Abstract
Fosfomycin is a broad-spectrum antibiotic which is established as therapy for uncomplicated lower urinary tract infections. In addition, preliminary data indicate that fosfomycin has a potential role in the treatment of soft tissue infections. However, the use of fosfomycin has not been established for this condition, and it is unclear whether the level of fosfomycin penetration into human soft tissues is high enough to eradicate relevant pathogens. To better characterize the antibiotic potential of fosfomycin, we applied a combined in vivo pharmacokinetic-in vitro pharmacodynamic model to human volunteers. For this purpose fosfomycin concentrations in vivo in the fluid of the interstitial space of human soft tissues were measured by microdialysis following intravenous infusion of 4 or 8 g of fosfomycin (n = 6). Subsequently, bacterial isolates with relevance for soft tissue infections were exposed to concentrations according to the in vivo pharmacokinetic profile in the interstitial space fluid obtained by microdialysis. Our experiments indicated a high degree of soft tissue penetration for fosfomycin, with ratios of the area under the concentration-time curve from 0 to 8 h for muscle (AUC0–8muscle)/AUC0–8serum of 0.48 ± 0.08 and 0.53 ± 0.04 and ratios of AUC0–8adipose tissue/AUC0–8serum of 0.74 ± 0.12 and 0.71 ± 0.11 following administration of 4 and 8 g, respectively. In corresponding in vitro simulation experiments with selected isolates of Staphylococcus aureus, Enterobacter cloacae, and Serratia marcescens for which MICs were 16 μg/ml, organisms were undetectable after a single dosing interval. Fosfomycin exhibits a strong ability to penetrate into the fluid of the interstitial space of soft tissues and reaches levels sufficient to substantially inhibit the growth of relevant bacteria at the target site. We therefore conclude that fosfomycin might qualify as an alternative candidate for the therapy of soft tissue infections.
Soft tissue infections are among the most frequent infections worldwide and may lead to serious (25) and even life-threatening (10, 14) complications. Therefore, current treatment guidelines suggest immediate empirical antibiotic therapy and subsequent modification of the antibiotic regimen depending on the bacterial culture result.
One important prerequisite for an antibiotic to be clinically effective in soft tissue infections is the ability to attain unbound concentrations in the interstitial fluid high enough to exceed the MICs for the relevant pathogens (16, 24). An antibiotic which might be particularly favorable in this regard is fosfomycin (6, 8). To date, fosfomycin is considered a broad-spectrum antibiotic with a wide therapeutic range and is established as therapy for uncomplicated lower urinary tract infections (21). Fosfomycin gains high concentrations in the cerebrospinal fluid of patients suffering from meningitis (5) and is occasionally applied in combination with other antibiotics in the therapy of osteomyelitis (15). Although promising but preliminary clinical data show the high degree of effectiveness of fosfomycin treatment for soft tissue infections (27), the administration of fosfomycin has still not been established for this condition. Due to its chemical structure, its pharmacokinetic properties, and antibacterial spectrum, however, fosfomycin may qualify as an alternative candidate for the treatment of soft tissue infections.
We set out to better characterize the antibiotic potential of fosfomycin to eradicate bacteria at the relevant target site by applying a previously described combined in vivo pharmacokinetic-in vitro pharmacodynamic approach (2, 3). This approach is based on the measurement of free, i.e., pharmacologically active (11, 16), fosfomycin concentrations in the interstitial fluid, i.e., the relevant effect site (23), by microdialysis (MD) and subsequent simulation of the pharmacokinetic profile in an in vitro bacterial culture.
MATERIALS AND METHODS
The study was approved by the local ethics committee. All volunteers were given a detailed description of the study, and their written consent was obtained. The study was performed in accordance with the Declaration of Helsinki and the Good Clinical Practice Guidelines of the European Commission.
Experimental subjects.
The study population included six healthy male volunteers receiving no medications (mean ± standard error weight, 79 ± 3 kg; mean ± standard error height, 182 ± 2 cm; age range, 23 to 29 years).
Study design.
The study was performed as a single-center, randomized, non-placebo-controlled, open, crossover study. Each volunteer was studied twice and was randomly assigned to receive an intravenous (i.v.) infusion of either 4 or 8 g of fosfomycin (Fosfomycin; Biochemie GmbH, Vienna, Austria) over 30 min on each occasion after an overnight fast. Between the two appointments there was a washout phase of 1 week.
Sampling procedures.
To measure fosfomycin concentrations in the interstitial fluid of human soft tissues in vivo we used in vivo MD (26). Briefly, MD is based on sampling of analytes from the interstitial space by means of a semipermeable membrane at the tip of a MD probe. The probe is constantly perfused with a physiological solution at a low flow rate (1.5 μl/min). Once the probe is implanted into the tissue, substances present in the interstitial fluid (concentration in tissue [Ctissue]) are filtered by diffusion out of the interstitial fluid into the probe, resulting in a concentration (Cdialysate) in the perfusion medium. Samples are collected and analyzed. For most analytes, equilibrium between the concentration in interstitial fluid of human soft tissue and the concentration in the perfusion medium is incomplete; therefore, Ctissue is greater than Cdialysate. The factor by which the concentrations are interrelated is termed in vivo recovery. Therefore, to obtain absolute concentrations in interstitial fluid from the concentrations in the dialysate, MD probe calibration was assessed by the retrodialysis method (16). The principle of this method relies on the assumption that the diffusion process is quantitatively equal in both directions through the semipermeable membrane. Therefore, fosfomycin was added to the perfusion medium at a concentration of 100 μg/ml, and the rate of disappearance through the membrane was taken as the in vivo recovery value. The in vivo recovery value was calculated as follows: percent recovery = 100 − (100 · fosfomycin Cdialysate · fosfomycin concentration in perfusate−1).
In the present study, one MD probe was inserted into a thigh muscle and a second MD probe was inserted into the subcutaneous layer of the thigh by a previously described procedure (26). The MD system was connected and perfused with Ringer's solution at a flow rate of 1.5 μl/min except during the in vivo calibration period. Continuous perfusion was performed with a microinfusion pump (Precidor; Infors-AG, Basel, Switzerland). At 30 min after probe insertion, an in vivo probe calibration was performed for 30 min, which was followed by a 30-min washout period. Thereafter, fosfomycin was randomly administered over 30 min at the doses mentioned above. Sampling was continued at 20-min intervals for up to 8 h. Microdialysates were immediately frozen and stored at −80°C until analyses. Venous blood was simultaneously taken at 20-min intervals according to the time of sampling of the microdialysates and was centrifuged at 1,600 × g for 5 min at 5°C. Thereafter, venous plasma was pipetted into polypropylene tubes and was immediately frozen and stored at −80°C.
Analyses. (i) Chemical analyses.
Fosfomycin was analyzed by a previously published, modified gas chromatographic method (4). Serum or perfusate samples were precipitated with 4 volumes of methanol containing 125 μg of ethylphosphonic acid per ml as the internal standard and were centrifuged at 16,000 × g for 5 min at room temperature. The supernatant was brought to dryness under reduced pressure and was derivatized with N,O-bis(trimethylsilyl)trifluoracetamide with 1% trimethylchlorsilane (Fluka 15238) for 15 min at 56°C. Fosfomycin was measured with a Hewlett-Packard (HP) 5890 Series II gas chromatograph equipped with an HP 7673 autosampler, a split injector, a nitrogen phosphorous detector, and an HP 1701 capillary column (14% cyanopropylphenylmethylpolysiloxane; 30 m by 0.32 mm [inner diameter]; film thickness, 0.25 μm) with helium at 70 kPa as the carrier gas. Data acquisition was performed with the HP 3396 Series II integrator and the HP 7673 controller. Chromatograms of blank samples did not show interfering peaks. Fosfomycin was quantitated with a calibration curve prepared with blank serum samples spiked with concentrations from 10 to 600 μg/ml. The relation between the ratio of the peak area of fosfomycin and the peak area of the internal standard and the concentrations of fosfomycin were linear, with a correlation coefficient of 0.9936. The coefficients of variation were 9.7% at 10 μg/ml, 8.7% at 200 μg/ml, and 3.1% at 600 μg/ml (n = 6). The limit of detection was 1 μg/ml.
(ii) Pharmacokinetic analysis.
Data were fitted by a commercially available computer program (Kinetika 2.0.2; INNAPHASE, Philadelphia, Pa.) according to a two-compartment model for values for serum and according to a one-compartment model for values for the peripheral compartment. The time to the maximum concentration of drug and the maximum concentration of drug (Cmax) were calculated for plasma, subcutaneous adipose tissue, and muscle. The area under the concentration-time curve (AUC) from 0 to 8 h (AUC0–8) was determined for plasma (AUC0–8plasma) and adipose tissue (AUC0–8adipose tissue) by the trapezoidal rule. As a measure of drug penetration into tissue, the AUC0–8muscle/AUC0–8plasma and AUC0–8adipose tissue/AUC0–8plasma ratios were determined.
(iii) Calculations and data analysis.
All values are expressed as means ± standard errors of the means (SEMs). Concentrations in interstitial fluid were calculated from the concentrations in the dialysate as described previously (17).
For comparisons between pharmacokinetic parameters for different compartments and fosfomycin dosages, Wilcoxon signed rank sum tests were conducted with a commercially available computer program (Statistica Statsoft; Statsoft Inc., Tulsa, Okla.). A P value of <0.05 was considered the level of significance.
(iv) In vivo pharmacokinetic-in vitro pharmacodynamic simulation.
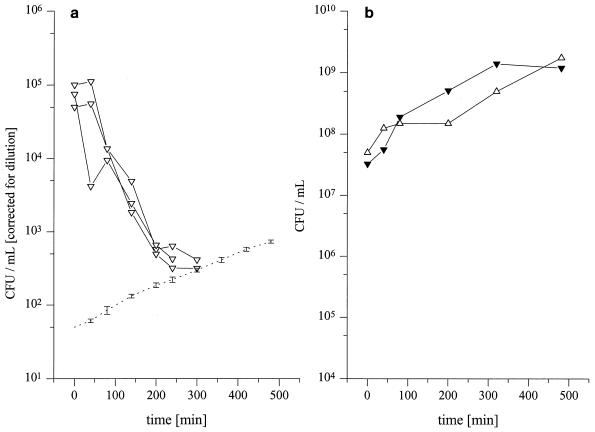
On the basis of the pharmacokinetic data obtained from the in vivo experiments we simulated the time-versus-concentration profile of fosfomycin in the fluid of the interstitial space in vitro in order to describe the antibacterial activity of fosfomycin at the target site. Therefore, 50-ml Falcon tubes with a starting volume of 2 ml of Mueller-Hinton broth (MHB) which were kept in a water bath at 37°C were inoculated with select clinical isolates commonly found in soft tissue infections at an approximate concentration of 5 × 105 CFU/ml. Subsequently, the time-versus-fosfomycin concentration profile obtained in vivo from the concentrations in serum and interstitial fluid were simulated in vitro by changing the fosfomycin concentrations in broth by adding MHB at 20-min intervals depending on the individual pharmacokinetic data by the following equation: V2 = (C1/C2) × V1 where C1 and V1 are the current fosfomycin concentration and the current MHB volume, respectively; C2 is the desired fosfomycin concentration; and V2 is the calculated MHB volume to be added to simulate the individual fosfomycin clearance. Therefore, the detection limits in Fig. 1a and Fig. 3 decreased over time.
FIG. 1.
(a) Reproducibility of the pharmacodynamic effect of the in vitro time-versus-concentration profiles obtained by in vivo MD in subcutaneous adipose tissue after i.v. administration of 4 g of fosfomycin for S. marcescens (n = 3). The dotted line indicates the detection limit. (b) Influence of dilution over time shown for S. marcescens (open triangles, corrected for dilution; closed triangles, nondiluted control group).
FIG. 3.
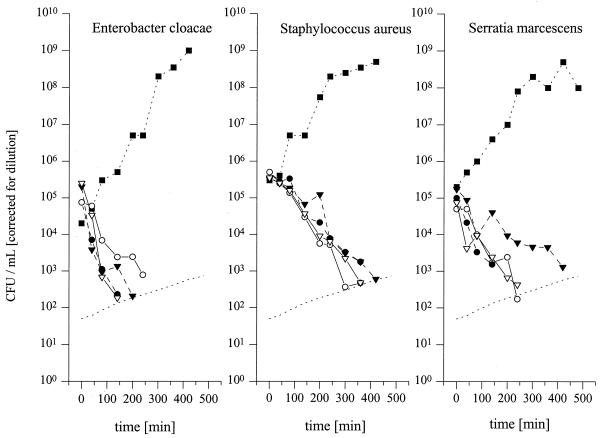
Time-kill curves for selected isolates of S. aureus, E. cloacae, and S. marcescens for which fosfomycin MICs are 16 μg/ml for an in vitro simulation model. The in vivo time course of fosfomycin concentrations in serum and fluid of the interstitial space obtained from the experiments whose results are shown in Fig. 2 was simulated. Solid symbols with dashed lines, time-kill data for profiles of concentration in serum after infusion of 4 g (closed triangles with dashed lines) and 8 g (closed circles with dashed lines) of fosfomycin; open symbols, time-kill data for concentrations in interstitial fluid of subcutaneous adipose tissue after infusion of 4 g (open triangles with solid lines) and 8 g (open circles and with solid lines) of fosfomycin. The CFU data shown were corrected for dilution. The detection limit over time is shown as the dotted line.
The influence of dilution on growth control curves is depicted in Fig. 1b, and the CFU data were corrected for dilution. To assess the similarity of the pharmacokinetic profile in vitro to that observed in vivo, we checked the variability introduced by dilution at several select time points. The reproducibility during our experiments is shown for Serratia marcescens in Fig. 1a. Samples for determination of bacterial counts were drawn at fixed time points (control and 0, 40, 80, 140, 200, 260, 320, 380, 440, and 480 min). After vortexing of the culture tube, 50 μl of sample was taken and twofold serially diluted with 0.9% sodium chloride. Samples of 20 μl obtained at each dilution step were then plated onto Columbia agar plates and the plates were incubated at 37°C for 24 h. Subsequently, the colonies were counted and the counts were backextrapolated to the original volume.
The MICs of fosfomycin were determined twice, before and after 24 h of pharmacokinetic modeling, by a twofold serial MD method in MHB. Isolates of Staphylococcus aureus, Enterobacter cloacae, and S. marcescens were precultured overnight in brain heart infusion broth and were introduced into MHB containing fosfomycin at an inoculum of approximately 5 × 105 CFU/ml. All bacteria were stored frozen in liquid nitrogen at −196°C until they were used.
RESULTS
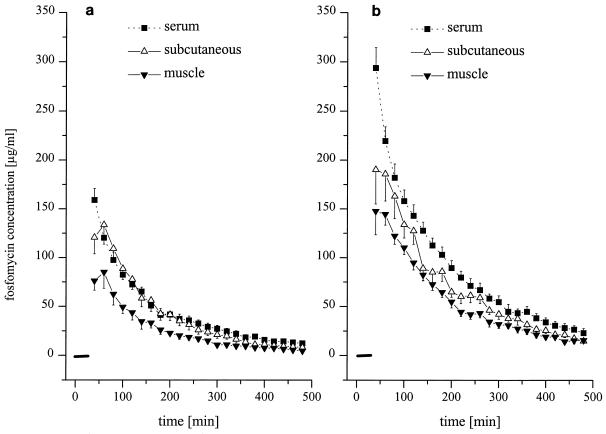
The time-versus-concentration profiles and the pharmacokinetic parameters of fosfomycin following i.v. infusion of 4 and 8 g in serum and in the fluid of the interstitial space of subcutaneous adipose tissue and skeletal muscle are shown in Fig. 2 and Table 1, respectively. The AUC0–8serum values were significantly higher than AUC0–8muscle and AUC0–8adipose tissue values for both dosages.
FIG. 2.
Time versus fosfomycin concentration profiles in serum (closed squares) and fluid of the interstitial space of skeletal muscle (closed triangles) and subcutaneous adipose tissue (open triangles) following intravenous administration of 4 g (a) and 8 g (b) of fosfomycin to healthy volunteers (n = 6). The black bar indicates the time of infusion (30 min). Results are presented as means ± SEMs.
TABLE 1.
Pharmacokinetic parameters for serum, skeletal muscle, and subcutaneous adipose tissue following administration of fosfomycin to healthy volunteersa
| Fosfomycin dosage (g) |
Cmax (μg/ml)
|
C8 (μg/ml)a
|
AUC (μg · min · ml−1)
|
AUCmuscle/AUCserum | AUCadipose tissue/ AUCserum | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscle | Subcutis | Serum | Muscle | Subcutis | Serum | Muscle | Subcutis | Serum | |||
| 4 | 97 ± 13 | 144 ± 19 | 202 ± 20 | 10.7 ± 1.9 | 4.8 ± 0.9 | 2.7 ± 1.5 | 12,113 ± 3,424 | 18,838 ± 2,641 | 26,594 ± 2,482 | 0.48 ± 0.08 | 0.74 ± 0.12 |
| 8 | 156 ± 16 | 208 ± 30 | 395 ± 46 | 22.1 ± 3.6 | 13.7 ± 3.7 | 9.8 ± 2.3 | 27,644 ± 2,403 | 35,822 ± 2,917 | 53,199 ± 4,245 | 0.53 ± 0.04 | 0.71 ± 0.11 |
Data are means ± SEMs. See text for dosages and administration schedules. C8, concentration at 8 h.
Time-kill curves for the in vitro simulation model are shown in Fig. 3. For E. cloacae no colonies were visible after 200 and 300 min following simulation of the in vivo time-versus-concentration profile in the interstitial space of subcutaneous adipose tissue following administration of 4 and 8 g of fosfomycin, respectively. Following administration of the two dosages no colonies of S. marcescens and S. aureus were visible after 300 and 420 min, respectively. The lowest concentration of fosfomycin which inhibited bacterial growth after incubation for 24 h at 37°C was determined to be the MIC and was 16 mg/liter for each of the three bacteria tested. The coefficient of variation during simulation of the in vitro pharmacokinetic profile was 11%.
DISCUSSION
In the case of soft tissue infections bacteria are almost exclusively localized in the fluid of the interstitial space (22). Thus, the efficacy of antimicrobial therapy for soft tissue infections is dependent on the ability of an antibiotic to penetrate the fluid of the interstitial space. Recent research confirmed the relevance of these considerations, particularly for soft tissue infections, and impressively demonstrated that the pharmacokinetics for plasma insufficiently mirror the pharmacokinetics for the target site (2, 17, 18, 20) and also the ability of an antibiotic to eradicate the causative pathogen (2). Therefore, antibiotic therapy should be tailored to achieve concentrations in interstitial fluid high enough to exceed the MIC for the causative pathogen.
Besides a high degree of in vitro antibiotic activity, an ideal antibiotic for soft tissue infections should be characterized by several physicochemical attributes, particularly a low level of protein binding, i.e., the ability to attain high unbound concentrations at the target site, and hydrophilicity, i.e., the ability to selectively penetrate extracellular water spaces. These characteristics are typical of several antibiotics currently recommended as treatments for soft tissue infections, particularly beta-lactams (24). An alternative, promising candidate in this regard appears to be fosfomycin, which is negligibly bound to serum proteins, has a water-soluble, hydrophilic structure, and does not undergo metabolism to an inactive portion. Although the pharmacokinetics of fosfomycin in serum are well documented (9), the drug's ability to penetrate the fluid of the interstitial space of human soft tissues has not been characterized to date. The present study was therefore aimed at measuring in vivo fosfomycin pharmacokinetics in the fluid of the interstitial space of adipose tissue and skeletal muscle.
The main finding of our study was that the AUC0–8 of fosfomycin in the interstitial fluid of soft tissues reached 50 to 70% of the corresponding AUC0–8 for serum, which indicates a high degree of penetration into the target site. Our data are in clear contrast to those from previous studies on fosfomycin concentrations in biopsy specimens, which reported concentrations of almost 30% in adipose and muscle tissue compared with those in serum (8, 22). With knowledge of the recent data, these contradictory findings may be explained by the fact that some drugs attain especially high concentrations in the fluid of the interstitial space, whereas their access to the intracellular space may be substantially limited (17). In particular, measurement of drug concentrations by tissue homogenization after biopsy allows no discrimination between different compartments. As shown previously (17), tissue homogenization may lead to relative dilution of the compartment of interest, i.e., the fluid of the interstitial space, and therefore to underestimation of the concentrations in interstitial fluid. Our present findings, however, which demonstrated the strong ability of fosfomycin to penetrate tissues, are in accordance with data obtained previously with animal models with implanted tissue cages (13) and skin blister data (12), although these were shown to poorly reflect the pharmacokinetics in the fluid of the interstitial spaces of target tissues (19).
Despite favorable tissue penetration characteristics, therapy with fosfomycin may be limited by the emergence of resistant isolates, a view which was corroborated by in vitro studies in which different isolates of bacteria were exposed to static antibiotic concentrations (1, 7). A pharmacodynamic effect in vivo, however, is the result of a dynamic exposure of the infective agent to the unbound antibiotic drug fraction at the relevant effect site, i.e., the fluid of the interstitial space. Thus, static conditions in an in vitro setting do not accurately reflect a dynamic situation in a target organ under in vivo conditions. To relate our findings on the pharmacokinetics in tissue to the effect of fosfomycin on clinical isolates of bacteria relevant to soft-tissue infection, we used a previously described in vivo pharmacokinetic-in vitro pharmacodynamic model (2). For this purpose, we exposed in vitro different isolates of bacteria to fosfomycin concentrations according to the time-versus-concentration profile in interstitial fluid obtained by microdialysis to obtain a pharmacokinetic-pharmacodynamic model. As shown in Fig. 2, no visible bacterial growth could be observed after single-dose exposure of selected bacterial isolates to fosfomycin at concentrations determined from the pharmacokinetic profile of the drug in interstitial fluid, and no resistant isolate emerged. Thus, our study not only provides evidence for the fact that fosfomycin attains high concentrations in tissues but also provides evidence that it has the ability to substantially inhibit the growth of relevant infectious agents at the effect site.
In conclusion, we have shown that fosfomycin attains high concentrations in the interstitial fluid of human soft tissues and reaches levels sufficient to eradicate relevant bacteria. Therefore, fosfomycin might qualify as an alternative candidate for the treatment of soft tissue infections in humans.
ACKNOWLEDGMENTS
This work was supported in part by Biochemie Austria.
We are grateful to Edith Lackner and Gernot Steffen for contributions.
Footnotes
Corresponding author. Mailing address: Department of Clinical Pharmacology, Division of Clinical Pharmacokinetics, University of Vienna Medical School, Allgemeines Krankenhaus, Währinger Gürtel 18-20, A-1090 Vienna, Austria. Phone: 43-1-40400-2981. Fax: 43-1-40400-2998. E-mail: markus.mueller@univie.ac.at.
REFERENCES
- 1.Arca P, Reguera G, Hardisson C. Plasmid-encoded fosfomycin resistance in bacteria isolated from the urinary tract in a multicenter survey. J Antimicrob Chemother. 1997;40:393–399. doi: 10.1093/jac/40.3.393. [DOI] [PubMed] [Google Scholar]
- 2.Brunner M, Hollenstein U, Delacher S, Jäger D, Schmid R, Lackner E, Georgopoulos A, Eichler H G, Müller M. Distribution and antimicrobial activity of ciprofloxacin in human soft tissues. Antimicrob Agents Chemother. 1999;43:1307–1309. doi: 10.1128/aac.43.5.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dalla Costa D, Nolting A, Rand K, Derendorf H. Pharmacokinetic-pharmacodynamic modeling of the in vitro antiinfective effect of piperacillin-tazobactam combinations. Int J Clin Pharamacol Ther. 1997;35:426–433. [PubMed] [Google Scholar]
- 4.Dios-Vieitez M C, Goñi M M, Renedo M J, Fos D. Determination of fosfomycin in human urine by capillary gas chromatography: application to clinical pharmacokinetic studies. Chromatographia. 1996;43:293–295. [Google Scholar]
- 5.Drobnic L, Quiles M, Rodriguez A. A study of the levels of fosfomycin in the cerebrospinal fluid in adult meningitis. Chemotherapy (Basel) 1997;23(Suppl. 1):180–188. doi: 10.1159/000222045. [DOI] [PubMed] [Google Scholar]
- 6.Gallardo A J, Saez J M, Enriquez G, Cobacho A R, Toronteras R, Recordan C, Del Moral A, Arroyo A, Curiel A G. Surgical suppurating infections and surgical abdominal infections treated with fosfomycin. Chemotherapy (Basel) 1977;23(Suppl. 1):392–398. doi: 10.1159/000222080. [DOI] [PubMed] [Google Scholar]
- 7.Hamilton-Miller J M T. In vitro activity of fosfomycin against problem gram-positive cocci. Microbios. 1992;71:95–103. [PubMed] [Google Scholar]
- 8.Hirt S W, Alken A, Müller H, Haverich A, Vömel W. Die perioperative Antibiotikaprophylaxe mit Fosfomycin in der Herzchirurgie: Serumkinetik unter extrakorporaler Zirkulation und Konzentrationsbestimmungen im Herzklappengewebe. Z Kardiol. 1990;79:615–620. [PubMed] [Google Scholar]
- 9.Kirby W M M. Pharmacokinetics of fosfomycin. Chemotherapy (Basel) 1977;23(Suppl. 1):141–151. doi: 10.1159/000222040. [DOI] [PubMed] [Google Scholar]
- 10.Kotrappa K S, Bansal R S, Amin N M. Necrotizing fasciitis. Am Fam Physician. 1996;53:1691–1697. [PubMed] [Google Scholar]
- 11.Kunin C M, Craig W A, Kornguth M, Monson R. Influence of binding on the pharmacologic activity of antibiotics. Ann N Y Acad Sci. 1973;26:214–224. doi: 10.1111/j.1749-6632.1973.tb20483.x. [DOI] [PubMed] [Google Scholar]
- 12.Lastra C F, Marino E L, Dominguez-Gil A, Tabernero J M, Gonzalez Lopez A, Yuste Chaves M. The influence of uremia on the accessibility of phosphomycin into interstitial tissue fluid. Eur J Clin Pharmacol. 1983;25:333–338. doi: 10.1007/BF01037944. [DOI] [PubMed] [Google Scholar]
- 13.Lastra C F, Marino E L, Dominguez-Gil A. Linearity of the pharmacokinetics of phosphomycin in serum and interstitial tissue fluid in rabbits. Drug Res. 1986;36:1518–1520. [PubMed] [Google Scholar]
- 14.Lewis R T. Soft tissue infections. World J Surg. 1998;22:146–151. doi: 10.1007/s002689900362. [DOI] [PubMed] [Google Scholar]
- 15.Meißner A, Haag R, Rahmanzadeh R. Adjuvant fosfomycin medication in chronic osteomyelitis. Infection. 1989;17:146–151. doi: 10.1007/BF01644014. [DOI] [PubMed] [Google Scholar]
- 16.Merrikin D J, Briant J, Rolinson G N. Effect of protein binding on antibiotic activity in vivo. J Antimicrob Chemother. 1983;11:233–238. doi: 10.1093/jac/11.3.233. [DOI] [PubMed] [Google Scholar]
- 17.Müller M, Haag O, Burgdorff T, Georgopoulos A, Weninger W, Jansen B, Stanek G, Agneter E, Pehamberger H, Eichler H G. Characterization of peripheral compartment kinetics of antibiotics by in vivo microdialysis in humans. Antimicrob Agents Chemother. 1996;40:2703–2709. doi: 10.1128/aac.40.12.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Müller M, Stass H, Brunner M, Möller J G, Lackner E, Eichler H G. Penetration of moxifloxacin into peripheral compartments in humans. Antimicrob Agents Chemother. 1999;43:2345–2349. doi: 10.1128/aac.43.10.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Müller M, Brunner M, Schmid R, Putz E M, Schmiedberger A, Wallner I, Eichler H G. Comparison of three different experimental methods for the assessment of peripheral compartment pharmacokinetics in humans. Life Sci. 1998;62:PL227–PL234. doi: 10.1016/s0024-3205(98)00071-x. [DOI] [PubMed] [Google Scholar]
- 20.Müller M, Brunner M, Hollenstein U, Joukhadar C, Schmid R, Minar E, Ehringer H, Eichler H G. Penetration of ciprofloxacin into the interstitial space of inflamed foot lesions in non-insulin-dependent diabetes mellitus patients. Antimicrob Agents Chemother. 1999;43:2056–2058. doi: 10.1128/aac.43.8.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel S S, Balfour J A, Bryson H M. Fosfomycin tromethamine. A review of its antibacterial activity, pharmacokinetic properties and therapeutic efficacy as a single-dose oral treatment for acute uncomplicated lower urinary tract infections. Drugs. 1997;53:637–656. doi: 10.2165/00003495-199753040-00007. [DOI] [PubMed] [Google Scholar]
- 22.Plaue R, Müller O, Fabricius K, Oellers B. Untersuchungen über die Diffusionsrate von Fosfomycin in verschiedene menschliche Gewebe. Therapiewoche. 1980;30:8329–8333. [Google Scholar]
- 23.Ryan D M. Pharmacokinetics of antibiotics in natural and experimental superficial compartments in animals and humans. J Antimicrob Chemother. 1993;31(Suppl. D):1–16. doi: 10.1093/jac/31.suppl_d.1. [DOI] [PubMed] [Google Scholar]
- 24.Ryan D M, Cars O. A problem of the interpretation of betalactam antibiotic levels in tissues. J Antimicrob Chemother. 1983;12:281–284. doi: 10.1093/jac/12.3.281. [DOI] [PubMed] [Google Scholar]
- 25.Smith A J, Daniels T, Bohnen J M A. Soft tissue infections and the diabetic foot. Am J Surg. 1996;172(Suppl. 6A):7S–12S. doi: 10.1016/s0002-9610(96)00344-3. [DOI] [PubMed] [Google Scholar]
- 26.Ungerstedt U. Microdialysis—principles and applications for studies in animal and man. J Intern Med. 1991;230:365–373. doi: 10.1111/j.1365-2796.1991.tb00459.x. [DOI] [PubMed] [Google Scholar]
- 27.Wildling E, Stauffer F, Breyer S, Janata O, Burgmann H, Georgopoulos A, Graninger W. Fosfomycin, eine therapeutische Alternative bei schwer zu behandelnden Infektionen. Antibiotika Monitor. 1992;8:87–90. [Google Scholar]