Abstract
The preferential blood supply from the hepatic artery to liver tumors allows for the regional delivery of chemotherapy, commonly referred to as hepatic artery infusion chemotherapy via a subcutaneous pump. Hepatic artery infusion chemotherapy has been demonstrated to improve overall survival in select patients with colorectal liver metastasis and is a promising treatment for unresectable intrahepatic cholangiocarcinoma. This review focuses on the technical aspects of hepatic artery infusion pump placement.
Keywords: colorectal neoplasms, hepatic artery infusion chemotherapy, liver neoplasms, pump, techniques
1 |. INTRODUCTION
1.1 |. Background
Colorectal cancer is the third most common cancer in the United States.1 Although colorectal cancer incidence is declining overall,2 it is increasing among those under 50 years old, who often present with more advanced disease.3 Fifteen percent of patients with colorectal cancer present with synchronous liver metastasis and up to 60% will eventually develop liver metastasis during follow-up.4 However, unlike many other cancers, patients with liver-only colorectal metastasis can be cured with complete resection and it is hypothesized that colorectal cancers progress in a stepwise pattern of metastatic progression via the portal vein.5 Intrahepatic cholangiocarcinoma can similarly be regionally confined to the liver and early trials have suggested a potential benefit to hepatic arterial chemotherapy.6 These concepts have led to the development of regional chemotherapy, primarily in the form of intra-arterial chemotherapy, for colorectal liver metastasis.
The anatomic principles that support the use of regional chemotherapy for hepatic malignancy, and in particular metastatic colorectal cancer, are based on the fact that liver metastasis is perfused almost exclusively by the hepatic artery.7 From a pharmacologic standpoint selected chemotherapeutic agents to have up to a 99% first-pass metabolism by the liver,8 thus allowing for much higher and more selective concentrations of chemotherapy delivery to liver metastasis than in conventional systematic therapy. Although various chemotherapeutic regimens have been trialed in the last 50 years, the most widely utilized drug in the United States currently for liver-directed therapies is 5-Fluoro 2-deoxyuridine (FUDR) due to its high first-pass extraction by the liver followed by rapid clearance. These principles, along with the success of a few small early clinical trials performed during the 1970’s and 1980’s, led to the development of what is now the most common technique for the delivery of regional chemotherapy to the liver, hepatic artery infusion chemotherapy (HAIC), utilizing a surgically implanted subcutaneous pump.9,10 Infusion of hepatic artery chemotherapy through percutaneously placed ports will not be discussed in this review.
1.2 |. Indications and contraindications
The indications for HAIC have significantly expanded over the last few decades. Initial studies involving the use of HAIC primarily involved patients with extensive, unresectable, metastatic colorectal cancer to the liver.11–13 In the 1990’s and more recently, adjuvant HAIC has also been utilized to decrease recurrence rates in patients with resected colorectal liver metastasis.14–17 There is also strong evidence to support the use of HAIC in the converting patients with initially unresectable disease, to resectable disease.18–20
The initial CALGB randomized trial comparing HAIC versus systemic 5-fluorouracil and leucovorin for chemotherapy-naïve unresectable colorectal liver metastasis demonstrated response rates of 47% with HAIC (vs 24% systemic chemotherapy) with slight improvements in overall survival.13 A phase I study including 23 chemotherapy-naive patients with unresectable colorectal liver metastasis treated with HAIC and systemic chemotherapy (oxaliplatin and irinotecan) demonstrated a 100% response rate and a median survival of 51 months.19 Notably, 57% of the initially unresectable patients went on to resection. A subsequent trial of previously chemotherapy-naïve patients treated with systemic chemotherapy and HAIC demonstrated an 82% response rate with median survival not yet being met at the 38 months follow up.21 In comparison previously treated patients had a 72% to 85% response rate in these two studies19,21 and even in those patients refractory to all standard chemotherapies, a response rate of 33% can be seen with HAIC.22 A more recent trial of 64 patients with unresectable colorectal liver metastasis treated with best systemic therapy and HAIC demonstrated response rates of 73% overall (67% with prior chemotherapy and 86% chemotherapy-naïve) with 52% of all patients being converted to complete resection and 14% being cancer-free at median 94 months from initial diagnosis.20 In comparison, untreated patients typically survive less than 1 year and treatment with modern systemic chemotherapy is typically associated with a median survival of 2 years and is rarely, if ever, curative.23
Intrahepatic cholangiocarcinoma (ICC) is the second most common primary malignancy of the liver and although 85% of patients present with unresectable disease, extrahepatic disease is relatively rare.24 This, along with the low response rates to systemic chemotherapy (response rate 25% and median survival less than 1 year),25 has led to the evolution of HAIC as a promising treatment option for patients with ICC. The initial phase two trial of 26 unresectable ICC patients treated with HAIC with FUDR demonstrated a response rate of 47% and overall survival of 29.5 months.6 A subsequent review of 104 patients with liver-only, unresectable ICC treated with HAIC demonstrated a response rate of 59% and overall survival of 30.8 months, with eight patients going on to resection.26 A subsequent phase two clinical trial included 38 unresectable ICC patients treated with HAIC combined with systemic gemcitabine and oxaliplatin suggested that the combination approach was associated with further improvements in progression-free survival.27
The primary contraindications to HAIC include poor hepatic function, due to underlying liver disease, prolonged systemic chemotherapy, or extensive hepatic replacement by the tumor. A tumor burden of greater than 70% of the liver is a relative contraindication. Portal hypertension, portal vein thrombus, or hepatic artery occlusion also preclude placement. Although not an absolute contraindication, there is limited data on the safety of pump placement after external beam radiation or radioembolization. Patients must also be able to tolerate general anesthesia and the laparotomy for placement of the catheter (nonoperative techniques exist via percutaneous placement but they are only performed by a select few centers and outside of the scope of this review).28 Lastly aberrant or replaced arterial anatomy, although not a contraindication, can increase the difficulty of the operation and is discussed below. Extra-hepatic disease outside of the primary is considered a relative contraindication but the treatment can be considered in selected cases.
2 |. OPERATIVE TECHNIQUES
2.1 |. Preoperative planning
Numerous factors must be considered before HAIC pump placement. Most importantly the patient must have access to a medical oncologist with experience administering HAIC as well as an institution with the infrastructure (appropriately trained surgeons, interventional radiology, nuclear medicine, gastroenterology, and diagnostic radiology) to administer HAIC and manage the pump postoperatively. From the surgical standpoint, the patient’s prior abdominal surgical history must be reviewed as extensive adhesions or prior hepatobiliary surgery can increase the complexity of the operation. All patients being considered for HAIC pump placement must undergo an arterial phase CT to evaluate their vascular anatomy. This is particularly important because one-third of patients have abnormal hepatic artery anatomy.29 Rarely, a formal angiogram is necessary to delineate detailed anatomy and flow. The preferred location for the placement of the catheter is into the gastroduodenal artery as this avoids the risk of thrombus of the hepatic artery and is associated with the lowest complication rate.30 In general, the rule of thumb is to place the catheter in the gastroduodenal artery (GDA), ligate all accessory/replaced vessels and rely on hepatic cross perfusion (see below). All patients receiving a HAIC Pump must also undergo a cholecystectomy to prevent chemotherapy-induced cholecystitis.
2.2 |. How we do it
The patient is placed under general anesthesia and prophylactic antibiotics are given. A subcostal, midline, or right subcostal hockey stick incision can be used at the surgeon’s discretion and is often influenced by the need for concurrent colectomy or liver resection. Typically, the primary tumor is removed at the time of pump placement and this has been found to be safe. Some primary tumors, particularly rectal primaries, are left in place if there has been a dramatic response to chemotherapy and/or chemoradiation. In general, the pump is placed first, the pump pocket incision is closed and then the primary tumor is removed. To begin the operation the abdomen is explored to document both the extent of liver disease and rule out extra-hepatic disease. Historically this was done via staging laparoscopy given the high rate of extrahepatic disease, however, careful patient selection and high-quality cross-sectional imaging have decreased the rate of unexpected extrahepatic disease and this is not always required. It is important to document the extent of hepatic disease utilizing intra-operative ultrasonography as this may influence future resections.
Next, a standard cholecystectomy is performed. The common hepatic artery is subsequently identified superior to the pancreas and the first portion of the duodenum utilizing the hepatic artery lymph node as a landmark. The hepatic artery lymph node is removed as it provides good exposure to the artery and can provide pathologic staging information. Dissection is continued along the hepatic artery to identify the GDA and any suprapyloric branches, as well as the right gastric artery, are ligated to prevent perfusion of the duodenum. The entire length of the GDA is circumferentially exposed from its origin to where it enters the substance of the pancreas. A long-distance of exposed artery is necessary for accurate catheter placement. Along the distal GDA, branches to the retroperitoneum are commonly encountered and should be ligated. There is typically a dominant branch coursing to the right just before the GDA enters the pancreas (supraduodenal branch) that is a good landmark for the distal extent of dissection. The common hepatic artery is circumferentially exposed 1 cm proximally and the proper hepatic (and right or left hepatic depending on the length of the proper hepatic) 2 cm distally from the GDA to ensure no gastro-duodenal perfusion. This distance has been shown to be where most of the vessels that cause postoperative extrahepatic perfusion arise from.31 Dissection is continued along the first portion of the duodenum anterior to the bile duct taking any small branches to the duodenum to prevent extrahepatic perfusion. Although not necessary in every case, it is reasonable to remove the portacaval lymph node for staging and to prevent confusion on pstoperative perfusion scans since it is perfused by the hepatic artery. Care is taken not to devascularize the bile duct. Papaverine is then dripped on the GDA to induce vasodilation which will assist with catheter placement later. Additional nuances of the arterial dissection based on abnormal anatomy are discussed below.
Attention is then turned to the abdominal wall. The lower left abdomen is the most common location for pump placement if there are no contraindications (eg, stoma) since future incisions for liver resection may involve the right subcostal area. One should take care to avoid the pump abutting the rib or iliac crest as this can be painful. In obese patients, the pump may be placed on the left chest wall over the ribs to ensure it is accessible and less likely to flip. A transverse incision is made large enough to fit the pump and dissection is carried down to the fascia. Depending on the thickness of the abdominal wall additional fat may need to be excised to ensure that the pump will be accessible. It is generally advisable to make a tight pump pocket to prevent flipping. The device is then primed, assembled and the catheter is trimmed to the last tying ring and flushed. The catheter is then pulled through the middle of the pump pocket through the abdominal wall into the abdominal cavity. The catheter exit site should be directly posterior to the pump to avoid the possibility of it later being injured by the injection needle. The pump is secured to the abdominal wall fascia with permanent sutures through the rings and any excess tubing should be intra-abdominal (Figure 1).
FIGURE 1.
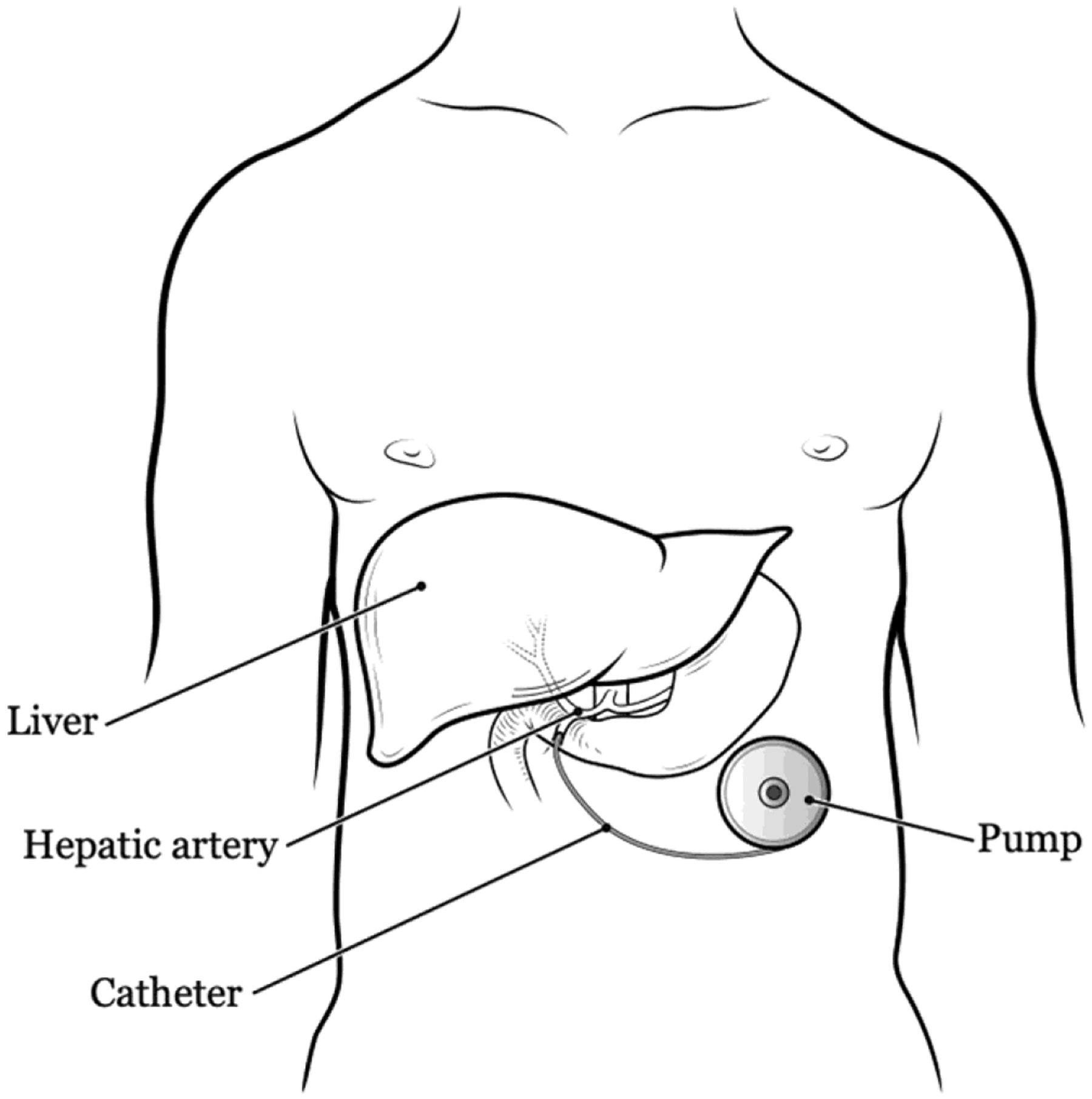
Standard placement location for hepatic artery infusion chemotherapy (HAIC) pump
Attention is again turned to the GDA which is ligated distally with a silk tie as close to the pancreas as possible. Vascular control is obtained using a bulldog clamp at the proximal GDA, or the common and proper hepatic arteries can be clamped separately if needed. A transverse atriotomy is made in the distal GDA allowing enough room between the arteriotomy and the takeoff of the proper hepatic to allow the catheter to be secured and the catheter is placed. The catheter tip should lie just at the origin of the GDA. It should not protrude into the hepatic artery, nor should it terminate a long distance to the hepatic artery. The former can cause thrombosis and the latter can cause pooling of chemotherapy and arteritis. The catheter is then secured at least two times to the GDA using non-absorbable suture around the tying rings, taking care not to occlude the catheter (Figure 2).
FIGURE 2.
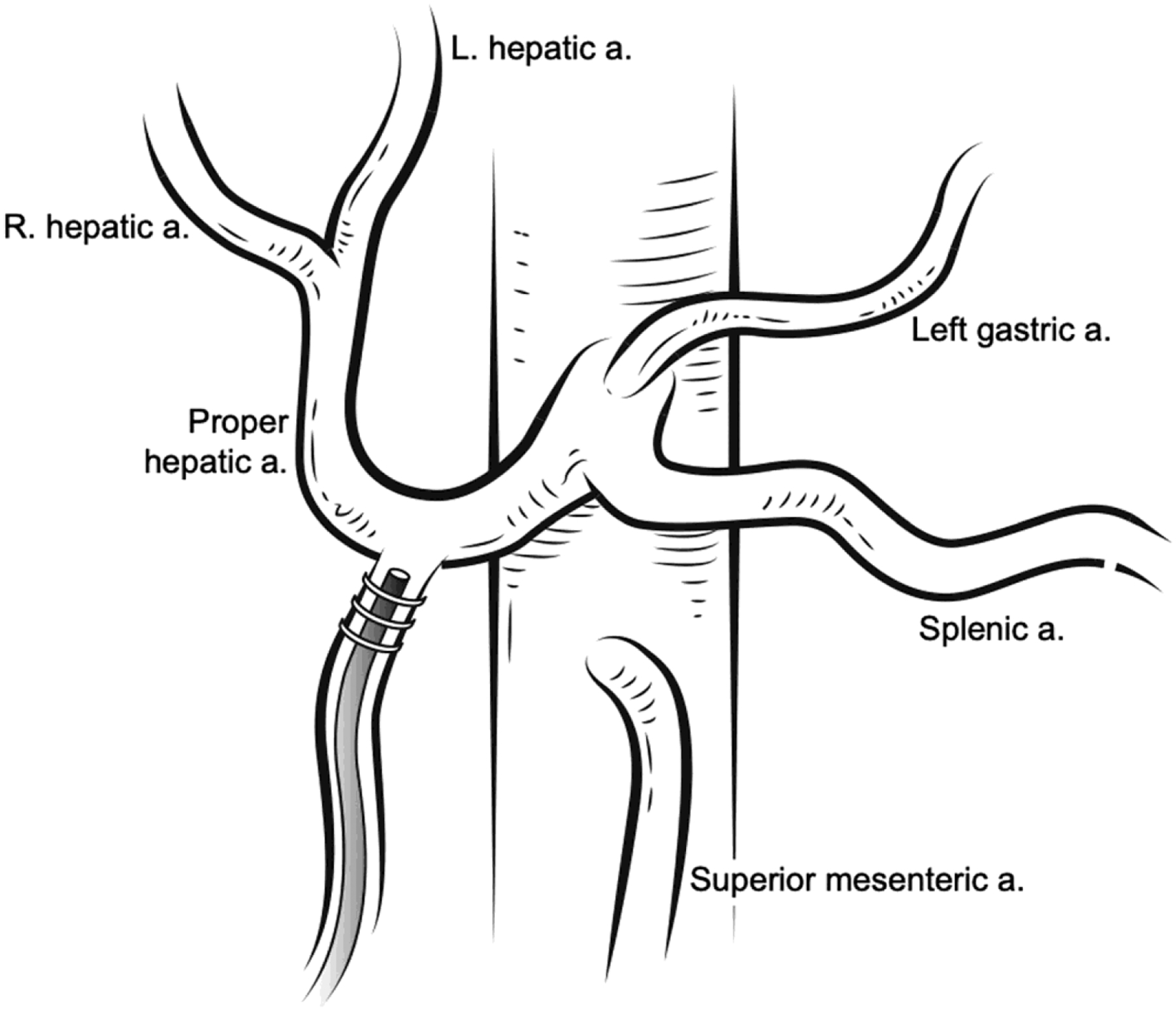
Standard placement of the catheter into the gastroduodenal artery in the setting of normal anatomy
Once the pump is placed perfusion is tested using a side port injection of methylene blue (preferred) or fluorescein (with a Woods lamp). This is used to rule out extrahepatic perfusion and to ensure complete perfusion of the liver (Figure 3). The system is then flushed with low dose heparin and the port pocked and celiotomy are closed in standard fashion.
FIGURE 3.
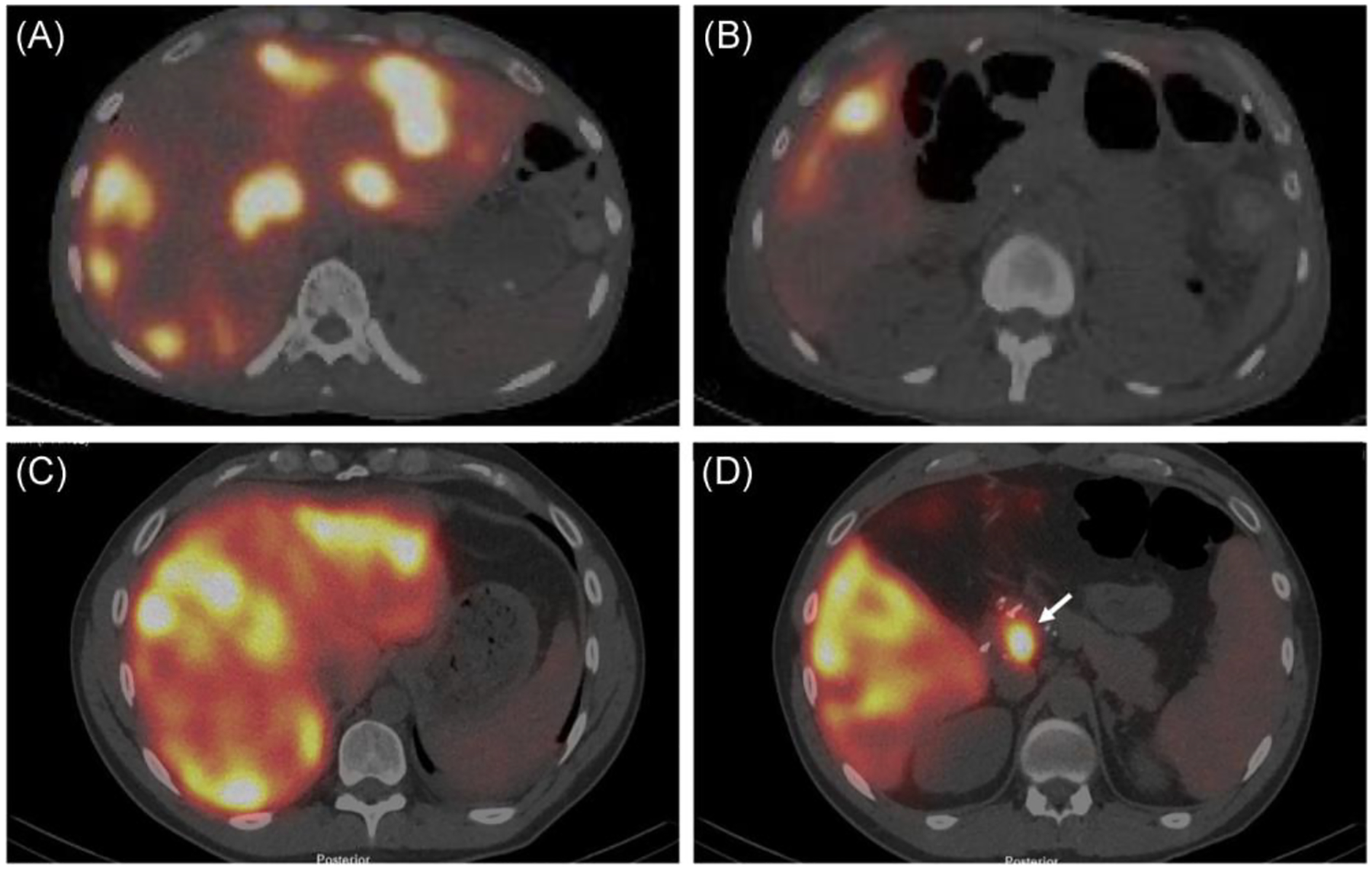
Postoperative Tc99-labeled macroaggregated albumin injection scan utilizing SPECT/CT of a patient with normal bilobar perfusion of the liver (A) and no extra-hepatic perfusion (B) compared to a second patient with normal bilobar perfusion of the liver (C) but with extra-hepatic perfusion of the pancreas (arrow, D)
2.3 |. Aberrant hepatic arterial anatomy
Aberrant hepatic arterial anatomy is seen in 38% of patients undergoing pump placement.23 Although rarely an absolute contraindication, aberrant anatomy can complicate both the placement and future function of the pump and requires careful preoperative planning.22 In general, the preferred technique in most cases of aberrant hepatic arterial anatomy is to place the catheter in the GDA with ligation of accessory/replaced vessels as cross perfusion resulting in complete hepatic perfusion is reliable. Anecdotally, we have encountered a few cases of poor cross perfusion related to large central tumors. In cases where there is not immediate cross perfusion it usually occurs over the course of a month and can be documented by serial flow studies. However, each of the most common anomalies a surgeon might encounter, and how to approach them, will be detailed herein.
Aberrant origin of the GDA from either the right hepatic artery left hepatic artery or as a trifurcation occurs in 6% to 11% of patients. In the case of a trifurcation, the catheter should be placed in the GDA and perfusion tested intraoperatively. If bilobar perfusion is adequate, then no additional ligation is necessary. If a lobe is not perfused, then one of the hepatic artery branches must be ligated. Cross-perfusion will typically occur immediately. When the GDA inserts into either the right or left hepatic artery the catheter should similarly be placed into the GDA, the flow should be tested, and one of the hepatic arteries must be similarly ligated. In these situations, the catheter tip must be situated at the point of contact with the vessel flowing to the liver. Rarely placement into the splenic artery is necessary, however, this is can be technically challenging and is associated with higher rates of postoperative complications.
Accessory left and right hepatic arteries occur in 2% to 10% and 4% to 16% of cases respectively. In these cases the approach is to place the catheter into the native GDA and ligate the accessory artery, again relying on cross perfusion. A replaced left hepatic artery with no native left (4%–16% of cases) is similarly managed by standard placement with ligation of the replaced left haptic artery. A similar approach can be used in the case of the replaced the right hepatic artery with no native right (6%–16% of cases). There are many combinations of left/right accessory/replaced hepatic artery branches. In general, the most reliable and effective strategy is nearly always to place the catheter in the GDA and ligate the accessory/replaced vessels. This even includes a situation where there is a replaced right and an accessory left hepatic artery with only a small branch to segment IV of the liver coming off of the GDA. Cross perfusion is still reliable in these cases in our experience. One situation that can result in issues with cross perfusion is a large tumor or resection cavity located centrally in the liver. When performing the ligation procedures one can consider clamping and testing cross perfusion with side port dye injections if there is a major concern. Placement of a second catheter and pump or placement of a catheter directly into a replaced hepatic artery has been described but is rarely indicated and can be associated with thrombosis.29 When there is no GDA to use, either due to injury or embolization, there are a couple of potential strategies. The preferred strategy is to place the catheter in a ligated right or left hepatic artery up to its origin. Again, this relies on cross perfusion. If this approach is not possible (eg, a replaced right and left hepatic artery with no branches to the liver off of the GDA) the best method is to suture a side branch to the largest branch to the liver and ligate the contralateral branch. We have used ePTFE, saphenous vein or gonadal vein grafts with success although complication rates have been higher in these situations. One other approach is to use branches of the left gastric artery as a conduit to a replaced left hepatic artery. It is important to note that as the complexity of catheter placement increases the risk of complications also increases.
In summary, when dealing with aberrant anatomy the general principle is to use the GDA and ligated all accessory/replaced vessels. The next best approach is to use the native right or left hepatic artery as the conduit. The last approach is to suture side branches to form a conduit. As one progresses along this continuum the risk of complications and pumps failure increases.
2.4 |. Minimally invasive pump placement
Numerous institutions have reported successful minimally invasive placement of HAIC pumps.32–34 However, this operation should only be attempted by surgeons with significant experience with both open pump placement and experience with advanced minimally invasive techniques. The technique for both the laparoscopic and robotic approaches to HAIC pump placement mimics that of open placement and to date appears to be feasible and safe.
2.5 |. Pump placement after hepatectomy
Minor hepatic resections typically do not impact HAIC pump placement beyond the previously mentioned considerations. However, in the case of pump placement after major hepatectomy, the perfusion to the remnant liver must be considered. In the case of standard anatomy, placement into the GDA is recommended. If the GDA arises from the left or right hepatic artery care must be taken to ligate the resected hepatic artery distal to the takeoff of the GDA. In the presence of accessory hepatic arteries, one must consider the condition of the remnant liver before ligation. If there is poor cross perfusion to a liver remnant one should strongly consider leaving the accessory vessel in situ and embolizing that branch in a separate setting with percutaneous techniques once there has been adequate liver regeneration. Placement of HAIC pump in the setting of a replaced hepatic artery supplying the remnant liver requires special considerations. For example, if the remnant right is supplied by a replaced hepatic artery direct cannulation of the replaced right hepatic is not recommended due to the high risk of thrombosis. One could consider the placement of a sutured conduit to the replaced right but this can be somewhat risky in the case of a regenerating liver due to the risk of thrombosis. We rarely, if ever, consider this approach. In the setting of a replaced left hepatic artery supplying the remnant left one can similarly suture a conduit to the left hepatic artery or the catheter can be placed in a branch of the left gastric artery. As stated above, the risk of complications and pump failure are higher as the complexity of placement increases.
2.6 |. Postoperative care
The Postoperative care of patients undergoing HAIC pump placement is often dictated by the concurrent liver or colon resections and otherwise involves routine post-laparotomy care. Prophylactic antibiotics in the postoperative setting are not indicated. All patients should receive a Tc99-labeled macroaggregated albumin injection scan through the pump to identify any extrahepatic perfusions before filling the pump with chemotherapy (Figure 3). We typically perform this scan just before discharge. Extrahepatic perfusion or other issues are typically dealt with immediately while in the hospital but can be considered for outpatient management.
3 |. TROUBLESHOOTING
When performed at high volume centers, pump placement can be done with low mortality (<1%) and morbidity (22%).30 However, even at experienced centers, there remains an almost 10% rate of a pump failure at 1 year. Intra-operative complications, including bleeding, are rare. Postoperative pump pocket infections, even in the setting of concurrent colon resections, are also uncommon (2%). However, care should be taken to avoid any contamination of the pump and pump pocket during the operation including closing and excluding the pocket before bowel work. The pump pocket should be closely monitored postoperatively and any signs of infection require early intervention to prevent hardware infection. Minor cellulitis in the pump pocket incision can be managed with antibiotics. True pump pocket infections with gross infection of the pump are a difficult situation. Attempts at washing out the pump with antibacterial solutions have been attempted, however, these inevitably result in very short-lived success. One can consider re-siting the pump. This requires removal of the infected pump, isolation of the catheter through a small laparotomy and connecting that catheter to a new pump (with its own catheter) at a separate site with a catheter repair kit over a small metal joining piece.
Postoperative bleeding, either intra-abdominally or into the pump pocket, occurs in less than 1% of patients. Minor bleeding into the pump pocket can be managed conservatively. It is somewhat common to get a flank ecchymosis but as long as there is no hematoma in the pocket these typically resolve with conservative therapy. In the case of a significant pump pocket hematoma, the patient should be returned to the operating room for the evacuation of the clot.
Flipping of the pump within the pump pocket is another problem that can be encountered. This most commonly happens in obese patients and has resulted in the practice of chest wall placement for grossly overweight patients. A flipped pump is often noted by staff tying to access the pump or can be identified on imaging studies. This generally requires resuturing of the pump in place or resiting the pump on the chest wall.
If there is extrahepatic perfusion in the operating room, one can often identify the culprit vessel(s), ligate them and document resolution with repeat dye testing. Occasionally, there is persistent extrahepatic perfusion in the operating room and ongoing ligation and dissection of the porta hepatis cannot resolve it. In these situations, it is sometimes too risky to continue searching for vessels (biliary injury, biliary ischemia, etc) and is best to close and evaluate with postoperative flow studies (sometimes these will be normal in these situations) and angiography. Despite normal intraoperative tests, extrahepatic flow can be found on postoperative perfusion scans. These occur form missed hepatic artery branches and can be treated with angioembolization (Figure 3).29,31 These most commonly arise from the right hepatic artery and usually within 2 cm of the GDA origin.31 Experienced interventional radiologists are invaluable in these situations as culprit vessels can be very small and difficult to embolize. If no vessels are seen on angiography then the scan should be repeated in a few weeks as the malperfusion occasionally resolves. It is critical to understand that there will be situations where there is uncorrectable extrahepatic perfusion and an inability to safely use the pump. Patients must be counseled about this possibility. If extrahepatic perfusion is not properly identified, the patients are at risk of gastroduodenal ulcers and/or pancreatitis and may develop significant pain upon the administration of chemotherapy. Incomplete perfusion of the liver occurs in 2% of patients and is typically due to failure to ligate replaced or accessory hepatic arteries and can often be managed by angioembolization and/or improves with time as collateral flow across the liver develops.
During placement of the pump catheter, there can be varying degrees of arterial dissection. Sometimes this is immediately obvious and can be recognized by difficulty passing the catheter forward into the lumen of the GDA. Occasionally, this is noted in the dye study when the dye is clearly noted within the wall of the arteries. Management of this situation is highly individualized. If there is a loss of the GDA, the strategies mentioned above can be employed. If the dissection is more diffuse and there is no obvious strategy to move the catheter, it is often prudent to just leave the catheter in place and wait for assessment on postoperative flow studies, which often show the flow has normalized, but may also result in a nonfunctional pump.
An arterial or catheter-related thrombus, although rare, can often be salvaged with anticoagulation or lytic therapies through the pump catheter. Alternatively, delayed thrombosis is particularly troublesome and is associated with high rates of pump failure. Pseudoaneurysms are sometimes found at the GDA origin incidentally on follow up scans. In general, these should be managed with angiography and usually require stenting across the GDA orifice to prevent bleeding. If a stent is not feasible, embolization is necessary. Frank bleeding from the catheter can also occur and typically presents with epigastric pain and a large upper abdominal hematoma. The management is similar and requires angiography and stenting or embolization. These situations result in loss of the pump.
Lastly, the risk of biliary sclerosis has been well documented and can be a devastating long-term consequent of HAIC. Although initial studies reported biliary sclerosis rates as high as 30% to 50%35,36 modern series report rates at closer to 5% long-term.37 Biliary sclerosis is associated with abnormal flow scans, postoperative infections, and higher chemotherapy doses. Concurrent dexamethasone in the pump and early recognition of biliary sclerosis can help prevent this complication.36,37
4 |. THE LEARNING CURVE
For most experienced hepatobiliary surgical oncologists the technical factors involved in placing a HAIC pump are often not the primary limitation in developing an HAIC program. However, an in-depth understanding of the unique anatomic considerations and postoperative complications is necessary. In addition, the primary limitation in developing an HAIC program is often related to the significant amount of specialized training and support that is needed from colleagues in radiology, interventional radiology, medical oncology, gastroenterology, and nursing. Although there remains is no published data on the learning curve, a series by Allen et al demonstrated increased rates of complications when the pump placement was performed by surgeons that placed fewer than 25 pumps.30 Surgeons who have not learned pump placement in their training should spend time at an experienced center to see cases and learn directly from experienced surgeons.
Abbreviations:
- FUDR
5-Fluoro 2-deoxyuridine
- GDA
gastroduodenal artery
- HAIC
hepatic artery infusion chemotherapy
- ICC
intrahepatic cholangiocarcinoma
Footnotes
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. [DOI] [PubMed] [Google Scholar]
- 2.Cronin KA, Lake AJ, Scott S, et al. Annual report to the nation on the status of cancer, part I: national cancer statistics. Cancer. 2018;124: 2785–2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davis DM, Marcet JE, Frattini JC, Prather AD, Mateka JJL, Nfonsam VN. Is it time to lower the recommended screening age for colorectal cancer? J Am Coll Surg. 2011;213:352–361. [DOI] [PubMed] [Google Scholar]
- 4.Daly JM, Kemeny N. Therapy of colorectal hepatic metastases. Important Adv Oncol. 1986:251–256. 3330537. [PubMed] [Google Scholar]
- 5.Weiss L, Grundmann E, Torhorst J, et al. Haematogenous metastatic patterns in colonic carcinoma: an analysis of 1541 necropsies. J Pathol. 1986;150:195–203. [DOI] [PubMed] [Google Scholar]
- 6.Jarnagin WR, Schwartz LH, Gultekin DH, et al. Regional chemotherapy for unresectable primary liver cancer: results of a phase II clinical trial and assessment of DCE-MRI as a biomarker of survival. Ann Oncol Off J Eur Soc Med Oncol. 2009;20:1589–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breedis C, Young G. The blood supply of neoplasms in the liver. Am J Pathol. 1954;30:969–977. [PMC free article] [PubMed] [Google Scholar]
- 8.Ensminger WD, Gyves JW. Clinical pharmacology of hepatic arterial chemotherapy. Semin Oncol. 1983;10:176–182. [PubMed] [Google Scholar]
- 9.Johnson LP, Rivkin SE. The implanted pump in metastatic colorectal cancer of the liver: risk versus benefit. Am J Surg. 1985;149: 595–598. [DOI] [PubMed] [Google Scholar]
- 10.Weiss GR, Garnick MB, Osteen RT, et al. Long-term hepatic arterial infusion of 5-fluorodeoxyuridine for liver metastases using an implantable infusion pump. J Clin Oncol. 1983;1:337–344. [DOI] [PubMed] [Google Scholar]
- 11.Kerr DJ, McArdle CS, Ledermann J, et al. Intrahepatic arterial versus intravenous fluorouracil and folinic acid for colorectal cancer liver metastases: a multicentre randomised trial. Lancet. 2003;361: 368–373. [DOI] [PubMed] [Google Scholar]
- 12.Lorenz M, Müller HH. Randomized, multicenter trial of fluorouracil plus leucovorin administered either via hepatic arterial or intravenous infusion versus fluorodeoxyuridine administered via hepatic arterial infusion in patients with nonresectable liver metastases from colorectal carcinoma. J Clin Oncol. 2000;18:243–254. [DOI] [PubMed] [Google Scholar]
- 13.Kemeny NE, Niedzwiecki D, Hollis DR, et al. Hepatic arterial infusion versus systemic therapy for hepatic metastases from colorectal cancer: a randomized trial of efficacy, quality of life, and molecular markers (CALGB 9481). J Clin Oncol. 2006;24:1395–1403. [DOI] [PubMed] [Google Scholar]
- 14.Kusano M, Honda M, Okabayashi K, et al. Randomized controlled Phase III study comparing hepatic arterial infusion with systemic chemotherapy after curative resection for liver metastasis of colorectal carcinoma: JFMC 29–0003. J Cancer Res Ther. 2017;13:84. [DOI] [PubMed] [Google Scholar]
- 15.Goéré D, Benhaim L, Bonnet S, et al. Adjuvant chemotherapy after resection of colorectal liver metastases in patients at high risk of hepatic recurrence. Ann Surg. 2013;257:114–120. [DOI] [PubMed] [Google Scholar]
- 16.Kemeny N, Huang Y, Cohen AM, et al. Hepatic arterial infusion of chemotherapy after resection of hepatic metastases from colorectal cancer. N Engl J Med. 1999;341:2039–2048. [DOI] [PubMed] [Google Scholar]
- 17.Kemeny NE, Gonen M. Hepatic arterial infusion after liver resection. N Engl J Med. 2005;352:734–735. [DOI] [PubMed] [Google Scholar]
- 18.Datta J, Narayan RR, Kemeny NE, D’Angelica MI. Role of hepatic artery infusion chemotherapy in treatment of initially unresectable colorectal liver metastases. JAMA Surg. 2019;154:768. [DOI] [PubMed] [Google Scholar]
- 19.Kemeny NE, Melendez FDH, Capanu M, et al. Conversion to resectability using hepatic artery infusion plus systemic chemotherapy for the treatment of unresectable liver metastases from colorectal carcinoma. J Clin Oncol. 2009;27:3465–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pak LM, Kemeny NE, Capanu M, et al. Prospective phase II trial of combination hepatic artery infusion and systemic chemotherapy for unresectable colorectal liver metastases: long term results and curative potential. J Surg Oncol. 2018;117:634–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D’Angelica MI, Correa-Gallego C, Paty PB, et al. Phase II trial of hepatic artery infusional and systemic chemotherapy for patients with unresectable hepatic metastases from colorectal cancer: conversion to resection and long-term outcomes. Ann Surg. 2015;261:353–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cercek A, Boucher TM, Gluskin JS, et al. Response rates of hepatic arterial infusion pump therapy in patients with metastatic colorectal cancer liver metastases refractory to all standard chemotherapies. J Surg Oncol. 2016;114:655–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loupakis F, Cremolini C, Masi G, et al. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med. 2014; 371:1609–1618. [DOI] [PubMed] [Google Scholar]
- 24.Nathan H, Aloia TA, Vauthey J-N, et al. A proposed staging system for intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2009;16:14–22. [DOI] [PubMed] [Google Scholar]
- 25.Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N EnglJ Med. 2010;362:1273–1281. [DOI] [PubMed] [Google Scholar]
- 26.Konstantinidis IT, Koerkamp BG, Do RKG, et al. Unresectable intrahepatic cholangiocarcinoma: Systemic plus hepatic arterial infusion chemotherapy is associated with longer survival in comparison with systemic chemotherapy alone. Cancer. 2016;122:758–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cercek A, Boerner T, Tan BR, et al. Assessment of hepatic arterial infusion of floxuridine in combination with systemic gemcitabine and oxaliplatin in patients with unresectable intrahepatic cholangiocarcinoma: a phase 2 clinical trial. JAMA Oncol. 2020;6(1):60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Castaing D, Azoulay D, Fecteau AH, Bismuth H. Implantable hepatic arterial infusion device: placement without laparotomy via an intercostal artery. J Am Coll Surg. 1998;187:565–568. [DOI] [PubMed] [Google Scholar]
- 29.Allen PJ, Stojadinovic A, Ben-Porat L, et al. The management of variant arterial anatomy during hepatic arterial infusion pump placement. Ann Surg Oncol. 2002;9:875–880. [DOI] [PubMed] [Google Scholar]
- 30.Allen PJ, Nissan A, Picon AI, et al. Technical complications and durability of hepatic artery infusion pumps for unresectable colorectal liver metastases: an institutional experience of 544 consecutive cases. J Am Coll Surg. 2005;201:57–65. [DOI] [PubMed] [Google Scholar]
- 31.Perez DR, Kemeny NE, Brown KT, et al. Angiographic identification of extrahepatic perfusion after hepatic arterial pump placement: implications for surgical prevention. HPB. 2014;16:744–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng J, Glasgow RE, O’Rourke RW, Swanstrom LL, Hansen PD. Laparoscopic radiofrequency ablation and hepatic artery infusion pump placement in the evolving treatment of colorectal hepatic metastases. Surg Endosc. 2003;17:61–67. [DOI] [PubMed] [Google Scholar]
- 33.Qadan M, D’Angelica MI, Kemeny NE, Cercek A, Kingham TP. Robotic hepatic arterial infusion pump placement. HPB. 2017;19: 429–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dhir M, Zenati MS, Padussis JC, et al. Robotic assisted placement of hepatic artery infusion pump is a safe and feasible approach. J Surg Oncol. 2016;114:342–347. [DOI] [PubMed] [Google Scholar]
- 35.Hohn DC, Rayner AA, Economou JS, Ignoffo RJ, Lewis BJ, Stagg RJ. Toxicities and complications of implanted pump hepatic arterial and intravenous floxuridine infusion. Cancer. 1986;57:465–470. [DOI] [PubMed] [Google Scholar]
- 36.Kemeny N, Seiter K, Niedzwiecki D, et al. A randomized trial of intrahepatic infusion of fluorodeoxyuridine with dexamethasoneversus fluorodeoxyuridine alone in the treatment of metastatic colorectal cancer. Cancer. 1992;69:327–334. [DOI] [PubMed] [Google Scholar]
- 37.Ito K, Ito H, Kemeny NE, et al. Biliary sclerosis after hepatic arterial infusion pump chemotherapy for patients with colorectal cancer liver metastasis: incidence, clinical features, and risk factors. Ann Surg Oncol. 2012;9:1609–1617. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.


