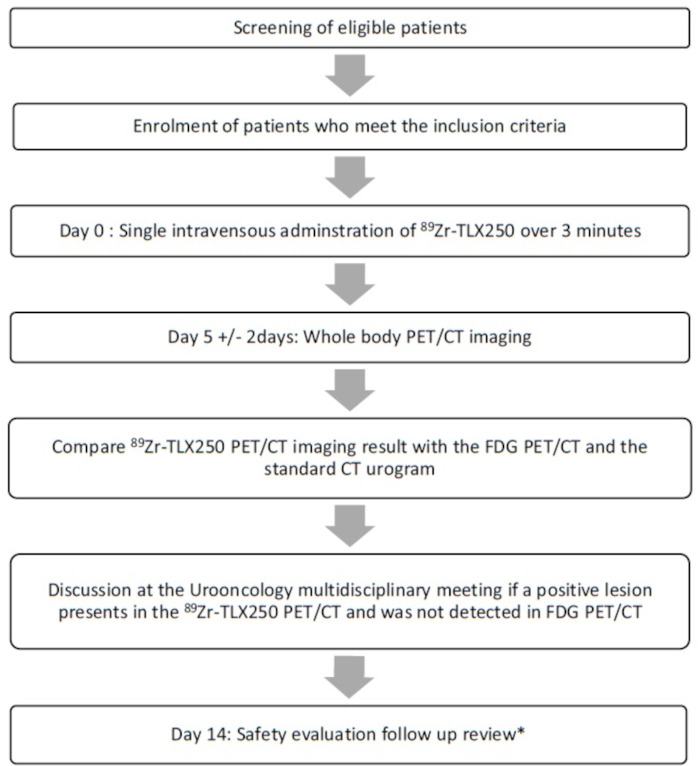
The eligibility criteria are listed in box 1 and the trial schema is outlined in figure 1. Eligible patients are also required to have undergone FDG PET/CT scanning (a part of standard of care) within the proceeding 28 days to allow accurate comparison between the two modalities. All participants will provide written informed consent.
Box 1. Key inclusion and exclusion criteria.
Inclusion criteria
Age ≥18 years old.
Able to provide informed consent.
Histologically diagnosed with urothelial carcinoma or bladder cancer (or upper tract urothelial carcinoma diagnosed based on standard imaging and malignant urine cytology or direct visualisation on ureteroscopy) or known metastatic bladder or other urothelial carcinoma (based on previous imaging and/or histopathology).
Negative serum pregnancy test in female patients of childbearing potential at screening. Confirmation of negative pregnancy test result from urine within 24 hours prior to receiving investigational product.
Consent to practise double-barrier contraception until a minimum of 42 days after 89Zr-TLX250 administration.
Exclusion criteria
Active malignancy other than urothelial carcinoma or bladder cancer.
Administration of a radioisotope within 10 physical half-lives of 89Zr prior to study enrolment.
Administration of chemotherapy, radiotherapy or immunotherapy within 4 weeks prior to planned administration of 89Zirconium-labelled girentuximab (89Zr-TLX250) or continuing adverse effects from such therapy.
Planned antineoplastic therapies for the period between administration of 89Zr-TLX250 and imaging.
Serious non-malignant disease that may interfere with the objectives of the study (eg, advanced liver disease).
Renal insufficiency with glomerular filtration rate ≤45 mL/min/1.73 m2.
Pregnancy or lactation.
Exposure to murine or chimeric antibodies within the last 5 years.
Known hypersensitivity to or human antichimeric antibodies against girentuximab.
Exposure to any experimental diagnostic or therapeutic agent in the 30 days prior to the date of planned administration of 89Zr-TLX250.
Contraindications to 18F-fluorodeoxyglucose positron emitting tomography/CT.
Figure 1.
Trial schema, schema showing the pathway for patients recruited into the phase I trial of 89Zirconium-labelled girentuximab (89Zr-TLX250) positron emitting tomography (PET) in 89Zirconium-labelled girentuximab PET in Urothelial Cancer Patients (ZiPUP). *Vital signs, standard laboratory (full blood count, renal function, basic electrolytes and liver function test), 12-lead ECG and concomitant medication recording and adverse event recording (NCI-CTCAE V.5.0). NCI-CTCAE V.5.0, National Cancer Institute-Common Terminology Criteria for Adverse Effects version V.5.0
Screening
Procedures performed during the screening visit include a review of patient eligibility criteria (box 1) and the obtaining of informed consent for trial enrolment. Participants will then undergo screening assessments including physical examination, recording of Eastern Cooperative Oncology Group Performance Status, vital signs, 12-lead ECG, review of prior/concomitant medications, and clinical laboratory tests (full blood count, urea and electrolytes, liver function test, serum beta-human chorionic gonadotropin if applicable and urine analysis) as summarised in the schedule of study assessments (table 1).
Table 1.
The schedule of study assessments is set out as follows
| Visit name | Screening | IMP administration | Imaging | Follow-up |
| Time point | Day −28 to −1 | Day 0 | Day 5±2 | Day 14 (Or before starting chemotherapy or undergoing surgery) |
| Informed consent | X | |||
| Eligibility criteria | X | |||
| 18F-FDG-PET/CT | X | |||
| Physical exam | X | |||
| ECOG status | X | |||
| Vital signs | X | X Preinjection and postinjection |
X | |
| 12-lead ECG | X | X Postinjection |
||
| Haematology Biochemistry |
X | |||
| Liver function tests | X | |||
| Serum β-HCG | X | |||
| Urine analysis | X | |||
| Urine pregnancy test | X | |||
| PET/CT | X | |||
| Adverse events | X | X | X | |
| Concomitant medications | X | X | X | X |
ECOG, Eastern Cooperative Oncology Group; 18F-FDG-PET/CT, 18F-fluorodeoxyglucose positron emitting tomography/CT; β-HCG, beta-human chorionic gonadotropin.
89Zr-TLX250 administration (day 0)
On the day of injection, a urine pregnancy test will be performed to confirm ongoing non-pregnant status in all premenopausal women. A slow intravenous administration of 37 Mega-Becquerel.
(MBq) (±10%) 89Zr-TLX250, containing a mass dose of 10 mg of TLX250 (this dosage has been arrived at based on a previous trial15), will be delivered over 3 min. Vital signs and a 12-lead ECG will be performed before and after the intravenous injection. Adverse event recording according to the National Cancer Institute-Common Terminology Criteria for Adverse Effects version 5.0 (NCI-CTCAE V.5.0) will be performed following administration of the investigational agent.
Imaging (day 5±2)
As part of PET/CT hybrid acquisition, whole-body PET static and low-dose CT including brain to mid-thigh will be performed over a maximum of 45 min in four bed positions at a single time point 5±2 days postadministration of 89Zr-TLX250. Vital signs will be recorded. Those who have potentially significant lesions with increased uptake on 89Zr-TLX250 PET/CT but not on FDG PET/CT will have their imaging discussed at the next available uro-oncology multidisciplinary team meeting to determine if further investigation or a deviation in management plan is indicated. Adverse event recording will be performed as previously discussed.
Follow-up (day 14)
Participants will receive a phone consultation 2 weeks following the administration of the 89Zr-TLX250 (or before commencement of treatment) that will include a symptom enquiry, recording of concomitant medications and adverse event recording (NCI-CTC V.5.0).
Endpoints
The primary endpoint is the feasibility of using 89Zr-TLX250 PET/CT as a staging modality for urothelial carcinoma or bladder cancer. The feasibility will be ascertained by the ability to recruit to the target sample size, deliver the 89Zr-TLX250 PET/CT and generate diagnostic grade images.
Secondary endpoints are the safety, tolerability, sensitivity and specificity of 89Zr-TLX250 PET/CT as compared with FDG PET/CT. Safety and tolerability will be assessed according to vital signs, 12-lead ECGs, adverse event records and the requirement for new medications. As part of the effectiveness analysis, tumour versus mediastinal uptake ratios will be calculated and compared for both the primary tumour and any other lesions identified for both modalities. The sensitivity and specificity of 89Zr-TLX250 PET/CT for detecting lymph node metastases will be calculated as compared with both FDG PET/CT and pathological lymph node status, where the patient proceeds to radical cystectomy and pelvic lymph node dissection.
A pragmatic target sample size of 20 patients has been chosen for this phase I feasibility study, based on the target population and other logistical factors. Descriptive statistics will be used in the reporting of the primary and secondary endpoints for this pilot study using appropriate parametric and non-parametric tests.
Patient and public involvement
Patients or the public are not involved in the design, conduct, reporting or dissemination plans of our research.
Ethics and dissemination
Ethics approval has been obtained from the South Metropolitan Health Service Human Research Ethics Committee (RGS0000003940).
Eligible patients will only be enrolled, and study-related procedures carried out after providing written informed consent. Patients will be given a full explanation, in lay terms, of the aims of the study and potential risks including as a written patient information sheet. It will be explained that they may refuse to take part in or withdraw from the study without prejudice to their future care and treatment at any time. In any case where the patient is not fluent in English, an interpreter will be present during the consenting process. Participants will be issued with a copy of the information provided and their signed consent to participate in the study.
Supplementary Material
Footnotes
Correction notice: This article has been corrected since it was published Online First. The author affiliation section has been updated.
Contributors: MA-Z: Write and reviewing the protocol as per BMJ Open requirement. PV: Write the initial protocol draft and review the final draft. SM: Review and edit the final draft. EL: Participated in protocol draft writing. NL: Review and edit the final draft. TF: Participated in protocol draft writing. ADR: Review and edit the final draft. RG: Write the protocol draft. DH: Review the protocol draft, main supervisor of the study.
Funding: The study is sponsored by the South Metropolitan Health Service, Western Australia and is funded by Telix pharmaceuticals, who will also supply the 89Zr-TLX250 at no cost.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.IARC, Cancer Today . Estimated number of new cases in 2020, worldwide, both sexes, all ages, 2021. [Google Scholar]
- 2.Harsanyi S, Ziaran S, Bevizova K, et al. The prognostic value of E-cadherin and Ki-67 compared to standard histopathologic examination in non-muscle invasive bladder cancer. Bratisl Lek Listy 2020;121:444–9. 10.4149/BLL_2020_072 [DOI] [PubMed] [Google Scholar]
- 3.Herr HW. Routine CT scan in cystectomy patients: does it change management? Urology 1996;47:324–5. 10.1016/S0090-4295(99)80446-4 [DOI] [PubMed] [Google Scholar]
- 4.Donat MD, Herr HW. Transitional cell carcinoma of the renal pelvis and ureter: diagnosis, staging, management, and prognosis. In: Oncology U, Osterling JE, Richie JP, eds. WB Saunders Harcourt Brace & Co. Philadelphia, 1997: 215. [Google Scholar]
- 5.Jana S, Blaufox MD. Nuclear medicine studies of the prostate, testes, and bladder. Semin Nucl Med 2006;36:51–72. 10.1053/j.semnuclmed.2005.09.001 [DOI] [PubMed] [Google Scholar]
- 6.Nayak B, Dogra PN, Naswa N, et al. Diuretic 18F-FDG PET/CT imaging for detection and locoregional staging of urinary bladder cancer: prospective evaluation of a novel technique. Eur J Nucl Med Mol Imaging 2013;40:386–93. 10.1007/s00259-012-2294-6 [DOI] [PubMed] [Google Scholar]
- 7.Ivanov S, Liao SY, Ivanova A, et al. Expression of hypoxia-inducible cell-surface transmembrane carbonic anhydrases in human cancer. Am J Pathol 2001;158:905–19. 10.1016/S0002-9440(10)64038-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klatte T, Seligson DB, Rao JY, et al. Carbonic anhydrase IX in bladder cancer: a diagnostic, prognostic, and therapeutic molecular marker. Cancer 2009;115:1448–58. 10.1002/cncr.24163 [DOI] [PubMed] [Google Scholar]
- 9.de Martino M, Lucca I, Mbeutcha A, et al. Carbonic anhydrase IX as a diagnostic urinary marker for urothelial bladder cancer. Eur Urol 2015;68:552–4. 10.1016/j.eururo.2015.06.015 [DOI] [PubMed] [Google Scholar]
- 10.Hofman MS, Violet J, Hicks RJ, et al. [177Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study. Lancet Oncol 2018;19:825–33. 10.1016/S1470-2045(18)30198-0 [DOI] [PubMed] [Google Scholar]
- 11.van de Watering FCJ, Rijpkema M, Perk L, et al. Zirconium-89 labeled antibodies: a new tool for molecular imaging in cancer patients. Biomed Res Int 2014;2014:1–13. 10.1155/2014/203601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Dongen GA, Huisman MC, Boellaard R, et al. 89Zr-immuno-PET for imaging of long circulating drugs and disease targets: why, how and when to be applied? Q J Nucl Med Mol Imaging 2015;59:18–38. [PubMed] [Google Scholar]
- 13.Hekman MCH, Rijpkema M, Aarntzen EH, et al. Positron emission tomography/computed tomography with 89Zr-girentuximab can aid in diagnostic dilemmas of clear cell renal cell carcinoma suspicion. Eur Urol 2018;74:257–60. 10.1016/j.eururo.2018.04.026 [DOI] [PubMed] [Google Scholar]
- 14.Gaykema SBM, Brouwers AH, Lub-de Hooge MN, et al. 89Zr-bevacizumab PET imaging in primary breast cancer. J Nucl Med 2013;54:1014–8. 10.2967/jnumed.112.117218 [DOI] [PubMed] [Google Scholar]
- 15.Evaluation of Safety, Biodistibution and Sensitivity/Specificity of PET/CT Imaging With 89Zr-TLX250 in Subjects With RCC - Full Text View - ClinicalTrials.gov.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.