ABSTRACT
Lipid droplets (LDs) are ubiquitous organelles that store and supply lipids for energy metabolism, membrane synthesis and production of lipid-derived signaling molecules. While compositional differences in the phospholipid monolayer or neutral lipid core of LDs impact their metabolism and function, the proteome of LDs has emerged as a major influencer in all aspects of LD biology. The perilipins (PLINs) are the most studied and abundant proteins residing on the LD surface. This Cell Science at a Glance and the accompanying poster summarize our current knowledge of the common and unique features of the mammalian PLIN family of proteins, the mechanisms through which they affect cell metabolism and signaling, and their links to disease.
KEY WORDS: Lipid droplets, Lipid metabolism, Lipid signaling, Perilipins
Summary: Perilipins coat lipid droplets to regulate their metabolism and signaling, influencing disease development.
Introduction
Research on lipid droplet (LD) biology has burgeoned in the past 30 years. From these studies, we know that LDs are formed within the endoplasmic reticulum (ER) phospholipid bilayer and then bud off into the cytosol. The core of LDs is comprised largely of triacylglycerols (TAGs) and cholesterol esters (CEs) in most cell types and is surrounded by a phospholipid monolayer. In addition to the many TAGs, CEs and phospholipid species present, hundreds of other lipids are found in lower abundance (Bartz et al., 2007; Chitraju et al., 2012). LDs may undergo expansion or fusion, which contribute to the heterogeneity of LD sizes observed in most cells. Catabolism of LDs occurs through the action of cytosolic lipases or the autophagic degradation of LDs, a process termed lipophagy (Singh et al., 2009). Many studies have also identified roles for LDs beyond energy storage and shown that LDs are central mediators of many aspects of cell signaling and function, which will be discussed in depth in the following sections.
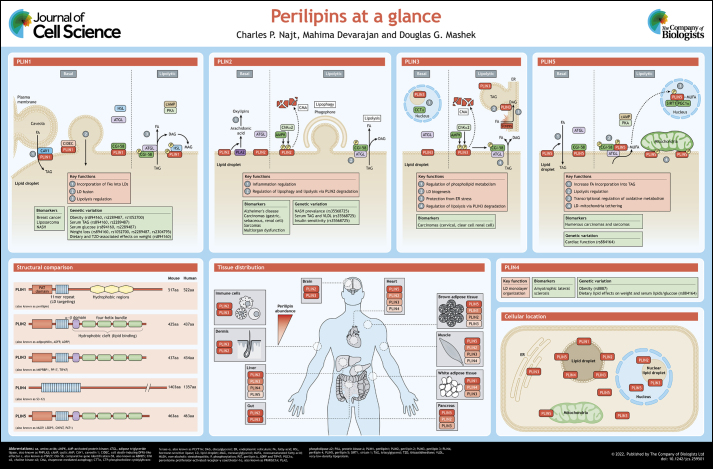
The seminal event that accelerated the field of LD biology forward was the discovery of perilipin 1 (PLIN1) – the first identified LD protein – in adipocytes (Greenberg et al., 1991). There are four splice variants of PLIN1 (PLIN1a–PLIN1d), which feature carboxyl termini of various lengths (Kimmel and Sztalryd, 2016); for the purposes of this review, we will focus on PLIN1a, which is the primary form expressed in adipocytes, unless otherwise noted. PLIN1 is a member of a larger family of perilipin proteins (PLINs) that are grouped together based on their sequence homology and their affinity for LDs. The five mammalian PLIN proteins have been numbered in order of their discovery; four of the proteins share a conserved amino-terminal perilipin A, ADRP and TIP47 (PAT) domain (PLIN1, -2, -3 and -5), and all five contain an 11mer repeat sequence of various lengths that is predicted to form amphipathic helices – human PLIN4 contains the largest, at nearly 1000 amino acids in length with 29 tandem repeats of a 33mer sequence (Kimmel et al., 2010) (see poster). The latter region, conserved in all members of the PLIN family of proteins, has been shown to direct targeting of recombinant human PLIN proteins to LDs (Rowe et al., 2016). While the 11mer repeat region anchors the PLIN proteins, it is unclear what permits selective LD localization (Giménez-Andrés et al., 2021; Hansen et al., 2017; McManaman et al., 2003; Nakamura and Fujimoto, 2003). The major isoform of PLIN1 (PLIN1a), PLIN2 and PLIN5 preferentially associate with LDs enriched in TAG, whereas shorter isoforms of PLIN 1 (PLIN1c and PLIN1d) and PLIN 4 preferentially associate with LDs enriched in CE (Hsieh et al., 2012). Retinoid-containing LDs of hepatic stellate cells and retinal pigment epithelial cells have been shown to be coated with PLIN2 and PLIN5 (Blaner et al., 2009; Orban et al., 2011). Strong hydrophobicity clearly promotes LD binding, but specificity to the LD as opposed to membrane bilayers is lost if the protein hydrophobic region is too large; this suggests that there might exist a range of optimal hydrophobicity and hydrophobic moments that enables certain PLINs to target specific LDs of a given lipid composition (Chorlay and Thiam, 2020; Hsieh et al., 2012; Prévost et al., 2018). In addition to hydrophobicity of the LD-targeting sequence, the acyl chain saturation of phospholipids affects the interaction of recombinant forms of human PLIN3 with phospholipid monolayers of varying composition in vitro (Mirheydari et al., 2016). Several studies have suggested that phospholipid packing and surface protein crowding differ between LDs and membrane bilayers, conferring unique properties that likely impact protein targeting to LDs (Bacle et al., 2017; Kory et al., 2015; Thiam et al., 2013); a detailed review of LD targeting has recently been published (Dhiman et al., 2020).
PLIN1 has an additional three sequences of hydrophobic amino acids with central proline residues in its carboxyl terminus that mediate its targeting to LDs in cultured cells independent of the 11mer repeat sequences (Garcia et al., 2003; Subramanian et al., 2004). The hydrophobic amino acids coupled to the central proline are thought to form a hydrophobic hairpin structure that embeds into the core of the LD, similar to predicted hairpin structures of other LD-targeted proteins, such as diacylglycerol O-acyltransferase 2 and long chain acyl-CoA synthetase 3 (also known as ACSL3) (Kory et al., 2016). In contrast to PLIN1, the perilipins PLIN2, PLIN3 and PLIN5 contain a carboxyl-terminal hydrophobic cleft consisting of a four-helix bundle and a unique α–β domain, which has been highlighted in the crystal structure of PLIN3 (Hickenbottom et al., 2004; PDB:1SZI) and modeled in PLIN2 (Najt et al., 2014) and PLIN5 (Najt et al., 2020). It is this proposed carboxyl-terminal structure that is responsible for binding non-membrane-associated lipids, such as cholesterol and various fatty acids (FAs) (Hickenbottom et al., 2004; Najt et al., 2014, 2020). In addition, the carboxyl-terminal region in PLIN1 and PLIN5 bind the lipase co-activator comparative gene identification-58 (CGI-58, also known as ABHD5), whereas no study has shown CGI-58 binding to PLIN2 or PLIN3 (Patel et al., 2014). While much remains to be elucidated regarding the unique structural features of the PLINs, the regions outlined above contribute to the shared and unique biological functions of each of the PLINs, which we discuss below, together with their roles in disease (see also Boxes 1 and 2).
Box 1. Expression of PLINs as disease biomarkers.
LD accumulation is typically associated with detrimental cellular effects, such as insulin resistance, mitochondrial dysfunction and inflammation, among others. Consequently, the expression of PLINs is commonly linked with a plethora of diseases, especially cancer (Cruz et al., 2020; Zhang et al., 2017). Because of their tight associations with disease prevalence, some PLINs have been proposed as biomarkers for various diseases (see poster). PLIN1 expression is diagnostic for breast cancer (Zhou et al., 2016), liposarcoma (Westhoff et al., 2017) and NASH (Carr et al., 2017). PLIN2 has been proposed as a biomarker for Alzheimer's disease (Conte et al., 2021), as well as numerous types of carcinomas (Ostler et al., 2010; Sun et al., 2020; Tolkach et al., 2017) and sarcomas (Straub et al., 2019). Serum PLIN2 is also predictive of multi-organ dysfunction in critically ill patients (Kurt et al., 2021), which is consistent with its general inflammatory effects. PLIN3 has been linked to several forms of carcinoma (Szigeti et al., 2009; Wang et al., 2018), and PLIN4 is proposed as a biomarker for amyotrophic lateral sclerosis (Zhu et al., 2021). Finally, PLIN5 has been proposed as a diagnostic marker for numerous types of carcinomas and sarcomas (Asimakopoulou et al., 2019; Hashani et al., 2018). Given the intimate relationship between the expression levels of various PLINs and the abovementioned diseases, defining the mechanisms through which these proteins contribute to disease etiology warrants research attention.
Box 2. Genetic variation of PLINs and disease.
Numerous polymorphisms of various PLINs have been identified that associate with the prevalence of several diseases or disease biomarkers (see poster). Frameshift mutations in the PLIN1 gene result in partial lipodystrophy in humans, which is characterized by an abnormal distribution of adipose tissue, highlighting the importance of this protein in systemic lipid homeostasis (Gandotra et al., 2011). Numerous variants of PLIN1 are associated with obesity (Qi et al., 2004, 2005), resistance to weight loss (Aller et al., 2017; Corella et al., 2005; Ruiz et al., 2011; Smith et al., 2008), as well as serum levels of TAG and glucose (Qi et al., 2004). In addition, the rs894160 gene variant of PLIN1 mediates the effects of diet composition on weight gain (Smith et al., 2008). Specifically, subjects with the minor allele of this variant have lower incidence of obesity when consuming diets with higher complex carbohydrate intakes, whereas in subjects with lower carbohydrate intake, the minor allele is associated with increased obesity. Similarly, the PLIN4 rs8887 gene variant is associated with obesity, and both rs8887 and rs884164 gene variants of PLIN4 differentially impact obesity and serum lipid profiles based upon dietary lipid composition (Richardson et al., 2011). The S251P variant of PLIN2 is linked to NASH development (Faulkner et al., 2020) and increased serum lipids (Magné et al., 2013), but surprisingly associates with insulin sensitivity (Sentinelli et al., 2016). An extension of the genomic repeat sequence of the PLIN4 amphipathic helices, resulting in an additional 33 amino acids, is associated with increased aggregation of PLIN4 and aggrephagy in a rare form of myopathy (autosomal-dominant progressive myopathy with rimmed ubiquitin-positive autophagic vacuolation; Ruggieri et al., 2020). While alterations in PLIN5 have not been extensively studied, the PLIN5 rs884164 gene variant is linked to alterations in cardiac function (Drevinge et al., 2016). Collectively, these studies further highlight the importance of PLINs as drivers of disease development rather than simply markers of disease presence.
PLIN1
PLIN1 is expressed primarily in adipocytes found in adipose and breast tissue, but lower levels of expression are also detected in steroidogenic cells and in hepatocytes from subjects with non-alcoholic steatohepatitis (NASH), a liver pathology consisting of fat buildup, inflammation and fibrosis (Servetnick et al., 1995; Straub et al., 2008). Perhaps the most studied function of PLIN1 is its role in lipolysis, a highly orchestrated process entailing the catabolism of esterified lipids to their FA constituents (Coleman and Mashek, 2011). In adipocytes, adipose triglyceride lipase (ATGL, also known as PNPLA2) catalyzes the initial step in the lipolytic cascade – the hydrolysis of TAG – and is followed by hormone-sensitive lipase (HSL), which facilitates diacylglycerol (DAG) hydrolysis into monoacylglycerol (MAG). ATGL is regulated by interactions with numerous proteins, including its primary co-activator CGI-58. Under basal (unstimulated) conditions, PLIN1 interacts with CGI-58 at the LD surface and renders it inaccessible to ATGL (Granneman et al., 2007) (see poster). Upon lipolytic stimulation following activation of the cyclic AMP–protein kinase A (PKA) pathway, PKA phosphorylates PLIN1 (Zhang et al., 2003) as well as CGI-58 (Sahu-Osen et al., 2015) and HSL (Anthonsen et al., 1998). Following the phosphorylation of PLIN1, its interactions with CGI-58 are disrupted, allowing CGI-58 to bind and recruit ATGL to the LD surface (Granneman et al., 2007, 2009) (see poster). Phosphorylation of PLIN1 also drives its interactions with HSL (Granneman et al., 2007; Miyoshi et al., 2007), whereby cytosolic HSL is recruited to the LD surface to increase lipolysis (Shen et al., 2009). Through its direct interaction with HSL and CGI-58, and its indirect effects on promoting ATGL activity, PLIN1 is recognized as a major regulator of lipolysis. Consistent with this role, ablation of PLIN1 leads to higher rates of basal lipolysis but blunts the effects of β-adrenergic stimulation of lipolysis (Martinez-Botas et al., 2000; Tansey et al., 2001). The higher rates of basal lipolysis are thought to drive the increase in thermogenesis and browning observed in adipose tissue in PLIN1-knockout mice (Martinez-Botas et al., 2000; Saha et al., 2004; Tansey et al., 2001). PLIN1 is also required for autophagic proteins to recognize and catabolize LDs during lipophagy, which provides an additional mechanism through which PLIN1 coordinates LD turnover (Lizaso et al., 2013). Converse to its anti-lipolytic effects, PLIN1 may aid the synthesis and growth of LDs by enhancing FA uptake. PLIN1 interacts with caveolin 1 (CAV1) in caveolae – plasma membrane invaginations that act as hubs for signaling and metabolism – to coordinate the transfer and incorporation of exogenous FAs into TAG stored within LDs (Ost et al., 2005; Simard et al., 2010) (see poster). PLIN1 also interacts with cell death-inducing DFFA-like effector c (CIDEC, also known as FSP27), a key LD protein that drives fusion of LDs, to facilitate the formation of unilocular LDs that are characteristic of white adipocytes (Sun et al., 2013).
Consistent with higher rates of basal lipolysis, PLIN1-knockout mice are resistant to diet-induced obesity (Tansey et al., 2001). Ablation of PLIN1 also reduces the expression of lipid synthetic genes and increases expression of oxidative genes, suggesting that PLIN1 can bidirectionally regulate lipid metabolism through transcriptional signaling (Castro-Chavez et al., 2003). Despite the resistance to obesity, PLIN1-knockout mice display adipose tissue inflammation and insulin resistance, which may be mediated by the recruitment of pro-inflammatory macrophages (Sohn et al., 2018). Surprisingly, overexpression of PLIN1 in adipose tissue results in reduced adipose tissue and also conveys resistance to diet-induced obesity (Miyoshi et al., 2010; Sawada et al., 2010). Although knockout and overexpression models have similar anti-obesity effects, adipose overexpression of PLIN1 improves glucose tolerance and insulin sensitivity, unlike the effects seen in mice lacking PLIN1 (Miyoshi et al., 2010). These studies suggest that PLIN1 plays a key role in balancing adipose tissue lipid homeostasis with insulin sensitivity, although the detailed mechanisms defining how PLIN1 links lipid metabolism to insulin signaling remains to be elucidated.
PLIN2
PLIN2 is ubiquitously expressed and plays a more significant role in non-adipose tissues. Although PLIN2 is not known to interact with or sequester proteins involved in lipolysis, it reduces ATGL localization to LDs and slows rates of lipolysis (Listenberger et al., 2007; Sapiro et al., 2009). While the exact mechanism explaining this reduced lipolysis is not known, PLIN2 may impact other LD-targeted proteins through its alteration of the phospholipid monolayer; indeed, PLIN2 interacts with the components of the monolayer but not the neutral core of LDs (McIntosh et al., 2012; Storey et al., 2011). PLIN2 interactions with the phospholipid monolayer may determine which proteins are able to target the LD; therefore, PLIN2 turnover is a key determinant of protein targeting and LD metabolism. Under nutrient deprivation, AMP-activated protein kinase (AMPK) becomes activated and catalyzes the phosphorylation of PLIN2 (Kaushik and Cuervo, 2016) (see poster). This modification leads to the recognition of PLIN2 by the heat shock cognate protein of 70 kDa (HSC70, also known as HSPA8), which facilitates the degradation of PLIN2 via chaperone-mediated autophagy (CMA). PLIN2 is also phosphorylated by choline kinase α2 (encoded by CHKA) in response to glucose deprivation, leading to CMA-mediated degradation (Liu et al., 2021). The loss of PLIN2 allows ATGL and proteins involved in lipophagy to access LDs and initiate the hydrolysis of lipid stores (Kaushik and Cuervo, 2015; Liu et al., 2021). In addition, amino-terminal acetylation of non-LD-bound PLIN2 promotes its ubiquitylation and subsequent degradation when insufficient LDs are available for stabilization of the protein (Nguyen et al., 2019). Thus, degradation of PLIN2 appears to be a key node in regulating LD catabolism.
Loss-of-function studies have highlighted various physiological roles of PLIN2. The first mouse knockout model of PLIN2 showed reduced hepatic TAG levels and protection against hepatic steatosis, but not protection from obesity (Chang et al., 2006). However, interpretation of the findings from this model was complicated by the discovery of an incomplete deletion of PLIN2 resulting in expression of a truncated form of the protein. A subsequent global knockout of PLIN2 with no truncated form validated the original finding that PLIN2 ablation leads to reduced hepatic TAG levels, but also revealed a resistance to diet-induced obesity (Orlicky et al., 2018). Upon high-carbohydrate feeding, PLIN2-knockout mice exhibit increased body temperature and browning of subcutaneous adipose tissue, which could also contribute to the obesity resistance (Libby et al., 2018). Further studies have shown that PLIN2-knockout mice display reduced enterocyte LDs in response to dietary lipids, lower lipid absorption, increased fecal lipid content, as well as alterations in the gut microbiome (Frank et al., 2015; Xiong et al., 2017). Taken together, these results suggest that PLIN2 in the intestine may contribute to systemic energy metabolism. Perhaps the most studied effects of PLIN2 loss have been in the liver, where tissue-specific deletion of PLIN2 robustly reduces diet- or genetically-driven steatosis, inflammation and fibrosis (Griffin et al., 2021; Imai et al., 2007, 2012; Najt et al., 2016). Moreover, PLIN2 ablation alters phospholipid metabolism and signaling to promote very-low-density lipoprotein (VLDL) secretion from mouse liver (Magnusson et al., 2006; Martínez-Uña et al., 2015; Najt et al., 2016; Tsai et al., 2017), enhance FA oxidation (Griffin et al., 2021; Najt et al., 2016; Tsai et al., 2017) and suppress de novo lipogenesis (Libby et al., 2016; Varela et al., 2008), all of which may contribute to the effects of reduced PLIN2 expression in lowering levels of TAG. As expected, overexpression of PLIN2 leads to LD accumulation in numerous tissues including heart (Ueno et al., 2017), liver (Tsai et al., 2017) and muscle (Bosma et al., 2012).
While many mechanisms likely underlie the different effects of PLIN2, its role in inflammation is undoubtedly a major contributor (see poster). PLIN2 expression and the coinciding accumulation of LDs are increased in response to lipopolysaccharide (LPS) or other inflammatory stimuli (Khatchadourian et al., 2012; Llorente-Cortés et al., 2007; Wang et al., 2012). PLIN2 expression is also increased in tissues or cells characterized by chronic inflammation, including microglia from aged mice (Marschallinger et al., 2020), foam cell and atherosclerotic plaques (Paul et al., 2008), non-alcoholic steatohepatitic livers (Carr et al., 2017) and numerous cancers (Cruz et al., 2020; Straub et al., 2010). PLIN2 ablation attenuates, and overexpression exacerbates, LPS-mediated inflammation (Cho and Kang, 2015; Wang et al., 2012). Tissue-specific or global deletion of PLIN2 broadly reduces inflammation associated with obesogenic diets (McManaman et al., 2013; Najt et al., 2016) and protects against atherosclerosis (Paul et al., 2008). Further linking PLIN2 and inflammation, recombinant murine PLIN2 binds various lipids but shows the highest affinity for cholesterol and arachidonic acid (Najt et al., 2014). Cholesterol and its metabolites are known to have pro-inflammatory effects (Tall and Yvan-Charvet, 2015), and arachidonic acid is the initial substrate for the generation of eicosanoids, a diverse family of oxidized lipids (oxylipins) that drive inflammation. As LDs have emerged as major sites of eicosanoid generation (Accioly et al., 2008; Dichlberger et al., 2016; Moreira et al., 2009), these data implicate a potential role of PLIN2 as a key player in this important lipid-mediated signaling pathway. PLIN2 ablation, likely through enhanced lipolysis and FA mobilization, also commonly increases signaling through the PPAR family of transcription factors (Bosma et al., 2012; Feng et al., 2017; Sapiro et al., 2009). While the PPARs have profound effects on metabolism, they also increase the expression of numerous anti-inflammatory genes (Straus and Glass, 2007), which may also contribute to the beneficial effects of PLIN2 ablation.
PLIN3
PLIN3 is ubiquitously expressed and plays significant roles in the formation of TAG and LD biogenesis (Bulankina et al., 2009; Nose et al., 2013; Wolins et al., 2001). PLIN3 is rapidly recruited to LDs upon FA exposure, and knockdown of PLIN3 attenuates LD formation (Bulankina et al., 2009; Wolins et al., 2001). PLIN3 binding to DAG, which is enriched at sites of LD biogenesis on the ER, is instrumental to this recruitment (Skinner et al., 2009) (see poster). Consistent with its role in LD formation, PLIN3 knockdown prevents diet-induced hepatic steatosis and reduces VLDL secretion (Carr et al., 2012; Ferguson et al., 2017). Interestingly, PLIN3 is also required for replication of hepatitis C virus and the steatosis associated with viral infection (Ferguson et al., 2017; Ploen et al., 2013; Vogt et al., 2013). Another role of PLIN3 is to help protect cells from ER stress and apoptosis in response to exogenous FAs or alcohol, both of which promote TAG synthesis (Gu et al., 2019; Urahama et al., 2008). This is in line with earlier findings showing that FA incorporation into LDs is important to prevent ER stress and lipotoxicity (see poster; Listenberger et al., 2003). A further role of PLIN3 is to regulate phospholipid metabolism. Specifically, PLIN3 competes with CTP:phosphocholine cytidylyltransferase-α (CCTα, also known as PCYT1A), which catalyzes a key step in phosphatidylcholine synthesis, for binding to nucleoplasmic LDs as a means to antagonize phosphatidylcholine synthesis (Sołtysik et al., 2019) (see poster). Interestingly, this competition occurs on nuclear LDs, which have only been recently identified and are the subject of intense research (Uzbekov and Roingeard, 2013). Thus, PLIN3 impacts TAG synthesis and LD biogenesis through numerous mechanisms.
Unlike PLIN1 and PLIN5, the effects of PLIN3 on lipolysis are less clear. Several studies suggest that PLIN3 ablation enhances lipolysis and can activate PPAR-α (also known as PPARA) and oxidative metabolism similar to PLIN2 ablation (Bell et al., 2008; Lee et al., 2018). In support of these studies, and like PLIN2, PLIN3 is phosphorylated by AMPK and choline kinase α2 and is subsequently degraded via CMA in fasting conditions to allow LD degradation (Kaushik and Cuervo, 2015; Liu et al., 2021) (see poster). PLIN3 abundance in muscle correlates with FA oxidation and exercise training (Chow et al., 2017; Covington et al., 2014, 2015; MacPherson et al., 2013; Shepherd et al., 2017). Collectively, these data suggest that PLIN3 promotes LD biogenesis and protects LDs from degradation until lipolytic stimuli are present, at which time it is degraded to allow for LD catabolism.
PLIN4
PLIN4 is the least studied member of the PLIN family. In adipocytes, PLIN4 is rapidly recruited to LDs upon FA exposure (Wolins et al., 2003), and its expression is increased during adipocyte differentiation (Nimura et al., 2015; Wolins et al., 2005). Global deletion of PLIN4 does not affect adipose tissue but does lower cardiac TAG levels; however, cardiac PLIN5 expression is also reduced in this model, making it difficult to ascertain whether these effects are direct or indirect (Chen et al., 2013). PLIN4 also appears to be critical for phospholipid monolayer organization (Čopič et al., 2018). The oversized 11mer repeat region of the protein aids in stabilizing PLIN4 at the LD surface (Giménez-Andrés et al., 2021). PLIN4 function has also been associated with neurodegenerative disease (see Box 1); its levels are increased in neurons in an animal model of Parkinson's disease and its downregulation in cultured neurons reduces LDs and restores mitophagy to overcome mitochondrial damage and dysfunction (Han et al., 2018).
PLIN5
PLIN5 is arguably the most dynamic of the PLIN proteins as it appears to have a multitude of cellular functions. PLIN5 shows the highest expression in highly oxidative tissues, such as brown adipose tissue, heart, muscle and liver tissue, and is induced in response to exercise training (Louche et al., 2013; Shepherd et al., 2017) or fasting (Gemmink et al., 2016; Zhang et al., 2020). PLIN5 also regulates lipolysis through its direct interactions with ATGL and CGI-58 (see poster). Under basal conditions, PLIN5 sequesters CGI-58 to prevent its interaction with ATGL, similar to PLIN1 in adipose tissue (Granneman et al., 2009, 2011; Wang et al., 2011). In support of these mechanistic findings, global deletion of PLIN5 reduces LD accumulation in numerous oxidative tissues (Kuramoto et al., 2014; Mason et al., 2014; Wang et al., 2015), whereas overexpression of PLIN5 commonly promotes LD accumulation (Harris et al., 2015; Pollak et al., 2013). Despite its anti-lipolytic effects under basal conditions, PLIN5 enhances lipolysis under PKA-stimulated conditions (Pollak et al., 2014; Wang et al., 2011). Indeed, PKA-mediated phosphorylation of murine PLIN5 at Ser155 is a critical modification that alters its binding to ATGL, resulting in a switch from anti-lipolytic to pro-lipolytic activity (Keenan et al., 2021).
PLIN5 expression is tightly linked to mitochondrial biogenesis and oxidative metabolism across tissues (Mason and Watt, 2015). There appear to be at least two mechanisms underlying this association. First, PLIN5 directly interacts with mitochondria through its carboxyl-terminal 20 amino acids, and acts as a tether between LDs and mitochondria (Wang et al., 2011) (see poster). This association increases in response to PKA signaling, similar to fasting, and is dependent on phosphorylation at Ser155 (Keenan et al., 2021). It has been proposed that these bridges would exist to allow for the direct transfer of FAs from LDs to mitochondria for oxidation during nutrient-deprived conditions (Rambold et al., 2015). However, other studies refute these findings and instead suggest that PLIN5 and LD–mitochondria interactions exist to facilitate lipid anabolic pathways, including TAG synthesis (Benador et al., 2018). In support, PLIN5 increases FA incorporation into TAG (Montgomery et al., 2019; Trevino et al., 2015; Zhang et al., 2020). Second, PLIN5 can facilitate mitochondrial biogenesis and oxidative metabolism by playing a key role as a transcriptional co-regulator. In response to PKA signaling and phosphorylation on Ser155, PLIN5 binds lipolytically derived FAs, especially monounsaturated FAs (MUFAs), and traffics them to the nucleus (Najt et al., 2020). Once inside the nucleus, PLIN5 forms a complex with peroxisome proliferator-activated receptor-γ coactivator-1α (PGC1α, also known as PPARGC1A) and sirtuin 1 (SIRT1) (Gallardo-Montejano et al., 2016; Najt et al., 2020), the latter of which is allosterically activated by the PLIN5-delivered MUFAs. SIRT1 is known to deacetylate PGC1α, which promotes mitochondrial biogenesis and oxidative metabolism in part through its co-activation of the transcription factor PPAR-α (Rodgers et al., 2005). SIRT1–PGC1α–PPARα-dependent signaling also regulates other key cellular pathways, including the induction of autophagy and suppression of inflammation (Zhang et al., 2020). Detailed studies have yet to parse apart the distinct contributions of the LD–mitochondria-tethering role and the transcriptional regulatory role of PLIN5 to its many downstream effects.
Conclusions and future perspectives
The PLINs have emerged as a key family of proteins orchestrating cell signaling and metabolism, well beyond their classically viewed roles as LD structural proteins that simply govern lipolysis. A large body of literature highlights links between PLINs, as well as other LD-associated proteins and lipids, and an expanding list of diseases. Despite these advances in our understanding of the PLINs, there are many aspects of PLIN biology that remain scantly explored. These include but are not limited to their unique structural aspects, including their role in lipid binding, and how alterations (expression level, post-translational modifications, variants) of PLINs alter LD metabolism and/or signaling and cell function. The links between LD accumulation and alterations in lipid metabolism in many metabolic and aging-related diseases justify future studies to further characterize the PLINs, identify novel mechanisms through which they impact cell and tissue homeostasis, and develop genetic or pharmaceutical approaches to manipulate their function to alleviate disease.
Poster Panels
Acknowledgements
The authors are grateful to our many colleagues and collaborators for their insightful discussions that aided in writing this Cell Science at a Glance article. We apologize to those whose work we have been unable to cite due to space restrictions.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Funding
Our work in this area is supported by grants to D.G.M. from the National Institutes of Health (AG069768, DK114401 and AG055452), and Eli Lilly and Company. C.P.N. was supported by grants from the National Institutes of Health (K99AG070104) and American Heart Association (20POST35180115). M.D. is supported by a National Institutes of Health grant AG029796. Deposited in PMC for release after 12 months.
Cell science at a glance
Individual poster panels are available for downloading at https://journals.biologists.com/jcs/article-lookup/doi/10.1242/jcs.259501#supplementary-data
References
- Accioly, M. T., Pacheco, P., Maya-Monteiro, C. M., Carrossini, N., Robbs, B. K., Oliveira, S. S., Kaufmann, C., Morgado-Diaz, J. A., Bozza, P. T. and Viola, J. P. B. (2008). Lipid bodies are reservoirs of cyclooxygenase-2 and sites of prostaglandin-E2 synthesis in colon cancer cells. Cancer Res. 68, 1732-1740. 10.1158/0008-5472.CAN-07-1999 [DOI] [PubMed] [Google Scholar]
- Aller, E. E. J. G., Mariman, E. C. M., Bouwman, F. G. and Van Baak, M. A. (2017). Genetic predictors of ≥5% weight loss by multidisciplinary advice to severely obese subjects. J. Nutrigenet. Nutrigenomics 10, 32-42. 10.1159/000469662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthonsen, M. W., Rönnstrand, L., Wernstedt, C., Degerman, E. and Holm, C. (1998). Identification of novel phosphorylation sites in hormone-sensitive lipase that are phosphorylated in response to isoproterenol and govern activation properties in vitro. J. Biol. Chem. 273, 215-221. 10.1074/jbc.273.1.215 [DOI] [PubMed] [Google Scholar]
- Asimakopoulou, A., Vucur, M., Luedde, T., Schneiders, S., Kalampoka, S., Weiss, T. S. and Weiskirchen, R. (2019). Perilipin 5 and lipocalin 2 expression in hepatocellular carcinoma. Cancers (Basel) 11, 385. 10.3390/cancers11030385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacle, A., Gautier, R., Jackson, C. L., Fuchs, P. F. J. and Vanni, S. (2017). Interdigitation between triglycerides and lipids modulates surface properties of lipid droplets. Biophys. J. 112, 1417-1430. 10.1016/j.bpj.2017.02.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartz, R., Li, W.-H., Venables, B., Zehmer, J. K., Roth, M. R., Welti, R., Anderson, R. G. W., Liu, P. and Chapman, K. D. (2007). Lipidomics reveals that adiposomes store ether lipids and mediate phospholipid traffic. J. Lipid Res. 48, 837-847. 10.1194/jlr.M600413-JLR200 [DOI] [PubMed] [Google Scholar]
- Bell, M., Wang, H., Chen, H., McLenithan, J. C., Gong, D.-W., Yang, R.-Z., Yu, D., Fried, S. K., Quon, M. J., Londos, C.et al. (2008). Consequences of lipid droplet coat protein downregulation in liver cells: abnormal lipid droplet metabolism and induction of insulin resistance. Diabetes 57, 2037-2045. 10.2337/db07-1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benador, I. Y., Veliova, M., Mahdaviani, K., Petcherski, A., Wikstrom, J. D., Assali, E. A., Acín-Pérez, R., Shum, M., Oliveira, M. F., Cinti, S.et al. (2018). Mitochondria bound to lipid droplets have unique bioenergetics, composition, and dynamics that support lipid droplet expansion. Cell Metab. 27, 869-885.e6. 10.1016/j.cmet.2018.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaner, W. S., O'Byrne, S. M., Wongsiriroj, N., Kluwe, J., D'Ambrosio, D. M., Jiang, H., Schwabe, R. F., Hillman, E. M. C., Piantedosi, R. and Libien, J. (2009). Hepatic stellate cell lipid droplets: a specialized lipid droplet for retinoid storage. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1791, 467-473. 10.1016/j.bbalip.2008.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosma, M., Hesselink, M. K. C., Sparks, L. M., Timmers, S., Ferraz, M. J., Mattijssen, F., Van Beurden, D., Schaart, G., De Baets, M. H., Verheyen, F. K.et al. (2012). Perilipin 2 improves insulin sensitivity in skeletal muscle despite elevated intramuscular lipid levels. Diabetes 61, 2679-2690. 10.2337/db11-1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulankina, A. V., Deggerich, A., Wenzel, D., Mutenda, K., Wittmann, J. G., Rudolph, M. G., Burger, K. N. J. and Höning, S. (2009). TIP47 functions in the biogenesis of lipid droplets. J. Cell Biol. 185, 641-655. 10.1083/jcb.200812042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr, R. M., Patel, R. T., Rao, V., Dhir, R., Graham, M. J., Crooke, R. M. and Ahima, R. S. (2012). Reduction of TIP47 improves hepatic steatosis and glucose homeostasis in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 302, R996-R1003. 10.1152/ajpregu.00177.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr, R. M., Dhir, R., Mahadev, K., Comerford, M., Chalasani, N. P. and Ahima, R. S. (2017). Perilipin staining distinguishes between steatosis and non-alcoholic steatohepatitis in adults and children. Clin. Gastroenterol. Hepatol. 15, 145-147. 10.1016/j.cgh.2016.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Chavez, F., Yechoor, V. K., Saha, P. K., Martinez-Botas, J., Wooten, E. C., Sharma, S., O'Connell, P., Taegtmeyer, H. and Chan, L. (2003). Coordinated upregulation of oxidative pathways and downregulation of lipid biosynthesis underlie obesity resistance in perilipin knockout mice: a microarray gene expression profile. Diabetes 52, 2666-2674. 10.2337/diabetes.52.11.2666 [DOI] [PubMed] [Google Scholar]
- Chang, B. H.-J., Li, L., Paul, A., Taniguchi, S., Nannegari, V., Heird, W. C. and Chan, L. (2006). Protection against fatty liver but normal adipogenesis in mice lacking adipose differentiation-related protein. Mol. Cell. Biol. 26, 1063-1076. 10.1128/MCB.26.3.1063-1076.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, W., Chang, B., Wu, X., Li, L., Sleeman, M. and Chan, L. (2013). Inactivation of Plin4 downregulates Plin5 and reduces cardiac lipid accumulation in mice. Am. J. Physiol. Endocrinol. Metab. 304, E770-E779. 10.1152/ajpendo.00523.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitraju, C., Trötzmüller, M., Hartler, J., Wolinski, H., Thallinger, G. G., Lass, A., Zechner, R., Zimmermann, R., Köfeler, H. C. and Spener, F. (2012). Lipidomic analysis of lipid droplets from murine hepatocytes reveals distinct signatures for nutritional stress. J. Lipid Res. 53, 2141-2152. 10.1194/jlr.M028902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho, K.-A. and Kang, P. B. (2015). PLIN2 inhibits insulin-induced glucose uptake in myoblasts through the activation of the NLRP3 inflammasome. Int. J. Mol. Med. 36, 839-844. 10.3892/ijmm.2015.2276 [DOI] [PubMed] [Google Scholar]
- Chorlay, A. and Thiam, A. R. (2020). Neutral lipids regulate amphipathic helix affinity for model lipid droplets. J. Cell Biol. 219, e201907099. 10.1083/jcb.201907099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow, L. S., Mashek, D. G., Wang, Q., Shepherd, S. O., Goodpaster, B. H. and Dubé, J. J. (2017). Effect of acute physiological free fatty acid elevation in the context of hyperinsulinemia on fiber type-specific IMCL accumulation. J. Appl. Physiol. 123, 71-78. 10.1152/japplphysiol.00209.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman, R. A. and Mashek, D. G. (2011). Mammalian triacylglycerol metabolism: Synthesis, lipolysis, and signaling. Chem. Rev. 111, 6359-6386. 10.1021/cr100404w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte, M., Medici, V., Malagoli, D., Chiariello, A., Cirrincione, A., Davin, A., Chikhladze, M., Vasuri, F., Legname, G., Ferrer, I.et al. (2021). Expression pattern of perilipins in human brain during aging and in Alzheimer's disease. Neuropathol. Appl. Neurobiol. 48, e12756. 10.1111/nan.12756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Čopič, A., Antoine-Bally, S., Giménez-Andrés, M., La Torre Garay, C., Antonny, B., Manni, M. M., Pagnotta, S., Guihot, J. and Jackson, C. L. (2018). A giant amphipathic helix from a perilipin that is adapted for coating lipid droplets. Nat. Commun. 9, 1332. 10.1038/s41467-018-03717-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corella, D., Qi, L., Sorlí, J. V., Godoy, D., Portolés, O., Coltell, O., Greenberg, A. S. and Ordovas, J. M. (2005). Obese subjects carrying the 11482G>A polymorphism at the perilipin locus are resistant to weight loss after dietary energy restriction. J. Clin. Endocrinol. Metab. 90, 5121-5126. 10.1210/jc.2005-0576 [DOI] [PubMed] [Google Scholar]
- Covington, J. D., Galgani, J. E., Moro, C., LaGrange, J.-M., Zhang, Z., Rustan, A. C., Ravussin, E. and Bajpeyi, S. (2014). Skeletal muscle perilipin 3 and coatomer proteins are increased following exercise and are associated with fat oxidation. PLoS ONE 9, e91675. 10.1371/journal.pone.0091675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covington, J. D., Noland, R. C., Hebert, R. C., Masinter, B. S., Smith, S. R., Rustan, A. C., Ravussin, E. and Bajpeyi, S. (2015). Perilipin 3 differentially regulates skeletal muscle lipid oxidation in active, sedentary, and type 2 diabetic males. J. Clin. Endocrinol. Metab. 100, 3683-3692. 10.1210/JC.2014-4125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz, A. L. S., Barreto, E. de A., Fazolini, N. P. B., Viola, J. P. B. and Bozza, P. T. (2020). Lipid droplets: platforms with multiple functions in cancer hallmarks. Cell Death Dis. 11, 105. 10.1038/s41419-020-2297-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhiman, R., Caesar, S., Thiam, A. R. and Schrul, B. (2020). Mechanisms of protein targeting to lipid droplets: a unified cell biological and biophysical perspective. Semin. Cell Dev. Biol. 108, 4-13. 10.1016/j.semcdb.2020.03.004 [DOI] [PubMed] [Google Scholar]
- Dichlberger, A., Schlager, S., Kovanen, P. T. and Schneider, W. J. (2016). Lipid droplets in activated mast cells – a significant source of triglyceride-derived arachidonic acid for eicosanoid production. Eur. J. Pharmacol. 785, 59-69. 10.1016/j.ejphar.2015.07.020 [DOI] [PubMed] [Google Scholar]
- Drevinge, C., Dalen, K. T., Mannila, M. N., Täng, M. S., Ståhlman, M., Klevstig, M., Lundqvist, A., Mardani, I., Haugen, F., Fogelstrand, P.et al. (2016). Perilipin 5 is protective in the ischemic heart. Int. J. Cardiol. 219, 446-454. 10.1016/j.ijcard.2016.06.037 [DOI] [PubMed] [Google Scholar]
- Faulkner, C. S., White, C. M., Shah, V. H. and Jophlin, L. L. (2020). A single nucleotide polymorphism of PLIN2 is associated with nonalcoholic steatohepatitis and causes phenotypic changes in hepatocyte lipid droplets: a pilot study. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1865, 158637. 10.1016/j.bbalip.2020.158637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng, Y. Z., Lund, J., Li, Y., Knabenes, I. K., Bakke, S. S., Kase, E. T., Lee, Y. K., Kimmel, A. R., Thoresen, G. H., Rustan, A. C.et al. (2017). Loss of perilipin 2 in cultured myotubes enhances lipolysis and redirects the metabolic energy balance from glucose oxidation towards fatty acid oxidation. J. Lipid Res. 58, 2147-2161. 10.1194/jlr.M079764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson, D., Zhang, J., Davis, M. A., Helsley, R. N., Vedin, L.-L., Lee, R. G., Crooke, R. M., Graham, M. J., Allende, D. S., Parini, P.et al. (2017). The lipid droplet-associated protein perilipin 3 facilitates hepatitis C virus-driven hepatic steatosis. J. Lipid Res. 58, 420-432. 10.1194/jlr.M073734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank, D. N., Bales, E. S., Monks, J., Jackman, M. J., MacLean, P. S., Ir, D., Robertson, C. E., Orlicky, D. J. and McManaman, J. L. (2015). Perilipin-2 modulates lipid absorption and microbiome responses in the mouse intestine. PLoS ONE 10, e0131944. 10.1371/journal.pone.0131944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallardo-Montejano, V. I., Saxena, G., Kusminski, C. M., Yang, C., McAfee, J. L., Hahner, L., Hoch, K., Dubinsky, W., Narkar, V. A. and Bickel, P. E. (2016). Nuclear Perilipin 5 integrates lipid droplet lipolysis with PGC-1α/SIRT1-dependent transcriptional regulation of mitochondrial function. Nat. Commun. 7, 12723. 10.1038/ncomms12723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandotra, S., Le Dour, C., Bottomley, W., Cervera, P., Giral, P., Reznik, Y., Charpentier, G., Auclair, M., Delépine, M., Barroso, I.et al. (2011). Perilipin deficiency and autosomal dominant partial lipodystrophy. N. Engl. J. Med. 364, 740-748. 10.1056/NEJMoa1007487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia, A., Sekowski, A., Subramanian, V. and Brasaemle, D. L. (2003). The central domain is required to target and anchor perilipin A to lipid droplets. J. Biol. Chem. 278, 625-635. 10.1074/jbc.M206602200 [DOI] [PubMed] [Google Scholar]
- Gemmink, A., Bosma, M., Kuijpers, H. J. H., Hoeks, J., Schaart, G., van Zandvoort, M. A. M. J., Schrauwen, P. and Hesselink, M. K. C. (2016). Decoration of intramyocellular lipid droplets with PLIN5 modulates fasting-induced insulin resistance and lipotoxicity in humans. Diabetologia 59, 1040-1048. 10.1007/s00125-016-3865-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giménez-Andrés, M., Emeršič, T., Antoine-Bally, S., D'ambrosio, J. M., Antonny, B., Derganc, J. and Čopič, A. (2021). Exceptional stability of a perilipin on lipid droplets depends on its polar residues, suggesting multimeric assembly. eLife 10, e61401. 10.7554/eLife.61401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granneman, J. G., Moore, H.-P. H., Granneman, R. L., Greenberg, A. S., Obin, M. S. and Zhu, Z. (2007). Analysis of lipolytic protein trafficking and interactions in adipocytes. J. Biol. Chem. 282, 5726-5735. 10.1074/jbc.M610580200 [DOI] [PubMed] [Google Scholar]
- Granneman, J. G., Moore, H.-P. H., Mottillo, E. P. and Zhu, Z. (2009). Functional interactions between Mldp (LSDP5) and Abhd5 in the control of intracellular lipid accumulation. J. Biol. Chem. 284, 3049-3057. 10.1074/jbc.M808251200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granneman, J. G., Moore, H.-P. H., Mottillo, E. P., Zhu, Z., Zhou, L., Granneman, J. G., Moore, H.-P. H., Mottillo, E. P., Zhu, Z. and Zhou, L. (2011). Interactions of perilipin-5 (plin5) with adipose triglyceride lipase. J. Biol. Chem. 286, 5126-5135. 10.1074/jbc.M110.180711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg, A. S., Egan, J. J., Wek, S. A., Garty, N. B., Blanchette-Mackie, E. J. and Londos, C. (1991). Perilipin, a major hormonally regulated adipocyte-specific phosphoprotein associated with the periphery of lipid storage droplets. J. Biol. Chem. 266, 11341-11346. 10.1016/S0021-9258(18)99168-4 [DOI] [PubMed] [Google Scholar]
- Griffin, J. D., Bejarano, E., Wang, X.-D. and Greenberg, A. S. (2021). Integrated action of autophagy and adipose tissue triglyceride lipase ameliorates diet-induced hepatic steatosis in liver-specific PLIN2 knockout mice. Cells 10, 1016. 10.3390/cells10051016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu, Y., Yang, Y., Cao, X., Zhao, Y., Gao, X., Sun, C., Zhang, F., Yuan, Y., Xu, Y., Zhang, J.et al. (2019). Plin3 protects against alcoholic liver injury by facilitating lipid export from the endoplasmic reticulum. J. Cell. Biochem. 120, 16075-16087. 10.1002/jcb.28889 [DOI] [PubMed] [Google Scholar]
- Han, X., Zhu, J., Zhang, X., Song, Q., Ding, J., Lu, M., Sun, S. and Hu, G. (2018). Plin4-dependent lipid droplets hamper neuronal mitophagy in the MPTP/p-induced mouse model of Parkinson's disease. Front. Neurosci. 12, 397. 10.3389/fnins.2018.00397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen, J. S., De Maré, S., Jones, H. A., Göransson, O. and Lindkvist-Petersson, K. (2017). Visualization of lipid directed dynamics of perilipin 1 in human primary adipocytes. Sci. Rep. 7, 15011. 10.1038/s41598-017-15059-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, L.-A. L. S., Skinner, J. R., Shew, T. M., Pietka, T. A., Abumrad, N. A. and Wolins, N. E. (2015). Perilipin 5-driven lipid droplet accumulation in skeletal muscle stimulates the expression of fibroblast growth factor 21. Diabetes 64, 2757-2768. 10.2337/db14-1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashani, M., Witzel, H. R., Pawella, L. M., Lehmann-Koch, J., Schumacher, J., Mechtersheimer, G., Schnölzer, M., Schirmacher, P., Roth, W. and Straub, B. K. (2018). Widespread expression of perilipin 5 in normal human tissues and in diseases is restricted to distinct lipid droplet subpopulations. Cell Tissue Res. 374, 121-136. 10.1007/s00441-018-2845-7 [DOI] [PubMed] [Google Scholar]
- Hickenbottom, S. J., Kimmel, A. R., Londos, C. and Hurley, J. H. (2004). Structure of a lipid droplet protein: The PAT family member TIP47. Structure 12, 1199-1207. 10.1016/j.str.2004.04.021 [DOI] [PubMed] [Google Scholar]
- Hsieh, K., Lee, Y. K., Londos, C., Raaka, B. M., Dalen, K. T. and Kimmel, A. R. (2012). Perilipin family members preferentially sequester to either triacylglycerol-specific or cholesteryl-esterspecific intracellular lipid storage droplets. J. Cell Sci. 125, 4067-4076. 10.1242/jcs.104943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai, Y., Varela, G. M., Jackson, M. B., Graham, M. J., Crooke, R. M. and Ahima, R. S. (2007). Reduction of hepatosteatosis and lipid levels by an adipose differentiation-related protein antisense oligonucleotide. Gastroenterology 132, 1947-1954. 10.1053/j.gastro.2007.02.046 [DOI] [PubMed] [Google Scholar]
- Imai, Y., Boyle, S., Varela, G. M., Caron, E., Yin, X., Dhir, R., Dhir, R., Graham, M. J. and Ahima, R. S. (2012). Effects of perilipin 2 antisense oligonucleotide treatment on hepatic lipid metabolism and gene expression. Physiol. Genomics 44, 1125-1131. 10.1152/physiolgenomics.00045.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushik, S. and Cuervo, A. M. (2015). Degradation of lipid droplet-associated proteins by chaperone-mediated autophagy facilitates lipolysis. Nat. Cell Biol. 17, 759-770. 10.1038/ncb3166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushik, S. and Cuervo, A. M. (2016). AMPK-dependent phosphorylation of lipid droplet protein PLIN2 triggers its degradation by CMA. Autophagy 12, 432-438. 10.1080/15548627.2015.1124226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan, S. N., De Nardo, W., Ou, J., Schittenhelm, R. B., Montgomery, M. K., Granneman, J. G., Hinde, E. and Watt, M. J. (2021). Perilipin 5 S155 phosphorylation by PKA is required for the control of hepatic lipid metabolism and glycemic control. J. Lipid Res. 62, 100016. 10.1194/jlr.RA120001126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatchadourian, A., Bourque, S. D., Richard, V. R., Titorenko, V. I. and Maysinger, D. (2012). Dynamics and regulation of lipid droplet formation in lipopolysaccharide (LPS)-stimulated microglia. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1821, 607-617. 10.1016/j.bbalip.2012.01.007 [DOI] [PubMed] [Google Scholar]
- Kimmel, A. R. and Sztalryd, C. (2016). The perilipins: major cytosolic lipid droplet-associated proteins and their roles in cellular lipid storage, mobilization, and systemic homeostasis. Annu. Rev. Nutr. 36, 471-509. 10.1146/annurev-nutr-071813-105410 [DOI] [PubMed] [Google Scholar]
- Kimmel, A. R., Brasaemle, D. L., McAndrews-Hill, M., Sztalryd, C. and Londos, C. (2010). Adoption of PERILIPIN as a unifying nomenclature for the mammalian PAT-family of intracellular lipid storage droplet proteins. J. Lipid Res. 51, 468-471. 10.1194/jlr.R000034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kory, N., Thiam, A.-R., Farese, R. V. and Walther, T. C. (2015). Protein crowding is a determinant of lipid droplet protein composition. Dev. Cell 34, 351-363. 10.1016/j.devcel.2015.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kory, N., Farese, R. V. and Walther, T. C. (2016). Targeting fat: mechanisms of protein localization to lipid droplets. Trends Cell Biol. 26, 535-546. 10.1016/j.tcb.2016.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramoto, K., Sakai, F., Yoshinori, N., Nakamura, T. Y., Wakabayashi, S., Kojidani, T., Haraguchi, T., Hirose, F. and Osumi, T. (2014). Deficiency of a lipid droplet protein, Perilipin 5, suppresses myocardial lipid accumulation, thereby preventing type 1 diabetes-induced heart malfunction. Mol. Cell. Biol. 34, 2721-2731. 10.1128/MCB.00133-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurt, B., Buendgens, L., Wirtz, T. H., Loosen, S. H., Schulze-Hagen, M., Truhn, D., Brozat, J. F., Jhaisha, S. A., Hohlstein, P., Koek, G.et al. (2021). Serum perilipin 2 (Plin2) predicts multiple organ dysfunction in critically ill patients. Biomedicines 9, 1210. 10.3390/biomedicines9091210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, Y. K., Sohn, J. H., Han, J. S., Park, Y. J., Jeon, Y. G., Ji, Y., Dalen, K. T., Sztalryd, C., Kimmel, A. R. and Kim, J. B. (2018). Perilipin 3 deficiency stimulates thermogenic beige adipocytes through PPARα activation. Diabetes 67, 791-804. 10.2337/db17-0983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby, A. E., Bales, E. S., Orlicky, D. J. and McManaman, J. L. (2016). Perilipin-2 deletion impairs hepatic lipid accumulation by interfering with SREBP activation and altering the hepatic lipidome. J. Biol. Chem. 291, 24231-24246. 10.1074/jbc.M116.759795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby, A. E., Bales, E. S., Monks, J., Orlicky, D. J. and McManaman, J. L. (2018). Perilipin-2 deletion promotes carbohydrate-mediated browning of white adipose tissue at ambient temperature. J. Lipid Res. 59, 1482-1500. 10.1194/jlr.M086249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Listenberger, L. L., Han, X., Lewis, S. E., Cases, S., Farese, R. V., Jr, Ory, D. S. and Schaffer, J. E. (2003). Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc. Natl. Acad. Sci. USA 100, 3077-3082. 10.1073/pnas.0630588100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Listenberger, L. L., Ostermeyer-Fay, A. G., Goldberg, E. B., Brown, W. J. and Brown, D. A. (2007). Adipocyte differentiation-related protein reduces the lipid droplet association of adipose triglyceride lipase and slows triacylglycerol turnover. J. Lipid Res. 48, 2751-2761. 10.1194/jlr.M700359-JLR200 [DOI] [PubMed] [Google Scholar]
- Liu, R., Lee, J.-H., Li, J., Yu, R., Tan, L., Xia, Y., Zheng, Y., Bian, X.-L., Lorenzi, P. L., Chen, Q.et al. (2021). Choline kinase alpha 2 acts as a protein kinase to promote lipolysis of lipid droplets. Mol. Cell 81, 2722-2735.e9. 10.1016/j.molcel.2021.05.005 [DOI] [PubMed] [Google Scholar]
- Lizaso, A., Tan, K.-T. and Lee, Y.-H. (2013). β-adrenergic receptor-stimulated lipolysis requires the RAB7-mediated autolysosomal lipid degradation. Autophagy 9, 1228-1243. 10.4161/auto.24893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorente-Cortés, V., Royo, T., Juan-Babot, O. and Badimon, L. (2007). Adipocyte differentiation-related protein is induced by LRP1-mediated aggregated LDL internalization in human vascular smooth muscle cells and macrophages. J. Lipid Res. 48, 2133-2140. 10.1194/jlr.M700039-JLR200 [DOI] [PubMed] [Google Scholar]
- Louche, K., Badin, P.-M., Montastier, E., Laurens, C., Bourlier, V., de Glisezinski, I., Thalamas, C., Viguerie, N., Langin, D. and Moro, C. (2013). Endurance exercise training up-regulates lipolytic proteins and reduces triglyceride content in skeletal muscle of obese subjects. J. Clin. Endocrinol. Metab. 98, 4863-4871. 10.1210/jc.2013-2058 [DOI] [PubMed] [Google Scholar]
- MacPherson, R. E. K., Ramos, S. V., Vandenboom, R., Roy, B. D. and Peters, S. J. (2013). Skeletal muscle PLIN proteins, ATGL and CGI-58, interactions at rest and following stimulated contraction. Am. J. Physiol. Regul. Integr. Comp. Physiol. 304, R644-R650. 10.1152/ajpregu.00418.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magné, J., Aminoff, A., Sundelin, J. P., Mannila, M. N., Gustafsson, P., Hultenby, K., Wernerson, A., Bauer, G., Listenberger, L., Neville, M. J.et al. (2013). The minor allele of the missense polymorphism Ser251Pro in perilipin 2 (PLIN2) disrupts an α-helix, affects lipolysis, and is associated with reduced plasma triglyceride concentration in humans. FASEB J. 27, 3090-3099. 10.1096/fj.13-228759 [DOI] [PubMed] [Google Scholar]
- Magnusson, B., Asp, L., Boström, P., Ruiz, M., Stillemark-Billton, P., Lindén, D., Borén, J. and Olofsson, S.-O. (2006). Adipocyte differentiation-related protein promotes fatty acid storage in cytosolic triglycerides and inhibits secretion of very low-density lipoproteins. Arterioscler. Thromb. Vasc. Biol. Arterioscler. Thromb. Vasc. Biol. 26, 1566-1571. 10.1161/01.ATV.0000223345.11820.da [DOI] [PubMed] [Google Scholar]
- Marschallinger, J., Iram, T., Zardeneta, M., Lee, S. E., Lehallier, B., Haney, M. S., Pluvinage, J. V., Mathur, V., Hahn, O., Morgens, D. W.et al. (2020). Lipid-droplet-accumulating microglia represent a dysfunctional and proinflammatory state in the aging brain. Nat. Neurosci. 23, 194-208. 10.1038/s41593-019-0566-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Botas, J., Anderson, J. B., Tessier, D., Lapillonne, A., Chang, B. H.-J., Quast, M. J., Gorenstein, D., Chen, K.-H. and Chan, L. (2000). Absence of perilipin results in leanness and reverses obesity in Lepr(db/db) mice. Nat. Genet. 26, 474-479. 10.1038/82630 [DOI] [PubMed] [Google Scholar]
- Martínez-Uña, M., Varela-Rey, M., Mestre, D., Fernández-Ares, L., Fresnedo, O., Fernandez-Ramos, D., Juan, V. G., De Martin-Guerrero, I., García-Orad, A., et al. (2015). S-Adenosylmethionine increases circulating very-low density lipoprotein clearance in non-alcoholic fatty liver disease. J. Hepatol. 62, 673-681. 10.1016/j.jhep.2014.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason, R. R. and Watt, M. J. (2015). Unraveling the roles of PLIN5: linking cell biology to physiology. Trends Endocrinol. Metab. 26, 144-152. 10.1016/j.tem.2015.01.005 [DOI] [PubMed] [Google Scholar]
- Mason, R. R., Mokhtar, R., Matzaris, M., Selathurai, A., Kowalski, G. M., Mokbel, N., Meikle, P. J., Bruce, C. R. and Watt, M. J. (2014). PLIN5 deletion remodels intracellular lipid composition and causes insulin resistance in muscle. Mol. Metab. 3, 652-663. 10.1016/j.molmet.2014.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh, A. L., Senthivinayagam, S., Moon, K. C., Gupta, S., Lwande, J. S., Murphy, C. C., Storey, S. M. and Atshaves, B. P. (2012). Direct interaction of Plin2 with lipids on the surface of lipid droplets: a live cell FRET analysis. Am. J. Physiol. Cell Physiol. 303, C728-C742. 10.1152/ajpcell.00448.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManaman, J. L., Zabaronick, W., Schaack, J. and Orlicky, D. J. (2003). Lipid droplet targeting domains of adipophilin. J. Lipid Res. 44, 668-673. 10.1194/jlr.C200021-JLR200 [DOI] [PubMed] [Google Scholar]
- McManaman, J. L., Bales, E. S., Orlicky, D. J., Jackman, M., MacLean, P. S., Cain, S., Crunk, A. E., Mansur, A., Graham, C. E., Bowman, T. A.et al. (2013). Perilipin-2-null mice are protected against diet-induced obesity, adipose infl ammation, and fatty liver disease. J. Lipid Res. 54, 1346-1359. 10.1194/jlr.M035063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirheydari, M., Rathnayake, S. S., Frederick, H., Arhar, T., Mann, E. K., Cocklin, S. and Kooijman, E. E. (2016). Insertion of perilipin 3 into a glycero(phospho)lipid monolayer depends on lipid headgroup and acyl chain species. J. Lipid Res. 57, 1465-1476. 10.1194/jlr.M068205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi, H., Perfield, J. W., II, Souza, S. C., Shen, W.-J., Zhang, H.-H., Stancheva, Z. S., Kraemer, F. B., Obin, M. S. and Greenberg, A. S. (2007). Control of adipose triglyceride lipase action by serine 517 of perilipin A globally regulates protein kinase A-stimulated lipolysis in adipocytes. J. Biol. Chem. 282, 996-1002. 10.1074/jbc.M605770200 [DOI] [PubMed] [Google Scholar]
- Miyoshi, H., Souza, S. C., Endo, M., Sawada, T., Perfield, J. W., Shimizu, C., Stancheva, Z., Nagai, S., Strissel, K. J., Yoshioka, N.et al. (2010). Perilipin overexpression in mice protects against diet-induced obesity. J. Lipid Res. 51, 975-982. 10.1194/jlr.M002352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery, M. K., Watt, M. J., Keenan, S. N., Meex, R. C., Lo, J. C. Y., Ryan, A. and Nie, S. (2019). Perilipin 5 deletion in hepatocytes remodels lipid metabolism and causes hepatic insulin resistance in mice. Diabetes 68, 543-555. 10.2337/db18-0670 [DOI] [PubMed] [Google Scholar]
- Moreira, L. S., Piva, B., Gentile, L. B., Mesquita-Santos, F. P., D'Avila, H., Maya-Monteiro, C. M., Bozza, P. T., Bandeira-Melo, C. and Diaz, B. L. (2009). Cytosolic phospholipase A2-driven PGE2 synthesis within unsaturated fatty acids-induced lipid bodies of epithelial cells. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1791, 156-165. 10.1016/j.bbalip.2009.01.003 [DOI] [PubMed] [Google Scholar]
- Najt, C. P., Lwande, J. S., McIntosh, A. L., Senthivinayagam, S., Gupta, S., Kuhn, L. A. and Atshaves, B. P. (2014). Structural and functional assessment of perilipin 2 lipid binding domain(s). Biochemistry 53, 7051-7066. 10.1021/bi500918m [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najt, C. P., Senthivinayagam, S., Aljazi, M. B., Fader, K. A., Olenic, S. D., Brock, J. R. L., Lydic, T. A., Jones, A. D. and Atshaves, B. P. (2016). Liver-specific loss of Perilipin 2 alleviates diet-induced hepatic steatosis, inflammation, and fibrosis. Am. J. Physiol. Gastrointest. Liver Physiol. 310, G726-G738. 10.1152/ajpgi.00436.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najt, C. P., Khan, S. A., Heden, T. D., Witthuhn, B. A., Perez, M., Heier, J. L., Mead, L. E., Franklin, M. P., Karanja, K. K., Graham, M. J.et al. (2020). Lipid droplet-derived monounsaturated fatty acids traffic via PLIN5 to allosterically activate SIRT1. Mol. Cell 77, 810-824.e8. 10.1016/j.molcel.2019.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura, N. and Fujimoto, T. (2003). Adipose differentiation-related protein has two independent domains for targeting to lipid droplets. Biochem. Biophys. Res. Commun. 306, 333-338. 10.1016/S0006-291X(03)00979-3 [DOI] [PubMed] [Google Scholar]
- Nguyen, K. T., Lee, C.-S., Mun, S.-H., Truong, N. T., Park, S. K. and Hwang, C.-S. (2019). N-terminal acetylation and the N-end rule pathway control degradation of the lipid droplet protein PLIN2. J. Biol. Chem. 294, 379-388. 10.1074/jbc.RA118.005556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimura, S., Yamaguchi, T., Ueda, K., Kadokura, K., Aiuchi, T., Kato, R., Obama, T. and Itabe, H. (2015). Olanzapine promotes the accumulation of lipid droplets and the expression of multiple perilipins in human adipocytes. Biochem. Biophys. Res. Commun. 467, 906-912. 10.1016/j.bbrc.2015.10.045 [DOI] [PubMed] [Google Scholar]
- Nose, F., Yamaguchi, T., Kato, R., Aiuchi, T., Obama, T., Hara, S., Yamamoto, M. and Itabe, H. (2013). Crucial role of Perilipin-3 (TIP47) in formation of lipid droplets and PGE2 production in HL-60-derived neutrophils. PLoS ONE 8, e71542. 10.1371/journal.pone.0071542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orban, T., Palczewska, G. and Palczewski, K. (2011). Retinyl ester storage particles (retinosomes) from the retinal pigmented epithelium resemble lipid droplets in other tissues. J. Biol. Chem. 286, 17248-17258. 10.1074/jbc.M110.195198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlicky, D. J., Libby, A. E., Bales, E. S., McMahan, R. H., Monks, J., La Rosa, F. G. and McManaman, J. L. (2018). Perilipin-2 promotes obesity and progressive fatty liver disease in mice through mechanistically distinct hepatocyte and extra-hepatocyte actions. J. Physiol. 597, 1565-1584. 10.1113/JP277140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ost, A., Ortegren, U., Gustavsson, J., Nystrom, F. H. and Stralfors, P. (2005). Triacylglycerol is synthesized in a specific subclass of caveolae in primary adipocytes. J. Biol. Chem. 280, 5-8. 10.1074/jbc.C400429200 [DOI] [PubMed] [Google Scholar]
- Ostler, D. A., Prieto, V. G., Reed, J. A., Deavers, M. T., Lazar, A. J. and Ivan, D. (2010). Adipophilin expression in sebaceous tumors and other cutaneous lesions with clear cell histology: an immunohistochemical study of 117 cases. Mod. Pathol. 23, 567-573. 10.1038/modpathol.2010.1 [DOI] [PubMed] [Google Scholar]
- Patel, S., Yang, W., Kozusko, K., Saudek, V. and Savage, D. B. (2014). Perilipins 2 and 3 lack a carboxy-terminal domain present in perilipin 1 involved in sequestering ABHD5 and suppressing basal lipolysis. Proc. Natl. Acad. Sci. USA 111, 9163-9168. 10.1073/pnas.1318791111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul, A., Chang, B. H.-J., Li, L., Yechoor, V. K. and Chan, L. (2008). Deficiency of adipose differentiation-related protein impairs foam cell formation and protects against Atherosclerosis. Circ. Res. 102, 1492-1501. 10.1161/CIRCRESAHA.107.168070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploen, D., Hafirassou, M. L., Himmelsbach, K., Sauter, D., Biniossek, M. L., Weiss, T. S., Baumert, T. F., Schuster, C. and Hildt, E. (2013). TIP47 plays a crucial role in the life cycle of hepatitis C virus. J. Hepatol. 58, 1081-1088. 10.1016/j.jhep.2013.01.022 [DOI] [PubMed] [Google Scholar]
- Pollak, N. M., Schweiger, M., Jaeger, D., Kolb, D., Kumari, M., Schreiber, R., Kolleritsch, S., Markolin, P., Grabner, G. F., Heier, C.et al. (2013). Cardiac-specific overexpression of perilipin 5 provokes severe cardiac steatosis via the formation of a lipolytic barrier. J. Lipid Res. 54, 1092-1102. 10.1194/jlr.M034710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak, N. M., Jaeger, D., Kolleritsch, S., Zimmermann, R., Zechner, R., Lass, A. and Haemmerle, G. (2014). The interplay of protein kinase A and perilipin 5 regulates cardiac lipolysis. J. Biol. Chem. 290, 1295-1306. 10.1074/jbc.M114.604744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prévost, C., Sharp, M. E., Kory, N., Lin, Q., Voth, G. A., Farese, R. V. and Walther, T. C. (2018). Mechanism and determinants of amphipathic helix-containing protein targeting to lipid droplets. Dev. Cell 44, 73-86.e4. 10.1016/j.devcel.2017.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi, L., Corella, D., Sorlí, J. V., Portolés, O., Shen, H., Coltell, O., Godoy, D., Greenberg, A. S. and Ordovas, J. M. (2004). Genetic variation at the perilipin (PLIN) locus is associated with obesity-related phenotypes in White women. Clin. Genet. 66, 299-310. 10.1111/j.1399-0004.2004.00309.x [DOI] [PubMed] [Google Scholar]
- Qi, L., Tai, E. S., Tan, C. E., Shen, H., Chew, S. K., Greenberg, A. S., Corella, D. and Ordovas, J. M. (2005). Intragenic linkage disequilibrium structure of the human perilipin gene (PLIN) and haplotype association with increased obesity risk in a multiethnic Asian population. J. Mol. Med. 83, 448-456. 10.1007/s00109-004-0630-4 [DOI] [PubMed] [Google Scholar]
- Rambold, A. S., Cohen, S. and Lippincott-Schwartz, J. (2015). Fatty acid trafficking in starved cells: regulation by lipid droplet lipolysis, autophagy, and mitochondrial fusion dynamics. Dev. Cell 32, 678-692. 10.1016/j.devcel.2015.01.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson, K., Louie-Gao, Q., Arnett, D. K., Parnell, L. D., Lai, C.-Q., Davalos, A., Fox, C. S., Demissie, S., Cupples, L. A., Fernandez-Hernando, C.et al. (2011). The plin4 variant rs8887 modulates obesity related phenotypes in humans through creation of a novel mir-522 seed site. PLoS ONE 6, e17944. 10.1371/journal.pone.0017944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers, J. T., Lerin, C., Haas, W., Gygi, S. P., Spiegelman, B. M. and Puigserver, P. (2005). Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature 434, 113-118. 10.1038/nature03354 [DOI] [PubMed] [Google Scholar]
- Rowe, E. R., Mimmack, M. L., Barbosa, A. D., Haider, A., Isaac, I., Ouberai, M. M., Thiam, A. R., Patel, S., Saudek, V., Siniossoglou, S.et al. (2016). Conserved amphipathic helices mediate lipid droplet targeting of perilipins 1-3. J. Biol. Chem. 291, 6664-6678. 10.1074/jbc.M115.691048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggieri, A., Naumenko, S., Smith, M. A., Iannibelli, E., Blasevich, F., Bragato, C., Gibertini, S., Barton, K., Vorgerd, M., Marcus, K.et al. (2020). Multiomic elucidation of a coding 99-mer repeat-expansion skeletal muscle disease. Acta Neuropathol.. 140, 231-235. 10.1007/s00401-020-02164-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz, J. R., Larrarte, E., Margareto, J., Ares, R., Alkorta, P. and Labayen, I. (2011). Preliminary findings on the role of PLIN1 polymorphisms on body composition and energy metabolism response to energy restriction in obese omen. Br. J. Nutr. 106, 486-490. 10.1017/S0007114511000432 [DOI] [PubMed] [Google Scholar]
- Saha, P. K., Kojima, H., Martinez-Botas, J., Sunehag, A. L. and Chan, L. (2004). Metabolic adaptations in the absence of perilipin: increased beta-oxidation and decreased hepatic glucose production associated with peripheral insulin resistance but normal glucose tolerance in perilipin-null mice. J. Biol. Chem. 279, 35150-35158. 10.1074/jbc.M405499200 [DOI] [PubMed] [Google Scholar]
- Sahu-Osen, A., Montero-Moran, G., Schittmayer, M., Fritz, K., Dinh, A., Chang, Y.-F., McMahon, D., Boeszoermenyi, A., Cornaciu, I., Russell, D.et al. (2015). CGI-58/ABHD5 is phosphorylated on Ser239 by protein kinase A: control of subcellular localization. J. Lipid Res. 56, 109-121. 10.1194/jlr.M055004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapiro, J. M., Mashek, M. T., Greenberg, A. S. and Mashek, D. G. (2009). Hepatic triacylglycerol hydrolysis regulates peroxisome proliferator-activated receptor alpha activity. J. Lipid Res. 50, 1621-1629. 10.1194/jlr.M800614-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada, T., Miyoshi, H., Shimada, K., Suzuki, A., Okamatsu-Ogura, Y., Perfield, J. W., II, Kondo, T., Nagai, S., Shimizu, C., Yoshioka, N.et al. (2010). Perilipin overexpression in white adipose tissue induces a brown fat-like phenotype. PLoS ONE 5, e14006. 10.1371/journal.pone.0014006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sentinelli, F., Capoccia, D., Incani, M., Bertoccini, L., Severino, A., Pani, M. G., Manconi, E., Cossu, E., Leonetti, F. and Baroni, M. G. (2016). The perilipin 2 (PLIN2) gene Ser251Pro missense mutation is associated with reduced insulin secretion and increased insulin sensitivity in Italian obese subjects. Diabetes. Metab. Res. Rev. 32, 550-556. 10.1002/dmrr.2751 [DOI] [PubMed] [Google Scholar]
- Servetnick, D. A., Brasaemle, D. L., Gruia-Gray, J., Kimmel, A. R., Wolff, J. and Londos, C. (1995). Perilipins are associated with cholesteryl ester droplets in steroidogenic adrenal cortical and Leydig cells. J. Biol. Chem. 270, 16970-16973. 10.1074/jbc.270.28.16970 [DOI] [PubMed] [Google Scholar]
- Shen, W. J., Patel, S., Miyoshi, H., Greenberg, A. S. and Kraemer, F. B. (2009). Functional interaction of hormone-sensitive lipase and perilipin in lipolysis. J. Lipid Res. 50, 2306-2313. 10.1194/jlr.M900176-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd, S. O., Cocks, M., Meikle, P. J., Mellett, N. A., Ranasinghe, A. M., Barker, T. A., Wagenmakers, A. J. M. and Shaw, C. S. (2017). Lipid droplet remodelling and reduced muscle ceramides following sprint interval and moderate-intensity continuous exercise training in obese males. Int. J. Obes. 41, 1745-1754. 10.1038/ijo.2017.170 [DOI] [PubMed] [Google Scholar]
- Simard, J. R., Meshulam, T., Pillai, B. K., Kirber, M. T., Brunaldi, K., Xu, S., Pilch, P. F. and Hamilton, J. A. (2010). Caveolins sequester FA on the cytoplasmic leaflet of the plasma membrane, augment triglyceride formation, and protect cells from lipotoxicity. J. Lipid Res. 51, 914-922. 10.1194/jlr.M900251-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, R., Kaushik, S., Wang, Y., Xiang, Y., Novak, I., Komatsu, M., Tanaka, K., Cuervo, A. M. and Czaja, M. J. (2009). Autophagy regulates lipid metabolism. Nature 458, 1131-1135. 10.1038/nature07976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner, J. R., Shew, T. M., Schwartz, D. M., Tzekov, A., Lepus, C. M., Abumrad, N. A. and Wolins, N. E. (2009). Diacylglycerol enrichment of endoplasmic reticulum or lipid droplets recruits perilipin 3/TIP47 during lipid storage and mobilization. J. Biol. Chem. 284, 30941-30948. 10.1074/jbc.M109.013995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, C. E., Tucker, K. L., Yiannakouris, N., Garcia-Bailo, B., Mattei, J., Lai, C.-Q., Parnell, L. D. and Ordovás, J. M. (2008). Perilipin polymorphism interacts with dietary carbohydrates to modulate anthropometric traits in hispanics of Caribbean origin. J. Nutr. 138, 1852-1858. 10.1093/jn/138.10.1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn, J. H., Lee, Y. K., Han, J. S., Jeon, Y. G., Kim, J. I., Choe, S. S., Kim, S. J., Yoo, H. J. and Kim, J. B. (2018). Perilipin 1 (Plin1) deficiency promotes inflammatory responses in lean adipose tissue through lipid dysregulation. J. Biol. Chem. 293, 13974-13988. 10.1074/jbc.RA118.003541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sołtysik, K., Ohsaki, Y., Tatematsu, T., Cheng, J. and Fujimoto, T. (2019). Nuclear lipid droplets derive from a lipoprotein precursor and regulate phosphatidylcholine synthesis. Nat. Commun. 10, 473. 10.1038/s41467-019-08411-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey, S. M., McIntosh, A. L., Senthivinayagam, S., Moon, K. C. and Atshaves, B. P. (2011). The phospholipid monolayer associated with perilipin-enriched lipid droplets is a highly organized rigid membrane structure. Am. J. Physiol. Endocrinol. Metab. 301, E991-E1003. 10.1152/ajpendo.00109.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub, B. K., Stoeffel, P., Heid, H., Zimbelmann, R. and Schirmacher, P. (2008). Differential pattern of lipid droplet-associated proteins and de novo perilipin expression in hepatocyte steatogenesis. Hepatology 47, 1936-1946. 10.1002/hep.22268 [DOI] [PubMed] [Google Scholar]
- Straub, B. K., Herpel, E., Singer, S., Zimbelmann, R., Breuhahn, K., Macher-Goeppinger, S., Warth, A., Lehmann-Koch, J., Longerich, T., Heid, H.et al. (2010). Lipid droplet-associated PAT-proteins show frequent and differential expression in neoplastic steatogenesis. Mod. Pathol. 23, 480-492. 10.1038/modpathol.2009.191 [DOI] [PubMed] [Google Scholar]
- Straub, B. K., Witzel, H. R., Pawella, L. M., Renner, M., Eiteneuer, E., Hashani, M., Schirmacher, P., Roth, W. and Mechtersheimer, G. (2019). Perilipin 1 expression differentiates liposarcoma from other types of soft tissue sarcoma. Am. J. Pathol. 189, 1547-1558. 10.1016/j.ajpath.2019.04.017 [DOI] [PubMed] [Google Scholar]
- Straus, D. S. and Glass, C. K. (2007). Anti-inflammatory actions of PPAR ligands: new insights on cellular and molecular mechanisms. Trends Immunol. 28, 551-558. 10.1016/j.it.2007.09.003 [DOI] [PubMed] [Google Scholar]
- Subramanian, V., Garcia, A., Sekowski, A. and Brasaemle, D. L. (2004). Hydrophobic sequences target and anchor perilipin A to lipid droplets. J. Lipid Res. 45, 1983-1991. 10.1194/jlr.M400291-JLR200 [DOI] [PubMed] [Google Scholar]
- Sun, Z., Gong, J., Wu, H., Xu, W., Wu, L., Xu, D., Gao, J., Wu, J.-W., Yang, H., Yang, M.et al. (2013). Perilipin1 promotes unilocular lipid droplet formation through the activation of Fsp27 in adipocytes. Nat. Commun. 4, 1594. 10.1038/ncomms2581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, X., Yang, S., Feng, X., Zheng, Y., Zhou, J., Wang, H., Zhang, Y., Sun, H. and He, C. (2020). The modification of ferroptosis and abnormal lipometabolism through overexpression and knockdown of potential prognostic biomarker perilipin2 in gastric carcinoma. Gastric Cancer 23, 241-259. 10.1007/s10120-019-01004-z [DOI] [PubMed] [Google Scholar]
- Szigeti, A., Minik, O., Hocsak, E., Pozsgai, E., Boronkai, A., Farkas, R., Balint, A., Bodis, J., Sumegi, B. and Bellyei, S. (2009). Preliminary study of TIP47 as a possible new biomarker of cervical dysplasia and invasive carcinoma. Anticancer Res. 29, 717-724. [PubMed] [Google Scholar]
- Tall, A. R. and Yvan-Charvet, L. (2015). Cholesterol, inflammation and innate immunity. Nat. Rev. Immunol. 15, 104-116. 10.1038/nri3793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tansey, J. T., Sztalryd, C., Gruia-Gray, J., Roush, D. L., Zee, J. V., Gavrilova, O., Reitman, M. L., Deng, C.-X., Li, C., Kimmel, A. R.et al. (2001). Perilipin ablation results in a lean mouse with aberrant adipocyte lipolysis, enhanced leptin production, and resistance to diet-induced obesity. Proc. Natl. Acad. Sci. USA 98, 6494-6499. 10.1073/pnas.101042998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiam, A. R., Antonny, B., Wang, J., Delacotte, J., Wilfling, F., Walther, T. C., Beck, R., Rothman, J. E. and Pincet, F. (2013). COPI buds 60-nm lipid droplets from reconstituted water–phospholipid–triacylglyceride interfaces, suggesting a tension clamp function. Proc. Natl. Acad. Sci. USA 110, 13244-13249. 10.1073/pnas.1307685110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolkach, Y., Lüders, C., Meller, S., Jung, K., Stephan, C. and Kristiansen, G. (2017). Adipophilin as prognostic biomarker in clear cell renal cell carcinoma. Oncotarget 8, 28672-28682. 10.18632/oncotarget.15639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevino, M. B., Mazur-Hart, D., Machida, Y., King, T., Nadler, J., Galkina, E. V., Poddar, A., Dutta, S. and Imai, Y. (2015). Liver Perilipin 5 expression worsens hepatosteatosis but not insulin resistance in high fat-fed mice. Mol. Endocrinol. 29, 1414-1425. 10.1210/me.2015-1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai, T.-H., Chen, E., Li, L., Saha, P., Lee, H.-J., Huang, L.-S., Shelness, G. S., Chan, L. and Chang, B. H.-J. (2017). The constitutive lipid droplet protein PLIN2 regulates autophagy in liver. Autophagy 13, 1130-1144. 10.1080/15548627.2017.1319544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno, M., Suzuki, J., Hirose, M., Sato, S., Imagawa, M., Zenimaru, Y., Takahashi, S., Ikuyama, S., Koizumi, T., Konoshita, T.et al. (2017). Cardiac overexpression of perilipin 2 induces dynamic steatosis: Prevention by hormone-sensitive lipase. Am. J. Physiol. Endocrinol. Metab. 313, E699-E709. 10.1152/ajpendo.00098.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urahama, Y., Ohsaki, Y., Fujita, Y., Maruyama, S., Yuzawa, Y., Matsuo, S. and Fujimoto, T. (2008). Lipid droplet-associated proteins protect renal tubular cells from fatty acid-induced apoptosis. Am. J. Pathol. 173, 1286-1294. 10.2353/ajpath.2008.080137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzbekov, R. and Roingeard, P. (2013). Nuclear lipid droplets identified by electron microscopy of serial sections. BMC Res. Notes 6, 386. 10.1186/1756-0500-6-386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela, G. M., Antwi, D. A., Dhir, R., Yin, X., Singhal, N. S., Graham, M. J., Crooke, R. M. and Ahima, R. S. (2008). Inhibition of ADRP prevents diet-induced insulin resistance. Am. J. Physiol. Gastrointest. Liver Physiol. 295, G621-G628. 10.1152/ajpgi.90204.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt, D. A., Camus, G., Herker, E., Webster, B. R., Tsou, C.-L., Greene, W. C., Yen, T.-S. B. and Ott, M. (2013). Lipid droplet-binding protein TIP47 regulates hepatitis C Virus RNA replication through interaction with the viral NS5A protein. PLoS Pathog.. 9, e1003302. 10.1371/journal.ppat.1003302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H., Sreenivasan, U., Hu, H., Saladino, A., Polster, B. M., Lund, L. M., Gong, D., Stanley, W. C., Sztalryd, C. and Sztalryd, C. (2011). Perilipin 5, a lipid droplet-associated protein, provides physical and metabolic linkage to mitochondria. J. Lipid Res. 52, 2159-2168. 10.1194/jlr.M017939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J., Si, Y., Wu, C., Sun, L., Ma, Y., Ge, A. and Li, B. (2012). Lipopolysaccharide promotes lipid accumulation in human adventitial fibroblasts via TLR4-NF-B pathway. Lipids Health Dis. 11, 139. 10.1186/1476-511X-11-139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, C., Zhao, Y., Gao, X., Li, L., Yuan, Y., Liu, F., Zhang, L., Wu, J., Hu, P., Zhang, X.et al. (2015). Perilipin 5 improves hepatic lipotoxicity by inhibiting lipolysis. Hepatology 61, 870-882. 10.1002/hep.27409 [DOI] [PubMed] [Google Scholar]
- Wang, K., Ruan, H. L., Song, Z. S., Cao, Q., Bao, L., Liu, D., Xu, T. B., Xiao, H. B., Wang, C., Cheng, G.et al. (2018). PLIN3 is up-regulated and correlates with poor prognosis in clear cell renal cell carcinoma. Urol. Oncol. Semin. Orig. Investig. 36, 343.e9-343.e19. 10.1016/j.urolonc.2018.04.006 [DOI] [PubMed] [Google Scholar]
- Westhoff, C. C., Mrozinski, J., Riedel, I., Heid, H. W. and Moll, R. (2017). Perilipin 1 is a highly specific marker for adipocytic differentiation in sarcomas with intermediate sensitivity. J. Cancer Res. Clin. Oncol. 143, 225-232. 10.1007/s00432-016-2263-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolins, N. E., Rubin, B. and Brasaemle, D. L. (2001). TIP47 associates with lipid droplets. J. Biol. Chem. 276, 5101-5108. 10.1074/jbc.M006775200 [DOI] [PubMed] [Google Scholar]
- Wolins, N. E., Skinner, J. R., Schoenfish, M. J., Tzekov, A., Bensch, K. G. and Bickel, P. E. (2003). Adipocyte protein S3-12 coats nascent lipid droplets. J. Biol. Chem. 278, 37713-37721. 10.1074/jbc.M304025200 [DOI] [PubMed] [Google Scholar]
- Wolins, N. E., Quaynor, B. K., Skinner, J. R., Schoenfish, M. J., Tzekov, A. and Bickel, P. E. (2005). S3-12, adipophilin, and TIP47 package lipid in adipocytes. J. Biol. Chem. 280, 19146-19155. 10.1074/jbc.M500978200 [DOI] [PubMed] [Google Scholar]
- Xiong, X., Bales, E. S., Ir, D., Robertson, C. E., McManaman, J. L., Frank, D. N. and Parkinson, J. (2017). Perilipin-2 modulates dietary fat-induced microbial global gene expression profiles in the mouse intestine. Microbiome 5, 117. 10.1186/s40168-017-0327-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H. H., Souza, S. C., Muliro, K. V., Kraemer, F. B., Obin, M. S. and Greenberg, A. S. (2003). Lipase-selective functional domains of Perilipin A differentially regulate constitutive and protein kinase A-stimulated lipolysis. J. Biol. Chem. 278, 51535-51542. 10.1074/jbc.M309591200 [DOI] [PubMed] [Google Scholar]
- Zhang, P., Meng, L., Song, L., Du, J., Du, S., Cui, W., Liu, C. and Li, F. (2017). Roles of perilipins in diseases and cancers. Curr. Genomics 19, 247-257. 10.2174/1389202918666170915155948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, E., Cui, W., Lopresti, M., Mashek, M. T., Najt, C. P., Hu, H. and Mashek, D. G. (2020). Hepatic PLIN5 signals via SIRT1 to promote autophagy and prevent inflammation during fasting. J. Lipid Res. 61, 338-350. 10.1194/jlr.RA119000336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, C., Wang, M., Zhou, L., Zhang, Y., Liu, W., Qin, W., He, R., Lu, Y., Wang, Y., Chen, X.-Z.et al. (2016). Prognostic significance of PLIN1 expression in human breast cancer. Oncotarget 7, 54488-54502. 10.18632/oncotarget.10239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, L., Hu, F., Li, C., Zhang, C., Hang, R. and Xu, R. (2021). Perilipin 4 protein: an impending target for amyotrophic lateral sclerosis. Mol. Neurobiol. 58, 1723-1737. 10.1007/s12035-020-02217-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.