Abstract
Purpose: We investigated whether COVID-19 patients on mechanical ventilation (MV) had a different extubation outcome compared to non-COVID-19 patients while identifying predictive factors of extubation failure in the former. Methods: A retrospective, single-center, and observational study was done on 216 COVID-19 patients admitted to an intensive care unit (ICU) between March 2020 and March 2021, aged ≥ 18 years, in use of invasive MV for more than 24 h, which progressed to weaning. The primary outcome that was evaluated was extubation failure during ICU stay. A statistical analysis was performed to evaluate the association of patient characteristics with extubation outcome, and a Poisson regression model determined the predictive value. Results: Seventy-seven patients were extubated; the mean age was 57.2 years, 52.5% were male, and their mean APACHE II score at admission was 17.8. On average, MV duration until extubation was 8.7 ± 3.7 days, with 14.9 ± 10.1 days of ICU stay and 24.6 ± 14.0 days with COVID-19 symptoms. The rate of extubation failure (ie, the patient had to be reintubated during their ICU stay) was 22.1% (n = 17), while extubation was successful in 77.9% (n = 60) of cases. Failure was observed in only 7.8% of cases when evaluated 48 hours after extubation. The mean reintubation time was 4.28 days. After adjusting the analysis for age, sex, during of symptoms, days under MV, dialysis, and PaO2/FiO2 ratio, some parameters independently predicted extubation failure: age ≥ 66 years (APR = 5.12 [1.35-19.46]; p = 0.016), ≥ 31 days of symptoms (APR = 5.45 [0.48-62.19]; p = 0.016), and need for dialysis (APR = 5.10 [2.00-13.00]; p = 0.001), while a PaO2/FiO2 ratio >300 decreased the probability of extubation failure (APR = 0.14 [0.04-0.55]; p = 0.005). The presence of three predictors (ie, age ≥ 66 years, time of symptoms ≥ 31 days, need of dialysis, and PaO2/FiO2 ratio < 200) increased the risk of extubation failure by a factor of 23.0 (95% CI, 3.34-158.5). Conclusion: COVID-19 patients had an extubation failure risk that was almost three times higher than non-COVID-19 patients, with the extubation of the former being delayed compared to the latter. Furthermore, an age ≥ 66 years, time of symptoms ≥ 31 days, need of dialysis, and PaO2/FiO2 ratio > 200 were independent predictors for extubation failure, and the presence of three of these characteristics increased the risk of failure by a factor of 23.0.
Keywords: COVID-19, mechanical ventilation, weaning, extubation, predictive factors
Introduction
Since the first reported case of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in humans in December 2019, the virus has spread globally, affecting the vast majority of countries and causing more than 4.5 million deaths to date.1 This virus is responsible for coronavirus disease 2019 (COVID-19), which can have different clinical presentations ranging from mild respiratory symptoms to extremely serious conditions that require intensive care, such as acute respiratory distress syndrome (ARDS).2,3
Intensive care unit (ICU) admission for COVID-19 patients ranges from 7% to 14%.4-9 In Brazil, 50.7% of patients admitted to ICUs required invasive mechanical ventilation (MV), with the majority (57.8%) requiring MV for more than 7 days, and the mortality rate of these patients was 65.7%.10
During the COVID-19 pandemic, hospitals around the world were forced to admit an unprecedented number of critically ill patients, leading to ICU overcrowding, shortages of equipment such as mechanical ventilators, and insufficiency of staff with the expertise required to manage the ventilators, mainly in patients with ARDS, which is associated with a higher mortality rate.11 In this context, it is of fundamental importance to manage the weaning of patients with COVID-19 in order to reduce their time on MV and increase the success of weaning and extubation, leading to shorter ICU and hospital admissions and increased bed availability.
In non-COVID-19 patients on MV, the extubation failure rate ranges from 5% to 30%, which doubles ICU stay duration and substantially increases the risk of death.12 There is still no consensus on the characteristics of COVID-19 patients that can predict the success or failure of weaning and extubation. Knowledge of these predictors would allow ICU teams to identify patients that are at a higher risk of extubation failure to avoid reintubation and all its associated risks.
The objective of this study was to analyze the extubation outcomes in COVID-19 patients on MV, identify predictive factors of extubation failure that can help ICU professionals identify COVID-19 patients that can be safely extubated, and compare the extubation outcome of COVID-19 patients with previous results from our group in non-COVID-19 patients.
Method
This retrospective, single center, and observational study took place at Santa Terezinha University Hospital (Hospital Universitário Santa Terezinha, HUST), which has 15 beds for COVID-19 patients. This study was approved by the local ethics committee (n° 3.040.334) and followed the STROBE statement for reporting observational studies.13
Inclusion criteria were patients admitted to ICU between March 2020 and March 2021, aged ≥ 18 years, with COVID-19 confirmed by reverse transcription polymerase chain reaction (RT-PCR) for SARS-CoV-2, and submitted to invasive MV for more than 24 h, which progressed to weaning. Patients who were accidentally or self-extubated, underwent tracheostomy, or died before weaning were excluded. Palliative extubation was an exclusion criterion, but none occurred in this sample.
The criteria to initiate weaning were improvement in the condition that caused the respiratory failure; a partial pressure of arterial oxygen (PaO2) higher than 60 mm Hg with a fraction of inspired oxygen (FiO2) lower than 0.4 and a positive end-expiratory pressure (PEEP) of 5 cm of water or less; hemodynamic stability (without or with a low dose of vasopressors and without decompensated coronary insufficiency or arrhythmias with hemodynamic repercussion); patient's ability to initiate an inspiratory effort; and the absence of a significant acid-base imbalance. Patients who tolerated pressure support ventilation (PSV) mode with a PEEP of 3–5 cmH2O and a pressure over PEEP of 7 cmH2O with adequate ventilation (volume of 4-6 mL/kg and respiratory rate [RR] < 35/min) underwent a spontaneous breathing trial (SBT) with a T piece. The criteria to consider patient intolerance to the SBT were RR ˃ 35/min; oxygen saturation (SpO2) < 90% with FiO2 ˃ 0.5; heart rate ˃ 140/min or greater than a 20% increase from baseline; and systolic blood pressure (SBP) ˃ 180 mm Hg, < 90 mm Hg, or development of arrhythmia, agitation, anxiety, or change in level of consciousness. Patients who tolerated the SBT for 30 min and presented a rapid shallow breath index (RSBI) < 105 breaths/min/L were extubated.14 Inclusion, exclusion, weaning, and extubation criteria were defined based on a previously published study,12 except for the inclusion of the COVID-19 diagnosis.
The primary outcome that was evaluated was extubation failure during ICU stay. The secondary outcomes were failure within 48 h after extubation, length of ICU and hospital stay, and ICU and hospital discharge. Clinical and demographic information were collected from patient records. Symptoms of COVID-19 were considered: fever or chills, cough, shortness of breath or difficulty breathing, fatigue, muscle or body aches, headache, new loss of taste or smell, sore throat, congestion or runny nose, nausea or vomiting, and diarrhea. The time of symptoms included symptomatic days prior to admission.15
A statistical analysis was performed using IBM SPSS Statistics v. 25. Categorical variables were presented as absolute numbers (n) and relative frequencies (%). The chi-square test or Fisher's exact test were used to evaluate the association between patient characteristics and extubation outcome. For quantitative variables, the Shapiro–Wilk test was used to evaluate the normality of distributions. Normally distributed variables were summarized as means (± standard deviation), and the independent samples Student's t-test was used to compare differences between groups. Patient characteristics associated with extubation outcome were evaluated for their independent capacity to predict extubation failure type using a Poisson regression model adjusted to potential confounders such as age, sex, time of symptoms, days on MV, use of dialysis, and PaO2/FiO2 ratio. The data are presented as prevalence ratios (PRs) and respective 95% confidence level (CI). An additional Poisson regression analysis was carried out to verify the probability of extubation failure considering the number of simultaneous predictors (0 to 3). The Fisher Exact test was used to evaluate the association of extubation outcome at 48 h and during ICU stay in this cohort compared to the cohort of our previous published study.12 The p-values were also presented considering a significance level of 5%. A post hoc power analysis yielded a statistical power of 0.96 (p2 = 0.78; p1 = 0.12; n = 77, α = 0.05 – Gpower 3.1).
Results
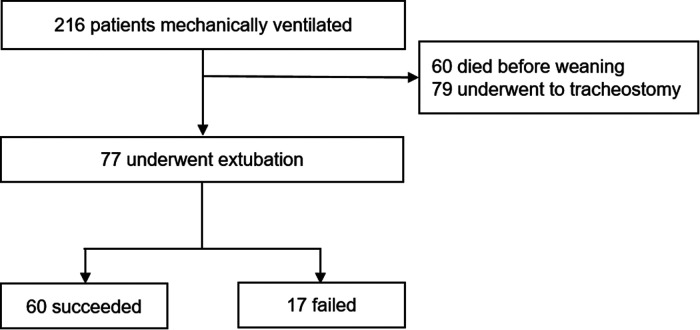
During the analyzed period, 216 COVID-19 patients aged ≥ 18 years were intubated and subjected to MV for more than 24 h. Sixty died before weaning, and 79 underwent tracheostomy, being excluded from the analysis. Seventy-seven patients were extubated and formed the final study sample (Figure 1).
Figure 1.
Flowchart of the derivation cohort.
The mean age of the extubated patients was 57.2 years, 52.5% were male, and the mean APACHE II score at admission was 17.8. The mean MV duration until extubation was 8.7 days, and the length of ICU stay was 14.9 days on average, while the mean duration of symptoms was of 24.6 days. The extubation failure rate was 22.1% (n = 17), demanding reintubation during the ICU stay, while extubation was successful in 77.9% (n = 60) of cases. The proportion of failures was reduced to 7.8% when evaluation was performed 48 h after extubation. The mean time for reintubation was 4.28 days. Of the extubated patients, 89.6% were discharged from the ICU, and 79.2% were discharged from the hospital (Table 1).
Table 1.
Characteristics of Mechanically Ventilated COVID-19 Patients.
| Total = 77 | Success = 60 | Failure = 17 | P value | |
|---|---|---|---|---|
| Age, years | 57.2 ± 16.7 | 55.5 ± 17.0 | 63.8 ± 15.6 | 0.073 |
| Male n (%) | 42 (52.5%) | 30 (50%) | 10 (58.8%) | 0.590 |
| APACHE II score, points | 17.8 ± 7.3 | 17.8 ± 7.5 | 17.9 ± 6.4 | 0.947 |
| Time of symptoms (days) | 24.6 ± 14.0 | 21.1 ± 10.3 | 36.6 ± 18.5 | 0.004 |
| Days of mechanical ventilation | 8.7 ± 3.7 | 8.3 ± 3.6 | 10.2 ± 4.1 | 0.069 |
| ICU length of stay (days) | 14.9 ± 10.1 | 11.9 ± 6.9 | 25.3 ± 12.7 | 0.001 |
| ICU discharge | 69 (89.6%) | 59 (98.3%) | 10 (58.8%) | <0.001 |
| Hospital discharge | 61 (79.2%) | 53 (88.3%) | 8 (47.1%) | <0.001 |
Chi-square test for categorical variables.
Student t test for quantitative variables.
There was no difference in age, sex, APACHE II score at admission, or days on MV between patients who experienced successful or failed extubation. However, patients with a failed extubation during their ICU stay showed symptoms for more days when also considering the period prior to admission (36.6 ± 18.5 days vs. 21.1 ± 10.3 days; p = 0.004), as well as a longer ICU stay (25.3 ± 12.7 days vs. 11.9 ± 6.9 days; p = 0.001). Furthermore, extubation failure was strongly associated with ICU (p < 0.001) and hospital (p < 0.001) discharge (Table 1).
The use of non-invasive ventilation, need for prone position, percentage of compromised lung evaluated by a CT scan after intubation, fluid balance in the last 24 h, dynamic lung compliance, partial pressure of carbon dioxide (PaCO2), estimated Glasgow coma scale, and RSBI during the SBT at 30 min did not differ between the failure and success groups, while need of dialysis (success = 1.7% vs. failure = 23.5%; p = 0.008) and a lower PaO2/FiO2 ratio (success = 299.7 ± 127.3 mm Hg vs. failure = 227.1 ± 86.1 mm Hg; p = 0.031) were associated with extubation failure (Table 2).
Table 2.
Comparison of Parameters Between the Success and Failure Extubation Groups.
| Success = 60 | Failure = 17 | P value | |
|---|---|---|---|
| NIV before IMV | 29 (49.2%) | 8 (47.1%) | 1.000 |
| Prone position | 26 (43.3%) | 6 (35.3%) | 0.591 |
| Dialysis | 1 (1.7%) | 4 (23.5%) | 0.008 |
| % of compromised lung by CT scan | 64.0 ± 14.5 | 66.8 ± 16.0 | 0.510 |
| Fluid balance - last 24 h (mL) | 244.8 ± 1403.8 | 300.1 ± 1336.1 | 0.889 |
| PaO2/FiO2 | 299.7 ± 127.3 | 227.1 ± 86.1 | 0.031 |
| Dynamic lung compliance (ml/cmH2O) | 64.3 ± 24.3 | 68.5 ± 36.7 | 0.674 |
| PaCO2 | 37.4 ± 11.1 | 38.3 ± 12.3 | 0.770 |
| eGCS | 14.0 ± 1.9 | 14.2 ± 1.5 | 0.731 |
| RSBI in SBT (breaths/min/L) | 47.4 ± 17.7 | 44.2 ± 15.9 | 0.511 |
eGCS: estimated Glasgow Coma Scale.
Chi-square test for categorical variables.
Student t test for quantitative variables.
A Poisson regression model was used to assess the predictive factors of extubation failure in COVID-19 patients on MV. After adjusting the analysis for age, sex, duration of symptoms, days of MV, dialysis, and PaO2/FiO2 ratio, some parameters independently predicted extubation failure: age ≥ 66 years (APR = 5.12 [1.35-19.46]; p = 0.016), ≥ 31 days with symptoms (APR = 5.45 [0.48-62.19]; p = 0.016), and need of dialysis (APR = 5.10 [2.00-13.00]; p = 0.001), while a PaO2/FiO2 ratio ˃ 300 decreased the risk of failed extubation (APR = 0.14 [0.04-0.55]; p = 0.005) (Table 3).
Table 3.
Crude and Adjusted Poisson Regression Model to Assess the Parameters Associated with Extubation Failure in COVID-19 Mechanically Ventilated Patients.
| Extubation Failure | ||
|---|---|---|
| CPR (95% CI) | APR# (95% CI) | |
| Age, years | p = 0.083* | p = 0.016* |
| 15-50 | 1.00 | 1.00 |
| 51-65 | 2.09 (0.60-7.32) | 4.49 (1.15-17.67) |
| ≥ 66 | 2.76 (0.80-9.49) | 5.12 (1.35-19.46) |
| Time of symptoms (days) | p = 0.001* | p = 0.016* |
| 5-15 | 1.00 | 1.00 |
| 16-30 | 3.16 (0.40-24.78) | 1.71 (1.14-21.40) |
| ≥31 | 10.53 (1.47-75.5) | 5.45 (0.48-62.19) |
| Days of mechanical ventilation | p = 0.054 | p = 0.387 |
| 1-7 | 1.00 | 1.00 |
| 8-14 | 1.72 (0.57-5.21) | 2.13 (0.44-10.39) |
| ≥15 | 3.87 (1.22-12.3) | 2.53 (0.66-9.65) |
| Dialysis | p < 0.001 | p = 0.001 |
| No | 1.00 | 1.00 |
| Yes | 4.43 (2.28-8.60) | 5.10 (2.00-13.00) |
| PaO2/FiO2 | p = 0.031* | p = 0.005* |
| <200 | 1.00 | 1.00 |
| 200-300 | 0.89 (0.38-2.07) | 0.69 (0.27-1.72) |
| >300 | 0.25 (0.06-1.08) | 0.14 (0.04-0.55) |
CPR, crude prevalence ratio; APR, adjusted prevalence ratio; (95% CI), 95% confidence interval.
Adjusted for age, sex, time of symptoms, days of mechanical ventilation, dialysis, and PaO2/FiO2.
*Trend test.
Considering the four abovementioned predictors of extubation failure, the presence of one, two, and three predictors increased its probability by a factor of 3.48 (95% CI, 0.43-28.30), 13.42 (95% CI, 1.83-98.0), and 23.0 (95% CI, 3.34-158.5), respectively (Table 4).
Table 4.
The Prevalence Rate of Extubation Failure Based on the Numbers of Predictive Characteristics Presented in the Mechanically Ventilated COVID-19 Patients.
| Total % (n) | Extubation Failure | ||
|---|---|---|---|
| % | PR (95% CI) | ||
| Number of predictors# | p < 0.001* | ||
| 0 | 31.9 (23) | 4.3 | 1.00 |
| 1 | 45.8 (33) | 15.2 | 3.48 (0.43-28.30) |
| 2 | 16.7 (12) | 58.3 | 13.42 (1.83-98.0) |
| 3 | 5.6 (4) | 100.0 | 23.0 (3.34-158.5) |
#Considering the predictors: age ≥ 66; time of symptoms ≥31 days; dialysis and PaO2/FiO2 <200.
PR, prevalence ratio; (95% CI), 95% confidence interval.
*Trend test.
Discussion
To our knowledge, this is the first study to date showing that the profile and predictors for successful MV discontinuation are different in COVID-19 patients compared to non-COVID-19 patients. Here, we present some important findings. First, we showed that COVID-19 patients undergoing MV presented a similar extubation failure rate when compared to non-COVID-19 patients when evaluated 48 h after extubation. However, when extubation outcome was analyzed during ICU stay, the extubation failure rate increased by almost three times, evidencing a physiological response associated with late extubation failure. Second, we found that an age ≥ 66 years, duration of symptoms ≥ 31 days, need of dialysis, and PaO2/FiO2 ratio ˂ 200 were independent predictors for extubation failure, and the presence of three of these characteristics increased the risk of extubation failure by 23.0 times.
Comparing this cohort of COVID-19 patients with a previously studied cohort of non-COVID-19 patients admitted to the ICU of the same hospital,12 we observed that there was no difference in the extubation failure rate at 48 h (COVID-19 = 7.8% [6/77] vs. non-COVID-19 = 8.2% [9/110]; p = 0.928); however, extubation failure throughout ICU stay was significantly more frequent in COVID-19 patients (COVID-19 = 22.1% [17/77] vs. non-COVID-19 = 10.0% [11/110]; p = 0.022). Furthermore, the time of mechanical ventilation until extubation was 8.7 ± 3.7 days (mean) in COVID-19 patients, while in non-COVID-19 patients it was 5 (2-7) days (median). It is important to note that patients with COVID-19 had to meet the same requirements as non-COVID-19 patients to be considered eligible for the SBT, and the criteria for SBT success were the same, as suggested by guidelines published before the pandemic. Yet the extubation outcomes were quite different, indicating that weaning and extubation of COVID-19 patients should be evaluated and conducted while taking other aspects into account, based on the physiological responses involved in the failure.
In our cohort, the mean time for reintubation was 4.28 days, much higher than previously reported for non-COVID-19 patients (median of 15 h [2-45 h]).16 The late extubation failure observed in COVID-19 patients may be due to different processes, or even a combination of them: 1) COVID-19 patients have an increased respiratory drive, leading to intense inspiratory effort and increased transpulmonary pressure,17 which may worsen lung damage by several mechanisms gathered under the name “patient self-inflicted lung injury” (P-SILI);18 2) due to the multisystem involvement, patients with COVID-19 have greater complications secondary to pulmonary infection, such as the risk of progression to sepsis or acute kidney failure, as well as a greater chance to develop antibiotic resistance, favoring reinfections with multiresistant microorganisms;19 3) beyond the systemic involvement, critically ill COVID-19 patients can show respiratory muscle failure due to ICU-acquired weakness (ICUAW), resulting from a combination of fatigue and rhabdomyolysis associated with sedation, neuromuscular blocking agents, corticosteroids, and immobilization.20,21
Four parameters were independently associated with extubation failure. An age ≥ 66 years increased the risk of extubation failure by a factor of 5.12. Previous studies on non-COVID-19 patients had already shown an association between age and the outcomes of weaning and extubation, focusing on the functional decline observed in this group of patients, mainly under disease conditions.22–25 The need of dialysis increased the risk of extubation failure by a factor of 5.10, which has been previously associated with extubation failure, most likely due to the close relationship between renal function, risk of pulmonary congestion, and respiratory failure, especially after withdrawal of positive pressure.26 The PaO2/FiO2 ratio has also been shown to be a weaning and extubation predictive parameter.22,27,28 Here, a PaO2/FiO2 ratio ˃ 200 decreased the risk of extubation failure to 0.69, and a PaO2/FiO2 ratio ˃ 300 decreased it to 0.14. Besides these, a duration of symptoms ≥ 31 days was associated with a 5.45 times greater risk of failure. Late weaning (ie, a long time after symptoms started) can be related to a non-resolution of the respiratory condition and increase the risk of secondary complications such as infections, ICUAW, or multi-organ dysfunction. Even more important than these individual parameters is a combination of three of them, which increased the risk of extubation failure by a factor of 23.0. Based on these results, and considering that extubation failure in COVID-19 patients is associated with long ICU stay and ICU and hospital mortality, it would be interesting to develop an instrument that considers these four parameters to be used at bedside, providing support to the ICU team in their decision to extubate COVID-19 patients.
This study has some limitations, such as its retrospective nature, single-center analysis, and small sample size. Furthermore, the comparison of the two cohorts has some limitations, such as the age difference between patients (COVID-19 = 57.2 ± 16.7; and non-COVID-19 = 67 (50-77) and the time-period bias. For this reason, we suggest the development of multicenter studies with larger sample sizes to confirm the findings presented here. Further, the specific context of a pandemic and a lack of ICU beds could change the way of managing weaning and extubation (ie, a faster process and with more therapeutic limitations).
Conclusion
In this article, we showed for the first time that COVID-19 patients have a different profile and predictors for MV discontinuation when compared to non-COVID-19 patients. The risk of failed extubation was nearly three times higher for COVID-19 patients compared to non-COVID-19 patients considering the whole ICU stay. Also, we found that an age ≥ 66 years, duration of symptoms ≥ 31 days, need of dialysis, and PaO2/FiO2 ratio ˂ 200 were independent predictors of extubation failure, and the presence of three of these characteristics increased the risk of extubation failure by 23.0 times. Finally, these four parameters can be used to develop an instrument to support the ICU team in their decision to extubate COVID-19 patients.
Acknowledgments
The authors would like to thank the ICU team from the Hospital Universitário Santa Terezinha and CNPq.
Footnotes
Funding: Scholarship PIBIC from CNPq to Natália Guzatti.
Authors’ Contributions: Conceptualization: Diego de Carvalho; João Rogério Nunes Filho; Antuani Rafael Baptistella; Methodology: Natália Godoy Guzatti; Fernanda Klein; Julia Almeida Oliveira; Gustavo Bruno Rático; Luana Patrícia Marmitt; Antuani Rafael Baptistella; Formal analysis and investigation: Luana Patrícia Marmitt; Diego de Carvalho; João Rogério Nunes Filho; Antuani Rafael Baptistella; Writing—original draft preparation: Natália Godoy Guzatti; Fernanda Klein; Julia Almeida Oliveira; Gustavo Bruno Rático; Antuani Rafael Baptistella; Writing—reviewing and editing: Marcos Freitas Cordeiro; Diego de Carvalho; João Rogério Nunes Filho; Antuani Rafael Baptistella; Funding acquisition: Antuani Rafael Baptistella; Supervision:
Diego de Carvalho; João Rogério Nunes Filho; Antuani Rafael Baptistella.
Ethics approval and consent to participate:
The local ethical committee (Comitê de Ética em Pesquisa UNOESC/HUST) approved the study (n° 3.040.334; CAAE: 1170718.5.0000.5367), which was performed in accordance with established ethical standards.
Availability of Data and Material: All data generated or analyzed during this study are available for consultation and can be requested from authors.
Code Availability: Not applicable.
Consent for Publication: Not applicable.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: Not applicable, because this article does not contain any studies with human or animal subjects.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Marcos Freitas Cordeiro https://orcid.org/0000-0002-2100-1614
Antuani Rafael Baptistella https://orcid.org/0000-0003-1708-9921
References
- 1.WHO (COVID-19). WHO Coronavirus (COVID-19) dashboard. https://covid19.who.int/ (2021).
- 2.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382(8):727-733. doi: 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mizera L, Rath D, Schoellmann A, et al. Deceleration capacity is associated with acute respiratory distress syndrome in COVID-19. Hear. Lung. 2021;50(6):914-918. doi: 10.1016/j.hrtlng.2021.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 2020;8:475-481. doi: 10.1016/S2213-2600(20)30079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in wuhan, China. JAMA 2020;323(11):1061. doi: 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in wuhan, China. Lancet. 2020;395(10223):497-506. doi: 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richardson S, Hirsch J, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. JAMA 2020;323(20): 2052. doi: 10.1001/jama.2020.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhatraju PK, Ghassemieh B, Nichols M, et al. COVID-19 in critically ill patients in the Seattle region — case series. N Engl J Med. 2020;382(21):2012-2022. doi: 10.1056/NEJMoa2004500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramírez P, Gordón M, Martín-Cerezuela M, et al. Acute respiratory distress syndrome due to COVID-19. Clinical and prognostic features from a medical critical care unit in valencia, Spain. Med. Intensiva. 2021;45(1):27-34. doi: 10.1016/j.medin.2020.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.AMIB- Associação de Medicina Intensiva Brasileira. UTIs brasileiras. Registro nacional de terapia intensiva http://www.utisbrasileiras.com.br/sari-covid-19/benchmarking-covid-19/ (2021).
- 11.Olivas-Martínez A, Cárdenas-Fragoso J, Jiménez J, et al. In-hospital mortality from severe COVID-19 in a tertiary care center in Mexico city; causes of death, risk factors and the impact of hospital saturation. PLoS One. 2021;16(2):e0245772. doi: 10.1371/journal.pone.0245772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baptistella AR, Mantelli L, Matte L, et al. Prediction of extubation outcome in mechanically ventilated patients: development and validation of the extubation predictive score (ExPreS). PLoS One. 2021;16(3):e0248868. doi: 10.1371/journal.pone.0248868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.von Elm E, Altman D, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J. Clin. Epidemiol. 2008;61(4): 344-349. doi: 10.1016/j.jclinepi.2007.11.008 [DOI] [PubMed] [Google Scholar]
- 14.AMIB- Associação de Medicina Intensiva Brasileira & SBPT. Diretrizes Brasileiras de Ventilação Mecânica. 2013.
- 15.National Center for Immunization and Respiratory Diseases (NCIRD), D. of V. D. Symptoms of COVID-19. Centers of Disease Control and prevention - CDChttps://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html2022.
- 16.Miltiades AN, Gershengorn H, Hua M, et al. Cumulative probability and time to reintubation in U. S. ICUs. Crit. Care Med. 2017;45(5):835-842. doi: 10.1097/CCM.0000000000002327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferrando C, Suarez-Sipmann F, Mellado-Artigas R, et al. Clinical features, ventilatory management, and outcome of ARDS caused by COVID-19 are similar to other causes of ARDS. Intensive Care Med. 2020;46(12):2200-2211. doi: 10.1007/s00134-020-06192-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carteaux G, Parfait M, Combet M, et al. Patient-Self inflicted lung injury: a practical review. J. Clin. Med. 2021;10(12): 2738. doi: 10.3390/jcm10122738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mughal MS, Kaur IP, Jaffery AR, et al. COVID-19 patients in a tertiary US hospital: assessment of clinical course and predictors of the disease severity. Respir. Med. 2020;172:106130. doi: 10.1016/j.rmed.2020.106130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Aerde N, Van den Berghe G, Wilmer A, Gosselink R, Hermans G. Intensive care unit acquired muscle weakness in COVID-19 patients. Intensive Care Med. 2020;46(11): 2083-2085. doi: 10.1007/s00134-020-06244-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mokhtari AK, Maurer LR, Christensen MA, et al. Rhabdomyolysis in severe COVID-19: male sex, high body mass Index, and prone positioning confer high risk. J. Surg. Res. 2021;266:35-43. doi: 10.1016/j.jss.2021.03.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Papanikolaou J, Makris D, Saranteas T, et al. New insights into weaning from mechanical ventilation: left ventricular diastolic dysfunction is a key player. Intensive Care Med. 2011;37(12):1976-1985. doi: 10.1007/s00134-011-2368-0 [DOI] [PubMed] [Google Scholar]
- 23.Capdevïla XJ, Perrigault PF, Perey PJ, Roust an JPA, D’Athis F. Occlusion pressure and its ratio to Maximum inspiratory pressure are useful predictors for successful extubation following T-piece weaning trial. Chest. 1995;108(2):482-489. doi: 10.1378/chest.108.2.482 [DOI] [PubMed] [Google Scholar]
- 24.Montgomery AB, Holle RHO, Neagley SR, Pierson DJ, Schoene RB. Prediction of successful ventilator weaning using airway occlusion pressure and hypercapnic challenge. Chest. 1987;91(4):496-499. doi: 10.1378/chest.91.4.496 [DOI] [PubMed] [Google Scholar]
- 25.Baptistella AR, Sarmento FJ, Silva KR, et al. Predictive factors of weaning from mechanical ventilation and extubation outcome: a systematic review. J. Crit. Care. 2018;48:56-62. doi: 10.1016/j.jcrc.2018.08.023 [DOI] [PubMed] [Google Scholar]
- 26.Muzaffar SN, Gurjar M, Baronia AK, et al. Predictors and pattern of weaning and long-term outcome of patients with prolonged mechanical ventilation at an acute intensive care unit in north India. Rev. Bras. Ter. Intensiva. 2017;29(1):23-33. doi: 10.5935/0103-507X.20170005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu Y-K, Kao K-C, Hsu K-H, Hsieh M-J, Tsai Y-H. Predictors of successful weaning from prolonged mechanical ventilation in Taiwan. Respir. Med. 2009;103(9):1189-1195. doi: 10.1016/j.rmed.2009.02.005 [DOI] [PubMed] [Google Scholar]
- 28.Savi A, Teixeira C, Silva JM, et al. Weaning predictors do not predict extubation failure in simple-to-wean patients. J. Crit. Care. 2012;27(2):e1-221. e8. [DOI] [PubMed] [Google Scholar]