Abstract
Extraintestinal manifestations (EIMs) are frequently observed in IBDs and contribute considerably to morbidity and mortality. They have long been considered a difficult to treat entity due to limited therapy options, but the increasing use of anti-tumour necrosis factors has dramatically changed the therapeutic approach to EIM in recent years. Newly emerging therapies such as JAK inhibitors and anti-interleukin 12/23 will further shape the available armamentarium. Clinicians dealing with EIMs in everyday IBD practice may be puzzled by the numerous available biological agents and small molecules, their efficacy for EIMs and their potential off-label indications. Current guidelines on EIMs in IBD do not include treatment algorithms to help practitioners in the treatment decision-making process. Herein, we summarise knowledge on emerging biological treatment options and small molecules for EIMs, highlight current research gaps, provide therapeutic algorithms for EIM management and shed light on future strategies in the context of IBD-related EIMs.
INTRODUCTION
Extraintestinal manifestations (EIMs) are frequently observed in IBDs. Their reported prevalence ranges from 6% to 47% depending on the studied population and the definition of EIM.1–7 Involvement of the following four organs are mostly considered as classical EIMs: joints (axial spondyloarthropathy, peripheral arthritis); skin (erythema nodosum (EN), pyoderma gangrenosum (PG), Sweet’s syndrome, oral aphthous ulcers); liver and biliary tract (primary sclerosing cholangitis (PSC)); and eyes (uveitis, episcleritis).8 9 In contrast, immune-mediated inflammatory diseases (IMIDs) such as psoriasis or rheumatoid arthritis are associated with IBD, but typically are not considered an EIM.10 The frequency of EIM increases with longer disease duration, and the presence of one EIM predisposes for the development of further EIMs.7 Some EIMs are associated with intestinal disease activity (such as pauciarticular peripheral arthritis, EN, Sweet’s syndrome, oral ulcers and episcleritis), while others do not parallel intestinal IBD activity.9 The latter group includes axial spondyolarthropathy and polyarticular peripheral arthritis of the small joints. The remaining EIMs may or may not parallel IBD activity, such as seen for PG, uveitis and PSC.9
EIMs should be treated since they considerably affect morbidity and mortality of patients with IBD.11 12 In addition, EIMs are associated with higher disease activity, increased risk of surgery and increased need for treatment escalation.13 14 Before the implementation of biologics, treatment options had been quite limited. The increasing use of anti-tumour necrosis factor (anti-TNF) and newly available biologics, small molecules have dramatically changed the therapeutic approach in EIM management. These drugs have been approved for various indications beyond IBD such as for rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis or uveitis. Nevertheless, data on their emerging use as EIM treatment are still sparse. Analysing EIM treatment in a prospective manner is difficult, since only few patients present with EIMs at study enrolment.8 To some degree, findings from IMIDs treated with biologics and small molecules—particularly randomised controlled trials in rheumatology and dermatology—might be extrapolated.
This review summarises knowledge on emerging biological treatment options and small molecules for EIM (table 1, figure 1), highlight current research gaps, provide therapeutic algorithms for EIM management and shed light on future strategies in the context of IBD-related EIMs.
Table 1.
Synopsis over current and emerging treatment options for different types of EIM
| Conventional treatment | Anti-TNF | Anti-integrins | JAK inhibitors | Anti-IL-12/23 | Comments | |
|---|---|---|---|---|---|---|
| Axial SpA | Short-term NSAIDs (COX-2) | Early use, particularly in refractory cases | No clinical data available | Efficacious in SpA, not approved yet | Efficacious in phase II trials, phase III trials early terminated | |
| Peripheral arthritis | Short-term NSAIDs, (COX-2), sulfasalazine MTX |
For resistant cases | Response in up to 50%, but also paradoxical arthritis possible | Approved for rheumatoid arthritis | Approved for psoriatic arthritis | Main goal: treatment of underlying IBD |
| Uveitis episcleritis | Steroids, immunosuppressants | Very efficacious, but small sample size | No data available | Successful use in two patients | Successful use in one patient | |
| EN | Steroids | Consider in severe or refractory cases | Resolution or partial response, but only case reports/series absence of MAdCAM1 expression in the skin | Approved for psoriatic arthritis, STAT3 expression in skin biopsies of patients with EN | Approved for psoriasis, high improvement rates based on a single case series | Main goal: treatment of underlying IBD |
| PG | Systemic steroids, CNI (local or systemic) | Consider early use | No resolution with VDZ (case report), absence of MAdCAM1 expression in the skin | Approved for psoriatic arthritis, resolution of PG in three patients | Approved for psoriasis, high improvement rates based on a single case series |
CNI, calcineurin inhibitor; EIM, extraintestinal manifestation; EN, erythema nodosum; IL, interleukin; JAK, Janus kinase; MTX, methotrexate; NSAID, non-steroidal anti-inflammatory drug; PG, pyoderma gangrenosum; SpA, axial spondyloarthropathy; TNF, tumour necrosis factor; VDZ, vedolizumab.
Figure 1.
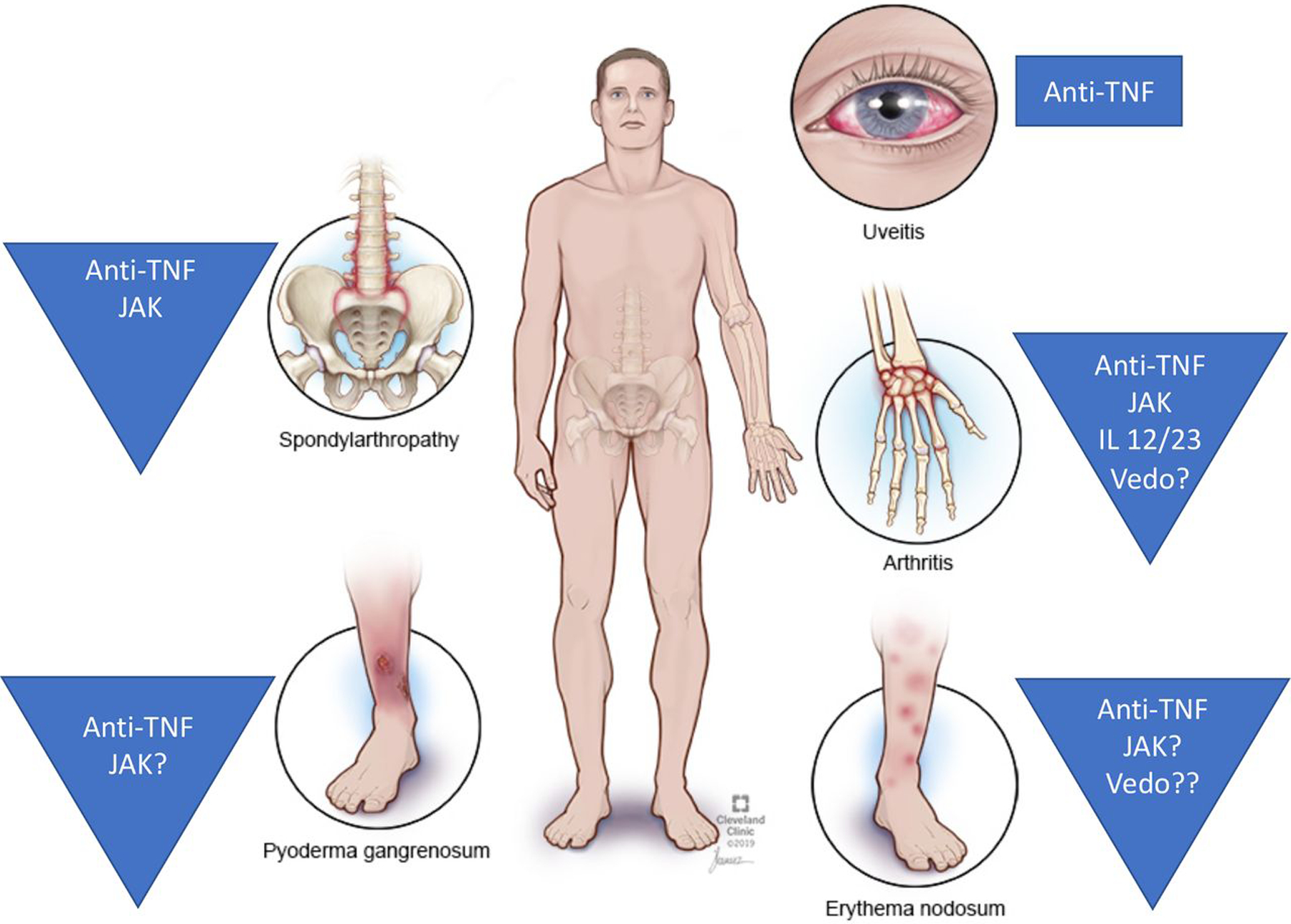
Overview of the most common EIM and their biological, small-molecule treatment options. EIM, extraintestinal manifestation; IL, interleukin; JAK, Januskinase; TNF, tumour necrosis factor; Vedo, vedolizumab.
ANTI-TNF
TNF-dependent mechanisms in EIM pathophysiology are the rationale for the use of anti-TNF therapeutic approaches. Clinical data suggest good response rates for cutaneous manifestations, arthritis and ocular EIM with several interventional and non-interventional studies available.15–17 Although no head-to-head comparisons have been performed, retrospective analyses suggest similar efficacy of anti-TNF agents.16 Slight, but non-significant differences appear to be attributed to the fact that adalimumab and certolizumab pegol are more often second-line and third-line treatments compared with infliximab.
Infliximab
So far, the only randomised placebo controlled trial in EIM has been conducted with infliximab (5 mg/kg), where 30 patients with PG (19 patients with IBD) were treated for 2 weeks.18 Clinical improvement was achieved in 46% versus 6%. In addition, several open-label studies have been published using infliximab (5 mg/kg) in EIMs with clinical remission rates of 33%–46% (arthritis, inflammatory arthralgia), 21%–25% (PG), 100% (uveitis, cutaneous manifestations), and improvement rates of 60%–80% (arthritis, inflammatory arthralgia), 63.6% (inflammatory back pain) and 69%–100% (PG).18–21 Generini et al further showed decreasing prevalence rates of arthritis after 6 months of infliximab treatment (from 58% down to 12.5%).22 Several retrospective studies (sample size 13–54 patients) are consistent with these data with improvement rates of 70%–100% for cutaneous and joint manifestations and remission rates of 25%–38% (cutaneous/joint manifestations) and 92%–100% (PG).23–25 A comprehensive analysis of infliximab-treated EIMs (musculoskeletal, ocular and cutaneous manifestations) in 189 patients enrolled in the Swiss IBD cohort study revealed clinical improvement rates of 74%.16
Adalimumab
Adalimumab (induction dose 160/80 mg, then 40 mg every 2 weeks) has been prospectively studied in the context of EIMs and demonstrated decreasing frequencies of arthritis (from 8.7% to 2.1%), sacroileitis (from 3.6% to 1.9%) and EN (from 2.4% to 0.4%) after a 20-week treatment.26 Six-month treatment resulted in improvement/remission rates of 61% (arthritis, n=7) and 100% (ankylosing spondylitis, n=1; uveitis, n=1; and PG, n=2).27 PG remission rates were 100% in the retrospective study conducted by Argüelles-Arias et al (n=7).24 The Swiss group revealed overall response rates of 70% for adalimumab-treated EIMs in 67 patients.16
Other anti-TNFs
The only study looking at IBD-related EIMs treated with certolizumab pegol was an analysis of the Swiss IBD cohort, where response rates were reported as 56%.16 The pegylated anti-TNF agent has been approved for other IMIDs such as rheumatoid arthritis, psoriatic arthritis and ankylosing spondylitis suggesting efficacy in IBD-associated musculoskeletal manifestations. Reported improvement rates from phase III trials were 57.3%–60.8% (vs placebo 8.7%–13.6%) for rheumatoid arthritis at week 24,28 29 51.9%–58.0% (vs placebo 24.3%) for psoriatic arthritis at week 1230 and 57.7%–63.6% (vs placebo 38.3%) for ankylosing spondylitis at week 12.31 A post hoc analysis of the RAPID-axSpA trial revealed significantly lower rates of uveitis flares with certolizumab pegol compared with placebo (3.0 vs 10.3 per 100 patient-years).32 In addition, uveitis was successfully treated in five of seven patients with previous failure to another anti-TNF agent.33
While no data are available for golimumab’s efficacy in EIM management, data from trials with patients suffering from other IMIDs may be extrapolated. In phase III trials, the following response rates have been described: 71.1% for ankylosing spondylitis at week 16 (vs placebo 40%)34; 51.0% for psoriatic arthritis at week 14 (vs placebo 9.0%)35; 61.6% for methotrexate (MTX)-naive rheumatoid arthritis after week 14 (vs 49.4% for MTX alone)36 and 55.1%–74.7% for MTX-experienced rheumatoid arthritis (vs 27.3%–33.1% for MTX alone).37 38 Golimumab treatment has been recently shown to significantly reduce the occurrence rate of acute uveitis in patients with ankylosing spondylitis (from 11.1 to 2.2/100 patient-years).39 More lately, there has been a case report demonstrating resolution of pyostomatitis vegetans in a patient with UC who under treatment with golimumab.40
Taken together, anti-TNFs are an efficacious treatment option for IBD-related EIMs. While anti-TNF agents are probably equally effective, best evidence is available for infliximab (online supplementary table).
ANTI-INTEGRINS
Anti-integrins block the interaction between ligands on lymphocytes and their corresponding receptors expressed on endothelial cells. Thereby, they interfere with leucocyte adhesion and migration, ultimately inhibiting T-lymphocyte trafficking to the site of inflammation. Two agents have been approved for the treatment of IBD: natalizumab and vedolizumab.
Vedolizumab
The gut selective mechanism of the integrin α4β7 antibody vedolizumab should restrict its activity to the gut, since the adhesion molecule ucosal addressin cell adhesion molecule 1 (MAdCAM1), which acts as the counterpart of α4β7, is exclusively expressed in the GI tract. A recent analysis of cutaneous EIMs did not reveal overexpression of MAdCAM1 in skin biopsies from patient with EN and PG.41 Nonetheless, vedolizumab treatment has been associated with successful resolution or at least improvement of EIMs, such as arthritis/arthralgia and EN in a recent prospective study.42 Two hundred and ninety-four vedolizumab-treated patients were followed through week 54; 49 of those patients had at least one EIM at baseline. Clinical remission rates were 44.7% for arthritis/inflammatory arthralgia (n=47) and 75% for cutaneous manifestations (n=4). These findings were partially replicated in a recent post hoc analysis of the vedolizumab trials in IBD43: vedolizumab-treated patients with Crohn’s disease (CD) were less likely to show new or worsening arthritis/arthralgia compared with placebo (HR=0.63). There are two possible explanations for these results: (1) vedolizumab has beneficial effects through a better control of intestinal disease therefore affecting EIMs that parallel intestinal disease activity. This is underscored by the fact that complete remission of musculoskeletal EIMs was associated with clinical remission of IBD in both studies42 43 and (2) the positive effect of vedolizumab on disease activity of EIM could occur, if lymphocytes require the α4β7–MAdCAM1 interaction to gain access to the gut where they are activated, followed by non-α4β7-dependent entry to extraintestinal sites. However, the post hoc analysis of the GEMINI trials did not reveal a positive effect of vedolizumab in UC and sustained resolution rates between for arthritis/arthralgia were not significantly different from placebo (GEMINI trial III 22% vs 16%).43 Moreover, a recent meta-analysis based on three interventional studies, five non-interventional studies and three case series concluded that as of yet there is no strong evidence for the efficacy of vedolizumab in the treatment of pre-existing EIMs.44 Lately, an ECCO CONFER series showed no response of PG, but partial response of EN.45 However, the latter is based on four patients only and should therefore be interpreted cautiously. Taken together, vedolizumab should be used for the treatment of intestinal disease activity and not for EIM. However, it might be considered for EIMs (such as peripheral arthritis) if they clearly parallel intestinal disease and if intestinal inflammation is indeed present.
Natalizumab
Natalizumab has been studied for the treatment of multiple sclerosis and CD. Although approved for CD management, it has rarely been used in clinical practice given the potentially fatal side effects of progressive multifocal leucoencephalopathy.46 Neither prospective nor retrospective studies have been conducted in IBD-associated EIMs. A study evaluating natalizumab for the treatment of rheumatoid arthritis failed to show efficacy and was terminated early (ClinicalTrials.gov NCT00083759). With regards to the potential of fatal side effects related to reactivation of the JC polyomavirus, natalizumab cannot be recommended in the setting of EIM.
Based on the available studies, anti-integrins are a possible treatment modality for IBD-related EIMs that parallel intestinal disease activity (online supplementary table). Natalizumab should not be used due to potentially deleterious side effects.
JANUS KINASE (JAK) INHIBITORS
JAK inhibitors are emerging oral agents for the treatment of IBD, rheumatological and dermatological disorders. Tofacitinib has been recently approved for UC in the USA and in Europe. Results from trials in rheumatoid arthritis and psoriasis are encouraging for its use in EIM management. Still, it should be kept in mind that positive results in the treatment of other IMIDs cannot be applied one to one to EIM management. Upregulation of STAT3 in EN and PG sheds light on the possible involvement of the JAK-STAT pathway in cutaneous EIMs and makes a response to JAK inhibitors reasonable to predict.41
Tofacitinib
Tofacitinib is approved for the treatment of UC, rheumatoid arthritis and psoriatic arthritis. Reported improvement rates from phase III trials for rheumatoid arthritis are 42%–71% after 3 to 6 months (placebo rate 24%–31%).47–51 For psoriatic arthritis they are 47%–61% after 3 months (placebo rate 24%–33%).52 53 For psoriasis they are 40%–64% after 12 to 16 weeks (placebo rates 6%–11%).54 55 For ankylosing spondylitis, a phase II trial showed promising results with 12-week improvement rates of 52%–81% (placebo rate 41%).56 A post hoc analysis of data from the OCTAVE Induction 1 and 2 and OCTAVE Sustain was recently published in abstract form suggesting some improvement of IBD-related peripheral arthritis by week 52, but low patient numbers did not permit any clear conclusions regarding the effect of tofacitinib on EIM symptoms (online supplementary table).57 Nevertheless, upregulation of the JAK-STAT pathway has been demonstrated in cutaneous manifestations. Immunohistochemical analyses of skin biopsies from patients with PG and EN revealed overexpression similar to what was seen in intestinal samples from patients with IBD.41 Indeed, upregulation of JAK-STAT was significantly higher in PG, EN compared with psoriasis. The use of tofacitinib in PG has further been supported by a case report and by a case series involving three patients.58 59 Very recently, tofacitinib has been successfully used to treat refractory uveitis and scleritis in two patients.60
Filgotinib
Filgotinib is a selective JAK-1 inhibitor currently under investigation for CD, rheumatoid arthritis, psoriatic arthritis and ankylosing spondylitis. Phase II trials in rheumatic diseases revealed promising results with improvement rates of 64%–79% for rheumatoid arthritis (at week 12, vs placebo rate of 29% and 44%), 80% for psoriatic arthritis (at week 16, vs placebo rate of 33%) and 76% for ankylosing spondylitis (at week 12, vs placebo rate of 40%).61–64
Despite the lack of direct evidence for the efficacy of tofacitinib in treatment of IBD-related EIMs, positive trials in rheumatology and dermatology make tofacitinib an appealing treatment modality for cutaneous and musculoskeletal EIMs. However, efficacy in the treatment of IMIDs such as rheumatoid arthritis does not necessarily imply guaranteed efficacy in the treatment of EIM (such as peripheral arthritis). In addition, its use is restricted to patients with UC, as it has not been shown to be efficacious in CD treatment.65
ANTI-INTERLEUKIN 12/23 (IL-12/23)
The anti-p40 (IL-12/23) antibody ustekinumab is approved for the treatment of CD, UC, psoriasis and psoriatic arthritis. Few case series and case reports revealed improvement of IBD-associated arthritis and cutaneous manifestations (online supplementary table). Still, there are no prospective studies published regarding the efficacy of ustekinumab in the treatment of IBD-associated EIMs.45 66–69 Phase III trials demonstrated improvement rates of psoriatic arthritis at week 24 of 42.4%–49.5% (compared with a placebo rate of 20.2%–22.8%).70–72 Despite some initially promising results in the treatment of ankylosing spondylitis with improvement rates after 24 week of 65%, the phase III programme was early terminated due to the lack of efficacy.73 74 Lately, ustekinumab has been successfully used in a patient with uveitis.75
Based on the available data (although not studied in true EIMs), ustekinumab appears to be a valuable option for musculoskeletal EIMs (peripheral arthritis) and might be considered for cutaneous and ocular manifestations in case other therapies fail. Given early termination of the phase III programme for ankylosing spondylitis, its use in this setting cannot be strongly supported.
OTHER ANTI-INTERLEUKINS
Further interleukins such as IL-17 or IL-6 have been successfully targeted in rheumatological and dermatological inflammatory disorders suggesting efficacy in EIM management also. The anti-IL-17 antibody secukinumab is approved for the treatment of psoriasis and psoriatic arthritis. Whether it has beneficial effects on joint and skin manifestations in IBD remains unknown. However—despite the pathogenic role of Th17 cells in the development of colitis—trials with anti-IL-17 have failed in IBD, reporting an even higher adverse rate than placebo.76 Moreover, in contrast to its efficacy in other inflammatory disorders, anti-IL-17 can even exacerbate IBD activity,77 which highlights a distinct involvement of the IL-17 pathway in these entities. The anti-IL-6 antibody tocilizumab is indicated in the treatment of rheumatoid arthritis. Anti-IL-6 antibodies might also be effective in IBD.78 Tocilizumab’s main limitation is the increased risk of intestinal perforation in patients with IBD.78
Given better options with a more favourable safety profile, off-label use of secukinumab and tocilizumab to treat EIMs in patients with IBD cannot be recommended.
TREATMENT ALGORITHM
In 2016, the first ECCO consensus guideline on EIMs was published with a detailed description of each EIM and discussion of possible treatment options.79 Except for axial spondyloarthropathy, these guidelines lack clear recommendations regarding a treatment algorithm and when, how to use biological and small-molecule therapies. A thorough meta-analysis of the efficacy of anti-TNFs for treatment of EIMs had not been available at that time.15 Data on vedolizumab and JAK-inhibitors have emerged only very recently. Other societies such as the American Gastroenterology Association, American College of Gastroenterology, World Gastroenterology Organization and the Asian Pacific Association of Gastroenterology do not have specific guidelines or recommendations for EIM management.
Treatment algorithms for musculoskeletal, cutaneous and ocular EIM
As a general principle, underlying intestinal disease activity should always be treated first, particularly for EIMs that clearly parallel IBD activity, such as pauciarticular arthritis or EN. Mild musculoskeletal disease can be treated using non-steroidal anti-inflammatory drugs (NSAIDs), but caution should be applied given their potential to worsen intestinal disease activity.80 81 Selective COX-2 inhibitors are an alternative since they have not been shown to exacerbate IBD.82 83 However, clinicians should keep their cardiovascular side effects in mind. Anti-TNFs should be initiated for refractory cases and/or intolerance to NSAIDs. Given the lack of clinical data on tofacitinib and ustekinumab in the treatment of IBD-associated EIMs, these drugs should preferably be used when initiated for underlying intestinal inflammation or in case of anti-TNF failure. For cutaneous manifestations, topical steroids might be sufficient. In case of severe disease, particularly PG, early use of anti-TNF should be considered regardless of intestinal disease activity. Best evidence is available for infliximab, but other anti-TNF agents are probably equally effective. Given the upregulation of the JAK-STAT pathway in both EN and PG, JAK inhibitors might be considered in the context of active intestinal inflammation, contraindications for anti-TNF and/or loss of response to anti-TNF. However, they represent an option in patients with UC only given their lack of efficacy in CD. Uveitis should always be seen as an ophthalmological emergency. Treatment should therefore be guided by an experienced ophthalmologist. Although uveitis may parallel intestinal disease activity, treatment of intestinal inflammation is not sufficient. Mild courses can be treated with topical corticosteroids, while more aggressive disease requires systemic steroids, immunosuppressive agents or anti-TNF. Possible therapeutic algorithms based on the above presented literature and the joint experience of the authors are shown in online supplementary figure 1 (musculoskeletal EIMs), online supplementary figure 2 (cutaneous EIMs) and online supplementary figure 3 (uveitis).
Multidisciplinary team (MDT) approach
In recent years, it has been increasingly recognised that an MDT approach is needed in IBD care. The complex nature of the disease, the presence of both intestinal and extraintestinal complications and the impact on quality of life are best dealt within dedicated IBD centres.84 85 Indeed, quality of care has been shown to be superior in specialised IBD centres compared with non-specialist general gastroenterology clinics.86 The UK IBD standard group recommends scheduled weekly MDT meetings to discuss complex IBD cases. Given the complex nature of EIM and the overlap with various non-GI specialties, an MDT approach with the additional inclusion of ophthalmologists, rheumatologists, dermatologists (depending on the present EIM) is crucial.10
FUTURE PERSPECTIVES
The diverse mechanisms underlying and perpetuating inflammation in EIMs are poorly defined, which limits the development of specific treatment strategies. Proposed pathophysiological mechanisms can be broken down into two main hypotheses: (1) EIMs as an extension of immune responses from the gut (due to molecular mimicry, T-cell trafficking and ectopic expression of gut-specific chemokines) and (2) EIMs as an independent inflammatory event (with systemic changes in innate immunity, changes in the microbiome and a general shift toward a proinflammatory state). The lack of specific animal models limits more mechanistic studies. However, newer technologies such as single-cell RNA sequencing may help to characterise the EIM inflammatory process in more detail.
Post hoc analyses of the randomised controlled trials with JAK inhibitors and ustekinumab will help to establish their role in EIM management. However, as for anti-TNF, proper and binding outcomes to assess EIM response are still lacking. Prospective IBD trials usually do not include systematic assessment of EIMs such as performed in rheumatology, dermatology or ophthalmology studies. Binding recommendations on how to assess EIMs and response to treatment have yet to be discussed. Given emerging evidence for the efficacy of anti-TNFs and the potential of newer treatment modalities, comparative trials are needed to define best therapeutic strategies, when EIMs are present. In addition, several open questions remain: should early use of biologics be recommended in the context of an IBD-related EIM? Are combination therapies superior for EIM treatment? And can drug target levels for IBD management be applied one to one for the treatment of EIMs?
CONCLUSIONS
The introduction of anti-TNF therapy has dramatically changed the management of EIMs over the last 10 years. Anti-TNF agents appear to be efficacious for most EIMs, although evidence for their efficacy mostly emerges from non-randomised controlled trials or is extrapolated from studies in rheumatology or dermatology. Despite their beneficial effects, anti-TNFs can also cause anti-TNF-induced skin lesions and therefore contribute to the burden of EIMs. Anti-integrins might have positive effects on joint and cutaneous manifestations, although underlying mechanisms remain unclear. Data for the use of JAK-inhibitors and anti-IL-12/23 in IBD-associated EIMs are currently limited, but their successful use in rheumatology and dermatology makes a response to these agents reasonable to predict (tables 2 and 3). The use of biological agents that are not approved for IBD cannot be recommended in treatment of EIMs given their possible deleterious effects on intestinal disease itself such as seen for anti-IL-6 and anti-IL-17.
Table 2.
Summary of biologics in IBD and their role in the treatment of IBD-associated EIM. Non-biological treatment options are not shown
| Anti-TNF | Anti-integrins | JAK | IL-12/23 | |||||
|---|---|---|---|---|---|---|---|---|
| IFX | ADA | CZP | Goli | VDZ | Natalizumab | Tofa | Ustekinumab | |
| Arthritis | ||||||||
| SpA | ||||||||
| EN | ||||||||
| PG | ||||||||
| Uveitis | ||||||||
 Should be considered.
Should be considered.
 May be considered.
May be considered.
 Cannot be recommended.
Cannot be recommended.
ADA, adalimumab; CZP, certolizumab; EN, erythema nodosum; Goli, golimumab; IFX, infliximab; IL, interleukin; JAK, Janus kinase; PG, pyoderma gangrenosum; SpA, axial spondyloarthropathy; TNF, tumour necrosis factor; Tofa, tofacitinib; VDZ, vedolizumab.
Table 3.
Current non-IBD FDA-approved indications for biological agents used in IBD management
| Biologics | Agent | Approved for the following non-IBD IMIDs |
|---|---|---|
| Anti-TNF | Infliximab | RA, SpA, PsA, Pso |
| Adalimumab | RA, JIA, PsA, SpA, hidradenitis suppurativa, Pso, uveitis | |
| Certolizumab pegol | RA, PsA, SpA, Pso | |
| Golimumab | RA, PsA, SpA | |
| Anti-integrin | Vedolizumab | None |
| Natalizumab | Multiple sclerosis | |
| JAK inhibitors | Tofacitinib | RA, PsA |
| IL-12/23 | Ustekinumab | Pso, PsA |
FDA, Food and Drug Administration; IL, interleukin; IMIDs, immune-mediated inflammatory diseases; JAK, Janus kinase; JIA, juvenile idiopathic arthritis; PsA, psoriatic arthritis; Pso, psoriasis; RA, rheumatoid arthritis; SpA, axial spondyloarthropathy; TNF, tumour necrosis factor.
Randomised controlled trials for the treatment of IBD-associated EIMs where activity of EIMs is properly assessed are urgently needed to establish clear and binding guidelines. Simple extraction of EIMs from index scores such as the CDAI in post hoc analyses are not satisfactory to sufficiently evaluate a drug’s potential in EIM management.
Supplementary Material
Acknowledgements
The authors thank Professor Alexander Navarini, MD (dermatology) and Professor Beat Michel, MD (rheumatology) for their critical review of the revised manuscript.
Funding
This work was supported by a grant from the Swiss National Science Foundation to TG (grant no: P2ZHP3_168561) and a research grant from the Novartis Foundation to TG.
Competing interests
TG has a consulting contract with Sanofi-Aventis, received a travel grant from Falk Pharma and Vifor, and an unrestricted research grant from Novartis. SRV received consultant fees and unrestricted research grants from Abbott, Celtrion, Ferring, MSD, Pfizer, Sanofi-Aventis, Takeda, Tillots, UCB, Vifor and Falk Pharma. FR has consulted with Allergan, AbbVie, Boehringer-Ingelheim, Celgene, Helmsley, Jannsen, Pliant, Receptos, RedX, Roche, Samsung, Takeda, Thetis, UCB and received research grants from Celgene, Pliant and UCB. TK received consultant fees and speaker honoraria from Abbott, Amgen, Biogen, Celtrion, Falk Pharma, Janssen, MSD, Takeda and UCB. LP-B reports personal fees from Merck, Abbvie, Janssen, Ferring, Tillots, Celltrion, Takeda, Pfizer, Amgen, Biogen, Samsung Bioepis, Genentech, Vifor, Pharmacosmos, Biogaran, Boerhinger-Ingelheim, Lilly, Index Pharmaceuticals, Sandoz, Celgene, Alma, Sterna, Nestlé and Enterome. AMS received consulting and/or speaker fees from Abbvie, Adare, Dr Falk Pharma, MSD, UCB, Pfizer, Takeda, Vifor, Receptos, Regeneron and received research grants Adare, Dr Falk Pharma, Receptos and Regeneron. No company representative was involved in conception, writing or financing of this study.
Footnotes
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
REFERENCES
- 1.Bernstein CN, Blanchard JF, Rawsthorne P, et al. The prevalence of extraintestinal diseases in inflammatory bowel disease: a population-based study. Am J Gastroenterol 2001;96:1116–22. [DOI] [PubMed] [Google Scholar]
- 2.Bernstein CN, Wajda A, Blanchard JF. The clustering of other chronic inflammatory diseases in inflammatory bowel disease: a population-based study. Gastroenterology 2005;129:827–36. [DOI] [PubMed] [Google Scholar]
- 3.Ricart E, Panaccione R, Loftus EV, et al. Autoimmune disorders and extraintestinal manifestations in first-degree familial and sporadic inflammatory bowel disease: a case-control study. Inflamm Bowel Dis 2004;10:207–14. [DOI] [PubMed] [Google Scholar]
- 4.Rankin GB, Watts HD, Melnyk CS, et al. National cooperative Crohn’s disease study: extraintestinal manifestations and perianal complications. Gastroenterology 1979;77:914–20. [PubMed] [Google Scholar]
- 5.Su CG, Judge TA, Lichtenstein GR. Extraintestinal manifestations of inflammatory bowel disease. Gastroenterol Clin North Am 2002;31:307–27. [DOI] [PubMed] [Google Scholar]
- 6.Veloso FT, Carvalho J, Magro F. Immune-related systemic manifestations of inflammatory bowel disease. A prospective study of 792 patients. J Clin Gastroenterol 1996;23:29–34. [DOI] [PubMed] [Google Scholar]
- 7.Vavricka SR, Brun L, Ballabeni P, et al. Frequency and risk factors for extraintestinal manifestations in the Swiss inflammatory bowel disease cohort. Am J Gastroenterol 2011;106:110–9. [DOI] [PubMed] [Google Scholar]
- 8.Lakatos PL, Lakatos L, Kiss LS, et al. Treatment of extraintestinal manifestations in inflammatory bowel disease. Digestion 2012;86(Suppl 1):28–35. [DOI] [PubMed] [Google Scholar]
- 9.Vavricka SR, Schoepfer A, Scharl M, et al. Extraintestinal manifestations of inflammatory bowel disease. Inflamm Bowel Dis 2015;21:1982–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hedin CRH, Vavricka SR, Stagg AJ, et al. The pathogenesis of extraintestinal manifestations: implications for IBD research, diagnosis, and therapy. J Crohns Colitis 2019;13:541–54. [DOI] [PubMed] [Google Scholar]
- 11.Monsén U, Sorstad J, Hellers G, et al. Extracolonic diagnoses in ulcerative colitis: an epidemiological study. Am J Gastroenterol 1990;85:711–6. [PubMed] [Google Scholar]
- 12.Das KM. Relationship of extraintestinal involvements in inflammatory bowel disease: new insights into autoimmune pathogenesis. Dig Dis Sci 1999;44:1–13. [DOI] [PubMed] [Google Scholar]
- 13.Conway G, Velonias G, Andrews E, et al. The impact of co-existing immune-mediated diseases on phenotype and outcomes in inflammatory bowel diseases. Aliment Pharmacol Ther 2017;45:814–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burisch J, Jess T, Egeberg A. Incidence of immune-mediated inflammatory diseases among patients with inflammatory bowel diseases in Denmark. Clin Gastroenterol Hepatol 2019;17:2704–12. e2703. [DOI] [PubMed] [Google Scholar]
- 15.Peyrin-Biroulet L, Van Assche G, Gómez-Ulloa D, et al. Systematic review of tumor necrosis factor antagonists in extraintestinal manifestations in inflammatory bowel disease. Clin Gastroenterol Hepatol 2017;15:25–36. e27. [DOI] [PubMed] [Google Scholar]
- 16.Vavricka SR, Gubler M, Gantenbein C, et al. Anti-TNF treatment for extraintestinal manifestations of inflammatory bowel disease in the Swiss IBD cohort study. Inflamm Bowel Dis 2017;23:1174–81. [DOI] [PubMed] [Google Scholar]
- 17.Greuter T, Bertoldo F, Rechner R, et al. Extraintestinal manifestations of pediatric inflammatory bowel disease: prevalence, presentation and anti-TNF treatment. J Pediatr Gastroenterol Nutr 2016;65:200–6. [DOI] [PubMed] [Google Scholar]
- 18.Brooklyn TN, Dunnill MGS, Shetty A, et al. Infliximab for the treatment of pyoderma gangrenosum: a randomised, double blind, placebo controlled trial. Gut 2006;55:505–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaufman I, Caspi D, Yeshurun D, et al. The effect of infliximab on extraintestinal manifestations of Crohn’s disease. Rheumatol Int 2005;25:406–10. [DOI] [PubMed] [Google Scholar]
- 20.Rispo A, Scarpa R, Di Girolamo E, et al. Infliximab in the treatment of extra-intestinal manifestations of Crohn’s disease. Scand J Rheumatol 2005;34:387–91. [DOI] [PubMed] [Google Scholar]
- 21.Herfarth H, Obermeier F, Andus T, et al. Improvement of arthritis and arthralgia after treatment with infliximab (remicade) in a German prospective, open-label, multicenter trial in refractory Crohn’s disease. Am J Gastroenterol 2002;97:2688–90. [DOI] [PubMed] [Google Scholar]
- 22.Generini S, Giacomelli R, Fedi R, et al. Infliximab in spondyloarthropathy associated with Crohn’s disease: an open study on the efficacy of inducing and maintaining remission of musculoskeletal and gut manifestations. Ann Rheum Dis 2004;63:1664–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caspersen S, Elkjaer M, Riis L, et al. Infliximab for inflammatory bowel disease in Denmark 1999–2005: clinical outcome and follow-up evaluation of malignancy and mortality. Clin Gastroenterol Hepatol 2008;6:1212–7. quiz 1176. [DOI] [PubMed] [Google Scholar]
- 24.Argüelles-Arias F, Castro-Laria L, Lobatón T, et al. Characteristics and treatment of pyoderma gangrenosum in inflammatory bowel disease. Dig Dis Sci 2013;58:2949–54. [DOI] [PubMed] [Google Scholar]
- 25.Regueiro M, Valentine J, Plevy S, et al. Infliximab for treatment of pyoderma gangrenosum associated with inflammatory bowel disease. Am J Gastroenterol 2003;98:1821–6. [DOI] [PubMed] [Google Scholar]
- 26.Löfberg R, Louis EV, Reinisch W, et al. Adalimumab produces clinical remission and reduces extraintestinal manifestations in Crohn’s disease: results from CARE. Inflamm Bowel Dis 2012;18:1–9. [DOI] [PubMed] [Google Scholar]
- 27.Barreiro-de-Acosta M, Lorenzo A, Domínguez-Muñoz JE. Efficacy of adalimumab for the treatment of extraintestinal manifestations of Crohn’s disease. Rev Esp Enferm Dig 2012;104:468–72. [DOI] [PubMed] [Google Scholar]
- 28.Smolen J, Landewé RB, Mease P, et al. Efficacy and safety of certolizumab pegol plus methotrexate in active rheumatoid arthritis: the RAPID 2 study. A randomised controlled trial. Ann Rheum Dis 2009;68:797–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keystone E, Heijde Dvander, Mason D, et al. Certolizumab pegol plus methotrexate is significantly more effective than placebo plus methotrexate in active rheumatoid arthritis: findings of a fifty-two-week, phase III, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Arthritis Rheum 2008;58:3319–29. [DOI] [PubMed] [Google Scholar]
- 30.Mease PJ, Fleischmann R, Deodhar AA, et al. Effect of certolizumab pegol on signs and symptoms in patients with psoriatic arthritis: 24-week results of a phase 3 double-blind randomised placebo-controlled study (RAPID-PsA). Ann Rheum Dis 2014;73:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Landewé R, Braun J, Deodhar A, et al. Efficacy of certolizumab pegol on signs and symptoms of axial spondyloarthritis including ankylosing spondylitis: 24-week results of a double-blind randomised placebo-controlled phase 3 study. Ann Rheum Dis 2014;73:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rudwaleit M, Rosenbaum JT, Landewé R, et al. Observed incidence of uveitis following Certolizumab pegol treatment in patients with axial spondyloarthritis. Arthritis Care Res 2016;68:838–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Llorenç V, Mesquida M, Sainz de la Maza M, et al. Certolizumab pegol, a new anti-TNF-α in the armamentarium against ocular inflammation. Ocul Immunol Inflamm 2016;24:167–72. [DOI] [PubMed] [Google Scholar]
- 34.Sieper J, van der Heijde D, Dougados M, et al. A randomized, double-blind, placebo-controlled, sixteen-week study of subcutaneous golimumab in patients with active nonradiographic axial spondyloarthritis. Arthritis Rheumatol 2015;67:2702–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kavanaugh A, McInnes I, Mease P, et al. Golimumab, a new human tumor necrosis factor alpha antibody, administered every four weeks as a subcutaneous injection in psoriatic arthritis: Twenty-four-week efficacy and safety results of a randomized, placebo-controlled study. Arthritis Rheum 2009;60:976–86. [DOI] [PubMed] [Google Scholar]
- 36.Emery P, Fleischmann RM, Moreland LW, et al. Golimumab, a human anti-tumor necrosis factor alpha monoclonal antibody, injected subcutaneously every four weeks in methotrexate-naive patients with active rheumatoid arthritis: twenty-four-week results of a phase III, multicenter, randomized, double-blind, placebo-controlled study of golimumab before methotrexate as first-line therapy for early-onset rheumatoid arthritis. Arthritis Rheum 2009;60:2272–83. [DOI] [PubMed] [Google Scholar]
- 37.Keystone EC, Genovese MC, Klareskog L, et al. Golimumab, a human antibody to tumour necrosis factor {alpha} given by monthly subcutaneous injections, in active rheumatoid arthritis despite methotrexate therapy: the GO-FORWARD Study. Ann Rheum Dis 2009;68:789–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tanaka Y, Harigai M, Takeuchi T, et al. Golimumab in combination with methotrexate in Japanese patients with active rheumatoid arthritis: results of the GO-FORTH study. Ann Rheum Dis 2012;71:817–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Bentum RE, Heslinga SC, Nurmohamed MT, et al. Reduced Occurrence Rate of Acute Anterior Uveitis in Ankylosing Spondylitis Treated with Golimumab - The GO-EASY Study. J Rheumatol 2019;46:153–9. [DOI] [PubMed] [Google Scholar]
- 40.Tursi A Concomitant hidradenitis suppurativa and Pyostomatitis vegetans in silent ulcerative colitis successfully treated with golimumab. Dig Liver Dis 2016;48:1511–2. [DOI] [PubMed] [Google Scholar]
- 41.Vavricka SR, Galván JA, Dawson H, et al. Expression patterns of TNFα, MAdCAM1, and STAT3 in intestinal and skin manifestations of inflammatory bowel disease. J Crohns Colitis 2018;12:347–54. [DOI] [PubMed] [Google Scholar]
- 42.Tadbiri S, Peyrin-Biroulet L, Serrero M, et al. Impact of vedolizumab therapy on extra-intestinal manifestations in patients with inflammatory bowel disease: a multicentre cohort study nested in the OBSERV-IBD cohort. Aliment Pharmacol Ther 2018;47:485–93. [DOI] [PubMed] [Google Scholar]
- 43.Feagan BG, Sandborn WJ, Colombel J-F, et al. Incidence of Arthritis/Arthralgia in inflammatory bowel disease with long-term Vedolizumab treatment: post hoc analyses of the gemini trials. J Crohns Colitis 2019;13:50–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chateau T, Bonovas S, Le Berre C, et al. Vedolizumab treatment in extra-intestinal manifestations in inflammatory bowel disease: a systematic review. J Crohns Colitis 2019;13:1569–77. [DOI] [PubMed] [Google Scholar]
- 45.Phillips FM, Verstockt B, Sebastian S, et al. Inflammatory cutaneous lesions in inflammatory bowel disease treated with Vedolizumab or ustekinumab: an ECCO CONFER multicentre case series. J Crohns Colitis 2020. doi: 10.1093/ecco-jcc/jjaa078. [Epub ahead of print: 22 Apr 2020]. [DOI] [PubMed] [Google Scholar]
- 46.Van Assche G, Van Ranst M, Sciot R, et al. Progressive multifocal leukoencephalopathy after natalizumab therapy for Crohn’s disease. N Engl J Med 2005;353:362–8. [DOI] [PubMed] [Google Scholar]
- 47.Lee EB, Fleischmann R, Hall S, et al. Tofacitinib versus methotrexate in rheumatoid arthritis. N Engl J Med 2014;370:2377–86. [DOI] [PubMed] [Google Scholar]
- 48.Fleischmann R, Kremer J, Cush J, et al. Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N Engl J Med 2012;367:495–507. [DOI] [PubMed] [Google Scholar]
- 49.Kremer J, Li Z-G, Hall S, et al. Tofacitinib in combination with nonbiologic disease-modifying antirheumatic drugs in patients with active rheumatoid arthritis: a randomized trial. Ann Intern Med 2013;159:253–61. [DOI] [PubMed] [Google Scholar]
- 50.van der Heijde D, Tanaka Y, Fleischmann R, et al. Tofacitinib (CP-690,550) in patients with rheumatoid arthritis receiving methotrexate: twelve-month data from a twenty-four-month phase III randomized radiographic study. Arthritis Rheum 2013;65:559–70. [DOI] [PubMed] [Google Scholar]
- 51.Burmester GR, Blanco R, Charles-Schoeman C, et al. Tofacitinib (CP-690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: a randomised phase 3 trial. Lancet 2013;381:451–60. [DOI] [PubMed] [Google Scholar]
- 52.Mease P, Hall S, FitzGerald O, et al. Tofacitinib or adalimumab versus placebo for psoriatic arthritis. N Engl J Med 2017;377:1537–50. [DOI] [PubMed] [Google Scholar]
- 53.Gladman D, Rigby W, Azevedo VF, et al. Tofacitinib for psoriatic arthritis in patients with an inadequate response to TNF inhibitors. N Engl J Med 2017;377:1525–36. [DOI] [PubMed] [Google Scholar]
- 54.Bachelez H, van de Kerkhof PCM, Strohal R, et al. Tofacitinib versus etanercept or placebo in moderate-to-severe chronic plaque psoriasis: a phase 3 randomised non-inferiority trial. Lancet 2015;386:552–61. [DOI] [PubMed] [Google Scholar]
- 55.Papp KA, Menter MA, Abe M, et al. Tofacitinib, an oral Janus kinase inhibitor, for the treatment of chronic plaque psoriasis: results from two randomized, placebo-controlled, phase III trials. Br J Dermatol 2015;173:949–61. [DOI] [PubMed] [Google Scholar]
- 56.van der Heijde D, Deodhar A, Wei JC, et al. Tofacitinib in patients with ankylosing spondylitis: a phase II, 16-week, randomised, placebo-controlled, dose-ranging study. Ann Rheum Dis 2017;76:1340–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rubin DT, Reinisch W, Greuter T, et al. Tu1868 the effect of tofacitinib on extraintestinal manifestations at baseline in patients with moderate to severe ulcerative colitis in the OCTAVE program. Gastroenterology 2020;158:S-1195–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gregory MH, Ciorba MA, Deepak P, et al. Successful treatment of pyoderma gangrenosum with concomitant tofacitinib and infliximab. Inflamm Bowel Dis 2019;25:e87–8. [DOI] [PubMed] [Google Scholar]
- 59.Kochar B, Herfarth N, Mamie C, et al. Tofacitinib for the treatment of pyoderma gangrenosum. Clin Gastroenterol Hepatol 2019;17:991–3. [DOI] [PubMed] [Google Scholar]
- 60.Paley MA, Karacal H, Rao PK, et al. Tofacitinib for refractory uveitis and scleritis. Am J Ophthalmol Case Rep 2019;13:53–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Westhovens R, Taylor PC, Alten R, et al. Filgotinib (GLPG0634/GS-6034), an oral JAK1 selective inhibitor, is effective in combination with methotrexate (MTX) in patients with active rheumatoid arthritis and insufficient response to MTX: results from a randomised, dose-finding study (DARWIN 1). Ann Rheum Dis 2017;76:998–1008. [DOI] [PubMed] [Google Scholar]
- 62.Kavanaugh A, Kremer J, Ponce L, et al. Filgotinib (GLPG0634/GS-6034), an oral selective JAK1 inhibitor, is effective as monotherapy in patients with active rheumatoid arthritis: results from a randomised, dose-finding study (DARWIN 2). Ann Rheum Dis 2017;76:1009–19. [DOI] [PubMed] [Google Scholar]
- 63.Mease P, Coates LC, Helliwell PS, et al. Efficacy and safety of filgotinib, a selective Janus kinase 1 inhibitor, in patients with active psoriatic arthritis (EQUATOR): results from a randomised, placebo-controlled, phase 2 trial. Lancet 2018;392:2367–77. [DOI] [PubMed] [Google Scholar]
- 64.van der Heijde D, Baraliakos X, Gensler LS, et al. Efficacy and safety of filgotinib, a selective Janus kinase 1 inhibitor, in patients with active ankylosing spondylitis (TORTUGA): results from a randomised, placebo-controlled, phase 2 trial. Lancet 2018;392:2378–87. [DOI] [PubMed] [Google Scholar]
- 65.Panés J, Sandborn WJ, Schreiber S, et al. Tofacitinib for induction and maintenance therapy of Crohn’s disease: results of two phase IIb randomised placebo-controlled trials. Gut 2017;66:1049–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Matsumoto S, Mashima H. Efficacy of ustekinumab against infliximab-induced psoriasis and arthritis associated with Crohn’s disease. Biologics 2018;12:69–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Spagnuolo R, Dastoli S, Silvestri M, et al. Anti-interleukin 12/23 in the treatment of erythema nodosum and Crohn disease: a case report. Dermatol Ther 2019;32:e12811. [DOI] [PubMed] [Google Scholar]
- 68.Piqueras-García J, Sahuquillo-Torralba AJ, Torres-Navarro I, et al. Pyoderma gangrenosum with ulcerative colitis successfully treated with ustekinumab. Actas Dermosifiliogr 2019;110:776–8. [DOI] [PubMed] [Google Scholar]
- 69.Nunes G, Patita M, Fernandes V. Refractory pyoderma gangrenosum in a patient with Crohn’s disease: complete response to ustekinumab. J Crohns Colitis 2019;13:812–3. [DOI] [PubMed] [Google Scholar]
- 70.McInnes IB, Kavanaugh A, Gottlieb AB, et al. Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trial. Lancet 2013;382:780–9. [DOI] [PubMed] [Google Scholar]
- 71.Kavanaugh A, Ritchlin C, Rahman P, et al. Ustekinumab, an anti-IL-12/23 p40 monoclonal antibody, inhibits radiographic progression in patients with active psoriatic arthritis: results of an integrated analysis of radiographic data from the phase 3, multicentre, randomised, double-blind, placebo-controlled PSUMMIT-1 and PSUMMIT-2 trials. Ann Rheum Dis 2014;73:1000–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kavanaugh A, Puig L, Gottlieb AB, et al. Efficacy and safety of ustekinumab in psoriatic arthritis patients with peripheral arthritis and physician-reported spondylitis: post-hoc analyses from two phase III, multicentre, double-blind, placebo-controlled studies (PSUMMIT-1/PSUMMIT-2). Ann Rheum Dis 2016;75:1984–8. [DOI] [PubMed] [Google Scholar]
- 73.Poddubnyy D, Hermann K-GA, Callhoff J, et al. Ustekinumab for the treatment of patients with active ankylosing spondylitis: results of a 28-week, prospective, open-label, proof-of-concept study (TOPAS). Ann Rheum Dis 2014;73:817–23. [DOI] [PubMed] [Google Scholar]
- 74.Deodhar A, Gensler LS, Sieper J, et al. Three multicenter, randomized, double-blind, placebo-controlled studies evaluating the efficacy and safety of ustekinumab in axial spondyloarthritis. Arthritis Rheumatol 2019;71:258–70. [DOI] [PubMed] [Google Scholar]
- 75.Mugheddu C, Atzori L, Del Piano M, et al. Successful ustekinumab treatment of noninfectious uveitis and concomitant severe psoriatic arthritis and plaque psoriasis. Dermatol Ther 2017;30. doi: 10.1111/dth.12527. [Epub ahead of print: 18 Aug 2017]. [DOI] [PubMed] [Google Scholar]
- 76.Hueber W, Sands BE, Lewitzky S, et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut 2012;61:1693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hohenberger M, Cardwell LA, Oussedik E, et al. Interleukin-17 inhibition: role in psoriasis and inflammatory bowel disease. J Dermatolog Treat 2017:1–6. [DOI] [PubMed] [Google Scholar]
- 78.Danese S, Vermeire S, Hellstern P, et al. Randomised trial and open-label extension study of an anti-interleukin-6 antibody in Crohn’s disease (ANDANTE I and II). Gut 2019;68:40–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Harbord M, Annese V, Vavricka SR, et al. The first European evidence-based consensus on extra-intestinal manifestations in inflammatory bowel disease. J Crohns Colitis 2016;10:239–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Meyer AM, Ramzan NN, Heigh RI, et al. Relapse of inflammatory bowel disease associated with use of nonsteroidal anti-inflammatory drugs. Dig Dis Sci 2006;51:168–72. [DOI] [PubMed] [Google Scholar]
- 81.Felder JB, Korelitz BI, Rajapakse R, et al. Effects of nonsteroidal antiinflammatory drugs on inflammatory bowel disease: a case-control study. Am J Gastroenterol 2000;95:1949–54. [DOI] [PubMed] [Google Scholar]
- 82.Sandborn WJ, Stenson WF, Brynskov J, et al. Safety of celecoxib in patients with ulcerative colitis in remission: a randomized, placebo-controlled, pilot study. Clin Gastroenterol Hepatol 2006;4:203–11. [DOI] [PubMed] [Google Scholar]
- 83.El Miedany Y, Youssef S, Ahmed I, et al. The gastrointestinal safety and effect on disease activity of etoricoxib, a selective cox-2 inhibitor in inflammatory bowel diseases. Am J Gastroenterol 2006;101:311–7. [DOI] [PubMed] [Google Scholar]
- 84.Louis E, Dotan I, Ghosh S, et al. Optimising the inflammatory bowel disease unit to improve quality of care: expert recommendations. J Crohns Colitis 2015;9:685–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ricci C, Lanzarotto F, Lanzini A. The multidisciplinary team for management of inflammatory bowel diseases. Dig Liver Dis 2008;40(Suppl 2):S285–8. [DOI] [PubMed] [Google Scholar]
- 86.Mawdsley JED, Irving PM, Makins RJ, et al. Optimizing quality of outpatient care for patients with inflammatory bowel disease: the importance of specialist clinics. Eur J Gastroenterol Hepatol 2006;18:249–53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


