Summary
A debate has emerged over the potential socio-ecological drivers of wildlife-origin zoonotic disease outbreaks and emerging infectious disease (EID) events. This Review explores the extent to which the incidence of wildlife-origin infectious disease outbreaks, which are likely to include devastating pandemics like HIV/AIDS and COVID-19, may be linked to excessive and increasing rates of tropical deforestation for agricultural food production and wild meat hunting and trade, which are further related to contemporary ecological crises such as global warming and mass species extinction. Here we explore a set of precautionary responses to wildlife-origin zoonosis threat, including: (a) limiting human encroachment into tropical wildlands by promoting a global transition to diets low in livestock source foods; (b) containing tropical wild meat hunting and trade by curbing urban wild meat demand, while securing access for indigenous people and local communities in remote subsistence areas; and (c) improving biosecurity and other strategies to break zoonosis transmission pathways at the wildlife-human interface and along animal source food supply chains.
Keywords: Emerging infectious disease, Zoonosis, Global food system, Livestock, Agriculture, Deforestation, Wild meat, One health
Introduction – trends and causes of wildlife-origin infectious diseases
Zoonoses are infectious diseases caused by pathogens (bacterial, viral or parasitic) transmitted from vertebrate animals (wild or domesticated) to humans, including those transmitted through an arthropod vector1 (Figure 1). Such diseases are classified as emerging infectious diseases (EIDs) when they have recently entered the human population for the first time, or when they have been present in humans historically but have recently increased in incidence or geographic range, or have re-emerged as a new variant2,3 (see Webappendix 1. Glossary of human infectious disease-related terms). Zoonotic disease outbreaks and EIDs can pose a significant threat to public health and global economies,4,5 and their frequency,2,3,6, 7, 8 capacity to geographically spread9 and economic impacts10,11 have been asserted to be on the rise.
Figure 1.
Zoonosis transmission pathways.
Throughout human history, major alterations in the human disease burden have largely resulted from anthropogenic (i.e. demographic and socio-economic) changes. The emergence of many of today's better known human contagious diseases was associated with the Neolithic revolution of 12,000 years ago, when small hunter-gatherer groups settled into agricultural villages to cultivate crops and raise domesticated animals, and later congregated in larger towns and cities.12,13 Several human-specific pathogens such as smallpox, measles, mumps and tuberculosis are believed to have originated from the domestication of mammalian and avian animals during this period.14, 15, 16 Although some other human-specific pathogens originated in wildlife much earlier, such as malaria's parasite (Plasmodium falciparum), which is likely to have its origins in western gorillas (Gorilla gorilla).17 Over time, the increase in incidence and spread of diseases have been facilitated by human and livestock population growth, urbanisation, travel, trade, wars, the colonization of territories and globalisation13,18, 19, 20 (see Webappendix 2. Human movement and infectious diseases). The Human Immunodeficiency Virus (HIV/AIDS) pandemic provides an illustration of some of these co-occurring processes (see Webappendix 3. The socio-economic origins of the HIV/AIDS pandemic).
Beginning in the second half of the 19th century, the infectious disease burden in human societies has been progressively reduced through public sanitation, improved nutrition, widespread childhood vaccination and the introduction of antimicrobials. Outbreak cases per capita (i.e. the total number of people infected as a proportion of a nation's population in an outbreak year) also appear to have been declining over time, thanks to global improvements in prevention, early detection, control and treatment.3 In the US, for example, in 1850 all 10 leading causes of death were infectious diseases (tuberculosis, cholera, malaria, etc.); by 2010, the 10 leading causes of death were non-communicable diseases (cancer, stroke, Alzheimer's disease, etc.), except for influenza/pneumonia.21 Non-communicable diseases such as diabetes and ischaemic heart disease are now the leading causes of premature mortality22 and disability23 in most regions of the globe.
Nevertheless, some analyses suggest that over the past half century or so the risk of zoonotic disease emergence (as measured by the number of first-occurrence zoonotic EID ‘events’2 and the frequency and richness of zoonotic disease outbreaks3) and subsequent epidemics and pandemics8,13 has been increasing. More specifically, Jones and colleagues2 observe an increasing incidence of zoonotic EID events between 1940 and 2004, with more than 70% of these events being caused by pathogens with a wildlife origin, such as Ebola virus, Nipah virus and SARS coronavirus (SARS-CoV). Smith and colleagues3 identify a rise in the number and richness of zoonotic disease outbreaks between 1980 and 2013 (12,012 in total, affecting every country in the world), including among the causes pathogens such as anthrax, chikungunya and tuberculosis. Although the veracity of such trends is still open to conjecture (see Webappendix 4. Limits of available zoonotic disease datasets and spatio-temporal trend analyses), numerous proponents have argued that zoonoses, and especially those from wildlife, may have been emerging and re-emerging at an unprecedented and increasing rate in recent decades.2,3,6, 7, 8,24 For example, of the seven coronaviruses known to infect humans, three (those causing Severe Acute Respiratory Syndrome (SARS), Middle East respiratory syndrome (MERS) and Coronavirus Disease-19 (COVID-19)) have emerged within the past 18 years25 (although it is difficult to assess trends from such small sample size). And of the 11 Ebola outbreaks that occurred in DRC since the 1970s, six took place in the past decade (some probably stemming from viral resurgence in survivors of previous outbreaks26 in a context of rising human population density and mobility27).
The ongoing occurrence and potentially increasing trend or increasing relative importance of wildlife-origin zoonotic outbreaks and EIDs are likely to represent a ‘hidden cost’ of socio-economic development and globalisation over the past century, being linked to faster human and livestock population growth, heightened urbanization and human crowding,13 accelerated encroachment into natural habitats and agricultural intensification,28 increased antibiotic use resulting in multi-drug resistance29 and the internationalisation of travel and trade.30 As an example of the latter, while in 2002–3 it took over two months for SARS-CoV (the coronavirus causing SARS) to move from China to Hong Kong and then to the rest of the world, in 2019-20 SARS-CoV-2 (the coronavirus causing COVID-19) spread out of China much more rapidly. This was due to SARS-CoV-2′s higher transmissibility31 and pre-symptomatic and asymptomatic infectiousness,32 but partly also to China's economic growth since 2003 and the related hastened expansion of domestic and international travel and trade.9
Some of the most important viral ‘reservoirs’ for infectious diseases of livestock and humans include bat, rodent and bird species that live in human-modified landscapes, and which are chronically or periodically infected with viruses that are either adapted to them, causing minimal or no disease, or that infect them asymptomatically, but which may be pathogenic for humans33, 34, 35 (Figure 1). Among these are hantaviruses, arenaviruses, avian arboviruses and coronaviruses. For example, current evidence indicates that fruit bats (Pteropus sp.) are a reservoir host for henipaviruses like Nipah and Hendra,36 that horseshoe bats (Rhinolophus sp.) are likely a natural host for betacoronaviruses like SARS-CoV and SARS-CoV-2,37, 38, 39 and that fruit bats may act as a natural host for filoviruses like Marburg40 and Ebola41,42 (although for some of these viruses the pathway of emergence and evolution from the ancestral strain to an epidemic human-to-human strain is not yet clear). Even though bats have been singled out as being particularly inclined to harbour zoonotic pathogens due to their flight-related immunological and ecological features,35,43 a recent study suggests that variation in the number of zoonoses among animal orders is simply a function of their species richness.44
Many viruses make use of an ‘intermediate’ vertebrate host through which they can evolve and transit from the reservoir species to humans45 (Figure 1). For instance, zoonotic spillover of Ebola has been associated by some analysts with the hunting, handling and consumption of meat from frugivorous animals (e.g. duiker antelopes, non-human primates) that may contract the virus by eating fruits partly consumed by infected bats.41,46 SARS-CoV is likely to have been transmitted from bats to wild animals such as the masked palm civet (Paguma larvata), and to have spilled from these into humans in live animal markets.37,47
However, direct wildlife—human zoonosis transmission is relatively rarely documented,34,48 in part because these types of spillover events are difficult to detect and therefore likely to be under-reported, and in part because humans are more regularly in contact with domestic and peri-domestic animals than with wild animals. Instead, intensive livestock production and transportation systems are known to play a major role in increasing the likelihood of domesticated animals acting as intermediate and amplifier hosts34,49,50 for wildlife-origin diseases such as anthrax, Q fever, campylobacteriosis and many others (Figure 1). In the Nipah epidemic that broke out in Malaysia in the late 1990s, for instance, the virus – which spilled over from bats to pigs through fruit and pigsties contaminated with bat saliva and urine – quickly spread among pigs, and from pigs to humans, facilitated by high pig population and farm densities.51
Other zoonotic diseases are transmitted from wild animals to humans through biting arthropod vectors such as mosquitoes, ticks and fleas (Figure 1). For example, evidence suggests that the four known Dengue virus serotypes were originally transmitted from monkeys to humans through Aedes mosquitoes in tropical Africa and Asia, and that there is potential for the re-emergence of sylvatic dengue in the human transmission cycle as a result of deforestation, climate change and geographic expansion of its mosquito vector.52 In the Western Ghats region of southern India, the tick-borne viral Kyasanur Forest Disease, which is believed to have monkey species as amplifying hosts and cattle as nutrition hosts, has been expanding rapidly beyond its endemic range over the past two decades, affecting an increasing number of low-income forest communities.53
Some EIDs believed to have their origin in wildlife have resulted in regional outbreaks or global pandemics that have caused extensive mortality, exceptional economic losses and widening social inequalities. Since its first detection in 1981, HIV-1/M has caused more than 32 million deaths,54 and to date the prospects of curative treatment or an effective vaccine remain uncertain.55 In 2020, the COVID-19 pandemic caused more than 1·8 million deaths in a year56 and resulted in the steepest global economic slowdown since the Great Depression of the 1930s,57 with developing countries the least capable of withstanding the economic shockwave.58
In this Review, we explore the emerging debate over some of the key potential socio-ecological drivers of wildlife-origin zoonotic disease outbreaks and EID events. Although this scholarship is still in its infancy, being mostly model-based and lacking sufficient empirical corroboration, it is nonetheless worthwhile to investigate the extent to which the incidence of wildlife-borne zoonosis outbreaks, which are likely to include devastating pandemics like HIV/AIDS59, 60, 61 and COVID-19,62, 63, 64 may be linked to forms of ecosystem degradation such as tropical deforestation for agricultural food production and over-harvesting of wild animals for food consumption, and as such may be related to contemporary ecological crises such as global warming and mass species extinction (Section 3). A second objective of the Review is to explore policy recommendations and social actions – such as a global transition to more plant-based diets and the curbing of tropical urban wild meat demand – which are increasingly deemed necessary to tackle climate change and biodiversity loss, and which, pending further research, may also prove beneficial for containing the risk of wildlife-borne infectious disease outbreaks and pandemics (sections 4–7, sections 4–7, sections 4–7, sections 4–7). Biosecurity and other strategies to break zoonosis transmission pathways at the wildlife—human interface and along animal source food supply chains are also discussed (Section 8).
Search strategy and selection criteria
In this Review we do not aim at providing a systematic literature review with quantitative synthesis of results, but rather a critical synthesis and detailed expert viewpoint of the issues related to wildlife-borne zoonotic disease risk and the global food system.
Information on zoonotic epidemic and pandemic risk, global livestock production systems and tropical wild meat hunting and trade was sourced by searches of Google Scholar and the reference lists of relevant peer-reviewed articles and books. Different combinations of multiple search terms relevant to the analysis were used, such as “emerging infectious disease”, “zoonosis”, “epidemic”, "pandemic", “cross-species transmissions”, “spillover”, “reservoir population”, “intermediate host”, “amplifier host”, “livestock”, “intensive farming”, “deforestation”, “forest encroachment”, “habitat fragmentation”, “forest edge”, “biodiversity dilution effect”, “wildlife”, “wild meat hunting”, “wild meat trade”, “global food system”, “plant-based diet”, “flexitarian diet”, “sustainable agriculture”, “sustainable intensification”, “organic farming”, “agroecology”, “livestock product taxation”, “wild meat ban”, “consumer campaign”, “community-based wildlife management”, “sustainable hunting”, “biosecurity” and “One Health”. Only articles and books published in English were included.
Socio-ecological drivers of wildlife-origin infectious diseases – tropical deforestation for livestock production and wild meat hunting and trade
Following the emergence of SARS-CoV-2 in China and its global spread resulting in the COVID-19 pandemic, a number of infectious disease and wildlife conservation scientists,11,65,66 as well as high-profile international bodies such as the World Health Organization (WHO), the World Organisation for Animal Health (OIE) and the United Nations Environment Programme (UNEP),67 pointed to tropical wild meat hunting and trade as a key driver of this and other disease outbreaks.
While game hunting for by-products and sport is practiced all over the world, in the tropical and subtropical forest regions of Africa, Asia and South America, over the past 30 years hunting pressure to supply the growing wild meat demand of rapidly expanding rural and urban populations has radically increased.68, 69, 70, 71 The progressive penetration of hunters into remote pristine forests, and their gradual depletion of local populations of game species, has caused significant wildlife population declines69,72 and sometimes led to a state of defaunation in which the trees are preserved but larger-bodied mammals are absent.70,71,73 A decline in large-bodied frugivores is also predicted to undermine seedling recruitment for bigger-seeded, heavy-wooded, long-lived tree species, which could reduce the carbon storage capacity of tropical forests.74 In addition to these ecological threats, it is argued, the hunting, transport, marketing, butchering and consumption of wild meat can also facilitate the emergence of zoonoses and so involve serious public health hazards.11,65,66,75
Other analysts have rebutted that the wild meat infection risk remains poorly understood, requiring cautious judgement about its role in zoonosis emergence,50,76,77 and that on a global scale such risk is likely to be significantly lower than that posed by intensive livestock production systems where high population density, low genetic diversity and immunosuppression enable rapid pathogen spread and mutation.28,50,78 For example, the 2009 swine influenza pandemic, which was caused by an A/H1N1 reassortant virus with gene segments from birds, North American pigs, Eurasian pigs and humans, is most likely to have originated in industrial pig farms in Mexico.79
However, although the infection risk from wild meat hunting and handling along the supply chain is unquantified and may be small, human diseases have nonetheless emerged and re-emerged from these practices, sometimes causing immense health and socio-economic damage. Such spillover events are likely to include some of the deadliest epidemic viral diseases, such as HIV60,80 and Ebola virus in Central and West Africa,41,42,46 Hepatitis E virus in Japan81 and SARS coronavirus in China.37,47 This, it is argued, justifies subscribing to the precautionary principle in developing wild meat policies aimed at containing the risk of infection. Whether a wild meat-related zoonotic pathogen is entirely transmitted to humans by spillover (e.g. rabies,82 toxoplasmosis83) or whether it may be transmitted by a combination of spillover and human-to-human epidemic dynamics (e.g. Lassa virus,84 Ebola virus,26 SARS-CoV-285), prevention of one rare spillover event could avert tens, hundreds or thousands of downstream human cases.11,86
It has been partly on the basis of such precautionary principle that blanket bans on wild meat have been invoked by some animal welfare and wildlife conservation groups87, 88, 89, 90 and implemented by some governments.91,92 Yet, policy interventions must be critically assessed in the broader context of related pros and cons to avoid counterproductive outcomes. Blanket bans tend to be ineffective in reducing wild meat hunting and trade in tropical regions,93, 94, 95, 96, 97, 98 and could have detrimental impacts on both Indigenous Peoples and Local Communities (IPLCs)99, 100, 101, 102 and wildlife conservation.103,104 Importantly, these bans also risk being counterproductive in reducing the threat of emergence and spread of wildlife-borne diseases, for two reasons: first, they may force trade underground where it is harder to enforce biosecurity measures, and second, they may detract attention from other critical intervention points such as the causes of human-to-human contagion.91,105
Other scientists have focused on broader ecosystemic dynamics, identifying natural habitat destruction as a potential key driver of wildlife-origin infectious disease outbreaks. Human-mediated changes to natural ecosystems can increase the risk of zoonotic spillover by altering reservoir host density, distribution and susceptibility; pathogen prevalence, survival and dissemination; and reservoir host—human contact rates.106 For example, tropical deforestation has been observed to disrupt local environmental conditions in ways that favour larval development and increase adult survivorship of mosquito vectors, and to cause a reduction in the abundance of animal species that prey on larvae and adults, so leading to an increased incidence of human malaria.107
Given the ubiquity of viral biodiversity, the geographic pervasiveness of intensive farming and the globalisation of travel and trade, a pandemic can conceivably originate on any part of the planet inhabited by people.76 Yet, it has been argued on the basis of some spatial models that the risk of zoonotic disease emergence (and, by extension, zoonotic-origin pandemics) may be more elevated in tropical areas undergoing high rates of deforestation.78,108, 109, 110, 111, 112 While there is need for cautious interpretation of results from these models (see Webappendix 4. Limits of available zoonotic disease datasets and spatio-temporal trend analyses), the potential implications of a correlation between an increase in wildlife-origin disease outbreaks and accelerated tropical deforestation are worth exploring.
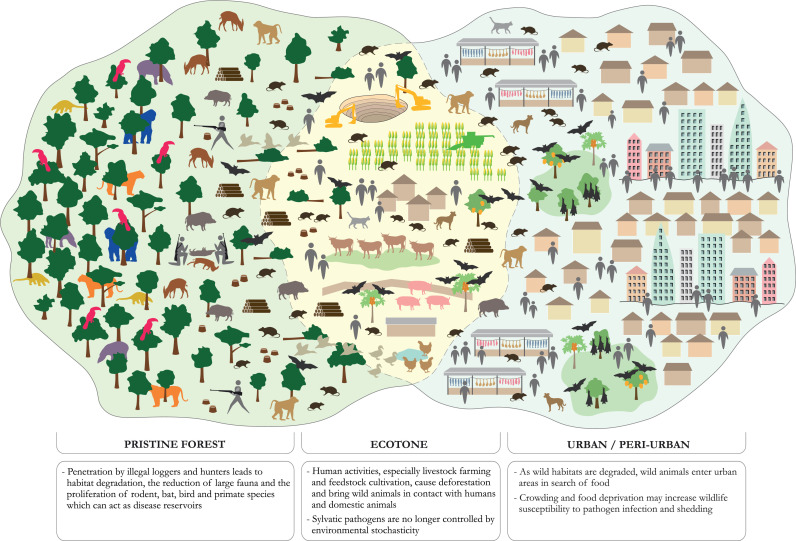
The line of reasoning behind such a correlation is that higher wild mammal species diversity in tropical forests may increase the ‘depth’ of the pathogen pool from which novel infectious diseases emerge. Deforestation then creates transition areas (or ‘ecotones’) where the remnants of this biodiversity come into closer proximity with humans and livestock, generating new opportunities for inter-species pathogen transmission,28,113, 114, 115 and where the sylvatic pathogens they harbour are no longer controlled by environmental stochasticity.111 The risk of zoonotic virus transmission has in fact been observed to be highest from domesticated mammals and wild rodent, bat, bird and primate species that have globally increased in abundance and/or expanded in range by adapting to human-dominated landscapes33,34 (Figure 2). Habitat fragmentation also increases the length of forest edges, which may increase the contact rate of humans and livestock with wildlife116,117 (although this is yet to be empirically validated).
Figure 2.
Tropical deforestation and overhunting as drivers of wildlife-origin zoonotic disease transmission.
For example, in Argentina, the emergence of Junin arenavirus (the causative agent of Argentine haemorrhagic fever) in the ‘humid pampa’ region has been linked to agricultural conversion and fragmentation of the prairie, which, by reducing the number of medium-size predators, may have enabled an upsurge in the virus’ reservoir host, the drylands vesper mouse (Calomys musculinus).118 On the east coast of Australia, fruit bats’ forest habitat has been degraded on a large scale, increasing their dependency on flowering and fruiting trees planted in suburban or urban gardens, and so raising opportunities for Hendra virus spillover in domestic horses and humans.36,119
There are also indications that habitat destruction and fragmentation and over-harvesting of wild animals worsen the incidence of certain wildlife-borne human diseases by reducing vertebrate biodiversity in ways that increase the prevalence and abundance of animal species known to host zoonotic and vector-borne viruses.120,121 For instance, in parts of Africa, a significant decrease in the number of predators and large herbivore competitors is predicted to result in an increase in the number of rodent reservoir species.122 In other words, habitat destruction and overhunting undermine biodiversity's capacity to ‘dilute’ pathogen spread.123 Moreover, habitat degradation induces environmental stressors, such as crowding and food deprivation, which may increase wildlife susceptibility to pathogen infection and shedding.121,124
Livestock production is the largest driver of natural habitat loss. About 40% of ice-free terrestrial surface is used for agricultural food production.125,126 Of this, around 75–80% is used as grazing land and cropland dedicated to cereal and soy feedstock production,125,127 and land dedicated to the cultivation of feedstock constitutes more than one third of total cropland,125,128 with about one third of global cereal production being fed to animals.129 Although since the 1950s overall rates of natural habitat conversion to agriculture have slowed (the net area used for food production remaining constant since then),130,131 agricultural frontiers have gradually shifted from the temperate regions of Europe, North America and Russia to the tropical regions of Latin America, Southeast Asia and Africa, where rates of conversion of tropical forests and savannahs to agriculture are high,132,133 and where agricultural use of the land is less efficient.134 In South America, for example, between 1990 and 2005 about 71% of rainforest conversion has been for cattle grazing and around 14% for commercial cropping including soya for animal feed.135
Grazing land and feedstock cultivation are therefore expanding into landscapes that host a significant share of the planet's remaining natural habitats and are particularly rich in biodiversity136 and carbon stocks.137 As such, there are concerns that in addition to being responsible for the majority of agricultural food-related greenhouse gas (GHG) emissions137, 138, 139, 140 and a prime driver of the current human-caused sixth mass extinction of species,141, 142, 143 livestock production may also increment the risk of wildlife-origin disease outbreaks by bringing humans and domestic animals into increasing contact with wild animal populations28,78,108,109,113,144 (Figure 2).
Although the pace of global population growth is slowing, the world population is projected to grow to around 9.7 billion people in 2050, and the most likely scenario is that it will continue growing throughout the present century.145 Despite large and growing inequalities (over 820 million people face chronic food deprivation and more than 2 billion people are micronutrientdeficient while 2.1 billion adults are overweight or obese),146 rapid economic growth in the world's most populous developing countries means that, on average, the global population is predicted to become more affluent.147 In advanced economies, per capita meat and dairy consumption has reached a plateau at environmentally unsustainable levels148,149 that often exceed health-based recommendations150 (see Webappendix 5. The human health, agronomic and food security benefits of predominantly plant-based (flexitarian) diets). For example, the total area of agricultural land used within the UK and abroad to rear lamb, beef and dairy cattle for consumption within the UK has been calculated to be larger than the entire landmass of Great Britain.151 A substantial increase in per capita meat and dairy consumption is also predicted in middle- and low-income countries152,153 (see Webappendix 6. Animal source food consumption levels across different socio-economic and ecological contexts). Furthermore, production of the amount of livestock meat that would be needed to replace urban wild meat consumption in the tropics would result in new large-scale land conversion.97,154 In the Congo Basin, for instance, replacement of an estimated wild meat yearly extraction of 4·5 million tonnes with local beef production would require the conversion of 25 million hectares of forest to pastures.155
These trends imply that, in the absence of targeted mitigation measures, there is a strong risk that increasing global livestock production will greatly destabilise the planetary ecosystem processes on which the web of life and human wellbeing depend.156,157 Climate change is already causing global crop production losses158 and affecting food security in many regions159 through increasing temperatures, changing precipitation patterns and greater frequency of some extreme events.140 Climate change phenomena have also been implicated in the expansion of disease vectors’ geographic ranges (e.g. summer temperature anomalies associated with outbreaks of West Nile Fever in Southeast Europe)160 and in the higher incidence of water-borne infectious diseases (e.g. rising sea surface temperature correlated with enhanced worldwide prevalence of Vibrio bacteria such as V. cholera).161 If current population growth and dietary trends continue, GHG emissions from agricultural food production are likely to increase by about 30–40% by 2050,140 and the target of keeping global warming well below 2 °C is unlikely to be met.140,157,162,163 Meanwhile, the rate of species extinction is at present 100–1000 times the historical average,164 with average species abundance likely to have fallen below the proposed 90% precautionary threshold.165,166 The negative impact of biodiversity loss on agricultural productivity alone has been estimated to warrant a moratorium on further land conversion.167 There are concerns from some quarters of the scientific community that zoonotic diseases of wildlife origin may soon join global warming and wild species extinction to constitute a third planetary ecological crisis.8,10,13
There is, as we have repeatedly emphasised, an urgent need for empirical research to clarify the trends in the risk of wildlife-origin zoonotic disease outbreaks and EID events, and the potential geographical link of such risk with contemporary high rates of deforestation and wild meat hunting and trade in the tropics77,168 (see Webappendix 4. Limits of available zoonotic disease datasets and spatio-temporal trend analyses). Nonetheless, based on the above considerations, a set of precautionary responses to the wildlife-origin zoonotic disease threat can be delineated. Such responses include: (a) limiting human encroachment into remaining tropical wildlands by promoting a global transition to diets low in livestock source foods; (b) containing tropical wild meat hunting and trade by curbing tropical urban wild meat demand while securing IPLC's access through community-based wild game management; and (c) improving biosecurity measures along animal source food supply chains, from hunting grounds and livestock and wildlife farms to rural and urban abattoirs and markets, and exploring other measures to break zoonosis transmission pathways at the wildlife—human interface. The remainder of this Review discusses each of this set of strategies.
Preventing natural habitat encroachment by combining sustainable agriculture and a global reduction of livestock source food production
Agricultural food production is one of the greatest sources of environmental degradation,133,169 and the global food system is responsible for up to one-third of all GHG emissions.170 Livestock production in particular has the highest environmental footprint (impact per unit product) of all foods in terms of land and water use, GHG emissions and eutrophication and acidification of aquatic and terrestrial systems.125,138,162,171, 172, 173 This is especially true for GHG emissions137,157 and pollutants that result in the formation of fine particulate matter (PM2.5).174 Due to livestock's low ‘feed to meat’ conversion ratios, manure-related emissions and ruminants’ enteric fermentation,128,138 livestock source foods are responsible for about 75% of total agricultural food-related methane and nitrous oxide emissions,157 and for about 15% of all anthropogenic GHG emissions, including carbon dioxide.137 PM2.5 poses a severe threat to human health,174 and is potentially also associated with COVID-19 morbidity and mortality rates.266
Crucially, expansion of grazing land and feedstock cultivation in tropical forests, grasslands and wetlands rich in biodiversity and carbon stocks is responsible for major biodiversity losses143,175 and GHG release into the atmosphere,137,140 as well as for the creation of ecotones that have been hypothesised to increase the risk of wildlife-borne infectious disease outbreaks.28,108,109,113,144 As seen in the previous section, not only does natural habitat clearing for livestock agriculture precipitate closer proximity of wildlife with humans and domestic animals.28,78,113,115 It also effects non-random patterns of defaunation, causing the loss of large- and medium-bodied herbivores and predators while favouring populations of rodent, bat, bird and primate species that are common zoonotic pathogen reservoirs33,34,121,176 (Figure 2).
This section therefore discusses the necessity of a global shift to predominantly plant-based diets in order to reduce the consumption and production of livestock source foods for environmental and public health purposes. It considers this strategy in relation to some of the main current approaches to sustainable agricultural production, namely sustainable intensification, organic farming and industrial-engineering initiatives such as soil-less vertical farming. The following section then explores the range of measures currently available to governments, civil society and the private sector to promote a reduction in the global consumption of livestock source foods.
In the past two decades, the global capacity to continue increasing the food supply appears to have been declining,177 as highlighted by slowing rates of yield increase for the world's major staple grains (maize, wheat, rice, soy), the increasing role of cropland expansion in their production growth, and recent episodes of abrupt spikes in their prices.178 These alarm bells urged a call for a sustainable intensification of global agriculture capable of increasing yields at the aggregate level (and so prevent further agricultural ‘extensification’ onto natural habitats) while also curbing environmental impacts per unit product. The use of the term ‘sustainable intensification’ has generated some controversy,179 but its core intention is to highlight that satisfactory agricultural yields are possible without detrimental environmental impacts, if the current agricultural system responsible for wasteful resource use and chemical pollution is improved through better application of management practices and technologies.180
However, although management practices and technologies exist that would enable improvements in agricultural yields with less environmental impact (e.g. drought- and pest-resistant cultivars, diversified cropping, minimum or zero tillage, improved slurry application, precision farming, enhanced livestock breeding, methane-reducing cattle feed additives, agroforestry, silvopasture, better water storage),181, 182, 183 their cost-effectiveness and level of adoption are uncertain and highly reliant on investment in public infrastructure, farmer incentive schemes and regulation.133,157,184 For instance, for what concerns precision farming innovations for the application of nutrients at the best rate, time and place, the high cost of technologies (e.g. detailed soil, GPS and drone mapping) and of obtaining information (e.g. weather and satellite data) has slowed adoption,133 and therefore limited private sector companies in their capacity to scale technologies up for affordability.162 Crucially, there is an intrinsic limit to the capacity of management and technology options to mitigate agricultural nitrogen run-off and especially livestock GHG emissions.140,153,157 At a global level, the mitigation benefits of improved grazing management (i.e. avoiding over-grazing and soil erosion) are greatly outweighed by the animals’ direct GHG emissions,185,186 and there are physiological and agronomic limits to the extent to which GHG emissions from ruminants’ enteric fermentation can be reduced through feed additives and manure management or herd management and breeding.138,140,182
Even assuming that at the global, aggregate level the adoption of improved management practices and technologies is sufficient to raise total yields and avoid a net increase in farmland, the latter may continue to contract in temperate regions and expand in tropical regions.133,135 This would limit the potential for sustainable intensification to mitigate climate change, biodiversity loss and zoonotic disease risk. More generally, there is growing consensus that even in the most optimistic scenario of widespread uptake of the best available management and technology options, the huge challenge of feeding an increasing human population without compromising the Earth's ecological stability will not be overcome without a radical shift in human diets away from their current heavy reliance on livestock source foods.143,151,157,162,169,183,184,187,188
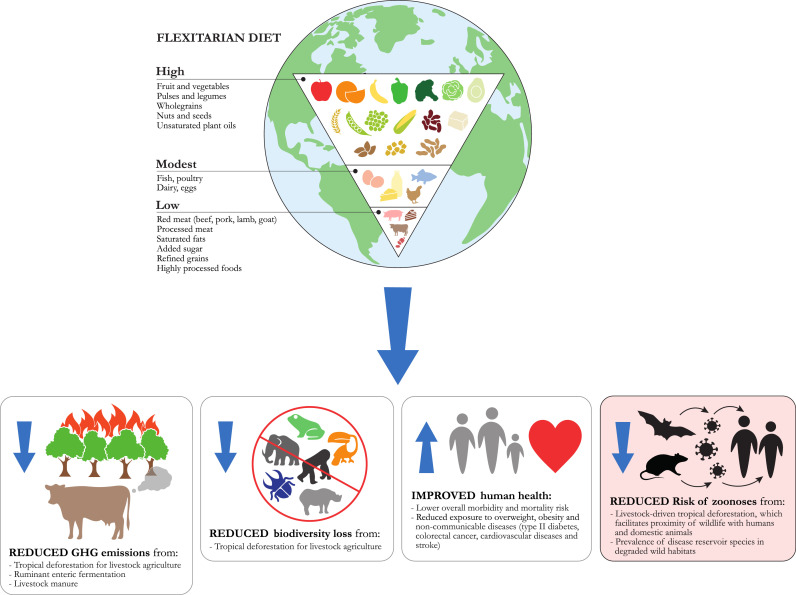
A global shift to predominantly plant-based diets has therefore been identified as crucial to reduce GHG emissions140,151,157 and biodiversity loss.141,143 These diets, sometimes referred to as ‘flexitarian diets’, are informed by current evidence on healthy eating and characterised by large amounts of plant foods (fruit, vegetables, plant source proteins like pulses, nuts and seeds, wholegrains and unsaturated plant oils), modest amounts of animal source proteins (fish, poultry, eggs, dairy), low amounts of red meat (beef, lamb, goat, pork) and processed meat, limited amounts of saturated fats, added sugar, refined grains and highly processed foods, and a balanced energy intake.162 As discussed in the previous section, such a dietary shift may contribute to also reducing the risk of wildlife-origin zoonotic disease outbreaks and EID events.78,108 In addition, a transition to more plant-based diets characterised by low shares of livestock source products would generate other important human health,22,162,172,189, 190, 191, 192, 193 agronomic and food security194, 195, 196 benefits (Figure 3) (see Webappendix 5. The human health, agronomic and food security benefits of predominantly plant-based (flexitarian) diets).
Figure 3.
Benefits of a global shift to plant-based (flexitarian) diets.
Predominantly plant-based, flexitarian diets have therefore been suggested as global reference diets for adults.193 Such diets allow for adaptation to personal dietary needs, preferences and cultural traditions, and include diverse dietary patterns such as omnivore, pescatarian, vegetarian and vegan. The features of a flexitarian diet are well established in the traditional diets of several regions (e.g. the Greek Mediterranean diet of mid-20th century and the traditional diets of India, China, Mexico and West African countries), which are largely plant-based, high in vegetable sources of protein like soy and nuts and low in red meat and dairy foods.162
Organic farming is one of the few legally regulated and internationally recognized approaches to sustainable agriculture that is known to deliver important local environmental benefits197, 198, 199, 200 (see Webappendix 7. Organic farming principles). It is also a rapidly growing food sector – the fastest in European and North American countries.201 As such, it is worth exploring whether there can be complementarities and synergies between policies promoting large scale conversion to organic farming and those pursuing a global shift to predominantly plant-based diets.
Organic farming has been widely reported to perform better than conventional farming against a range of environmental indicators, such as improved soil structure, biota activity and water holding and carbon sequestration capacities,202,203 reduced per unit area aquatic and terrestrial pollution from herbicide and pesticide leaching and nutrient run-off, and lower per unit area GHG emissions thanks to decreased production and use of synthetic fertilisers.199,204, 205, 206 Its more heterogeneous landscape features, together with decreased pressure from herbicides and insecticides and more diversified cropping practices, support a higher plant and animal biodiversity.207,208
However, organic crop systems have an average 8–20% lower yield productivity than conventional systems,209 and therefore tend to require larger stretches of land to produce the same quantity of product. Although at present only 1.4% of total global agricultural land is managed organically,201 if large scale conversion to organic farming were to occur, this could be at the expense of ‘extensification’ onto new land.184,210 If farmland expansion were to occur onto natural habitats (rather than degraded and secondary habitats), it could greatly increase biodiversity loss,175 GHG emission,211 eutrophication of natural ecosystems195 and potentially zoonosis transmission rates.78,108,109 In fact, biodiversity loss and GHG emissions are significantly higher when the same increase in food production is pursued through cropland extensification onto natural habitat than through intensified application of synthetic fertilisers on existing agricultural land.175,212 In other words, although organic production systems generate comparative local environmental benefits, widespread adoption without changes in total demand can involve trade-offs due to new conversion of natural ecosystems to meet overall food demand,184,210 with such conversion potentially occurring abroad (the so-called ‘telecoupling’ effect).134,213
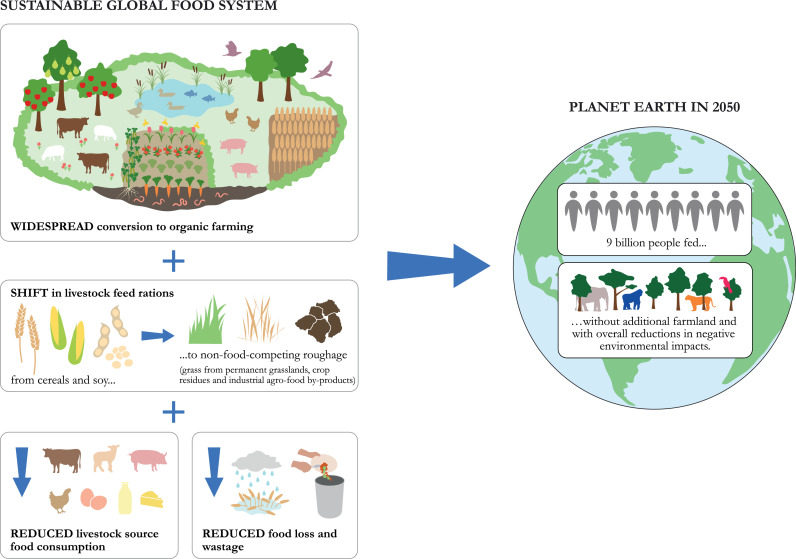
Yet, the pursuit of sustainability necessarily relies upon tackling both the production and consumption sides of food systems.157,214,215 Food system models187,216 suggest that complementing conversion to organic farming with a shift in livestock feed rations from cereals and soy to non-food-competing roughage (i.e. grass from permanent grasslands and by-products from food processing),217 a reduction in livestock source food consumption216 and cutbacks in food loss and wastage218,219 could feed more than 9 billion people in 2050 without additional farmland and with overall reductions in negative environmental impacts187 (Figure 4). In fact, considering that land used for plant source food production generates many more calories per hectare than land used for livestock source food production, reducing consumption of the latter is the single most effective strategy to improve the productivity of the land.151
Figure 4.
Global food system model complementing conversion to organic farming with a reduction in livestock source food consumption and cutbacks in food loss and wastage.
Moreover, greater investment in the development of crop varieties and animal breeds better-suited to low-input organic systems,220,221 and in management practices that improve soil health (e.g. diversified and cover cropping, conservation tillage),183,222,223 address yield-limiting factors (e.g. improved organic pest control, the use of bioeffectors such as mycorrhiza or rhizobia),206 and optimize nitrogen use efficiency and recycling (e.g. organic waste reusing)181,206 has the potential to significantly increase organic farming productivity. Recoupling cropping and livestock and allowing farm animals to graze on fallow land, as done in many smallholder224 and organic204,208 mixed farm systems, also offers a path to closing nutrient cycles, improving soil quality, raising land productivity and decreasing pollution from manure waste management.133,151,224 These yield-increasing measures would bring the further benefit of lowering the price of food produced through organic and other sustainable farming methods to levels that are closer to those of conventional farming and affordable also by less affluent segments of the population.151
Industrial-engineering crop farming approaches, such as soil-less vertical farming and crop-aquaculture, offer another mechanism to reduce agricultural extensification onto wildlands, by delinking food production from soil and natural environments while retaining its link to natural processes. These high-tech, capital-intensive options can be designed to maximise certain environmental sustainability targets such as closed nutrient cycling and recycling,225,226 and consumer acceptability may be less of an issue than generally assumed.227 However, their potential in terms of sustainable energy use and price competitiveness with conventional and organic farming is poorly known. Moreover, they are of limited relevance to poorer regions of the globe characterised by scarcity of capital and abundance of land.227
In developing countries that are rich in wildlands it is instead crucial that agricultural and land use policies continue to actively discourage livestock grazing and feed crop expansion from occurring onto natural and secondary habitats,165,216 directing such land uses towards lands that are already environmentally degraded such as abandoned agricultural areas and tree plantations. For example, in the Brazilian Amazon, between 2005 and 2012, reduced deforestation was successfully pursued through rural credit restrictions for counties with high deforestation rates, expansion of state protected areas and indigenous reserves in active agricultural frontiers, and Greenpeace-led embargos that excluded soy and beef grown on illegally cleared land from supply chains.228
Finally, in order to contain farmland expansion, major reductions in food losses and wastage across the food supply chain are also crucial; considering that at present more than a third of all food produced is either lost before it reaches the market or wasted by retailers, households and the hospitality industry.218,219 In rural areas of low- and middle-income countries, food loss at the initial production stages could be tackled through investment in improved harvest scheduling and techniques, post-harvest storage facilities, processing technologies (e.g. drying, packaging) and transport.218 Improvements are also necessary by the feedstock industry in their utilisation of crop residues, agro-industrial by-products and food waste.217 In urban areas, food waste by the public could be addressed through shelf-life prolonging packaging, clearer best-before labels, awareness campaigns promoting purchase planning and storage practices, and waste recycling back into production. Food manufacturers, retailers and services could also reduce portions162 (Figure 4).
Strategies for promoting a global transition to more plant-based diets
Promoting a global shift towards healthier and more environmentally sustainable plant-based diets is an enormous challenge as consumer preferences for animal source foods are rooted in biological and socio-cultural factors.229 Meat consumption is part of the human evolutionary heritage,230 and today, for a large share of the global population, the price of meat relative to income is lower than ever in history.153 Moreover, surveys in the US and Australia suggest that people who consume relatively higher quantities of meat and other livestock source foods tend to be generally less sensitive to ethical concerns in their food choices.231
Nonetheless, consumption habits and norms can and do change. This is indicated, for example, by dietary transitions from beef to pork and chicken in the US, Europe and other regions – which are partly a consequence of changes in their relative price,232 and the growing numbers of organic,201 restricted omnivore, vegetarian and vegan consumers233,234 in high-income countries – which are partly associated with rising concerns for environmental sustainability and farmed animal welfare.235,236 These trends can be further encouraged through coordinated initiatives by government, civil society and the private sector.153 Governments that dodge interventions to discourage unsustainable levels of livestock source food consumption for fear of public backlash may be overestimating such risk, as suggested by public expectation of government leadership in tackling this complex problem.151,237
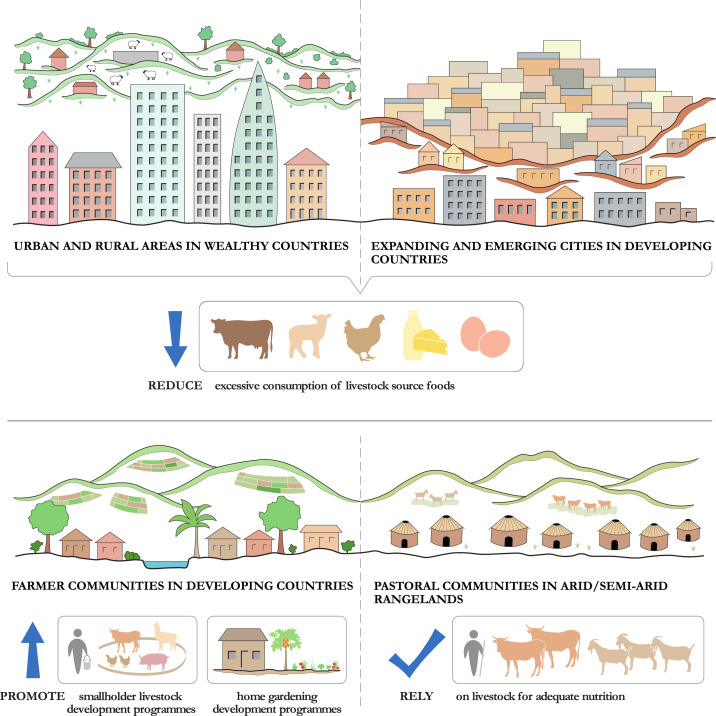
Obviously, current levels of consumption of livestock source foods and the degree of reliance of human communities on animal source proteins differ across countries and geographical regions. Efforts to reduce livestock production should prioritise excessive consumption levels in wealthier countries148,162 and in the expanding metropoles of developing countries.150,238 On the contrary, communities inhabiting harsh climates and marginal lands that are inhospitable to crop cultivation - such as hunter communities in tropical rainforests,102, 241 Inuit communities in Arctic regions230, 239 and pastoralist communities in arid and semi-arid rangelands240 - will necessarily continue to rely conspicuously on animal source foods for nutrition (Figure 5) (see Webappandix 6. Animal source food consumption levels across different socio-economic and ecological contexts).
Figure 5.
Approaches to sustainable livestock source food consumption in different geographical regions.
In regions characterised by high levels of consumption of livestock source foods, the available evidence indicates that providing consumers with information about the health and environmental impacts of food without additional contextual changes and regulatory or fiscal measures has limited effectiveness.242,243 In order to initiate a dietary transition away from heavy reliance on livestock source foods, the best strategy may therefore be to couple ‘soft’ informational and contextual interventions to raise consumers’ awareness and ‘nudge’ them towards dietary changes, and ‘harder’ fiscal measures towards which public resistance may progressively wane as people become increasingly aware of the risks of inaction and familiar with alternative dietary patterns153,242,244 (Figure 6). In fact, even when awareness and education are unlikely to produce major or sustained change in consumer demand for livestock source foods, they may still be an important precursor to harder regulatory measures and necessary to catalyse public support for policy change.152,245
Figure 6.
Strategies to promote a dietary transition away from heavy reliance on livestock source foods.
An important first step in this direction would be to better align national dietary guidelines, including those offered by paediatricians to families, with current evidence on healthy and environmentally sustainable diets.193 Targeting the young through education may be particularly important, since food tastes and eating habits tend to develop during the first years of life and to be persistent.162 To be enduring, education on healthy diets from sustainable food systems need to be integrated into maternal and childcare programmes, and into compulsory school curricula and textbooks.151,162 Training and clinical practice of physicians and paediatricians also need to include nutrition and ecosystem stability as determinants of human health162,244 (Figure 6). In Japan and South Korea, for example, diets remain higher in fish and plants and lower in meat and sugary drinks than in most developed countries, thanks to policies such as compulsory school lunches, government-funded lessons on traditional food preparation and strong campaigns promoting traditional cuisines.246
Tested informational and contextual interventions include front-of-pack eco-labels with simplified information on environmental impacts, which have been observed to encourage some consumers to shift towards more sustainable food choices.247,248 Campaigns such as the Meatless Monday and Veganuary have also had some success; through a ‘foot-in-the-door’ psychological technique, these initiatives enable consumers to commit to a small dietary adjustment with the possibility of later developing a desire for greater change, and also encourage businesses to launch new plant-based products and menu options244 (Figure 6).
Other interventions rely on unconscious behavioural responses to contextual cues. For instance, increasing the proportion of vegetarian meals offered at cafeterias,249 and programming restaurant menus and online meal registrations250 by offering vegetarian dishes as a default option, can increase the number of people choosing them.
The retail and hospitality sectors could be enticed to use their potent customer-choice influencing skills and enormous purchasing power to stir consumers and producers towards more environmentally sustainable and healthy foods. Such enticement could come from a statutory duty to publish an annual report open to public scrutiny on total sales of fruit, vegetables, plant source protein and livestock source foods together with their method of production accreditation (e.g. organically grown, pasture-fed, sustainably fished).151 Food procurement policies could also promote a higher percentage of plant-based, sustainably sourced meals at workplaces, schools, hospitals, barracks and prisons – when food is produced in bulk quantities, small changes (e.g. including minced mushrooms in beef burgers) can have large environmental and health impacts151,162,244 (Figure 6).
Moving on to fiscal policies, proposals have recently been made to tax livestock source foods not only to mitigate their environmental impacts, but also to account for the financial burden of meat-related chronic diseases borne by public health systems244,245 (see Webappendix 5. The human health, agronomic and food security benefits of predominantly plant-based (flexitarian) diets) and for the risk of zoonosis outbreaks involved in their production.251 In reality, efforts to fiscally regulate agricultural production to mitigate environmental impacts such as climate change have so far been limited in comparison to other sectors such as transport, energy, steel and cement,244 for several reasons. First, compared to other polluting industries, in agriculture the fonts of GHG emissions (e.g. crop residue decay, ruminants’ enteric digestion) are diffuse and highly variable and therefore difficult and costly to monitor.252 Second, agricultural GHG emissions associated with biological processes that are intrinsic to crop and livestock production (e.g. methane from flooded rice paddies and ruminants’ digestion) cannot be easily addressed by producers through technological and management mitigation options in response to taxation.138,163,182 Third, taxing food production for GHG emissions may penalise domestic products versus imported ones,244 unless arduous multilateral agreements for the regulation of meat and dairy supply are established.253
Therefore, although taxation works best if targeted to the intended externality at source, taxing consumption rather than production of livestock source foods is considered a more practical strategy (Figure 6), and some modelling studies have been carried out.237 One such study indicates that levying GHG taxes on the consumption of livestock source foods worldwide could reduce food-related emissions by about 9%. A portion of tax revenue would be available to compensate poorer consumers in low-income countries, who spend a larger proportion of their income on food, for potential budget losses. In this study, the mitigation potential of consumption-side GHG taxation measures compares favourably to that of supply-side measures like improved feed additives and manure management.254
The potential effectiveness of internalising external environmental and public health costs through consumption taxes warrants detailed assessment in specific jurisdictional contexts.255 A key factor is the price elasticity of taxed products, i.e. how strongly the quantities produced or demanded react to increases in price. For example, health-related taxes targeted at sugar-sweetened beverages255,256 and energy-dense257 and high-saturated fat258 foods have been observed to encourage food companies to reformulate and consumers to reduce consumption of these products, including a limited decrease in the consumption of certain beef and dairy products.259 In some countries, these taxes are estimated to have resulted in reduced cases of obesity and decayed and missing teeth.151,256 Yet, the environmental and health outcomes of such taxes critically depend on the cross-price elasticity of demand of other environmentally impactful and/or unhealthy products, and can be partly offset by consumers shifting their shopping to non-taxing jurisdictions.255,258
It is also important to avoid the potential unintended consequence that low-income households may respond to a rise in price of environmentally impactful and/or unhealthy foods by cutting back further on healthy foods such as fruit and vegetables. State taxation revenue could therefore be used to subsidize fruit and vegetable consumption,254 or invested in programmes for improving the nutrition of disadvantaged segments of the population.237 Examples of the latter include school free meals and holiday food clubs, vouchers for fresh fruit and vegetables for pregnant women and pre-school children, community initiatives (e.g. farmer markets, box schemes),151 and legislation that enables doctors to prescribe vouchers for fresh fruit and vegetables and government-funded classes on nutrition to people suffering from diet-related chronic health conditions.260
Taxation of livestock source foods, whether at the producer or at the consumer level, must also address likely challenges in terms of social acceptance.251 In most countries, livestock source food production, processing and retailing are conspicuous economic sectors with strong vested interests and political influence.153,253 Meanwhile, given widespread and strong consumer preferences for livestock source foods, the degree to which the state should intervene to limit their consumption for public health and environmental objectives is subject to controversy.153,244 Yet, in a recent public poll in England, a country with a strong meat and dairy culture, 62% of participants were either in favour or not opposed to the introduction of a tax on fresh and processed meat.151 An experimental study also indicates that in advanced economies, consumers’ support for a moderate consumption tax on livestock source foods could be enhanced by bundling it with popular policies such as enforcing stricter environmental standards on livestock farms and discounting plant-based alternative protein foods.237 One way to ease opposition by livestock farmers, on the other hand, may be to use the revenue obtained from consumption taxes to reward them for on-farm activities that further enhance the targeted ecosystem services (e.g. restoration of natural habitat for carbon sequestration and nutrient run-off regulation).261
Finally, over the past decade there has been significant investment in the development and marketing of meat, dairy and seafood protein analogues, including plant-based substitutes (both traditional and highly processed ones), cultured meats and edible insects262,263 (Figure 6). By decreasing land conversion that is primarily driven by demand for livestock source foods, these protein alternatives could contribute to reducing both environmental degradation and zoonotic spillover risk. However, these products vary significantly in their current and potential capacity to limit land conversion and other environmental impacts, and in their health benefits and consumer acceptability263, 264, 265, 266, 267 (see Webappendix 8. Protein analogues – opportunities and challenges).
Wild meat bans can be counter-productive for curbing trade and zoonotic disease risk
In tropical and subtropical regions, wild meat plays a crucial role in local livelihoods and food security,99,102,155,241,268,269 especially during periods of environmental and socio-economic crisis.101,270, 271, 272 In the past three decades, wildlife conservation policies have therefore been reframed around the principle of sustainable harvest and trade of wild animals and plants in order to accommodate the nutritional and livelihood needs of IPLCs.273 Social research culminating in the United Nations Sustainable Development Goals (SDGs) has highlighted the central economic and cultural role of wild meat for IPLCs.274 The 2016 Resolution on CITES (the Convention on International Trade in Endangered Species) and Livelihoods further recognized that building livelihoods from sustainable wildlife uses can contribute to the conservation of species and ecosystems.275
In recent years, though, the public health threat posed to an increasingly interconnected world by zoonoses of suspected wildlife-origin like HIV/AIDS, Ebola, SARS and most recently COVID-19 has raised new challenges for the fragile policy balance between local livelihood needs and global conservation priorities. During the 2013–16 Ebola outbreak in West Africa and in the early days of the COVID-19 pandemic in China, several animal welfare and wildlife conservation NGOs made calls for national governments and the World Health Organization (WHO) to forbid commercial wild meat trade.87,90,276,277 A primary component of public outbreak control measures was either the proscription of wild meat trade and consumption (e.g. in Sierra Leone and China),91,278 or the issuing of strong information campaigns linking wild meat to zoonoses (e.g. in Nigeria, Côte d'Ivoire, Cameroon).279 These events reignited debate over the effectiveness and acceptability of banning tropical wild meat hunting and trade for public health and conservation goals.87,280,281
However, blanket bans on wild meat can be problematic for a number of reasons. Firstly, as mentioned above, in many tropical and subtropical regions of Africa, Asia and South America, wild meat hunting and trade enables rural populations to meet their basic nutritional and income needs.102,155,239 For IPLCs in remote forest areas with little access to affordable domestic meat and fish, wild meat is often the main source of protein, fats and micronutrients.99,100,102 In the Congo and Amazon rainforest basins, for example, wild meat consumption satisfies between 60 and 80% of daily protein needs.155,241 In remote rural communities where economic opportunities are scarce, wild meat trade also makes important contributions to household income.268,269 In rural areas and provincial towns where it is not the primary source of protein, wild meat may still constitute a vital safety net during emergencies such as drought, epidemics, economic crisis and violent conflict.101,270, 271, 272 A blanket ban may have particularly negative impacts on poorer rural households who depend on wild meat for a large share of their protein intake and income generation.268,269 For many IPLCs, hunting and consumption of wild meat has been part of their livelihood for centuries and is an important constituent of their culture and identity.239,269,282
Secondly, bans are often futile and can even be counterproductive for reducing wild meat trade. The effect of a trade policy – be it a trade ban or trade regulation – on wildlife prices, demand and illegal supply is an empirical matter, influenced by wildlife biological and ecological features, market characteristics, and state enforcement and monitoring capacities that vary depending on the species, market segments and geographical locations involved.283,284 To be effective in stopping hunting and preventing the emergence of black markets, a trade ban requires extensive state enforcement and monitoring capacity, which in tropical and subtropical developing countries characterised by weak state institutions285 tends to be low.93,95 In developing countries, wildlife law enforcement is also particularly susceptible to corruption,286,287 with enforcement authorities turning a blind eye to illegal hunting for bribes, or reselling the seized wild meat to private clients.288 This is partly due to the low importance generally granted to wildlife crime compared to other forms of crime,289 and the small and irregular salaries received by wildlife and law enforcement officials290 (although there is encouraging evidence from other sectors that corruption can be reduced at the country and local project level).287,291
Thirdly, consumer preferences for wild meat are shaped not only by its price and household wealth, but also by non-price determinants (e.g. taste preferences, perceptions of healthiness, familiarity with protein substitutes, associations with tradition and social status) that constrain its price elasticity of demand.97,98,292 Where the non-price drivers of wild meat demand are strong, a trade ban that reduces supply and increases prices may fail to discourage consumption.93,94,98 In some cases, increased rarity and price may even raise the social status of consuming wild meat and consequently drive up demand.96,97 Further, some cases of species up-listing from CITES Appendix II to Appendix I have led to spikes in legal trade in the months prior to the ban taking effect – a factor that has caused serious exploitation for species like Kleinmann's tortoises and Geoffroyi cats.293 These considerations indicate that in the absence of effective law enforcement and demand reduction campaigns, a trade ban may fail to reduce and may even encourage illegal wild meat trade.
Fourthly, a blanket ban criminalises unregulated but sustainable forms of wild meat hunting and trade that target smaller, fast reproducing species (e.g. large rodents, pigs, small ungulates) that can withstand intensive hunting, and which can be important for local food security.294,295 In West and Central Africa, these species comprise up to 70% of subsistence hunting and trade.155,296 In several African countries, African giant pouched rats and cane rats are not only highly appreciated as food, but their harvest is also regarded as a form of pest control.72 A blanket ban would also undermine sustainable wild meat farming enterprises that in some parts of the tropics have substituted harvesting from the wild and contributed to the recovery of species like the capybara in Brazil,297 crocodiles in Africa and Central America275 and sea turtles on the Cayman Islands.298
Fifthly, tightening restrictions on local wild meat uses without enacting effective anti-corruption strategies and implementing wider policies of rural development that enable IPLCs to engage in alternative livelihoods, can alienate IPLCs and further entrench the divide between them and conservation authorities. In this way, blanket wild meat bans can be unfavourable to long term wildlife conservation goals.103,104
Finally, and crucially, a wild meat ban risks being counterproductive in both reducing the risk of emergence and re-emergence of wildlife-borne zoonoses, and in controlling human contagion once spillover has occurred. By forcing trade underground, a ban might encourage the proliferation of illegal wild meat markets where less attention is paid to biosecurity measures that are important to prevent zoonotic spillover.91,105,280,299 Wild meat traders and consumers might also be less likely to report illegal wild meat market transactions in outbreak investigations.
Once a virus has entered a human population and has adapted to its new human host, a wild meat ban cannot contain contagion that is driven by human-to-human transmission rather than by repeated contact with wild animals or their meat.300 Likewise, the risk of disease spreading to new areas lies with the movement of infected people, rather than the trade of infected wild animals and meat. For example, the unprecedented scale of the 2013–16 Ebola outbreak in West Africa has been linked to higher human population density and mobility compared to the more remote areas of Central Africa affected by previous outbreaks.27 A focus on wild meat prohibition may detract public attention from the more critical causes of interpersonal contagion, such as contact with infected people and perilous burial practices.301,302 In areas where wild meat is central to food security and livelihoods, a ban can also erode public confidence in government outbreak control measures. In these ways, a wild meat ban risks undermining the efficacy and legitimacy of government outbreak responses.91
Strategies to curb tropical urban wild meat demand while securing access for Indigenous Peoples and Local Communities (IPLCs)
In tropical regions, wild meat hunting and trade is increasingly driven by urban demand. In some large cities of the Congo and Amazon basins, although wild meat provides a minor proportion of per capita protein consumption, aggregate demand is very large.154,155 In many Asian countries, wild meat consumption is considered to symbolise high social status because of its relative rarity, cost and association with old elites, so that growing prosperity among the urban middle class has driven a rapid increase in wild meat demand.97,303 Considering that in most established metropoles of tropical regions, legume, fish and livestock source proteins are available in ample supply and at affordable prices, here wild meat consumption is no longer a nutritional necessity, but is rather driven by taste preferences, perceptions of freshness and healthiness, cultural motivations or associations with social status and prestige.97,98,292
Supplying cities with wild meat from tropical forests is not ecologically viable.154,155 In livestock-grazed rangelands where predators (e.g. dingos and wild dogs in Australia) have been partially removed and permanent water supplies have been expanded, commercial hunting of large wild herbivores (e.g. kangaroos) for their meat acts as a strategy to control total grazing pressure and preserve landscapes and biodiversity.304 The situation is starkly different in tropical and subtropical forests. Here, the interaction of the intrinsic low productivity of large wild herbivores305 with widespread loss of continuous habitat and large and growing urban populations means that even very low urban per capita consumption rates can result in an aggregate urban demand that would outstrip sustainable wild meat harvesting in the surrounding areas.154,155 In Kinshasa, for instance, consumption of only 1 kg of wild meat per inhabitant per year can result in massive wildlife harvests when multiplied by its 8 million residents.72
Consequently, in tropical regions the present scale of urban wild meat demand simultaneously threatens biodiversity69,98 and the food security of wild meat-reliant IPLCs.99,306 In addition, high levels of urban wild meat consumption may increase the risk of wildlife—human zoonotic disease transmission by incentivising hunter penetration of pristine forests,72,307 expansion of wildlife farming enterprises308,309 and increased wild meat sales in urban and peri-urban markets.310,311 A large and expanding wild meat sector poses a substantial strain on state capacity to enforce biosecurity measures and carry out disease surveillance.312
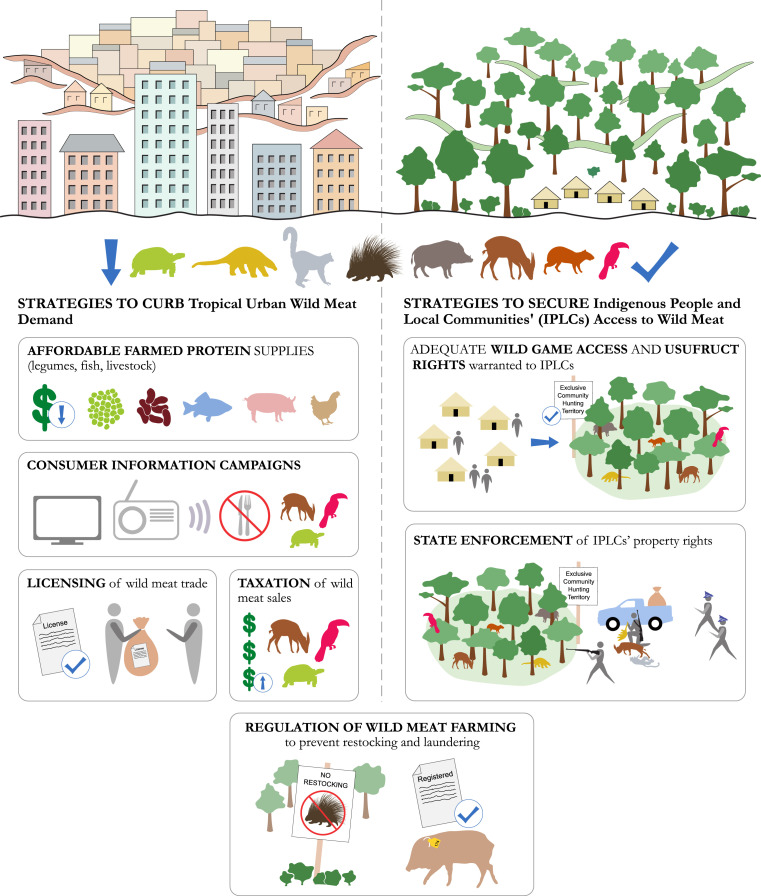
A balanced approach to tropical wild meat hunting and trade, addressing zoonotic disease threats and biodiversity losses while preserving local livelihoods, requires a holistic strategy that supports sustainable and biosecure wild game management in rural subsistence areas and curbs wild meat demand in established metropoles and emerging provincial towns where alternative protein sources are available or can be established72,269,274 (Figure 7).
Figure 7.
Strategies to curb tropical urban wild meat demand and secure indigenous people and local communities' (IPLCs) access to wild meat.
The few existing community-based wild game management initiatives indicate that local communities can have noticeable ecological knowledge on sustainable hunting, and strong incentives to respect internal hunting rules273,313 and carry out anti-poaching monitoring on their territories.314,315 However, in many tropical countries IPLCs often lack secure land tenure and adequate wildlife usufruct rights,103,316,317 which undercuts their incentive and capacity to undertake effective wild game management.318,319 In many of these countries, national laws on community hunting either criminalize most local wild meat hunting practices (e.g. with respect to target species, offtake levels, hunting areas, equipment), or are unclear about what forms of wild meat subsistence hunting and small-scale trade are permitted and by whom.72,317,320 The fact that many wildlife conservation groups continue to ascribe to a protectionist paradigm that views wild meat subsistence hunting and small-scale trade by village dwellers as inherently incompatible with wildlife conservation poses a further obstacle to the development of effective regulatory systems for sustainable community-based wild game management.268,320
A first priority is therefore to warrant communities in remote, wildlife rich areas with exclusive access rights to game on their customary lands and to entrust them with locally relevant usufruct and management rights.315 Adequate state enforcement of community property rights is also essential; without such backing, local communities may be unable to prevent outsiders from hunting illegally in their territories and may be exposed to violent attacks318,321 (Figure 7). As a starting point, state law enforcement capacity could be increased by investing in improving the proficiency of police, legal and judicial personnel in wild meat hunting and trade laws.72
Community-based wild game management institutions could also be used to introduce biosecurity measures for safer wild meat hunting and handling among local inhabitants (see Section 8 below).
Community-based initiatives can only be successful at promoting sustainable and biosecure wild game management on community lands if accompanied by efforts to reduce pressure from urban demand.155,309 To start with, in the growing number of settlements and provincial towns emerging near extractive, plantation and infrastructure construction sites, affordable supplies of locally farmed or imported legume, fish and livestock protein should be established or strengthened322 (Figure 7).
The next policy option to stem urban pressure would be to regulate the wild meat sector through a state-run trade license system that sets total wild meat offtake levels and permitted hunting zones and seasons. A parallel action would be to increase urban prices through sales taxation. However, in tropical and subtropical forest regions, regulation of the wild meat sector through trade licencing is extremely challenging due to high demand from large metropoles, the large number of rural and urban dwellers engaged in this commerce and the wide range of game species involved. States lack the resources and capacity to effectively regulate and monitor such trade, so that wild food harvesting and trade largely remains an informal, unregulated sector.72,98 Implementing a wild meat sales taxation system tends to be cost-ineffective and is similarly constrained by insufficient technological infrastructure and human resources.72 Moreover, since the non-price drivers of wild meat demand (e.g. taste, perceptions of healthiness, cultural identity, social status) tend to be strong among urban consumers, consumption by relatively affluent households may continue even when prices are raised through taxation and/or protein substitutes are made available at affordable prices.323
It is therefore likely that information campaigns are necessary to tackle urban wild meat consumption, and need to precede or accompany other policies attempting to regulate or tax the sector and fight the corruption of enforcement officers. Depending on what demand drivers and market segments are targeted, consumer campaigns might focus on the presence of preservative chemicals and the risks of spoilage and zoonotic disease transmission in wild meat,272,324 they might portray domestic and other meat substitutes as more modern/fashionable choices,262 or they might highlight the destructive impact of illegal wild meat trade on biodiversity and IPLCs (Figure 7). However, except for few studies,154,325 up to now it has been difficult to assess and draw lessons from wild meat consumer campaigns, as impact assessment has rarely been factored into their design.326
As mentioned in the previous section, not all forms of urban wild meat consumption pose sustainability threats. Some agricultural landscapes that have already lost much of their biodiversity, and are now mostly inhabited by fast-breeding, hunting-resilient species like large rodents, pigs and small ungulates, appear capable of supporting long-term wild meat urban trade even in the absence of strictly enforced hunting license and sales taxation systems.294,295
Wild meat farming has also rapidly expanded as a strategy to satisfy urban demand and so reduce pressure on wild animal populations,327,328 with some success cases.275,297 However, wild meat farming projects often suffer a low adoption rate among rural communities or struggle to reduce hunting from the wild.309,324 This is due to a number of challenges, such as: the unsuitability of some wild animal species to captive rearing, which encourages farm restocking through wild-caught individuals; the lower cost-efficiency of wild meat farming compared to hunting, whereby farmers charge higher prices than hunters;324,329 the difficulty for farm-reared wild meat to satisfy urban consumer preferences;97,329 and insufficient state capacity to prevent laundering of illegally hunted wild meat into legal farm-reared wild meat markets.72,309 Therefore, in order to contribute to reducing hunting pressure on wild populations, wild meat farming initiatives need to be informed by an in-depth understanding of consumer responses to farm-reared substitutes, and the sector must be effectively regulated to prevent restocking from the wild and laundering.97,309,324
Finally, in order to avoid becoming hubs for zoonotic disease outbreaks, legalised wild meat farms, abattoirs and markets need to comply with biosecurity measures discussed in the next section.
Improving biosecurity along animal source food supply chains and other strategies to break zoonosis transmission pathways
A more balanced approach to tropical wild meat hunting, farming and trade – acknowledging its contribution to local livelihoods and promoting biosecurity measures along supply chains – may not eliminate the risk of endemic and novel zoonotic disease transmission altogether, but neither will an ill-conceived anti-wild meat campaign that is likely to shove these activities deeper underground and erode public confidence in government outbreak control measures.91,280
In developing countries, wild meat hunters, farmers and butchers are highly exposed to bites and scratches from wild animals.330 Yet, those engaged in these occupations rarely perceive them to be hazardous, since they have been carried out for generations while information about potential zoonotic infection risk has been lacking. Moreover, many individuals engaged in wild meat hunting and trade perceive safety from zoonotic disease risk as a lower priority than earning a living from one of the few livelihood options available to them.330 In order to improve biosecurity in rural communities, interventions therefore need to focus on more than raising awareness about wild meat pathogen transmission; they should also provide local hunters, traders and butchers with training on safer ways to handle live and dead animals and inexpensive biosecurity measures that they can easily adopt to avoid infection.312
Biosecurity measures to prevent zoonotic disease transmission while hunting, butchering and handling wild animals and their meat include: wearing protective clothing (e.g. boots, long trousers and long-sleeved shirts – as many resist latex gloves for being slippery, flimsy and cost-prohibitive);312 averting bites by wild animals; avoiding the collection of dead or dying animals; wrapping recent kills in plastic or banana leaves to prevent animal blood from getting in contact with small cuts or micro-abrasions; washing hands regularly with soap and clean water; properly storing and preserving carcasses; preventing peri-domestic animals (e.g. feral dogs) from frequenting slaughtering locations and eating carcasses; keeping children away from butchering and cooking areas and from playing with dead animals; and thoroughly cooking wild meat before eating331,332 (Figure 8). Yet, seemingly simple measures like these can still be challenging in low-resource rural settings where even access to clean water and basic storage facilities may be problematic.312,333
Figure 8.
Biosecurity measures for hunting and handling wild meat in rural communities and in ‘wet markets’ selling domestic and wild animal products.
The trade-offs experienced by local communities between livelihood benefits and disease risk from wildlife resource use highlight the importance of involving such communities in the design of biosecurity strategies.53,334 Educational messages also need to reach rural and urban dwellers in the informal or illegal wild meat trade, for example through text messaging, radio and poster campaigns.312
Wild meat hunting is not the only pathway for wildlife-origin infectious disease emergence and transmission; instead, it is crucial that biosecurity measures are taken along the whole animal source food supply chain, including livestock and wildlife farms, animal transportation systems, abattoirs, food markets and restaurants.6,9,75 These measures include ensuring animal captive conditions that meet hygiene and welfare standards,278,311 minimising species mixing, worker use of protective and sanitary equipment, and routine veterinary care and disease inspection6,335 (Figure 8).
‘Wet markets’ selling fresh produce, including live or freshly slaughtered animals, underpin the informal food systems of fast growing rural and urban populations in tropical and subtropical regions.310,336 Given the tendency of governments to discourage wild meat trade in favour of wildlife conservation, provision of market infrastructure such as covered stalls and concrete/tiled counters, running water, drains and overnight and cold storage is generally lacking for this sector, with the consequence that wild animals and their meat are handled under particularly unhygienic conditions.312,333 Governments should proactively invest in providing markets with adequate water, sanitation, storage and waste management infrastructure, stalls with surfaces that can be easily disinfected, and possibly slaughtering facilities that are separate from the public. Local government agencies should provide suppliers and retailers with training in biosecurity regulations and monitor compliance337,338 (Figure 8). As these markets play an important role in the diet and livelihood of millions of low-income rural and urban dwellers,310,336 it is important that the introduction of new biosecurity standards does not disrupt their access to these markets. ‘Top-down’ biosecurity measures that do not take into account the interests and norms of these local actors also risk being ineffective.339 Hunters, farmers, traders, vendors, market managers, local administrators and public health scientists should therefore collaborate to identify locally-apt biosecurity regulations.338
Physical distancing measures should also be adopted on farms, pastures, abattoirs and live animal markets to reduce zoonosis transmission.86 For example, fencing is used to reduce bison—cattle contacts for brucellosis prevention.340 In Malaysia, policies requiring that fruit trees are planted at a distance from pig sties have averted new Nipah virus spillover from bats to pigs.341 In Bangladesh, Nipah virus spillover from bats to humans through drinking uncooked date palm sap infected with bat saliva and urine can be prevented by covering clay pots with bamboo skirts overnight.342 In live-bird markets, avian influenza outbreaks and viral genome reassortment can be minimised by limiting poultry population size and stay-time.335
Large-scale natural habitat restoration and reforestation programmes, which are mostly undertaken to pursue carbon sequestration343 and biodiversity conservation goals, could also be harnessed as a form of physical distancing that reduces the risk of wildlife-origin disease transmission by drawing reservoir wild animal populations away from livestock farms and human settlements.344 However, reforestation interventions require careful planning, as in some circumstances they may actually contribute to the risk of infectious disease emergence or range expansion. In the US and parts of Europe, for example, over the past two decades, a combination of reforestation of farmland, rebounding of deer populations and warmer temperatures is believed to have caused a range expansion of the deer tick (Ixodes scapularis) and the Lyme disease of which it is a vector.345 Moreover, planting trees in discontinuous land patches rather than to connect forest fragments, as well as planting monocultures within forest fragments, may have the effect of contributing to rather than decreasing forest fragmentation.115
Logging, mining, cash crop, ranching and infrastructure construction companies should be legally required to include zoonotic spillover risk reduction measures in their management plans. These measures would include minimising natural habitat fragmentation, adopting wild animal physical distancing measures, providing worker camps and emerging settlements with access to affordable and sustainably produced legume, fish and farm-reared animal protein, monitoring and sanctioning illegal wild meat hunting, sale and transportation at their sites, and promoting biosecurity measures among indigenous communities that practice legal hunting.108,322
Furthermore, interventions exist that target wild animal reservoirs and vectors, such as vaccination, culling and vector management. Owing to differences in reservoir and vector species ecologies, no strategy is likely to effectively tackle all wildlife-origin disease transmission pathways.86 For instance, while vaccination of domestic and wild carnivores has enabled the eradication of terrestrial rabies in most EU member states,346 it is not as effective against rapidly evolving strains of avian influenza.347 Culling can sometimes unintentionally enhance pathogen prevalence in the reservoir population by altering movement dynamics (as it may be the case with vampire bats culled to control rabies)348 or by reducing herd immunity (as observed with sick bison culled to control brucellosis).349 Integrated vector management (IVM) strategies that prioritize environmental management, biological control and personal protection over chemical interventions can yield long-term benefits while minimizing negative impacts on ecosystems and public health. However, countries in sub-Saharan Africa face significant human resource and infrastructure challenges to the deployment of IVM.350
The reintroduction and protection of natural predator and scavenger species has been proposed as an alternative to culling that controls wildlife-borne infectious diseases by reducing the reservoir population size, killing sick individuals and hastening consumption of infected carcasses.86,351 On rangelands, for instance, populations of vultures can significantly reduce the risk of bovine tuberculosis, anthrax and other pathogen spillover from uneaten carcasses.352 In Spain, wolf predation has been shown to reduce tuberculosis prevalence in wild boar without reducing its population density, as predation-induced mortality is compensated by a reduction in disease-induced mortality.353 However, in parts of the US, wolf reintroduction to control chronic wasting disease spillover from elks has met with resistance due to potential economic losses that may be caused by wolf predation on livestock, and the interest of sport hunter groups in maintaining large game populations.86
Overall, theoretical models of spillover prevention indicate that implementing biosecurity and physical distancing at the wildlife—human interface is a more effective strategy than targeting wild and domestic animals through veterinary treatment or culling. As such, biosecurity and physical distancing strategies constitute an investment priority, in concert with traditional public health measures targeting the human population such as surveillance for case detection, treatment and vaccination.86,354 Moreover, while international safety standards for food production and trade exist, such as the Hazard Analysis Critical Control Point (HACCP) standards and the Codex Alimentarius and OIE codes, these are insufficient by themselves to address zoonotic spillover risk occurring ‘upstream’ in the food supply chain. Instead, tackling zoonotic spillover risk requires interventions that target biosecurity, the physical environment and wild animal reservoirs directly at the wildlife—human interface.
Finally, a One Health approach has been endorsed by the WHO, FAO and OIE, based on recognition that human and animal health are interlinked and bound to the health of ecosystems.355 By integrating medical, veterinary, epidemiological, wildlife ecology, environmental and social science expertise, One Health aims at strengthening countries’ biosecurity and disease surveillance capacity. However, many zoonotic and vector-borne disease control programmes remain focused uniquely on targeting humans (e.g. through vaccination and drug treatment), lacking robust empirical knowledge of the underlying ecological and socio-cultural factors that lead to animal—human disease transmission. This is particularly true of neglected infectious diseases that primarily affect marginalised populations in low-income countries (e.g. leishmaniasis, leprosy, hydatidosis).86,330
Conclusions
The extensive literature examined in this Review highlights how the potentially increasing incidence of human infectious diseases originating in wild animals may be yet another sign of the way in which the current trajectory of ecosystem degradation is undermining the capacity of planet Earth to sustain human wellbeing. Considering the enormous health and socio-economic costs of wildlife-origin infectious disease epidemics and pandemics, preventing agricultural encroachment and over-harvesting of wild animals in the last remaining wildlands constitutes an important precautionary strategy to reduce such public health risk.
This strategy implies a global transition towards predominantly plant-based diets that are both healthier and more environmentally sustainable, and drastic reductions in tropical urban wild meat demand. Such actions are also key to the pressing need of mitigating global warming and biodiversity loss, and of safeguarding vulnerable farmer, pastoralist and hunter-gatherer communities who live in marginal landscapes and are strongly reliant on a stable climate and wildlife populations for food and income security. As such, tropical livestock-driven deforestation and urban-driven wild meat over-harvesting have become key issues in the multiple global agendas of environmental conservation, rural poverty relief and, pending further research, zoonotic infectious disease prevention.
Fifteen years of warnings from epidemiologists of the devastating consequences of increasing zoonotic disease risk have been ignored.25,356 Likewise, government responses to unprecedented global warming and wild species extinction crises have been inadequate and inconsistent.357,358 Yet, as a sustainable global future depends on urgent transformative change of current socio-cultural systems, there is no substitute for early action.213,359 It is to be hoped that the COVID-19 crisis will act as a trigger for global societies to recommit to what is most vital, prompting them to examine and act upon the scale of environmental disruption and the threat of zoonotic disease that we are facing. Part of recovery funding should be strategically allocated to implementing research and policies to promote radical shifts in human diets away from heavy reliance on livestock source foods and drastic reductions in tropical urban wild meat demand. This is crucial to simultaneously protect the environment, prevent the exacerbation of rural poverty and reduce the risk of a next zoonotic pandemic.
Contributors
Giulia I. Wegner is responsible for the conceptualisation of this scholarly Review paper (i.e. for the formulation and evolution of its overarching research goals and aims), for its overall design, and for writing the initial draft. She is also responsible for leadership and coordination of contributions by other co-authors, the illustrator and the photographer.
Kris A. Murray and David M. Morens contributed to the evolution of the aims of the paper.
Kris A. Murray, Marco Springmann, Adrian Muller, Susanne H. Sokolow, Karen Saylors and David M. Morens contributed to writing the final draft through critical review and commentary.
Emily Wright is responsible for the creation of all graphic illustrations. Emilia De Leonardis is responsible for the creation of the cover photo.
Funding
None.
Declaration of interests
The authors received no funding or other support for production of the manuscript and have no conflicts of interest.
Acknowledgments
The authors are particularly grateful to Emily Wright for the production of the illustrations in this Review paper, and to Emilia De Leonardis for the production of the cover photo. They owe gratitude to three anonymous reviewers for their insightful comments on how to improve earlier drafts of the paper. They are also thankful to Michael ’t Sas-Rolfes (Oxford Martin Programme on Wildlife Trade) for technical comments on wild meat trade policies, and to Sarah Olson (WCS Health Program) for sharing criticism of recently published grey literature on the roles of wildlife in emerging human infectious diseases. Giulia Wegner carried part of the preparatory research for this Review paper while supported by a doctoral studentship awarded by the Recanati-Kaplan Foundation. Marco Springmann acknowledges funding from the Wellcome Trust, Our Planet Our Health (Livestock, Environment and People (LEAP)), award number 205212/Z/16/Z. Susanne H. Sokolow acknowledges support from the NCEAS-SNAPP Ecological Levers for Health project.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2022.101386.
Appendix. Supplementary materials
References
- 1.WHO. Zoonoses. https://www.who.int/news-room/fact-sheets/detail/zoonoses. Published 2020. Accessed 14 October 2021.
- 2.Jones K.E., Patel N.G., Levy M.A., et al. Global trends in emerging infectious diseases. Nature. 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith K.F., Goldberg M., Rosenthal S., et al. Global rise in human infectious disease outbreaks. J R Soc Interface. 2014;11 doi: 10.1098/rsif.2014.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gatiso T.T., Ordaz-Németh I., Grimes T., et al. The impact of the Ebola virus disease (EVD) epidemic on agricultural production and livelihoods in Liberia. PLoS Negl Trop Dis. 2018;12(8) doi: 10.1371/journal.pntd.0006580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.UNCTAD . UNCTAD; 2020. The Covid-19 Shock for Developing Countries.https://unctad.org/system/files/official-document/gds_tdr2019_covid2_en.pdf [Google Scholar]
- 6.Morse S.S., Mazet J.A.K., Woolhouse M., et al. Prediction and prevention of the next pandemic zoonosis. Lancet. 2012;380(9857):1956–1965. doi: 10.1016/S0140-6736(12)61684-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.GPMB . GPMB; 2019. A World at Risk: Annual Report On Global Preparedness For Health Emergencies. Geneva, Switzerland. [Google Scholar]
- 8.Morens D.M., Fauci A.S. Emerging pandemic diseases: how we got to COVID-19. Cell. 2020;182(5):1077–1092. doi: 10.1016/j.cell.2020.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daszak P., Olival K.J., Li H. A strategy to prevent future epidemics similar to the 2019-nCoV outbreak. Biosaf Health. 2020;2:6–8. doi: 10.1016/j.bsheal.2020.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Marco M., Baker M.L., Daszak P., et al. Sustainable development must account for pandemic risk. Proc Natl Acad Sci. 2020;117(8):3888–3892. doi: 10.1073/pnas.2001655117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dobson A.P., Pimm S.L., Hannah L., et al. Ecology and economics for pandemic prevention. Science. 2020;369(6502):379–381. doi: 10.1126/science.abc3189. (80-) [DOI] [PubMed] [Google Scholar]
- 12.McMichael A.J. Environmental and social influences on emerging infectious diseases: past, present and future. Philos Trans R Soc B Biol Sci. 2004;359(1447):1049–1058. doi: 10.1098/rstb.2004.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morens D.M., Daszak P., Markel H., Taubenberger J.K. Pandemic COVID-19 joins history's pandemic legion. MBio. 2020;11(3):e00812–e00820. doi: 10.1128/mBio.00812-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pearce-Duvet JMC The origin of human pathogens: evaluating the role of agriculture and domestic animals in the evolution of human disease. Biol Rev. 2006;81(3):369–382. doi: 10.1017/S1464793106007020. [DOI] [PubMed] [Google Scholar]
- 15.Woolhouse M., Scott F., Hudson Z., Howey R., Chase-Topping M. Human viruses: discovery and emergence. Philos Trans R Soc B Biol Sci. 2012;367(1604):2864–2871. doi: 10.1098/rstb.2011.0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolfe N.D., Dunavan C.P., Diamond J. Origins of major human infectious diseases. Nature. 2007;477(7142):279–283. doi: 10.1038/nature05775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharp P.M., Plenderleith L.J., Hahn B.H. Ape origins of human malaria. Annu Rev Microbiol. 2020;74:39–63. doi: 10.1146/annurev-micro-020518-115628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murray K.A., Preston N., Allen T., Zambrana-Torrelio C., Hosseini P.R., Daszak P. Global biogeography of human infectious diseases. Proc Natl Acad Sci. 2015;112(41):12746–12751. doi: 10.1073/pnas.1507442112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hassell J.M., Begon M., Ward M.J., Fèvre E.M. Urbanization and disease emergence: dynamics at the wildlife–livestock–human interface. Trends Ecol Evol. 2017;32(1):55–67. doi: 10.1016/j.tree.2016.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crosby A.W. University of California Press; Berkeley, USA: 2019. The Columbian Exchange. (Dunn RE, Mitchell LJ, Ward K, eds.) [DOI] [Google Scholar]
- 21.O'Connell K.P. Defeating infectious disease. IQT Q. 2016;7(3):2–4. [Google Scholar]
- 22.Wang H., Naghavi M., Allen C., et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1459–1544. doi: 10.1016/S0140-6736(16)31012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vos T., Lim S.S., Abbafati C., et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sands P., El-Turabi A., Saynisch P., Morhard R. White Papers; Geneva, Switzerland: 2019. Outbreak Readiness and Business Impact: Protecting Lives and Livelihoods across the Global Economy. [Google Scholar]
- 25.Morens D.M., Breman J.G., Calisher C.H., et al. The origin of COVID-19 and why it matters. Am J Trop Med Hyg. 2020;103(3):955. doi: 10.4269/ajtmh.20-0849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fairhead J., Leach M., Millimouno D. Spillover or endemic? Reconsidering the origins of Ebola virus disease outbreaks by revisiting local accounts in light of new evidence from Guinea. BMJ Glob Health. 2021;6(4) doi: 10.1136/bmjgh-2021-005783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coltart C.E.M., Lindsey B., Ghinai I., Johnson A.M., Heymann D.L. The Ebola outbreak, 2013–2016: old lessons for new epidemics. Philos Trans R Soc B. 2017;372 doi: 10.1098/rstb.2016.0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones B.A., Grace D., Kock R., et al. Zoonosis emergence linked to agricultural intensification and environmental change. Proc Natl Acad Sci. 2013;110(21):8399–8404. doi: 10.1073/pnas.1208059110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Boeckel T.P., Brower C., Gilbert M., et al. Global trends in antimicrobial use in food animals. Proc Natl Acad Sci. 2015;112(18):5649–5654. doi: 10.1073/pnas.1503141112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu Y.G., Gillings M., Simonet P., Stekel D., Banwart S., Penuelas J. Microbial mass movements. Science. 2017;357(6356):1099–1100. doi: 10.1126/science.aao3007. (80-) [DOI] [PubMed] [Google Scholar]
- 31.Ke R., Romero-Severson E., Sanche S., Hengartner N. Estimating the reproductive number R0 of SARS-CoV-2 in the United States and eight European countries and implications for vaccination. J Theor Biol. 2021;517 doi: 10.1016/j.jtbi.2021.110621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sah P., Fitzpatrick M.C., Zimmer C.F., et al. Asymptomatic SARS-CoV-2 infection: a systematic review and meta-analysis. Proc Natl Acad Sci. 2021;118(34) doi: 10.1073/pnas.2109229118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gibb R., Redding D.W., Chin K., et al. Zoonotic host diversity increases in human-dominated ecosystems. Nature. 2020;584:398–402. doi: 10.1038/s41586-020-2562-8. [DOI] [PubMed] [Google Scholar]
- 34.Johnson C.K., Hitchens P.L., Pandit P.S., et al. Global shifts in mammalian population trends reveal key predictors of virus spillover risk. Proc R Soc B. 2020;287(1924) doi: 10.1098/rspb.2019.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nabi G., Wang Y., Lü L., et al. Bats and birds as viral reservoirs: a physiological and ecological perspective. Sci Total Environ. 2021;754 doi: 10.1016/j.scitotenv.2020.142372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Daszak P., Plowright R.K., Epstein J.H., Collinge S.K., Ray C., et al. Disease Ecology: Community Structure and Pathogen Dynamics. Oxford University Press; 2006. The emergence of Nipah and Hendra virus: pathogen dynamics across a wildlife-livestock-human continuum; pp. 186–201. [Google Scholar]
- 37.Cui J., Li F., Shi Z.L. Origin and evolution of pathogenic corona-viruses. Nat Rev Microbiol. 2019;17:181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Latinne A., Hu B., Olival K.J., et al. Origin and cross-species transmission of bat coronaviruses in China. Nat Commun. 2020;11(1):1–15. doi: 10.1038/s41467-020-17687-3. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39.Wacharapluesadee S., Tan C.W., Maneeorn P., et al. Evidence for SARS-CoV-2 related coronaviruses circulating in bats and pangolins in Southeast Asia. Nat Commun. 2021;12(1):1–9. doi: 10.1038/s41467-021-21240-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Towner J.S., Pourrut X., Albariño C.G., et al. Marburg virus infection detected in a common African bat. PLoS One. 2007;2(8):e764. doi: 10.1371/journal.pone.0000764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leroy E.M., Rouquet P., Formenty P., et al. Multiple Ebola virus transmission events and rapid decline of central African wildlife. Science. 2004;303(5656):387–390. doi: 10.1126/science.1092528. (80-) [DOI] [PubMed] [Google Scholar]
- 42.Leendertz S.A., Gogarten J.F., Dux A., Calvignac-Spencer S., Leendertz F.H. Assessing the evidence supporting fruit bats as the primary reservoirs for EBOLA viruses. Ecohealth. 2016;13:18–25. doi: 10.1007/s10393-015-1053-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Banerjee A., Baker M.L., Kulcsar K., Misra V., Plowright R., Mossman K. Novel insights into immune systems of bats. Front Immunol. 2020;11(26) doi: 10.3389/fimmu.2020.00026. 11, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mollentze N., Streicker D.G. Viral zoonotic risk is homogenous among taxonomic orders of mammalian and avian reservoir hosts. Proc Natl Acad Sci. 2020;117(17):9423–9430. doi: 10.1073/pnas.1919176117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parrish C.R., Holmes E.C., Morens D.M., et al. Cross-species virus transmission and the emergence of new epidemic diseases. Microbiol Mol Biol Rev. 2008;72(3):457–470. doi: 10.1128/MMBR.00004-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Olivero J., Fa J.E., Real R., et al. Mammalian biogeography and the Ebola virus in Africa. Mamm Rev. 2017;47:24–37. [Google Scholar]
- 47.Li W., Shi Z., Yu M., et al. Bats are natural reservoirs of SARS-like coronaviruses. Science. 2005;310(5748):676–679. doi: 10.1126/science.1118391. (80-) [DOI] [PubMed] [Google Scholar]
- 48.Sánchez CA, Li H, Phelps KL, et al. A strategy to assess spillover risk of bat SARS-related coronaviruses in Southeast Asia. medRxiv. 2021. [DOI] [PMC free article] [PubMed]
- 49.Graham J.P., Leibler J.H., Price L.B., et al. The animal-human interface and infectious disease in industrial food animal production: rethinking biosecurity and biocontainment. Public Health Rep. 2008;123(3):282–299. doi: 10.1177/003335490812300309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kock R., Caceres-Escobar H. IUCN; Gland, Switzerland: 2022. Situation Analysis on the Roles and Risks of Wildlife in the Emergence of Human Infectious Diseases. [Google Scholar]
- 51.Epstein J.H., Field H.E., Luby S., Pulliam J.R.C., Daszak P. Nipah virus: impact, origins, and causes of emergence. Curr Infect Dis Rep. 2006;8:59–65. doi: 10.1007/s11908-006-0036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aliaga-Samanez A., Cobos-Mayo M., Real R., et al. Worldwide dynamic biogeography of zoonotic and anthroponotic dengue. PLoS Negl Trop Dis. 2021;15(6) doi: 10.1371/journal.pntd.0009496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burthe S.J., Schäfer S.M., Asaaga F.A., et al. Reviewing the ecological evidence base for management of emerging tropical zoonoses: Kyasanur Forest Disease in India as a case study. PLoS Negl Trop Dis. 2021;15(4) doi: 10.1371/journal.pntd.0009243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.UNAIDS. Global HIV & AIDS statistics — 2020 fact sheet. Geneva, Switzerland; 2020. https://www.unaids.org/en/resources/fact-sheet.
- 55.Richman D.D., Margolis D.M., Delaney M., Greene W.C., Hazuda D., Pomerantz R.J. The challenge of finding a cure for HIV infection. Science. 2009;323:1304–1307. doi: 10.1126/science.1165706. (80-) [DOI] [PubMed] [Google Scholar]
- 56.Worldometers. COVID-19 coronavirus pandemic.
- 57.IMF . IMF; 2020. World Economic Outlook, April 2020: The Great Lockdown.https://www.imf.org/en/Publications/WEO/Issues/2020/04/14/weo-april-2020 [Google Scholar]
- 58.UNCTAD . UNCTAD; 2020. The Covid-19 Shock for Developing Countries. [Google Scholar]
- 59.Kalish M.L., Wolfe N.D., Ndongmo C.B., et al. Central African hunters exposed to simian immunodeficiency virus. Emerg Infect Dis. 2005;11(12):1928. doi: 10.3201/eid1112.050394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sharp P.M., Hahn B.H. Origins of HIV and the AIDS pandemic. Cold Spring Harb Perspect Med. 2011;1(1) doi: 10.1101/cshperspect.a006841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ayouba A., Akoua-Koffi C., Calvignac-Spencer S., et al. Evidence for continuing cross-species transmission of SIVsmm to humans: characterization of a new HIV-2 lineage in rural Côte d'Ivoire. AIDS. 2013;27(15) doi: 10.1097/01.aids.0000432443.22684.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou P., Yang X.L., Wang X.G., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Holmes E.C., Goldstein S.A., Rasmussen A.L., et al. The origins of SARS-CoV-2: a critical review. Cell. 2021;184(19):4848–4856. doi: 10.1016/j.cell.2021.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Worobey M. Dissecting the early COVID-19 cases in Wuhan. Science. 2021:eabm4454. doi: 10.1126/science.abm4454. (80-) [DOI] [PubMed] [Google Scholar]
- 65.Harypursat V., Chen Y.K. Six weeks into the 2019 coronavirus disease outbreak: it is time to consider strategies to impede the emergence of new zoonotic infections. Chin Med J Engl. 2020;133(9):1118–1120. doi: 10.1097/CM9.0000000000000760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang N., Liu P., Li W., Zhang L. Permanently ban wildlife consumption. Science. 2020;367(6485):1434–1435. doi: 10.1126/science.abb1938. (80-) [DOI] [PubMed] [Google Scholar]
- 67.OiE, WHO, UNEP . World Health Organization; 2021. Reducing Public Health Risks Associated With the Sale of Live Wild Animals of Mammalian Species in Traditional Food Markets: Interim Guidance, 12 April 2021.https://cdn.who.int/media/docs/default-source/food-safety/ig–121-1-food-safety-and-covid-19-guidance-for-traditional-food-markets-2021-04-12-en.pdf?sfvrsn=921ec66d_1&download=true [Google Scholar]
- 68.Canale G.R., Peres C.A., Guidorizzi C.E., Gatto C.A.F., Kierulff M.C.M. Pervasive defaunation of forest remnants in a tropical biodiversity hotspot. PLoS One. 2012;7(8):e41671. doi: 10.1371/journal.pone.0041671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Harrison R.D., Sreekar R., Brodie J.F., et al. Impacts of hunting on tropical forests in Southeast Asia. Conserv Biol. 2016;30:972–981. doi: 10.1111/cobi.12785. [DOI] [PubMed] [Google Scholar]
- 70.Schulze K., Knights K., Coad L., et al. An assessment of threats to terrestrial protected areas. Conserv Lett. 2018;11(3):e12435. [Google Scholar]
- 71.Benítez-López A., Santini L., Schipper A.M., Busana M., Huijbregts M.A.J. Intact but empty forests? Patterns of hunting-induced mammal defaunation in the tropics. PLoS Biol. 2019;17(5) doi: 10.1371/journal.pbio.3000247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Coad L., Fa J.E., Abernethy K., et al. CIFOR; Bogor, Indonesia: 2019. Towards a Sustainable, Participatory and Inclusive Wild Meat Sector. [Google Scholar]
- 73.Canale G.R., Peres C.A., Guidorizzi C.E., Gatto C.A.F., Kierulff M.C.M. Pervasive defaunation of forest remnants in a tropical biodiversity hotspot. PLoS One. 2012;7(8):e41671. doi: 10.1371/journal.pone.0041671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Peres C.A., Thaise E., Schietti J., Desmoulieres S.J.M., Levi T. Dispersal limitation induces long-term biomass collapse in overhunted Amazonian forests. Proc Natl Acad Sci. 2016;113(4):892–897. doi: 10.1073/pnas.1516525113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huong N.Q., Nga N.T.T., Van Long N, et al. Coronavirus testing indicates transmission risk increases along wildlife supply chains for human consumption in Viet Nam, 2013-2014. PLoS One. 2020;15(8) doi: 10.1371/journal.pone.0237129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Eskew E.A., Carlson C.J. Overselling wildlife trade bans will not bolster conservation or pandemic preparedness. Lancet Planet Health. 2020;4(6):e215–e216. doi: 10.1016/S2542-5196(20)30123-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fisher M.C., Murray K.A. Emerging infections and the integrative environment-health sciences: the road ahead. Nat Rev Microbiol. 2021:1–3. doi: 10.1038/s41579-021-00510-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rohr J.R., Barrett C.B., Civitello D.J., et al. Emerging human infectious diseases and the links to global food production. Nat Sustain. 2019;2:445–456. doi: 10.1038/s41893-019-0293-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mena I., Nelson M.I., Quezada-Monroy F., et al. Origins of the 2009 H1N1 influenza pandemic in swine in Mexico. Elife. 2016;5:e16777. doi: 10.7554/eLife.16777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Keele B.F., Van Heuverswyn F., Li Y., et al. Chimpanzee Reservoirs of Pandemic and Nonpandemic HIV-1. Science. 2006;313(5786):523–526. doi: 10.1126/science.1126531. (80-) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Takahashi K., Kitajima N., Abe N., Mishiro S. Complete or near-complete nucleotide sequences of hepatitis E virus genome recovered from a wild boar, a deer, and four patients who ate the deer. Virology. 2004;330(2):501–510. doi: 10.1016/j.virol.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 82.Ducrocq J., Proulx J., Lévesque B., De Serres G., Wood H., Lemire M. Assessment of naturally acquired neutralizing antibodies against rabies Lyssavirus in a subset of Nunavik's Inuit population considered most at risk of being exposed to rabid animals. Zoonoses Public Health. 2019;66(5):533–539. doi: 10.1111/zph.12561. [DOI] [PubMed] [Google Scholar]
- 83.Gaulin C., Ramsay D., Thivierge K., et al. Acute toxoplasmosis among Canadian deer hunters associated with consumption of undercooked deer meat hunted in the United States. Emerg Infect Dis. 2020;26(2):199. doi: 10.3201/eid2602.191218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lo Iacono G., Cunningham A.A., Fichet-Calvet E., et al. A unified framework for the infection dynamics of zoonotic spillover and spread. PLoS Negl Trop Dis. 2016;10(9) doi: 10.1371/journal.pntd.0004957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Frutos R., Gavotte L., Devaux C.A. Understanding the origin of COVID-19 requires to change the paradigm on zoonotic emergence from the spillover model to the viral circulation model. Infect Genet Evol. 2021 doi: 10.1016/j.meegid.2021.104812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sokolow S.H., Nova N., Pepin K.M., et al. Ecological interventions to prevent and manage zoonotic pathogen spillover. Philos Trans R Soc B. 2019;374(1782) doi: 10.1098/rstb.2018.0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pooley S., Nasi R., Fa J.E. No conservation silver lining to Ebola. Conserv Biol. 2015;29(3):965–967. doi: 10.1111/cobi.12454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Coalition to end the trade. Sign the petition to #ENDTHETRADE. https://endthetrade.com/. Published 2020. Accessed 23 April 2020.
- 89.WCS. Summary of WCS policy and messaging on COVID-19.https://c532f75abb9c1c021b8c-e46e473f8aadb72cf2a8ea564b4e6a76.ssl.cf5.rackcdn.com/2020/04/01/8294efiuzg_COVID_19_Summary_of_WCS_Policies_and_Messaging_March29.2.pdf. Published 2020. Accessed 23 November 2020.
- 90.World animal protection. End the global wildlife trade. Forever.
- 91.Bonwitt J., Dawson M., Kandeh M., et al. Unintended consequences of the ‘bushmeat ban’ in West Africa during the 2013-2016 Ebola virus disease epidemic. Soc Sci Med. 2018;200:166–173. doi: 10.1016/j.socscimed.2017.12.028. [DOI] [PubMed] [Google Scholar]
- 92.Gorman J. China's ban on wildlife trade a big step, but has loopholes, conservationists say. New York Times. https://www.nytimes.com/2020/02/27/science/coronavirus-pangolin-wildlife-ban-china.html. Published February 27, 2020.
- 93.Conrad K. Trade bans: a perfect storm for poaching? Trop Conserv Sci. 2012;5(3):245–254. [Google Scholar]
- 94.Challender D.W.S., Harrop S.R., MacMillan D.C. Towards informed and multi-faceted wildlife trade interventions. Glob Ecol Conserv. 2015;3:129–148. doi: 10.1016/j.gecco.2014.11.010. [DOI] [Google Scholar]
- 95.Weber D.S., Mandler T., Dyck M., De Groot P.J.V.C., Lee D.S., Clark D.A. Unexpected and undesired conservation outcomes of wildlife trade bans - an emerging problem for stakeholders? Glob Ecol Conserv. 2015;3:389–400. [Google Scholar]
- 96.Chen F. Poachers and snobs: demand for rarity and the effects of antipoaching policies. Conserv Lett. 2016;9(1):65–69. [Google Scholar]
- 97.Shairp R., Veríssimo D., Fraser I., Challender D., MacMillan D. Understanding urban demand for wild meat in Vietnam: implications for conservation actions. PLoS One. 2016;11(1) doi: 10.1371/journal.pone.0134787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wilkie D.S., Wieland M., Boulet H., et al. Eating and conserving bushmeat in Africa. Afr J Ecol. 2016;54(4):402–414. [Google Scholar]
- 99.Golden C.D., Fernald L.C.H., Brashares J.S., Rasolofoniaina B.J.R., Kremen C. Benefits of wildlife consumption to child nutrition in a biodiversity hotspot. Proc Natl Acad Sci. 2011;108(49):19653–19656. doi: 10.1073/pnas.1112586108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sarti F.M., Adams C., Morsello C., et al. Beyond protein intake: bushmeat as source of micronutrients in the Amazon. Ecol Soc. 2015;20(4):22. [Google Scholar]
- 101.Barboza R.R., Lopes S.F., Souto W.M.S., Fernandes-Ferreira H., Alves R.R.N. The role of game mammals as bushmeat in the Caatinga, northeast Brazil. Ecol Soc. 2016;21(2) [Google Scholar]
- 102.Van Vliet N., Moreno J., Gómez J., et al. Bushmeat and human health: assessing the evidence in tropical and sub-tropical forests. Ethnobiol Conserv. 2017;6(3):1–45. [Google Scholar]
- 103.Meer T., Schnurr M.A. The community versus community-based natural resource management: the case of Ndumo game reserve, South Africa. Can J Dev Stud Can d’études du Dév. 2013;34(4):482–497. doi: 10.1080/02255189.2013.849580. [DOI] [Google Scholar]
- 104.Cooney R., Roe D., Dublin H., et al. From poachers to protectors: engaging local communities in solutions to illegal wildlife trade. Conserv Lett. 2017;10(3):367–374. [Google Scholar]
- 105.Nguyen T.T.T., Fearnley L., Dinh X.T., et al. A stakeholder survey on live bird market closures policy for controlling highly pathogenic avian influenza in Vietnam. Front Vet Sci. 2017;4(136):136. doi: 10.3389/fvets.2017.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Plowright R.K., Parrish C.R., McCallum H., et al. Pathways to zoonotic spillover. Nat Rev Microbiol. 2017;15:502–510. doi: 10.1038/nrmicro.2017.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chaves L.S.M., Fry J., Malik A., Geschke A., Sallum M.A.M., Lenzen M. Global consumption and international trade in deforestation-associated commodities could influence malaria risk. Nat Commun. 2020;11(1):1258. doi: 10.1038/s41467-020-14954-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Allen T., Murray K.A., Zambrana-Torrelio C., et al. Global hotspots and correlates of emerging zoonotic diseases. Nat Commun. 2017;8(1124) doi: 10.1038/s41467-017-00923-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Olivero J., Fa J.E., Real R., et al. Recent loss of closed forests is associated with Ebola virus disease outbreaks. Sci Rep. 2017;7(1):1–9. doi: 10.1038/s41598-017-14727-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Burkett-Cadena N.D., Vittor A.Y. Deforestation and vector-borne disease: forest conversion favors important mosquito vectors of human pathogens. Basic Appl Ecol. 2018;26:101–110. doi: 10.1016/j.baae.2017.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wallace R., Chaves L.F., Bergmann L.R., et al. Springer; 2018. Clear-Cutting Disease Control: Capital-Led Deforestation, Public Health Austerity, and Vector-Borne Infection. [Google Scholar]
- 112.MacDonald A.J., Mordecai E.A. Amazon deforestation drives malaria transmission, and malaria burden reduces forest clearing. Proc Natl Acad Sci. 2019;116(44):22212–22218. doi: 10.1073/pnas.1905315116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Murray K.A., Daszak P. Human ecology in pathogenic landscapes: two hypotheses on how land use change drives viral emergence. Curr Opin Virol. 2013;3(1):79–83. doi: 10.1016/j.coviro.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Borremans B., Faust C., Manlove K.R., Sokolow S.H., Lloyd-Smith J.O. Cross-species pathogen spillover across ecosystem boundaries: mechanisms and theory. Philos Trans R Soc B. 2019;374(1782) doi: 10.1098/rstb.2018.0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rulli M.C., D'Odorico P., Galli N., Hayman D.T.S. Land-use change and the livestock revolution increase the risk of zoonotic coronavirus transmission from rhinolophid bats. Nat Food. 2021;2(6):409–416. doi: 10.1038/s43016-021-00285-x. [DOI] [PubMed] [Google Scholar]
- 116.Faust C.L., McCallum H.I., Bloomfield L.S.P., et al. Pathogen spillover during land conversion. Ecol Lett. 2018;21(4):471–483. doi: 10.1111/ele.12904. [DOI] [PubMed] [Google Scholar]
- 117.Bloomfield L.S.P., Tyler L.M., Lambin E.F. Habitat fragmentation, livelihood behaviors, and contact between people and non-human primates in Africa. Landsc Ecol. 2020;35:985–1000. [Google Scholar]
- 118.Sarute N., Ross S.R. New world arenavirus biology. Annu Rev Virol. 2017;4:141–158. doi: 10.1146/annurev-virology-101416-042001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Plowright R.K., Foley P., Field H.E., et al. Urban habituation, ecological connectivity and epidemic dampening: the emergence of Hendra virus from flying foxes (Pteropus spp.) Proc R Soc B Biol Sci. 2011;278(1725):3703–3712. doi: 10.1098/rspb.2011.0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Civitello D.J., Cohen J., Fatima H., et al. Biodiversity inhibits parasites: broad evidence for the dilution effect. Proc Natl Acad Sci. 2015;112(28):8667–8671. doi: 10.1073/pnas.1506279112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Glidden C.K., Nova N., Kain M.P., et al. Human-mediated impacts on biodiversity and the consequences for zoonotic disease spillover. Curr Biol. 2021;31(19):1342–1361. doi: 10.1016/j.cub.2021.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Young H.S., Dirzo R., Helgen K.M., et al. Declines in large wildlife increase landscape-level prevalence of rodent-borne disease in Africa. Proc Natl Acad Sci. 2014;111(19):7036–7041. doi: 10.1073/pnas.1404958111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rohr J.R., Civitello D.J., Halliday F.W., et al. Towards common ground in the biodiversity–disease debate. Nat Ecol Evol. 2020;4(1):24–33. doi: 10.1038/s41559-019-1060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Plowright R.K., Reaser J.K., Locke H., et al. Land use-induced spillover: a call to action to safeguard environmental, animal, and human health. Lancet Planet Health. 2021 doi: 10.1016/S2542-5196(21)00031-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Alexandratos N., Bruinsma J. FAO; Rome, Italy: 2012. World Agriculture towards 2030/2050: The 2012 Revision. [Google Scholar]
- 126. FAO. Land use in agriculture by the numbers. 2020. https://www.fao.org/sustainability/news/detail/en/c/1274219/.
- 127.Foley J.A., Ramankutty N., Brauman K.A., et al. Solutions for a cultivated planet. Nature. 2011;478(7369):337–342. doi: 10.1038/nature10452. [DOI] [PubMed] [Google Scholar]
- 128.Mottet A., de Haan C., Falcucci A., Tempio G., Opio C., Gerber P. Livestock: on our plates or eating at our table? A new analysis of the feed/food debate. Glob Food Secur. 2017;14:1–8. [Google Scholar]
- 129.Cassidy E.S., West P.C., Gerber J.S., Foley J.A. Redefining agricultural yields: from tonnes to people nourished per hectare. Environ Res Lett. 2013;8 doi: 10.1088/1748-9326/8/3/034015. [DOI] [Google Scholar]
- 130.Ramankutty N., Mehrabi Z., Waha K., et al. Trends in global agricultural land use: implications for environmental health and food security. Annu Rev Plant Biol. 2018;69(1):789–815. doi: 10.1146/annurev-arplant-042817-040256. [DOI] [PubMed] [Google Scholar]
- 131.NFS. The national food strategy - the plan. UK; 2021. file:///C:/Users/User/Downloads/1669_NFS_The_Plan_July21_S11 (1).pdf.
- 132.Gibbs H.K., Ruesch A.S., Achard F., et al. Tropical forests were the primary sources of new agricultural land in the 1980s and 1990s. Proc Natl Acad Sci. 2010;107(38):16732–16737. doi: 10.1073/PNAS.0910275107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ramankutty N., Mehrabi Z., Waha K., et al. Trends in global agricultural land use: implications for environmental health and food security. Annu Rev Plant Biol. 2018;69(1):789–815. doi: 10.1146/annurev-arplant-042817-040256. [DOI] [PubMed] [Google Scholar]
- 134.Roux N., Kastner T., Erb K.H., Haberl H. Does agricultural trade reduce pressure on land ecosystems? Decomposing drivers of the embodied human appropriation of net primary production. Ecol Econ. 2021;181 [Google Scholar]
- 135.De Sy V., Herold M., Achard F., et al. Land use patterns and related carbon losses following deforestation in South America. Environ Res Lett. 2015;10(12) [Google Scholar]
- 136.Tilman D., Clark M., Williams D.R., Kimmel K., Polasky S., Packer C. Future threats to biodiversity and pathways to their prevention. Nature. 2017;546:73–81. doi: 10.1038/nature22900. [DOI] [PubMed] [Google Scholar]
- 137.IPCC. Edenhofer O., Pichs-Madruga R., Sokona Y. Cambridge University Press; 2014. Climate Change 2014: Mitigation of Climate Change. Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel On Climate Change. et al., eds.). Cambridge, uk and New York, US. [Google Scholar]
- 138.Poore J., Nemecek T. Reducing food's environmental impacts through producers and consumers. Science. 2018;360:987–992. doi: 10.1126/science.aaq0216. (80-) [DOI] [PubMed] [Google Scholar]
- 139.Springmann M., Clark M., Mason-D'Croz D., et al. Options for keeping the food system within environmental limits. Nature. 2018;562(7728):519–525. doi: 10.1038/s41586-018-0594-0. [DOI] [PubMed] [Google Scholar]
- 140.Mbow C, Rosenzweig C, Barioni LG, et al. Food security. In: Shukla PR, Skea J, Buendia EC, et al., eds. Climate change and land: an IPCC special report on climate change, desertification, land degradation, sustainable land management, food security, and greenhouse gas fluxes in terrestrial ecosystems.; 2019.
- 141.Machovina B., Feeley K.J., Ripple W.J. Biodiversity conservation: the key is reducing meat consumption. Sci Total Environ. 2015;536:419–431. doi: 10.1016/j.scitotenv.2015.07.022. [DOI] [PubMed] [Google Scholar]
- 142.Newbold T., Hudson L.N., Arnell A.P., et al. Has land use pushed terrestrial biodiversity beyond the planetary boundary? A global assessment. Science. 2016;353(6296) doi: 10.1126/science.aaf2201. (80-)291-288. [DOI] [PubMed] [Google Scholar]
- 143.Roslyn C.H., Alexander P., Rabin S., Anthoni P., Rounsevell M.D.A., Arneth A. The role of global dietary transitions for safeguarding biodiversity. Glob Environ Chang. 2019:58. [Google Scholar]
- 144.Faust C.L., McCallum H.I., Bloomfield L.S.P., et al. Pathogen spillover during land conversion. Ecol Lett. 2018;21(4):471–483. doi: 10.1111/ele.12904. [DOI] [PubMed] [Google Scholar]
- 145.United Nations. World Population Prospects 2019: Data Booklet (ST/ESA/SER.A/424). 2019.
- 146.FAO, IFAD, UNICEF, WFP, WHO. State of Food security and nutrition in the World 2018: building climate resilience for food security and nutrition. 2018. http://www.fao.org/3/ca5162en/ca5162en.pdf.
- 147.Robinson S, Mason-D'Croz D, Sulser T, et al. The international model for policy analysis of agricultural commodities and trade (IMPACT): model description for Version 3.; 2015.
- 148.Micha R., Khatibzadeh S., Shi P., Andrews K.G., Engell R.E., Mozaffarian D. Global, regional and national consumption of major food groups in 1990 and 2010: a systematic analysis including 266 country-specific nutrition surveys worldwide. BMJ Open. 2015;5(9) doi: 10.1136/bmjopen-2015-008705. doi:e008705 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.FAO. FAOSTAT. http://www.fao.org/faostat/en/#home. Published 2020.
- 150.Bodirsky B.L., Pradhan P., Springmann M. Reducing ruminant numbers and consumption of animal source foods are aligned with environmental and public health demands. J Sustain Organ Agric Syst. 2019;69:25–30. [Google Scholar]
- 151.NFS . NFS; UK: 2021. The National Food Strategy - The Plan. [Google Scholar]
- 152.FAO . Food and Agriculture Organization of the United Nations (FAO); Rome, Italy: 2017. The State of Food and Agriculture: Leveraging Food Systems for Inclusive Rural Transformation. [Google Scholar]
- 153.Godfray H.C.J., Aveyard P., Garnett T., et al. Meat consumption, health, and the environment. Science. 2018;361(6399):eaam5324. doi: 10.1126/science.aam5324. (80-) [DOI] [PubMed] [Google Scholar]
- 154.Chaves W.A., Valle D.R., Monroe M.C., Wilkie D.S., Sieving K.E., Sadowsky B. Changing wild meat consumption: an experiment in the Central Amazon, Brazil. Conserv Lett. 2018;11:e12391. [Google Scholar]
- 155.Nasi R., Taber A., van Vliet N. Empty forests, empty stomachs? Bushmeat and livelihoods in the Congo and Amazon Basins. Int For Rev. 2011;13(3):355–368. [Google Scholar]
- 156.Steffen W., Richardson K., Rockstrom J., et al. Planetary boundaries: guiding human development on a changing planet. Science. 2015;347(6223) doi: 10.1126/science.1259855. (80-)1259855-1259855. [DOI] [PubMed] [Google Scholar]
- 157.Springmann M., Clark M., Mason-D'Croz D., et al. Options for keeping the food system within environmental limits. Nature. 2018;562(7728):519–525. doi: 10.1038/s41586-018-0594-0. [DOI] [PubMed] [Google Scholar]
- 158.Lesk C., Rowhani P., Ramankutty N. Influence of extreme weather disasters on global crop production. Nature. 2016;529(7584):84–87. doi: 10.1038/nature16467. [DOI] [PubMed] [Google Scholar]
- 159.Roy J., Tscharket P., Waisman H., Masson-Delmotte V., Zhai P., Pörtner H.O., et al. Global Warming of 1.5 °C. An IPCC Special Report. Cambridge University Press; 2018. Sustainable development, poverty eradication and reducing inequalities. [Google Scholar]
- 160.Semenza J.C., Suk J.E. Vector-borne diseases and climate change: a European perspective. FEMS Microbiol Lett. 2018;365(2):fnx244. doi: 10.1093/femsle/fnx244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Vezzulli L., Colwell R.R., Pruzzo C. Ocean warming and spread of pathogenic vibrios in the aquatic environment. Microb Ecol. 2013;65(4):817–825. doi: 10.1007/s00248-012-0163-2. [DOI] [PubMed] [Google Scholar]
- 162.Willett W., Rockström J., Loken B., et al. Food in the anthropocene: the EAT–Lancet commission on healthy diets from sustainable food systems. Lancet. 2019;393(10170):447–492. doi: 10.1016/S0140-6736(18)31788-4. [DOI] [PubMed] [Google Scholar]
- 163.Clark M.A., Domingo N.G.G., Colgan K., et al. Global food system emissions could preclude achieving the 1.5° and 2°C climate change targets. Science. 2020;370(6517):705–708. doi: 10.1126/science.aba7357. (80-) [DOI] [PubMed] [Google Scholar]
- 164.Ceballos G., Ehrlich P.R., Dirzo R. Biological annihilation via the ongoing sixth mass extinction signaled by vertebrate population losses and declines. Proc Natl Acad Sci. 2017;114(30):E6089–E6096. doi: 10.1073/pnas.1704949114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Steffen W., Richardson K., Rockstrom J., et al. Planetary boundaries: guiding human development on a changing planet. Science. 2015;347(6223) doi: 10.1126/science.1259855. (80-)1259855-1259855. [DOI] [PubMed] [Google Scholar]
- 166.Newbold T., Hudson L.N., Arnell A.P., et al. Has land use pushed terrestrial biodiversity beyond the planetary boundary? A global assessment. Science. 2016;353(6296) doi: 10.1126/science.aaf2201. (80-)291-288. [DOI] [PubMed] [Google Scholar]
- 167.Lanz B., Dietz S., Swanson T. The expansion of modern agriculture and global biodiversity decline: an integrated assessment. Ecol Econ. 2018;144:260–277. [Google Scholar]
- 168.Morand S., Lajaunie C. Outbreaks of vector-borne and zoonotic diseases are associated with changes in forest cover and oil palm expansion at global scale. Front Vet Sci. 2021;8:230. doi: 10.3389/fvets.2021.661063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Foley J.A., Ramankutty N., Brauman K.A., et al. Solutions for a cultivated planet. Nature. 2011;478(7369):337–342. doi: 10.1038/nature10452. [DOI] [PubMed] [Google Scholar]
- 170.Crippa M., Solazzo E., Guizzardi D., Monforti-Ferrario F., Tubiello F.N., Leip A. Food systems are responsible for a third of global anthropogenic GHG emissions. Nat Food. 2021;2(3):198–209. doi: 10.1038/s43016-021-00225-9. [DOI] [PubMed] [Google Scholar]
- 171.Mekonnen M.M., Hoekstra A.Y. A global assessment of the water footprint of farm animal products. Ecosystems. 2012;15(3):401–415. doi: 10.1007/s10021-011-9517-8. [DOI] [Google Scholar]
- 172.Clark M.A., Springmann M., Hill J., Tilman D. Multiple health and environmental impacts of foods. Proc Natl Acad Sci. 2019;116(46):23357–23362. doi: 10.1073/pnas.1906908116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Uwizeye A., de Boer I.J.M., Opio C.I., et al. Nitrogen emissions along global livestock supply chains. Nat Food. 2020;1(7):437–446. [Google Scholar]
- 174.Lavaine E., Majerus P., Treich N. Health, air pollution, and animal agriculture. Rev Agric Food Environ Stud. 2020:1–12. [Google Scholar]
- 175.Tilman D., Clark M., Williams D.R., Kimmel K., Polasky S., Packer C. Future threats to biodiversity and pathways to their prevention. Nature. 2017;546:73–81. doi: 10.1038/nature22900. [DOI] [PubMed] [Google Scholar]
- 176.Rohr J.R., Civitello D.J., Halliday F.W., et al. Towards common ground in the biodiversity–disease debate. Nat Ecol Evol. 2020;4(1):24–33. doi: 10.1038/s41559-019-1060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Tilman D., Balzer C., Hill J., Befort B.L. Global food demand and the sustainable intensification of agriculture. Proc Natl Acad Sci. 2011;108(50):20260–20264. doi: 10.1073/PNAS.1116437108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Cassman K., Grassini P. A global perspective on sustainable intensification research. Nat Sustain. 2020;3:262–268. doi: 10.1038/s41893-020-0507-8. [DOI] [Google Scholar]
- 179.Struik P.C., Kuyper T.W., Brussaard L., Leeuwis C. Deconstructing and unpacking scientific controversies in intensification and sustainability: why the tensions in concepts and values? Curr Opin Environ Sustain. 2014;8:80–88. [Google Scholar]
- 180.Godfray C.H.J., Garnett T. Food security and sustainable intensification. Philos Trans R Soc B. 2014;369(1639) doi: 10.1098/rstb.2012.0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Bramley R.G .V. Lessons from nearly 20 years of Precision Agriculture research, development, and adoption as a guide to its appropriate application. Crop Pasture Sci. 2009;60(3):197–217. [Google Scholar]
- 182.Herrero M., Henderson B., Havlík P., et al. Greenhouse gas mitigation potentials in the livestock sector. Nat Clim Chang. 2016;6:452–461. [Google Scholar]
- 183.Pretty J., Benton T.G., Bharucha Z.P., Al E. Global assessment of agricultural system redesign for sustainable intensification. Nat Sustain. 2018;1:441–446. [Google Scholar]
- 184.Cassman K., Grassini P. A global perspective on sustainable intensification research. Nat Sustain. 2020;3:262–268. doi: 10.1038/s41893-020-0507-8. [DOI] [Google Scholar]
- 185.Conant R.T., Cerri C.E.P., Osborne B.B., Paustian K. Grassland management impacts on soil carbon stocks: a new synthesis. Ecol Appl. 2017;27(2):662–668. doi: 10.1002/eap.1473. [DOI] [PubMed] [Google Scholar]
- 186.Garnett T, Godde C, Muller A, et al. Grazed and confused? Ruminating on cattle, grazing systems, methane, nitrous oxide, the soil carbon sequestration question – and what it all means for greenhouse gas emissions. Oxford, UK; 2017.
- 187.Muller A., Schader C., Scialabba N.E.H., et al. Strategies for feeding the world more sustainably with organic agriculture. Nat Commun. 2017;8:1290. doi: 10.1038/s41467-017-01410-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Hayek M.N., Garrett R.D. Nationwide shift to grass-fed beef requires larger cattle population. Environ Res Lett. 2018;13(8):84005. [Google Scholar]
- 189.Bouvard V., Loomis D., Guyton K.Z., et al. Carcinogenicity of consumption of red and processed meat. Lancet Oncol. 2015;16(16):1599–1600. doi: 10.1016/S1470-2045(15)00444-1. [DOI] [PubMed] [Google Scholar]
- 190.Afshin A., Sur P.J., Fay K.A., et al. Health effects of dietary risks in 195 countries, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2019;393(10184):1958–1972. doi: 10.1016/S0140-6736(19)30041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Bechthold A., Boeing H., Schwedhelm C., et al. Food groups and risk of coronary heart disease, stroke and heart failure: a systematic review and dose-response meta-analysis of prospective studies. Crit Rev Food Sci Nutr. 2019;59(7):1071–1090. doi: 10.1080/10408398.2017.1392288. [DOI] [PubMed] [Google Scholar]
- 192.Zheng Y., Li Y., Satija A., et al. Association of changes in red meat consumption with total and cause specific mortality among US women and men: two prospective cohort studies. BMJ. 2019:365. doi: 10.1136/bmj.l2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Springmann M., Spajic L., Clark M.A., et al. The healthiness and sustainability of national and global food based dietary guidelines: modelling study. BMJ. 2020:370. doi: 10.1136/bmj.m2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Eisler M.C., Lee M.R.F., Tarlton J.F., et al. Agriculture: steps to sustainable livestock. Nature. 2014;507(7490):32. doi: 10.1038/507032a. [DOI] [PubMed] [Google Scholar]
- 195.Clark M., Tilman D. Comparative analysis of environmental impacts of agricultural production systems, agricultural input efficiency, and food choice. Environ Res Lett. 2017;12(6):64016. [Google Scholar]
- 196.Van Hal O., De Boer I.J.M., Muller A., et al. Upcycling food leftovers and grass resources through livestock: impact of livestock system and productivity. J Clean Prod. 2019;219:485–496. [Google Scholar]
- 197.Skinner C., Gattinger A., Muller A., et al. Greenhouse gas fluxes from agricultural soils under organic and non-organic management — a global meta-analysis. Sci Total Environ. 2014;468-469:553–563. doi: 10.1016/j.scitotenv.2013.08.098. [DOI] [PubMed] [Google Scholar]
- 198.Tuck S.L., Winqvist C., Mota F., Ahnström J., Turnbull L.A., Bengtsson J. Land-use intensity and the effects of organic farming on biodiversity: a hierarchical meta-analysis. J Appl Ecol. 2014;51(3):746–755. doi: 10.1111/1365-2664.12219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Meier M.S., Stoessel F., Jungbluth N., Juraske R., Schader C., Stolze M. Environmental impacts of organic and conventional agricultural products – are the differences captured by life cycle assessment? J Environ Manag. 2015;149(1):193–208. doi: 10.1016/j.jenvman.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 200.Seufert V., Ramankutty N. Many shades of gray—the context-dependent performance of organic agriculture. Sci Adv. 2017;3(3) doi: 10.1126/sciadv.1602638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Willer H., Lernoud J. Frick and Bonn; 2019. The World of Organic Agriculture: Statistics and Emerging Trends 2019. [Google Scholar]
- 202.Nemecek T., Dubois D., Huguenin-Elie O., Gaillard G. Life cycle assessment of Swiss farming systems: I. Integrated and organic farming. Agric Syst. 2011;104(3):217–232. doi: 10.1016/j.agsy.2010.10.002. [DOI] [Google Scholar]
- 203.Gattinger A., Muller A., Haeni M., et al. Enhanced top soil carbon stocks under organic farming. Proc Natl Acad Sci. 2012;109(44):18226–18231. doi: 10.1073/pnas.1209429109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Nemecek T., Dubois D., Huguenin-Elie O., Gaillard G. Life cycle assessment of Swiss farming systems: I. Integrated and organic farming. Agric Syst. 2011;104(3):217–232. doi: 10.1016/j.agsy.2010.10.002. [DOI] [Google Scholar]
- 205.Skinner C., Gattinger A., Muller A., et al. Greenhouse gas fluxes from agricultural soils under organic and non-organic management — a global meta-analysis. Sci Total Environ. 2014;468-469:553–563. doi: 10.1016/j.scitotenv.2013.08.098. [DOI] [PubMed] [Google Scholar]
- 206.Seufert V., Ramankutty N. Many shades of gray—the context-dependent performance of organic agriculture. Sci Adv. 2017;3(3) doi: 10.1126/sciadv.1602638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Tuck S.L., Winqvist C., Mota F., Ahnström J., Turnbull L.A., Bengtsson J. Land-use intensity and the effects of organic farming on biodiversity: a hierarchical meta-analysis. J Appl Ecol. 2014;51(3):746–755. doi: 10.1111/1365-2664.12219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Feber R.E., Johnson P.J., Chamberlain D.E., Macdonald D.W., Feber R.E., et al. Wildlife Conservation on Farmland. Oxford University Press; Oxford, UK: 2015. Does organic farming affect biodiversity? pp. 108–132. [Google Scholar]
- 209.Ponisio L.C., M'Gonigle L.K., Mace K.C., Palomino J., de Valpine P., Kremen C. Diversification practices reduce organic to conventional yield gap. Proc R Soc B Biol Sci. 2015;282(1799) doi: 10.1098/rspb.2014.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Tilman D., Balzer C., Hill J., Befort B.L. Global food demand and the sustainable intensification of agriculture. Proc Natl Acad Sci. 2011;108(50):20260–20264. doi: 10.1073/PNAS.1116437108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Burney J.A., Davis S.J., Lobell D.B. Greenhouse gas mitigation by agricultural intensification. Proc Natl Acad Sci U S A. 2010;107(26):12052–12057. doi: 10.1073/pnas.0914216107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Burney J.A., Davis S.J., Lobell D.B. Greenhouse gas mitigation by agricultural intensification. Proc Natl Acad Sci U S A. 2010;107(26):12052–12057. doi: 10.1073/pnas.0914216107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Díaz S., Settele J., Brondízio E.S., et al. Pervasive human-driven decline of life on Earth points to the need for transformative change. Science. 2019;366(6471) doi: 10.1126/science.aax3100. (80-) [DOI] [PubMed] [Google Scholar]
- 214.Kummu M., de Moel H., Porkka M., Siebert S., Varis O., Ward P.J. Lost food, wasted resources: global food supply chain losses and their impacts on freshwater, cropland, and fertiliser use. Sci Total Environ. 2012;438:477–489. doi: 10.1016/j.scitotenv.2012.08.092. [DOI] [PubMed] [Google Scholar]
- 215.Cassidy E.S., West P.C., Gerber J.S., Foley J.A. Redefining agricultural yields: from tonnes to people nourished per hectare. Environ Res Lett. 2013;8 doi: 10.1088/1748-9326/8/3/034015. [DOI] [Google Scholar]
- 216.Erb K.H., Lauk C., Kastner T., Mayer A., Theurl M.C., Haberl H. Exploring the biophysical option space for feeding the world without deforestation. Nat Commun. 2016;7:11382. doi: 10.1038/ncomms11382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Schader C., Muller A., El-Hage Scialabba N., et al. Impacts of feeding less food-competing feedstuffs to livestock on global food system sustainability. J R Soc Interface. 2015;12(20150891) doi: 10.1098/rsif.2015.0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Gustavsson J, Cederberg C, Sonesson U. Global food losses and food waste: extent, causes and prevention. Rome, Italy; 2011.
- 219.Kummu M., de Moel H., Porkka M., Siebert S., Varis O., Ward P.J. Lost food, wasted resources: global food supply chain losses and their impacts on freshwater, cropland, and fertiliser use. Sci Total Environ. 2012;438:477–489. doi: 10.1016/j.scitotenv.2012.08.092. [DOI] [PubMed] [Google Scholar]
- 220.Vanloqueren G., Baret P.V. How agricultural research systems shape a technological regime that develops genetic engineering but locks out agroecological innovations. Res Policy. 2009;38(6):971–983. doi: 10.1016/j.respol.2009.02.008. [DOI] [Google Scholar]
- 221.Van Bueren E.T.L., Jones S.S., Tamm L., et al. The need to breed crop varieties suitable for organic farming, using wheat, tomato and broccoli as examples: a review. NJAS Wagening J Life Sci. 2011;58(3–4):193–205. doi: 10.1016/j.njas.2010.04.001. [DOI] [Google Scholar]
- 222.Isbell F., Adler P.R., Eisenhauer N., et al. Benefits of increasing plant diversity in sustainable agroecosystems. J Ecol. 2017;105(4):871–879. [Google Scholar]
- 223.Ponisio L.C., M'Gonigle L.K., Mace K.C., Palomino J., de Valpine P., Kremen C. Diversification practices reduce organic to conventional yield gap. Proc R Soc B Biol Sci. 2015;282(1799) doi: 10.1098/rspb.2014.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Thornton P.K., Herrero M. Adapting to climate change in the mixed crop and livestock farming systems in sub-Saharan Africa. Nat Clim Chang. 2015;5(9):830–836. [Google Scholar]
- 225.Specht K., Siebert R., Hartmann I., et al. Urban agriculture of the future: an overview of sustainability aspects of food production in and on buildings. Agric Hum Values. 2014;31(1):33–51. [Google Scholar]
- 226.Goddek S., Delaide B., Mankasingh U., Ragnarsdottir K.V., Jijakli H., Thorarinsdottir R. Challenges of sustainable and commercial aquaponics. Sustainability. 2015;7(4):4199–4224. [Google Scholar]
- 227.Muller A., Ferré M., Engel S., et al. Can soil-less crop production be a sustainable option for soil conservation and future agriculture? Land Use Policy. 2017;69:102–105. [Google Scholar]
- 228.Nepstad D., McGrath D., Stickler C., et al. Slowing Amazon deforestation through public policy and interventions in beef and soy supply chains. Science. 2014;344(6188):1118–1123. doi: 10.1126/science.1248525. (80-) [DOI] [PubMed] [Google Scholar]
- 229.Macdiarmid J.I., Douglas F., Campbell J. Eating like there's no tomorrow: public awareness of the environmental impact of food and reluctance to eat less meat as part of a sustainable diet. Appetite. 2016;96:487–493. doi: 10.1016/j.appet.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 230.Smil V. Eating meat: evolution, patterns, and consequences. Popul Dev Rev. 2002;28(4):599–639. [Google Scholar]
- 231.Piazza J., Ruby M.B., Loughnan S., et al. Rationalizing meat consumption. The 4Ns. Appetite. 2015;91:114–128. doi: 10.1016/j.appet.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 232.FAO. FAOSTAT. www.fao.org/faostat/en/?#data.
- 233.Mensink G.B.M., Barbosa C.L., Brettschneider A.K. Prevalence of persons following a vegetarian diet in Germany. J Health Monit. 2016;1(2):2–14. doi: 10.17886/RKI-GBE-2016-039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Neff R.A., Edwards D., Palmer A., Ramsing R., Righter A., Wolfson J. Reducing meat consumption in the USA: a nationally representative survey of attitudes and behaviours. Public Health Nutr. 2018;21(10):1835–1844. doi: 10.1017/S1368980017004190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Clark B., Stewart G.B., Panzone L.A., Kyriazakis I., Frewer L.J. Citizens, consumers and farm animal welfare: a meta-analysis of willingness-to-pay studies. Food Policy. 2017;68:112–127. [Google Scholar]
- 236.Kushwah S., Dhir A., Sagar M., Gupta B. Determinants of organic food consumption. A systematic literature review on motives and barriers. Appetite. 2019;143 doi: 10.1016/j.appet.2019.104402. [DOI] [PubMed] [Google Scholar]
- 237.Fesenfeld L.P., Wicki M., Sun Y., Bernauer T. Policy packaging can make food system transformation feasible. Nat Food. 2020;1(3):173–182. [Google Scholar]
- 238.Rivers C.J., McCoskey S. Does global meat consumption follow an environmental Kuznets curve. SSPP. 2013;9:26–36. [Google Scholar]
- 239.Bennett J., Gathering R.S., Bennett J., et al. McGill-Queen's University Press; 2004. Uqalurait: An Oral History of Nunavu; pp. 78–85. [Google Scholar]
- 240.Catley A., Lind J., Scoones I. Routledge and Earthscan; New York, USA: 2013. Pastoralism and Development in Africa: Dynamic Change At the Margins. [DOI] [Google Scholar]
- 241.Fa J.E., van Vliet N., Nasi R., Aguirre AA, Sukumar R. Tropical Conservation: Perspectives on Local and Global Priorities. Oxford University Press; Oxford, UK: 2016. Bushmeat, food security, and conservation in African rainforests. [Google Scholar]
- 242.Mozaffarian D., Afshin A., Benowitz N.L., et al. Population approaches to improve diet, physical activity, and smoking habits: a scientific statement from the American Heart Association. Circulation. 2012;126(12):1514–1563. doi: 10.1161/CIR.0b013e318260a20b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Garnett T, Mathewson S, Angelides P, Borthwick F, House C. Policies and actions to shift eating patterns: what works? London, UK; 2015. http://africacsa.org/#proposed-. Accessed 23 December 2020.
- 244.Bonnet C., Bouamra-Mechemache Z., Réquillart V., Treich N. Viewpoint: regulating meat consumption to improve health, the environment and animal welfare. Food Policy. 2020;97 [Google Scholar]
- 245.Springmann M., Mason-D'Croz D., Robinson S., et al. Health-motivated taxes on red and processed meat: a modelling study on optimal tax levels and associated health impacts. PLoS One. 2018;13(11) doi: 10.1371/journal.pone.0204139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Walton S, Hawkes C. What we can learn: a review of food policy innovations in six countries.; 2020. https://openaccess.city.ac.uk/id/eprint/24918/.
- 247.Vlaeminck P., Jiang T., Vranken L. Food labeling and eco-friendly consumption: experimental evidence from a Belgian supermarket. Ecol Econ. 2014;108:180–190. [Google Scholar]
- 248.Muller L., Lacroix A., Ruffieux B. Environmental labelling and consumption changes: a food choice experiment. Environ Resour Econ. 2019;73:871–897. doi: 10.1007/s10640-019-00328-9. [DOI] [Google Scholar]
- 249.Garnett E.E., Balmford A., Sandbrook C., Pilling M.A., Marteau T.M. Impact of increasing vegetarian availability on meal selection and sales in cafeterias. Proc Natl Acad Sci. 2019;116(42):20923–20929. doi: 10.1073/pnas.1907207116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Kurz V. Nudging to reduce meat consumption: immediate and persistent effects of an intervention at a university restaurant. J Environ Econ Manag. 2018;90:317–341. [Google Scholar]
- 251.Espinosa R., Tago D., Treich N. Infectious diseases and meat production. Environ Resour Econ. 2020;76:1019–1044. doi: 10.1007/s10640-020-00484-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Smith P., Martino D., Cai Z., et al. Policy and technological constraints to implementation of greenhouse gas mitigation options in agriculture. Agric Ecosyst Environ. 2007;118(1–4):6–28. [Google Scholar]
- 253.Mehrabi Z., Gill M., van Wijk M., Herrero M., Ramankutty N. Livestock policy for sustainable development. Nat Food. 2020;1(3):160–165. [Google Scholar]
- 254.Springmann M., Mason-D'Croz D., Robinson S., et al. Mitigation potential and global health impacts from emissions pricing of food commodities. Nat Clim Chang. 2017;7:69–74. doi: 10.1038/nclimate3155. [DOI] [Google Scholar]
- 255.Cawley J., Thow A.M., Wen K., Frisvold D. The economics of taxes on sugar-sweetened beverages: a review of the effects on prices, sales, cross-border shopping, and consumption. Annu Rev Nutr. 2019;39:317–338. doi: 10.1146/annurev-nutr-082018-124603. [DOI] [PubMed] [Google Scholar]
- 256.Hernández-F M., Cantoral A., Colchero M.A. Taxes to unhealthy food and beverages and oral health in Mexico: an observational study. Caries Res. 2021;55(3):183–192. doi: 10.1159/000515223. [DOI] [PubMed] [Google Scholar]
- 257.Taillie L.S., Rivera J.A., Popkin B.M., Batis C. Do high vs. low purchasers respond differently to a nonessential energy-dense food tax? Two-year evaluation of Mexico's 8% nonessential food tax. Prev Med Baltim. 2017;105:S37–S42. doi: 10.1016/j.ypmed.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Smed S., Scarborough P., Rayner M., Jensen J.D. The effects of the Danish saturated fat tax on food and nutrient intake and modelled health outcomes: an econometric and comparative risk assessment evaluation. Eur J Clin Nutr. 2016;70(6):681–686. doi: 10.1038/ejcn.2016.6. [DOI] [PubMed] [Google Scholar]
- 259.Jensen J.D., Smed S., Aarup L., Nielsen E. Effects of the Danish saturated fat tax on the demand for meat and dairy products. Public Health Nutr. 2016;19(17):3085–3094. doi: 10.1017/S1368980015002360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Slagel N., Newman T., Sanville L., et al. The effects of a fruit and vegetable prescription program (FVRx)® for low-income individuals on fruit and vegetable intake and food purchasing practices. J Nutr Educ Behav. 2018;50(7):S103. [Google Scholar]
- 261.Gren M., Höglind L., Jansson T. Refunding of a climate tax on food consumption in Sweden. Food Policy. 2021;100 [Google Scholar]
- 262.Luiselli L., Hema E.M., Segniagbeto G.H., et al. Understanding the influence of non-wealth factors in determining bushmeat consumption: results from four West African countries. Acta Oecol. 2019;94:47–56. [Google Scholar]
- 263.Santo R.E., Kim B.F., Goldman S.E., et al. Considering plant-based meat substitutes and cell-based meats: a public health and food systems perspective. Front Sustain Food Syst. 2020;4:134. [Google Scholar]
- 264.Smetana S., Mathys A., Knoch A., Heinz V. Meat alternatives: life cycle assessment of most known meat substitutes. Int J Life Cycle Assess. 2015;20(9):1254–1267. [Google Scholar]
- 265.Baiano A. Edible insects: an overview on nutritional characteristics, safety, farming, production technologies, regulatory framework, and socio-economic and ethical implications. Trends Food Sci Technol. 2020;100:35–50. [Google Scholar]
- 266.Mishyna M., Chen J., Benjamin O. Sensory attributes of edible insects and insect-based foods–future outlooks for enhancing consumer appeal. Trends Food Sci Technol. 2020;95:141–148. [Google Scholar]
- 267.Rubio N.R., Xiang N., Kaplan D.L. Plant-based and cell-based approaches to meat production. Nat Commun. 2020;11(1):1–11. doi: 10.1038/s41467-020-20061-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Kümpel N.F., Milner-Gulland E.J., Cowlishaw G., Rowcliffe J.M. Assessing sustainability at multiple scales in a rotational bushmeat hunting system. Conserv Biol. 2010;24:861–871. doi: 10.1111/j.1523-1739.2010.01505.x. [DOI] [PubMed] [Google Scholar]
- 269.Nielsen M.R., Meilby H., Smith-Hall C., Pouliot M., Treue T. The importance of wild meat in the global South. Ecol Econ. 2018;146:696–705. [Google Scholar]
- 270.Brashares J.S., Arcese P., Sam M.K., Coppolillo P.B., Sinclair A.R.E., Balmford A. Bushmeat hunting, wildlife declines, and fish supply in West Africa. Science. 2004;306:1180–1183. doi: 10.1126/science.1102425. (80-) [DOI] [PubMed] [Google Scholar]
- 271.Jambiya J, Milledge S, Mtango N. Night time spinach: conservation and livelihood implications of wild meat use in refugee situations in North-Western Tanzania. Dar Es Salaam, Tanzania; 2007.
- 272.Ordaz-Németh I., Arandjelovic M., Boesch L., et al. The socio-economic drivers of bushmeat consumption during the West African Ebola crisis. PLoS Negl Trop Dis. 2017:11. doi: 10.1371/journal.pntd.0005450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Gardner C.J., Davies Z.G. Rural bushmeat consumption within multiple-use protected areas: qualitative evidence from southwest Madagascar. Hum Ecol. 2014;42:21–34. [Google Scholar]
- 274.CBD. Sustainable wildlife management: guidance for a sustainable wild meat sector. 2017.
- 275.Stahl J., De Meulenaer T. CITES and the international trade in wildlife. Unasylva. 2017;68(1):17–26. [Google Scholar]
- 276.WCS. Summary of WCS policy and messaging on COVID-19.
- 277.Coalition to end the trade. Sign the petition to #ENDTHETRADE.
- 278.Whitfort A. COVID-19 and wildlife farming in China: legislating to protect wild animal health and welfare in the wake of a global pandemic. J Environ Law. 2021;33(1):57–84. [Google Scholar]
- 279.Akani G.C., Dendi D., Luiselli L. Ebola virus effects on the bushmeat trade in West Africa. Afr J Ecol. 2015;53(4):613–615. [Google Scholar]
- 280.‘t Sas-Rolfes M., Challender D.W.S., Hinsley A., Veríssimo D., Milner-Gulland E.J. Illegal wildlife trade: scale, processes, and governance. Annu Rev Environ Resour. 2019;44:201–228. [Google Scholar]
- 281.Challender D, Hinsley A, Veríssimo D, t Sas-Rolfes M. Coronavirus: why a blanket ban on wildlife trade would not be the right response. The Conversation. https://theconversation.com/coronavirus-why-a-blanket-ban-on-wildlife-trade-would-not-be-the-right-response-135746?fbclid=IwAR1k_PcQ90Xx5zp5kfYmrOndH8eZ-oivZWwaqXM3lSeCnPK_a8v6xDqJ4vc. Published April 8, 2020.
- 282.Alves R.R.N., van Vliet N., Alves R.R.N., Albuquerque U.P. Ethnozoology. Elsevier; Oxford, UK: 2018. Wild fauna on the menu; pp. 167–194. [Google Scholar]
- 283.Bulte E.H., Barbier E.B. Trade and renewable resources in a second best world: an overview. Environ Resour Econ. 2005;30:423–463. [Google Scholar]
- 284.Fischer C. Does trade help or hinder the conservation of natural resources? Rev Environ Econ Policy. 2010;4(1):103–121. [Google Scholar]
- 285.Nelson F., Nelson F. Community Rights, Conservation and Contested Land: The Politics of Natural Resource Governance in Africa. Earthscan Publications; London, UK: 2010. Introduction: the politics of natural resource governance in Africa. [Google Scholar]
- 286.Bennett E.L. Another inconvenient truth: the failure of enforcement systems to save charismatic species. Oryx. 2011;45(4):476–479. [Google Scholar]
- 287.Williams DA, Parry-Jones R, Roe D. The resource bites back: entry-points for addressing corruption in wildlife crime. U4 Issue. 2016;(2).
- 288.WWF & TRAFFIC. Strategies for fighting corruption in wildlife conservation: a primer. Gland, Switzerland; 2015. https://www.traffic.org/site/assets/files/9025/wci_strategies_for_fighting_corruption_wildlife_conservation.pdf.
- 289.Johnson A., Goodrich J., Hansel T., et al. To protect or neglect? Design, monitoring, and evaluation of a law enforcement strategy to recover small populations of wild tigers and their prey. Biol Conserv. 2016;202:99–109. [Google Scholar]
- 290.Massé F., Gardiner A., Lubilo R., Themba M.N. Inclusive anti-poaching? Exploring the potential and challenges of community-based anti-poaching. S. Afr. Crime Q. 2017;60:19–27. [Google Scholar]
- 291.Transparency International. Keeping REDD+ clean: a step-by-step guide to preventing corruption. 2012.
- 292.Chausson A.M., Rowcliffe J.M., Escouflaire L., Wieland M., Wright J.H. Understanding the sociocultural drivers of urban bushmeat consumption for behavior change interventions in Pointe Noire, Republic of Congo. Hum Ecol. 2019;47:179–191. [Google Scholar]
- 293.Rivalan P., Delmas V., Angulo E., et al. Can bans stimulate wildlife trade? Nature. 2007;447:529–530. doi: 10.1038/447529a. [DOI] [PubMed] [Google Scholar]
- 294.Cowlishaw G., Mendelson S., Rowcliffe J.M. Evidence for post-depletion sustainability in a mature bushmeat market. J Appl Ecol. 2005;42(3):460–468. [Google Scholar]
- 295.Gill D.J.C., Fa J.E., Rowcliffe J.M., Kümpel N.F. Drivers of change in hunter offtake and hunting strategies in Sendje, equatorial guinea. Conserv Biol. 2012;26(6):1052–1060. doi: 10.1111/j.1523-1739.2012.01876.x. [DOI] [PubMed] [Google Scholar]
- 296.Fa J.E., Brown D. Impacts of hunting on mammals in African tropical moist forests: a review and synthesis. Mamm Rev. 2009;39:231–264. [Google Scholar]
- 297.Nogueira-Filho S.L.G., da Cunha Nogueira S.S. Capybara meat: an extraordinary resource for food security in South America. Meat Sci. 2018;145:329–333. doi: 10.1016/j.meatsci.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 298.Nuno A., Blumenthal J.M., Austin T.J., et al. Understanding implications of consumer behavior for wildlife farming and sustainable wildlife trade. Conserv Biol. 2018;32(2):390–400. doi: 10.1111/cobi.12998. [DOI] [PubMed] [Google Scholar]
- 299.Challender D, Hinsley A, Veríssimo D, ’t Sas-Rolfes M. Coronavirus: why a blanket ban on wildlife trade would not be the right response. The Conversation. April 2020.
- 300.Gire S.K., Goba A., Andersen K.G., et al. Genomic surveillance elucidates Ebola virus origin and transmission during the 2014 outbreak. Science. 2014;345(6202):1369–1372. doi: 10.1126/science.1259657. (80-) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Nielsen C.F., Kidd S., Sillah A.R.M., Davis E., Mermin J., Kilmarx P.H. Improving burial practices and cemetery management during an Ebola virus disease epidemic—Sierra Leone, 2014. MMWR Morb Mortal Wkly Rep. 2015;64(1):20. [PMC free article] [PubMed] [Google Scholar]
- 302.Lokuge K., Caleo G., Greig J., et al. Successful control of Ebola virus disease: analysis of service based data from rural Sierra Leone. PLoS Negl Trop Dis. 2016;10(3) doi: 10.1371/journal.pntd.0004498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Volpato G., Fontefrancesco M.F., Gruppuso P., Zocchi D.M., Pieroni A. Baby pangolins on my plate: possible lessons to learn from the COVID-19 pandemic. J Ethnobiol Ethnomed. 2020;16(19) doi: 10.1186/s13002-020-00366-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Hacker R.B., Sinclair K., Pahl L. Prospects for ecologically and socially sustainable management of total grazing pressure in the southern rangelands of Australia. Rangel J. 2020;41(6):581–586. [Google Scholar]
- 305.Robinson J.G., Bennett E.L., Robinson J.G., Bennett E.L. Hunting For Sustainability in Tropical Forests. Columbia University Press; New York, USA: 2000. Carrying capacity limits to sustainable hunting in tropical forests; pp. 13–30. [Google Scholar]
- 306.van Vliet N., Quiceno-Mesa M.P., Cruz-Antia D., et al. From fish and bushmeat to chicken nuggets: the nutrition transition in a continuum from rural to urban settings in the Colombian Amazon region. Ethnobiol Conserv. 2015;4 [Google Scholar]
- 307.Laurance W.F., Clements G.R., Sloan S., et al. A global strategy for road building. Nature. 2014;513:229–232. doi: 10.1038/nature13717. [DOI] [PubMed] [Google Scholar]
- 308.F.A.O. Wildlife farm census to ensure safe and healthy wild animal products in Viet Nam. 2015. http://www.fao.org/vietnam/news/detail-events/en/c/276998/.
- 309.Wicander S., Coad L. University of Oxford and IUCN; Oxford, UK and Gland, Switzerland: 2015. Learning Our Lessons. A Review of Alternative Livelihood Projects in Central Africa. [Google Scholar]
- 310.FAO. Urban food systems and COVID-19: the role of cities and local governments in responding to the emergency. 2020.
- 311.Baker S.E., Cain R., van Kesteren F., Zommers Z.A., D'Cruze N., Macdonald D.W. Rough trade: animal welfare in the global wildlife trade. Bioscience. 2013;63(2):928–938. [Google Scholar]
- 312.Saylors K.E., Mouiche M.M., Lucas A., et al. Market characteristics and zoonotic disease risk perception in Cameroon bushmeat markets. Soc Sci Med. 2021;268 doi: 10.1016/j.socscimed.2020.113358. [DOI] [PubMed] [Google Scholar]
- 313.Vieira MAR de M., von Muhlen E.M., Shepard G.H. Participatory monitoring and management of subsistence hunting in the Piagaçu-Purus reserve, Brazil. Conserv Soc. 2015;13(3):254–264. [Google Scholar]
- 314.Fernandes D. Proceedings of the Eleventh Conference of the International Association for the Study of Common Property (ASCP) Digital Library of the Commons; Bloomington, IN: 2006. More eyes watching..." Community-based management of the arapaima (Arapaima gigas) in Central Guyana; pp. 1–19. [Google Scholar]
- 315.Vitos M., Stevens M., Lewis J., Haklay M. Proceedings of the 3rd ACM Symposium on Computing for Development, ACM DEV ’13. 2013. Making local knowledge matter: supporting non-literate people to monitor poaching in Congo; pp. 1–10. Vol 13. [Google Scholar]
- 316.Ribot J.C., Lund J.F., Treue T. Democratic decentralization in sub-Saharan Africa: its contribution to forest management, livelihoods, and enfranchisement. Environ Conserv. 2010;37(1):35–44. doi: 10.1017/S0376892910000329. [DOI] [Google Scholar]
- 317.Anderson J., Mehta S. USAID; Washington DC, USA: 2013. A Global Assessment of Community Based Natural Resource Management: Addressing the Critical Challenges of the Rural Sector. [Google Scholar]
- 318.Haller T., Merten S., Haller T. Disputing the Floodplains: Institutional Change and the Politics of Resource Management in African Wetlands. Brill; Boston: 2010. We had cattle and did not fish and hunt anyhow!”: institutional change and contested commons in the Kafue Flats Floodplains, Zambia. [Google Scholar]
- 319.Mogende E., Kolawole O. Dynamics of local governance in natural resource conservation in the Okavango Delta, Botswana. Nat Resour Forum. 2016;40(3):93–102. doi: 10.1111/1477-8947.12098. [DOI] [Google Scholar]
- 320.Smith H., Marrocoli S., Lozano A.G., Basurto X. Hunting for common ground between wildlife governance and commons scholarship. Conserv Pract Policy. 2018;33(1):9–21. doi: 10.1111/cobi.13200. [DOI] [PubMed] [Google Scholar]
- 321.Wilkie D, Painter M, Jacob I. Rewards and risks associated with community engagement in anti-poaching and anti-trafficking. 2016.
- 322.Poulsen J.R., Clark C.J., Mavah G., Elkan P.W. Bushmeat supply and consumption in a tropical logging concession in northern Congo. Conserv Biol. 2009;23(6):1597–1608. doi: 10.1111/j.1523-1739.2009.01251.x. [DOI] [PubMed] [Google Scholar]
- 323.Sandalj M., Treydte A.C., Ziegler S. Is wild meat luxury? Quantifying wild meat demand and availability in Hue, Vietnam. Biol Conserv. 2016;194:105–112. [Google Scholar]
- 324.Tensen L. Under what circumstances can wildlife farming benefit species conservation? Glob Ecol Conserv. 2016;6:286–298. [Google Scholar]
- 325.Veríssimo D., Schmid C., Kimario F.F., Eves H.E. Measuring the impact of an entertainment-education intervention to reduce demand for bushmeat. Anim Conserv. 2018;21(4):324–331. [Google Scholar]
- 326.Veríssimo D., Wan A.K.Y. Characterizing efforts to reduce consumer demand for wildlife products. Conserv Biol. 2019;33(3):623–633. doi: 10.1111/cobi.13227. [DOI] [PubMed] [Google Scholar]
- 327.Wang W., Yang L., Wronski T., Chen S., Hu Y., Huang S. Captive breeding of wildlife resources—China's revised supply-side approach to conservation. Wildl Soc Bull. 2019;43(3):425–435. doi: 10.1002/wsb.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.F.A.O. Wildlife farm census to ensure safe and healthy wild animal products in Viet Nam. 2015.
- 329.Brooks E.G.E., Roberton S.I., Bell D.J. The conservation impact of commercial wildlife farming of porcupines in Vietnam. Biol Conserv. 2010;143(11):2808–2814. [Google Scholar]
- 330.Saylors K., Wolking D.J., Hagan E., et al. Socializing One Health: an innovative strategy to investigate social and behavioral risks of emerging viral threats. One Health Outlook. 2021;3(1):1–16. doi: 10.1186/s42522-021-00036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.LeBreton M., Prosser A.T., Tamoufe U., et al. Healthy hunting in central Africa. Anim Conserv. 2006;9(4):372–374. [Google Scholar]
- 332.Bardosh K.L., El Berbri I., Ducrotoy M., Bouslikhane M., Ouafaa F.F., Welburn S.C. Zoonotic encounters at the slaughterhouse: pathways and possibilities for the control of cystic echinococcosis in northern Morocco. J Biosoc Sci. 2016;48(S1):S92–S115. doi: 10.1017/S0021932015000486. [DOI] [PubMed] [Google Scholar]
- 333.Greatorex Z.F., Olson S.H., Singhalath S., et al. Wildlife trade and human health in Lao PDR: an assessment of the zoonotic disease risk in markets. PLoS One. 2016;11(3) doi: 10.1371/journal.pone.0150666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Bardosh K.L. Towards a science of global health delivery: a socio-anthropological framework to improve the effectiveness of neglected tropical disease interventions. PLoS Negl Trop Dis. 2018;12(7) doi: 10.1371/journal.pntd.0006537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Zhou X., Wang Y., Liu H., et al. Effectiveness of market-level biosecurity at reducing exposure of poultry and humans to avian influenza: a systematic review and meta-analysis. J Infect Dis. 2018;218(12):1861–1875. doi: 10.1093/infdis/jiy400. [DOI] [PubMed] [Google Scholar]
- 336.Lin B., Dietrich M.L., Senior R.A., Wilcove D.S. A better classification of wet markets is key to safeguarding human health and biodiversity. Lancet Planet Health. 2021;5(6):e386–e394. doi: 10.1016/S2542-5196(21)00112-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Aiyar A., Pingali P. Pandemics and food systems - towards a proactive food safety approach to disease prevention & management. Food Secur. 2020;12(4):749–756. doi: 10.1007/s12571-020-01074-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.OiE W.H.O. World Health Organization; 2021. UNEP. Reducing Public Health Risks Associated with the Sale of Live Wild Animals of Mammalian Species in Traditional Food Markets: Interim Guidance; p. 2021. 12 April. [Google Scholar]
- 339.Leach M., Scoones I. The social and political lives of zoonotic disease models: narratives, science and policy. Soc Sci Med. 2013;88:10–17. doi: 10.1016/j.socscimed.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 340.Ndengu M., Matope G., de Garine-Wichatitsky M., et al. Seroprevalence of brucellosis in cattle and selected wildlife species at selected livestock/wildlife interface areas of the Gonarezhou National Park, Zimbabwe. Prev Vet Med. 2017;146:158–165. doi: 10.1016/j.prevetmed.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 341.Pulliam J.R.C., Epstein J.H., Dushoff J., et al. Agricultural intensification, priming for persistence and the emergence of Nipah virus: a lethal bat-borne zoonosis. J R Soc Interface. 2012;9(66):89–101. doi: 10.1098/rsif.2011.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Nahar N., Mondal U.K., Hossain M.J., et al. Piloting the promotion of bamboo skirt barriers to prevent Nipah virus transmission through date palm sap in Bangladesh. Glob Health Promot. 2014;21(4):7–15. doi: 10.1177/1757975914528249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Bastin J.F., Finegold Y., Garcia C., et al. The global tree restoration potential. Science. 2019;365(6448):76–79. doi: 10.1126/science.aax0848. (80-) [DOI] [PubMed] [Google Scholar]
- 344.Reaser J, Hunt BE, Ruiz-Aravena M, et al. Reducing land use-induced spillover risk by fostering landscape immunity: policy priorities for conservation practitioners. EcoEvoRxiv. 2020. doi:10.32942/osf.io/7 gd6a
- 345.Kilpatrick A.M., Dobson A.D.M., Levi T., et al. Lyme disease ecology in a changing world: consensus, uncertainty and critical gaps for improving control. Philos Trans R Soc B Biol Sci. 2017;372(1722) doi: 10.1098/rstb.2016.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.ECDC . ECDC; Stockholm, Sweden: 2016. Rabies - Annual Epidemiological Report 2016 [2014 Data] [Google Scholar]
- 347.Domenech J., Dauphin G., Rushton J., et al. Experiences with vaccination in countries endemically infected with highly pathogenic avian influenza: the Food and Agriculture Organization perspective. Rev Sci Tech. 2009;28(1):293–305. doi: 10.20506/rst.28.1.1865. Int Des Epizoot. [DOI] [PubMed] [Google Scholar]
- 348.Streicker D.G., Recuenco S., Valderrama W., et al. Ecological and anthropogenic drivers of rabies exposure in vampire bats: implications for transmission and control. Proc R Soc B Biol Sci. 2012;279(1742):3384–3392. doi: 10.1098/rspb.2012.0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Ebinger M., Cross P., Wallen R., White P.J., Treanor J. Simulating sterilization, vaccination, and test-and-remove as brucellosis control measures in bison. Ecol Appl. 2011;21(8):2944–2959. [Google Scholar]
- 350.Chanda E., Ameneshewa B., Bagayoko M., Govere J.M., Macdonald M.B. Harnessing integrated vector management for enhanced disease prevention. Trends Parasitol. 2017;33(1):30–41. doi: 10.1016/j.pt.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 351.Wild M.A., Hobbs N.T., Graham M.S., Miller M.W. The role of predation in disease control: a comparison of selective and nonselective removal on prion disease dynamics in deer. J Wildl Dis. 2011;47(1):78–93. doi: 10.7589/0090-3558-47.1.78. [DOI] [PubMed] [Google Scholar]
- 352.Ogada D.L., Torchin M.E., Kinnaird M.F., VO Ezenwa. Effects of vulture declines on facultative scavengers and potential implications for mammalian disease transmission. Conserv Biol. 2012;26(3):453–460. doi: 10.1111/j.1523-1739.2012.01827.x. [DOI] [PubMed] [Google Scholar]
- 353.Tanner E., White A., Acevedo P., Balseiro A., Marcos J., Gortázar C. Wolves contribute to disease control in a multi-host system. Sci Rep. 2019;9(1):1–12. doi: 10.1038/s41598-019-44148-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Roche B., Garchitorena A., Guégan J., et al. Was the COVID-19 pandemic avoidable? A call for a “solution-oriented” approach in pathogen evolutionary ecology to prevent future outbreaks. Ecol Lett. 2020;23(11):1557–1560. doi: 10.1111/ele.13586. [DOI] [PubMed] [Google Scholar]
- 355.Berthe F.C.J., Bouley T., Karesh W.B., et al. World Bank Group; Washington DC, USA: 2018. One Health: Operational Framework For Strengthening Human, Animal, and Environmental Public Health Systems At Their Interface.https://documents.worldbank.org/en/publication/documents-reports/documentdetail/961101524657708673/one-health-operational-framework-for-strengthening-human-animal-and-environmental-public-health-systems-at-their-interface [Google Scholar]
- 356.Kock R.A., Karesh W.B., Veas F., et al. 2019-nCoV in context: lessons learned? Lancet Planet Health. 2020;4(3):e87–e88. doi: 10.1016/S2542-5196(20)30035-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Watson R, McCarthy JJ, Canziani P, Nakicenovic N, Hisas L. The truth behind the climate pledges. 2019.
- 358.Scown M.W., Brady M.V., Nicholas K.A. Billions in misspent EU agricultural subsidies could support the sustainable development goals. One Earth. 2020;3:237–250. doi: 10.1016/j.oneear.2020.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Balmford A., Fisher B., Mace G.M., Wilcove D.S., Balmford B. Analogies and lessons from COVID-19 for tackling the extinction and climate crises. Curr Biol. 2020;30(17):R969–R971. doi: 10.1016/j.cub.2020.06.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.