Abstract
Objective:
To examine the concentration of C-reactive protein (CRP) in relation to gestational weeks during pregnancy among Chinese women.
Methods:
From a randomized control trial of prenatal supplementation with folic acid, iron-folic acid, and multiple micronutrients in China, we examined 834 pregnant women with CRP measured initially between 5 and 20 weeks and at follow-up between 28 and 32 weeks gestation. We calculated and plotted CRP geometric means by gestational weeks. The same analysis was repeated for women who had normal pregnancies (624 women) by excluding women with stillbirth, preterm, small for gestational age, body mass index <18.5 kg/m2 or >30 kg/m2 at enrollment, and hypertension or anemia during pregnancy.
Results:
We observed a significant positive trend between log-transformed CRP and gestational age from 5 to 20 weeks and from 28 to 32 weeks both in the full sample and in the subset of women who had normal pregnancies. CRP geometric mean was 0.81 mg/l at 5–7 weeks of gestation, 2.85 mg/l at 19–20 weeks of gestation, and 3.89 mg/l at 32 weeks of gestation. A similar increasing trend in the CRP median or percentage of elevated CRP were also observed.
Conclusion:
We concluded that CRP increased with gestational age among healthy Chinese women who delivered healthy infants. Am. J. Hum. Biol. 28:574–579, 2016.
C-reactive protein (CRP), an acute-phase protein, is an important component of the innate immune system and is initially produced in the liver in response to inflammatory stimuli (Black et al., 2004). CRP is frequently used as a biomarker to monitor the course of infectious and inflammatory diseases (Lobo et al., 2003). In the general population even a mild elevation of CRP is associated with a variety of medical conditions and may be evidence of inflammation or distressed or injured cells (Kushner et al., 2006). Among pregnant women, studies have consistently shown that elevated CRP levels are associated with adverse outcomes, including preterm delivery (Catov et al., 2007; Han et al., 2011; Hvilsom et al., 2002; Lohsoonthorn et al., 2007; Pitiphat et al., 2005; Vogel et al., 2005), preeclampsia (Guven et al., 2009; Rebelo et al., 2013; Tjoa et al., 2003), gestational diabetes (Rota et al., 2005; Wolf et al., 2003), fetal growth restriction, and risk of neonatal complications (Ernst et al., 2011). Elevated CRP levels in umbili cal cord blood have been reported among infants born small for gestational age (SGA) (Amarilyo et al., 2011; Trevisanuto et al., 2007). In addition, elevated maternal CRP may play a significant role in relation to developing childhood autism (Brown et al., 2014) and schizophrenia (Canetta et al., 2014). However, elevated CRP is also a normal component of pregnancy that reflects adaptations in maternal immunity that allow the mother to tolerate the fetus. Some elevation in CRP during pregnancy may be expected and likely necessary for healthy pregnancy (Miller, 2009; Watts et al., 1991).
Previous studies showed that pregnant women had significantly higher CRP levels than those who were not pregnant. However, these studies did not examine the CRP trend during pregnancy (Miller, 2009; Picklesimer et al., 2008; Sacks et al., 2004; von Versen-Hoeynck et al., 2009; Watts et al., 1991). Early studies using qualitative methods showed either no significant increase from the first trimester through delivery or a decline (Connell and Connell, 1971; Yeh, 1982). Connell and Connell (1971) studied 611 pregnant women in the United States and found that the percentages of positive (detectable) tests of CRP, taken from the first trimester through delivery, were not significantly different from one another. However, the method used for that study was only qualitative and could not determine CRP value. Yeh (1982) examined 203 healthy pregnant women in Taiwan and found that the percentage of positive CRP tests decreased from the first trimester to the third trimester. However, Yeh used a qualitative method and could not determine CRP value.
Only three studies examined the CRP trend during pregnancy using the quantitative method for CRP measurement (Belo et al., 2005; Kuzawa et al., 2013; Romem and Artal, 1985). Romem and Artal (1985) studied CRP among healthy pregnancies in the United States (n = 215), and found a positive linear correlation (r = 0.21, P < 0.05) between CRP values and gestational age from 8 to 42 weeks. The second study was conducted by Kuzawa et al. (2013) and found that among healthy, young, adult, Filipino women, women late in gestation had 10-fold higher CRP levels compared with nonpregnant women and among pregnant women (n = 101); median CRP levels were increased by trimesters from 0.6 mg/l in the first trimester to 2.0 mg/l in the third trimester. The third study was conducted by Belo et al. (2005) who longitudinally followed 23 pregnant Portuguese women and found that the median CRP values were consistently elevated throughout pregnancy. However, all the three studies were conducted with relatively small sample sizes.
Because elevated CRP levels are a normal component of pregnancy and are also associated with adverse outcomes during pregnancy, the level of elevated CRP and the duration of pregnancy (gestational age) are important. This study was based on a large, randomized control trial of prenatal supplementation with folic acid (FA), iron-folic acid (IFA), and multiple micronutrients (MMs) in China. The trial was conducted among an educated population with good access to health care and low levels of anemia at enrollment (approximately 5–6%; Liu et al., 2013). A subset of women with CRP measurements from 5 to 20 weeks gestation and from 28 to 32 weeks gestation were analyzed to explore the relationship between CRP and gestational weeks among Chinese women. This study examines the pattern of CRP and gestational weeks among pregnant Chinese women.
METHODS
Study population and sample selection
The trial took place in five rural counties in Hebei Province, China. Eligible pregnant women were enrolled from May 2006 to April 2009, and individually randomized in a 1:1:1 ratio to receive a daily supplement containing FA (400 mcg) (control), IFA (FA plus 30 mg iron), or MM formula from the United Nations International MM Preparation (FA, 30 mg iron, plus 13 additional MMs) provided before 20 weeks of gestation to delivery. Women were followed monthly from early pregnancy through delivery and at 4–8 weeks postpartum. Their infants were followed monthly from birth until age 1 year. To be eligible, pregnant women (a) recorded dates of their menstruation for 2 or more months before they became pregnant, were (b) nulliparous, (c) aged 20 years or older, (d) at 20 weeks or less gestation, (e) legally competent, (f) had not consumed iron supplements or other micronutrient supplements (other than FA) during the prior 6 months, (g) had a hemoglobin (Hb) level of 100 g/l or more, (h) resided in and received prenatal care in one of five counties, and (i) consented to participate. Randomization of individual women was stratified by county and random block sizes of three, six, and nine to ensure geographic balance with an approximately equal distribution of treatments within and across study counties.
In this study, 18,775 nulliparous pregnant women with Hb higher than 100 g/l were enrolled and randomized before 20 weeks gestational age and followed monthly through delivery and at 4–8 weeks postpartum (Liu et al., 2013). The primary outcome of this trial was perinatal mortality. Secondary outcomes included neonatal and infant mortality, preterm delivery, birthweight, birth length, gestational duration, and maternal hemoglobin concentration and anemia (Hb <110 g/l). Detailed information on this trial is published elsewhere (Liu et al., 2013).
To understand the physiological mechanisms underlying any beneficial or deleterious treatment effects observed during the trial, venous blood was collected from a subsample of women at two points during pregnancy: during enrollment into the study before 20 weeks gestation and at 28–32 weeks gestation. All women from two of the five study sites (Mancheng and Xianghe counties) were invited to participate in this substudy. A modified consent form was used during the enrollment of the subsample during March 2008 to February 2009, and the study protocol was approved by the Institutional Review Boards of the US Centers for Disease Control and Prevention and the Peking University Health Science Center, China.
A standardized training was conducted, including cold chain procedures. The blood samples were centrifuged, and serum was separated from the red cells within 2 h after collection; the serum was aliquoted into frozen tubes and stored at −20°C for a week at field sites. Serum samples were shipped on dry ice to the laboratory of the Peking University Institute of Reproductive and Child Health, and then stored at −80°C until analysis could be performed.
Laboratory analysis, anthropometric measurement, and case definition
Serum CRP (mg/l) was determined by using a Sandwich ELISA assay (Labsystem Multiscan MS type 352, Helsinki, Finland). The antigen and antibodies were as follows: CRP (Cat. No. 8C72, Hytest), monoclonal mouse anti-human CRP (Cat. No. 4C28, Hytest), and monoclonal mouse anti-human CRP HRP-conjugated (Cat. No. 4C28C, Hytest). Aliquots from a pool of quality control samples were prepared from serum samples with a low, medium, and high level of serum CRP. The CVs of three levels for serum CRP in these quality control specimens were 5.5, 4.8, and 6.0%; and the biases of the three levels were 14.6, 9.0, and 1.0%, respectively. Elevated CRP was defined as higher than 5 mg/l (Dati et al., 1996).
Hb concentration was measured from venous whole blood using the HemoCue system (HemoCue AB, Angelholm, Sweden) at enrollment, and at 28–32 weeks gestation. Maternal anemia was defined as a Hb concentration of less than 110.0 g/l (Centers for Disease Control and Prevention, 1998).
Maternal weight was measured at enrollment using an electronic scale (BW 150, UWE, Beijing, China) with precision to the nearest 50 g, and height was measured at enrollment by a collapsible height board to the nearest 0.1 cm. Body mass index (BMI) was calculated using body weight in kilograms divided by height in meters squared.
Blood pressure was measured during routine prenatal care visits by trained local health workers using a mercury sphygmomanometer. The women selected for this analysis had their blood pressure measured an average of 7.19 (standard deviation = 2.54) times from enrollment to delivery. Hypertension was defined as at least one systolic blood pressure at 140 mm Hg or more, or diastolic blood pressure at 90 mm Hg or more.
All women’s menstrual cycles were monitored for 2 or more months before enrollment to calculate the exact gestational age. Gestational age at delivery was defined as the number of weeks from the first day of the woman’s last recorded menstrual period to the day of delivery. Stillbirth was defined as the death of a fetus at any time after 28 weeks gestation. Preterm delivery was defined as live birth less than 37 weeks gestation from the first day of the last menstrual period. SGA is defined as being smaller in size than normal for gestational age, defined as a weight below the 10th percentile for the gestational age (Alexander et al., 1996). Normal pregnancy was defined in our study as the birth not being stillbirth, preterm, SGA, and the women not having a BMI less than 18.5 kg/m2 or a BMI more than 30 kg/m2 at enrollment (<20 weeks gestation), or not having hypertension, or being anemic during pregnancy. We used BMI more than 30 kg/m2 at enrollment as a criteria because there was no prepregnancy weight measured in this study, and the first weight measurement was their first prenatal visit before 20 weeks of gestation.
Statistical analysis
First, we log-transformed CRP (ln[CRP]) to normalize the distributions, because CRP concentrations were positively skewed. Second, we explored whether CRP may have contributed to differences in the three treatment groups by comparing the CRP status across the three treatment groups. Finally, we calculated and plotted CRP geometric means by gestational weeks. (We grouped every two gestational weeks together between 5 and 20 weeks to increase the sample size). Median and percentage of elevated CRP by each gestational week were also examined. In addition, we repeated our above analyses among a subset of women who had normal pregnancies. Because CRP was measured twice for each woman, (first measurement from 5 to 20 weeks gestation [Measurement period1] and second measurements from 28 to 32 weeks gestation [Measurement period 2]), all the above analyses were performed separately by each measurement period.
We used SAS (Version 9.3; SAS, Inc., Cary, NC) for all analyses. CRP was reported as geometric means (95% CIs). Linear regression was used to test the CRP trend by gestational age. Significance was set at P < 0.05.
RESULTS
Blood samples were collected from 1,145 women at enrollment and 834 from 28 to 32 weeks gestation. For this analysis, we only included women with both blood samples collected. Therefore, there were 834 pregnant women with 1,668 CRP measurements in the final analysis.
At enrollment, the mean maternal age was 23.3 ± 2.4 years, gestational age was 11.6 ± 4.5 weeks, and Hb was 121.8 ± 8.3 g/l. In addition, 97.8% of the women were of Han ethnicity, 1.9% had a primary or less education, and 91.1% were farmers. Only 5.6% of women had mild anemia (Hb level 100–109 g/l). Detailed information by study group is presented in Table 1.
TABLE 1.
Baseline maternal characteristics at enrollment by study groupa
| Characteristics | Folic acid (n = 282) | Iron-folic acid (n = 278) | Multiple micronutrient (n = 274) | P valueb |
|---|---|---|---|---|
| Maternal age, y | 23.4 ± 2.5 | 23.1 ± 2.2 | 23.5 ± 2.5 | 0.13 |
| Education (%) | 0.83 | |||
| Primary or less | 1.4 | 1.8 | 2.6 | |
| Secondary | 85.5 | 83.1 | 83.9 | |
| High school or above | 13.1 | 15.1 | 13.5 | |
| Ethnicity (%) | 0.06 | |||
| Han | 96.1 | 98.6 | 98.9 | |
| Other | 3.9 | 1.4 | 1.1 | |
| Occupation (%) | 0.33 | |||
| Farmer | 90.4 | 93.2 | 89.8 | |
| Other | 9.6 | 6.8 | 10.2 | |
| Body-mass index (kg/m2, %) | 0.87 | |||
| <18.5 | 6.4 | 6.9 | 8.0 | |
| 18.5–24.9 | 74.8 | 74.8 | 75.2 | |
| 25.0–29.9 | 16.0 | 14.0 | 14.2 | |
| ≥30.0 | 2.8 | 4.3 | 2.6 | |
| Height (cm) | 159.7 ± 4.8 | 160.4 ± 5.4 | 160.1 ± 4.9 | 0.32 |
| Gestational week (%) | 0.43 | |||
| <12 | 50.4 | 55.0 | 55.1 | |
| ≥12 | 49.6 | 45.0 | 44.9 | |
| Hemoglobin (g/l, %) | 0.10 | |||
| 100–109 | 5.3 | 7.2 | 3.3 | |
| 110–119 | 25.2 | 18.7 | 18.6 | |
| 120–129 | 39.0 | 45.7 | 47.1 | |
| ≥130 | 30.5 | 28.4 | 31.0 |
Plus–minus values are means ±SD.
Chi-square tests were used to examine statistical differences in categorical variables and analysis of variance to examine differences in means among study groups.
All the subjects in the three treatment groups were combined in our final analyses, as the geometric mean CRPs by three treatment groups were comparable both at baseline and follow-up, although the mean CRPs at follow-up were significantly higher than at baseline (Table 2).
TABLE 2.
C-reactive protein (CRP, mg/l) geometric mean, prevalence of elevated CRP (>5 mg/l) and 95% confidence intervals (CI) at baseline (4–19 weeks gestational age, [GA]) and follow-up (28–32 weeks GA) by study group
| Group | n | Baselinea mean (95% CI) | Follow-upb mean (95% CI) | P valuec | |
|---|---|---|---|---|---|
| Mean | Folic acid | 282 | 1.53 (1.33, 1.77) | 3.41 (3.04, 3.81) | <0.001 |
| Iron-folic acid | 278 | 1.60 (1.39, 1.86) | 3.52 (3.15, 3.94) | <0.001 | |
| Multiple micronutrient | 274 | 1.72 (1.49, 1.99) | 3.36 (3.01, 3.77) | <0.001 | |
| Prevalence | Folic acid | 282 | 17.7 (13.3, 22.2) | 36.5 (30.9, 42.2) | <0.001 |
| Iron-folic acid | 278 | 18.7 (14.1, 23.3) | 38.1 (32.4, 43.9) | <0.001 | |
| Multiple micronutrient | 274 | 21.9 (17.0, 26.8) | 33.9 (28.3, 39.6) | <0.001 |
At baseline, means or prevalence across groups were not significantly different (p > 0.05, 2-tailed t-test).
At follow-up, means or prevalence across groups were not significantly different after adjustment by baseline CRP level, gestational age, and interaction term (P > 0.05, Generalized Linear Model, GLM).
Within each row, 2-tailed t-test were used for testing the means and McNemar’s test was used for the prevalence.
The CRP geometric mean was 0.81 mg/l at 5–7 weeks of gestation but increased to 2.85 mg/l at 19–20 weeks of gestation, and by 32 weeks of gestation, the geometric mean of CRP was 3.89 mg/l (Table 3). A similar increasing trend in the median or percentage of elevated CRP was also observed (Table 3). The geometric means were increased with gestational age from 5 to 20 weeks of gestation, and again from 28 to 32 weeks of gestation (Fig. 1A). Linear regression was used for the trend tests in each period (P < 0.001).
TABLE 3.
Geometric means and 95% confidence intervals (CI), medians, and percentage of elevated values of C-reactive protein (CRP, mg/l) by gestational age
| Gestational age (weeks) | n | Geometric meana (95% CI) | Median | % >5.0 mg/l |
|---|---|---|---|---|
| Measurement period 1 | ||||
| 6 | 119 | 0.81 (0.65, 1.01) | 0.72 | 5.9 |
| 8 | 136 | 0.99 (0.81, 1.22) | 0.85 | 10.3 |
| 10 | 106 | 1.62 (1.32, 1.98) | 1.77 | 12.3 |
| 12 | 88 | 1.94 (1.54, 2.45) | 1.79 | 21.6 |
| 14 | 111 | 2.07 (1.64, 2.60) | 2.30 | 23.4 |
| 16 | 93 | 2.18 (1.71, 2.78) | 2.31 | 26.9 |
| 18 | 97 | 2.18 (1.73, 2.75) | 2.15 | 28.9 |
| 20 | 84 | 2.85 (2.23, 3.65) | 3.31 | 35.7 |
| Measurement period 2 | ||||
| 28 | 247 | 3.22 (2.86, 3.62) | 2.82 | 32.4 |
| 29 | 212 | 3.43 (3.00, 3.92) | 3.82 | 36.8 |
| 30 | 155 | 3.52 (3.00, 4.14) | 3.43 | 40.7 |
| 31 | 121 | 3.42 (2.88, 4.06) | 3.31 | 37.2 |
| 32 | 99 | 3.89 (3.31, 4.57) | 3.96 | 36.4 |
Linear regression for trend tests, P < 0.0001.
Fig. 1.
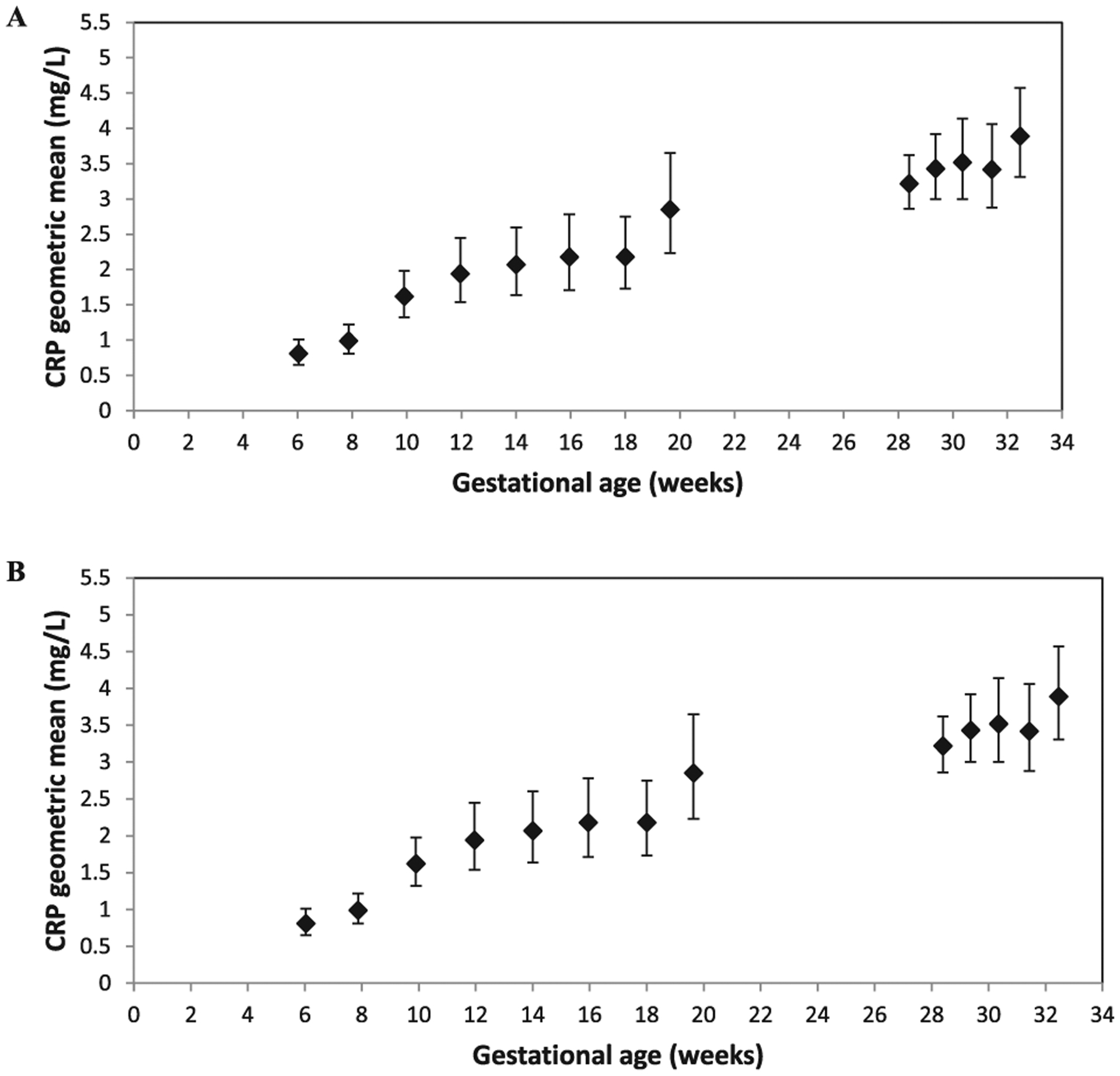
CRP geometric means with 95% confident intervals by gestational weeks among pregnant Chinese women from a randomized, double-blind trial in China, 2008–2010. Panel A, full sample (n = 834); Panel B, subsample for women with normal pregnancy (n = 624).
In the repeated analysis among a subset of women with normal pregnancies, we excluded pregnant women with stillbirth, preterm, SGA, a BMI of less than 18.5 kg/m2 or more than 30 kg/m2 at enrollment, hypertension, or anemia during pregnancy. The final sample for this analysis was 624 pregnant women with 1,248 CRP measurements. The geometric means by gestational age (Table 4) and the positive trend (Fig. 1B) were almost the same as the results from the full sample.
TABLE 4.
Geometric means and 95% confidence intervals (CI), medians, and percentage of elevated values of C-reactive protein (CRP, mg/l) by gestational age after excluding women with stillbirth, preterm, BMI >30 kg/m2 at enrollment, hypertension, or anemia during pregnancy
| Gestational age (weeks) | n | Geometric meana (95% CI) | Median | % > 5.0 mg/l |
|---|---|---|---|---|
| Measurement period 1 | ||||
| 6 | 85 | 0.82 (0.62, 1.07) | 0.70 | 7.1 |
| 8 | 104 | 1.00 (0.78, 1.27) | 0.81 | 10.6 |
| 10 | 80 | 1.41 (1.12, 1.78) | 1.69 | 8.6 |
| 12 | 67 | 2.05 (1.62, 2.59) | 2.03 | 20.9 |
| 14 | 85 | 1.87 (1.46, 2.41) | 1.85 | 20.0 |
| 16 | 72 | 2.34 (1.76, 3.11) | 2.50 | 30.6 |
| 18 | 72 | 2.21 (1.70, 2.88) | 2.30 | 30.1 |
| 20 | 59 | 2.81 (2.11, 3.74) | 2.93 | 37.3 |
| Measurement period 2 | ||||
| 28 | 189 | 3.09 (2.70, 3.54) | 2.86 | 31.2 |
| 29 | 160 | 3.54 (3.05, 4.11) | 3.91 | 36.9 |
| 30 | 119 | 3.45 (2.87, 4.14) | 3.41 | 39.5 |
| 31 | 89 | 3.55 (2.94, 4.30) | 3.86 | 39.3 |
| 32 | 67 | 4.06 (3.27, 5.04) | 4.04 | 38.8 |
Linear regression for trend tests, P < 0.0001.
DISCUSSION
In this study of pregnant Chinese women living north of Beijing, we found a significant positive trend between log-transformed CRP and gestational ages from 5 to 20 weeks and from 28 to 32 weeks. This trend remained significant after excluding women with stillbirth, preterm, SGA, a BMI of less than 18.5 kg/m2 or more than 30 kg/m2 at enrollment (<20 weeks gestation), hypertension, or anemia during pregnancy. In the repeated measurements analysis, we also observed an increased trend in changes between the two CRP measurements in relation to gestational weeks.
We did not observe significant differences between the full sample and the subsample with normal pregnancy, maybe because of our unique study population. Our study population was largely non-anemic (>93%) or only mildly anemic. Although predominantly rural (91% were farmers), nearly all women had at least a secondary education, and few were undernourished as measured by anthropometry, and mean maternal age was 23.3 ± 2.4 years. In addition, having monitored their menstrual cycle for 2 or more months before enrollment to calculate the exact gestational age, women were cognizant of their last menstrual period and prenatal health. Once enrolled, women were followed monthly, and all delivered in the hospital.
The increasing trend in CRP by gestational age from our study is similar to the study conducted by Kuzawa and colleagues among young adult Filipino women (Kuzawa et al., 2013); although, in general, the CRP median values by gestational among our Chinese women are higher than among the Filipino women. However, our study has a large sample size. Our results cannot be directly compared to the results from Yeh (1982), even though both studied Chinese women. Yeh’s study only used qualitative methods to detect CRP and, thus, cannot determine CRP values. They found that the percentages of positive CRP tests actually decreased from the first trimester to the third trimester. Our results cannot be directly compared to the results from Connell and Connell (1971) either because, again, a qualitative method was used, and it cannot determine CRP value. They found that the percentage of positive tests of CRP from the first trimester through delivery were not significantly different. The inconsistency of our results compared with both Yeh’s and Connell’s studies may be, at least partially, caused by the different laboratory methods used for detecting CRP.
Because elevated CRP is a normal component of pregnancy but also associated with adverse outcomes during pregnancy, we compared our sample of women with elevated CRP (>5 mg/l) to those without elevated CRP (≤5 mg/l) at 28–32 gestational age to examine whether there were any differences in terms of stillbirth, preterm, or SGA. No pregnant woman had a stillbirth after 32 weeks GA (data were not collected for women who had a stillbirth during the second measurement interval between 28 and 32 GA). Unlike previous studies which found an increased prevalence of preterm delivery (Catov et al., 2007; Han et al., 2011; Hvilsom et al., 2002; Lohsoonthorn et al., 2007; Pitiphat et al., 2005; Vogel et al., 2005) or SGA (Amarilyo et al., 2011; Trevisanuto et al., 2007) among women with elevated CRP, our study found no difference in the prevalence of preterm delivery or SGA by CRP level.
Our study also has limitations. Although each woman in our analysis contributed two CRP measurements from 5 to 20 weeks and from 28 to 32 gestational weeks, there are no CRP measurements at 21–27 gestational weeks or after 32 gestational weeks. Thus, we cannot examine the CRP trend throughout the pregnancy. Our study has several strengths, too. First, all women in our study had monitored their menstrual cycle for 2 or more months before enrollment, thus improving the accuracy of the gestational determination. Second, each woman had two CRP measurements from 5 to 19 weeks and from 28 to 32 weeks with relatively large sample sizes for each gestational week, which allowed us to examine the weekly trend. Third, both parametric and nonparametric methods were used for our data analysis so that our results can be directly compared with previous studies. And, finally, our study was able to examine a very homogenous and healthy population.
In summary, our study concluded that CRP increases with gestational weeks during pregnancy from 5 to 20 weeks and from 28 to 32 weeks in Chinese pregnant women. This same increasing trend in CRP across gestational age during pregnancy was also observed among a subsample of healthy women with normal pregnancy outcomes. Thus, mild elevation in CRP later in pregnancy may be expected to occur and reflects the immune adaptations during pregnancy.
Footnotes
Clinical trial identification number: NCT00137744. URL: www.clinical-trials.gov.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
The authors report no conflict of interest.
LITERATURE CITED
- Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. 1996. A United States national reference for fetal growth. Obstet Gynecol 87:163–168. [DOI] [PubMed] [Google Scholar]
- Amarilyo G, Oren A, Mimouni FB, Ochshorn Y, Deutsch V, Mandel D. 2011. Increased cord serum inflammatory markers in small-for-gestational-age neonates. J Perinatol 31:30–32. [DOI] [PubMed] [Google Scholar]
- Belo L, Santos-Silva A, Rocha S, Caslake M, Cooney J, Pereira-Leite L, Quintanilha A, Rebelo I. 2005. Fluctuations in C-reactive protein concentration and neutrophil activation during normal human pregnancy. Eur J Obstet Gynecol Reprod Biol 123:46–51. [DOI] [PubMed] [Google Scholar]
- Black S, Kushner I, Samols D. 2004. C-reactive protein. J Biol Chem 279: 48487–48490. [DOI] [PubMed] [Google Scholar]
- Brown AS, Sourander A, Hinkka-Yli-Salomäki S, McKeague IW, Sundvall J, Surcel HM. 2014. Elevated maternal C-reactive protein and autism in a national birth cohort. Mol Psychiatry 19:259–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canetta S, Sourander A, Surcel HM, Hinkka-Yli-Salomäki S, Leiviskä J, Kellendonk C, McKeague IW, Brown AS. 2014. Elevated maternal C-reactive protein and increased risk of schizophrenia in a national birth cohort. Am J Psychiatry 171:960–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catov JM, Bodnar LM, Ness RB, Barron SJ, Roberts JM. 2007. Inflammation and dyslipidemia related to risk of spontaneous preterm birth. Am J Epidemiol 166:1312–1319. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. 1998. Recommendations to prevent and control iron deficiency in the United States. MMWR Recomm Rep 47(RR-3):1–29. [PubMed] [Google Scholar]
- Connell BE, Connell TJ. 1971. C-reactive protein in pregnancy and contraception. Am J Obstet Gynecol 110:633–639. [DOI] [PubMed] [Google Scholar]
- Dati F, Schumann G, Thomas L, Aguzzi F, Baudner S, Bienvenu J, Blaabjerg O, Blirup-Jensen S, Carlström A, Petersen PH, Johnson AM, Milford-Ward A, Ritchie EF, Svendsen PJ, Whicher J. 1996. Consensus of a group of professional societies and diagnostic companies on guidelines for interim reference ranges for 14 proteins in serum based on the standardization against the IFCC/BCR/CAP reference material (CRM 470). Eur J Clin Chem Clin Biochem 34:517–520. [PubMed] [Google Scholar]
- Ernst GD, de Jonge LL, Hofman A, Lindemans J, Russcher H, Steegers EA, Jaddoe VW. 2011. C-reactive protein levels in early pregnancy, fetal growth patterns, and the risk for neona tal complications: the Generation R Study. Am J Obstet Gynecol 205:132.e1–132.e12. [DOI] [PubMed] [Google Scholar]
- Guven MA, Coskun A, Ertas IE, Aral M, Zencirci B, Oksuz H. 2009. Association of maternal serum CRP, IL-6, TNF-alpha, homocysteine, folic acid and vitamin B12 levels with the severity of preeclampsia and fetal birth weight. Hypertens Pregnancy 28:190–200. [DOI] [PubMed] [Google Scholar]
- Han YS, Ha EH, Park HS, Kim YJ, Lee SS. 2011. Relationships between pregnancy outcomes, biochemical markers and pre-pregnancy body mass index. Int J Obes 35:570–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hvilsom GB, Thorsen P, Jeune B, Bakketeig LS. 2002. C-reactive protein: a serological marker for preterm delivery? Acta Obstet Gynecol Scand 81:424–429. [DOI] [PubMed] [Google Scholar]
- Kushner I, Rzewnicki D, Samols D. 2006. What does minor elevation of C-reactive protein signify? Am J Med 119:166.e17–166.e28. [DOI] [PubMed] [Google Scholar]
- Kuzawa CW, Adair LS, Borja J, McDade TW. 2013. C-reactive protein by pregnancy and lactational status among Filipino young adult women. Am J Hum Biol 25:131–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JM, Mei Z, Ye R, Serdula MK, Ren A, Cogswell ME. 2013. Micronutrient supplementation and pregnancy outcomes: double-blind randomized controlled trial in China. JAMA Intern Med 173:276–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo SM, Lobo FR, Bota DP, Lopes-Ferreira F, Soliman HM, Mélot C, Vincent JL. 2003. C-reactive protein levels correlate with mortality and organ failure in critically ill patients. Chest 123:2043–2049. [DOI] [PubMed] [Google Scholar]
- Lohsoonthorn V, Qiu C, Williams MA. 2007. Maternal serum C-reactive protein concentrations in early pregnancy and subsequent risk of preterm delivery. Clin Biochem 40:330–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EM. 2009. Changes in serum immunity during pregnancy. Am J Hum Biol 21:401–403. [DOI] [PubMed] [Google Scholar]
- Picklesimer AH, Jared HL, Moss K, Offenbacher S, Beck JD, Boggess KA. 2008. Racial differences in C-reactive protein levels during normal pregnancy. Am J Obstet Gynecol 199:523.e1–523.e6. doi: 10.1016/j.ajog.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitiphat W, Gillman MW, Joshipura KJ, Williams PL, Douglass CW, Rich-Edwards JW. 2005. Plasma C-reactive protein in early pregnancy and preterm delivery. Am J Epidemiol 162:1108–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebelo F, Schlüssel MM, Vaz JS, Franco-Sena AB, Pinto TJ, Bastos FI, Adegboye AR, Kac G. 2013. C-reactive protein and later preeclampsia: systematic review andmeta-analysis taking into account the weight status. J Hypertens 31:16–26. [DOI] [PubMed] [Google Scholar]
- Romem Y, Artal R. 1985. C-reactive protein in pregnancy and in the postpartum period. Am J Obstet Gynecol 151:380–383. [DOI] [PubMed] [Google Scholar]
- Rota S, Yildirim B, Kaleli B, Aybek H, Duman K, Kaptanoğlu B. 2005. C-reactive protein levels in non-obese pregnant women with gestational diabetes. Tohoku J Exp Med 206:341–345. [DOI] [PubMed] [Google Scholar]
- Sacks GP, Seyani L, Lavery S, Trew G. 2004. Maternal C-reactive protein levels are raised at 4 weeks gestation. Hum Reprod 19:1025–1030. [DOI] [PubMed] [Google Scholar]
- Tjoa ML, van Vugt JM, Go AT, Blankenstein MA, Oudejans CB, van Wijk IJ. 2003. Elevated C-reactive protein levels during first trimester of pregnancy are indicative of preeclampsia and intrauterine growth restriction. J Reprod Immunol 59:29–37. [DOI] [PubMed] [Google Scholar]
- Trevisanuto D, Doglioni N, Altinier S, Zaninotto M, Plebani M, Zanardo V. 2007. High-sensitivity C-reactive protein in umbilical cord of small-for-gestational-age neonates. Neonatology 91:186–189. [DOI] [PubMed] [Google Scholar]
- Vogel I, Grove J, Thorsen P, Moestrup SK, Uldbjerg N, Møller HJ. 2005. Preterm delivery predicted by soluble CD163 and CRP in women with symptoms of preterm delivery. BJOG 112:737–742. [DOI] [PubMed] [Google Scholar]
- von Versen-Hoeynck FM, Hubel CA, Gallaher MJ, Gammill HS, Powers RW. 2009. Plasma levels of inflammatory markers neopterin, sialic acid, and C-reactive protein in pregnancy and preeclampsia. Am J Hypertens 22:687–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts DH, Krohn MA, Wener MH, Eschenbach DA. 1991. C-reactive protein in normal pregnancy. Obstet Gynecol 77:176–180. [DOI] [PubMed] [Google Scholar]
- Wolf M, Sandler L, Hsu K, Vossen-Smirnakis K, Ecker JL, Thadhani R. 2003. First-trimester C-reactive protein and subsequent gestational diabetes. Diabetes Care 26:819–824. [DOI] [PubMed] [Google Scholar]
- Yeh CS. 1982. A study on CRP in Chinese pregnant women and newborns. J Formosan Med Assoc 81:112–118. [PubMed] [Google Scholar]


