Abstract
Connective tissue diseases (CTDs) demonstrating features of interstitial lung disease (ILD) include systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), systemic sclerosis (SSc), dermatomyositis (DM) and polymyositis (PM), ankylosing spondylitis (AS), Sjogren syndrome (SS), and mixed connective tissue disease (MCTD). On histopathology of lung biopsy in CTD-related ILDs (CTD-ILDs), multi-compartment involvement is an important clue, and when present, should bring CTD to the top of the list of etiologic differential diagnoses. Diverse histologic patterns including nonspecific interstitial pneumonia (NSIP), usual interstitial pneumonia (UIP), organizing pneumonia, apical fibrosis, diffuse alveolar damage, and lymphoid interstitial pneumonia can be seen on histology in patients with CTD-ILDs. Although proportions of ILDs vary, the NSIP pattern accounts for a large proportion, especially in SSc, DM and/or PM and MCTD, followed by the UIP pattern. In RA patients, interstitial lung abnormality (ILA) is reported to occur in approximately 20–60% of individuals of which 35–45% will have progression of the CT abnormality. Subpleural distribution and greater baseline ILA involvement are risk factors associated with disease progression. Asymptomatic CTD-ILDs or ILA patients with normal lung function and without evidence of disease progression can be followed without treatment. Immunosuppressive or antifibrotic agents for symptomatic and/or fibrosing CTD-ILDs can be used in patients who require treatment.
Abbreviations: CTD, Connective tissue disease; CTD-ILD, Connective tissue disease-related interstitial lung disease; DM, Dermatomyositis; IIP, Idiopathic interstitial pneumonia; ILA, Interstitial lung abnormality; ILD, Interstitial lung disease; IPF, Idiopathic pulmonary fibrosis; IPAF, Interstitial pneumonitis with autoimmune features; LIP, Lymphoid interstitial pneumonia; MCTD, Mixed connective tissue disease; NSIP, Nonspecific interstitial pneumonia; OP, Organizing pneumonia; PM, Polymyositis; RA, Rheumatoid arthritis; SLE, Systemic lupus erythematosus; SS, Sjogren’s syndrome; SSc, Systemic sclerosis; UCTD, Undifferentiated connective tissue disease; UIP, Usual interstitial pneumonia
Keywords: Connective tissue disease, Interstitial lung abnormality, Interstitial lung disease
1. Introduction
Interstitial lung disease (ILD), or more properly diffuse parenchymal lung disease [1], encompasses a heterogeneous group of lung diseases characterized by diffuse involvement of the pulmonary parenchyma by varying degrees of inflammation and/or fibrosis [1], [2], [3]. ILDs can be of known cause, e.g., drug related, environmental and/or occupational, and systemic diseases; or of unknown cause, e.g., idiopathic interstitial pneumonia (IIP), granulomatous diseases, and other disorders such as lymphangioleiomyomatosis. Among systemic diseases, connective tissue disease (CTD) is one of the most common causes of ILD [4].
CTDs are systemic autoimmune disorders caused by excessive immune activated inflammation that targets the connective tissues of the body as seen in rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), Sjogren’s syndrome (SS), idiopathic inflammatory myopathies such as dermatomyositis (DM) and polymyositis (PM), systemic sclerosis (SSc), and mixed connective tissue disease (MCTD) [5]. Classification criteria for a given CTD must be met in order to establish the diagnosis of a specific CTD. ILD exists in approximately 40–50% of patients with CTDs, and is the main cause of morbidity and mortality [6]. The reported prevalence of ILDs in patients with CTDs (CTD-ILDs) varies by classification criteria and study registries for specific diagnoses, with higher frequency in SSc and idiopathic inflammatory myopathies (DM and PM) and lower frequency in SLE [7]. The prevalence of ILD in CTDs is: up to 58% in RA (RA-ILD), up to 13% in SLE (SLE-ILD), up to 27% in SS (SS-ILD), up to 80% in DM/PM (DM- and PM-ILD), up to 91% in SSc (SSc-ILD), and up to 67% in MCTD (MCTD-ILD) [8]. Relative prevalence of different CTD-ILDs and proportion estimates of patients with progressive fibrosing phenotype (percentages of individuals having each phenotype of CTD-ILD and percentages of individuals among whom a progressive fibrosing phenotype is developing) have been recently published: RA-ILD accounted for 39% of the whole CTD-ILDs and 40% of RA-ILDs showed progressive fibrosing phenotype; SSc-ILD accounted 31% and 32% of SSc-ILDs showed progressive fibrosis; SLE-ILD accounted 17% and 24% of SLE-ILDs showed progressive fibrosis; PM-ILD and MCTD-ILD each accounted for 6% and 16% of PM-ILDs and 24% of MCTD-ILDs, respectively, demonstrated progressive fibrosis and SS-ILD accounted for 1% and 24% of SS-ILDs showed progressive fibrosis [9].
The histopathologic and radiologic features of ILDs associated with CTDs are identical to those of their idiopathic counterparts [1], [10]. However, some histopathologic findings such as lymphoid hyperplasia (follicular hyperplasia), although not specific, are suggestive of association with CTDs [1], [10].
Prior studies have shown that the presence of CTD in ILD patients has an impact on prognosis [11], [12], and that treatment options are dependent upon the underlying CTD. Guidelines emphasize the classification of ILD based on etiologies and have uniformly recommended search for evidence of CTD in newly diagnosed ILDs [1], [13], [14]. However, due to complexities in diagnosis and treatment of CTD itself and lack of evidence, current guidelines do not clearly provide strategies for evaluation and management of CTD-ILD despite their significance.
Recently, clinical importance of interstitial lung abnormalities (ILA) incidentally detected on CT is recognized, which occurs in 4–9% of smokers and 2–7% of non-smokers [15], [16], [17]. ILA is defined as follows: incidental identification of non-dependent abnormalities including ground-glass, reticular abnormalities, architectural distortion, traction bronchiectasis, and non-emphysematous cysts involving at least 5% of a lung zone in individuals in whom ILD is not suspected [16], [17]. Management of ILA in patient with CTD (CTD-ILA) is yet to be determined [16], [17].
In this article, we demonstrate thin-section CT (TSCT) findings of ILD in association with CTD, and correlate the TSCT findings with histopathologic findings. Additionally, we discuss the evolution and management of interstitial lung abnormalities (ILA) to ILD in CTD [17].
2. TSCT and its technique and extent analysis of CTD-ILD and CTD-ILA
TSCT, obtained by the use of thin (1–1.5-mm) slice thickness and high-frequency reconstruction algorithms, provides detailed images of the lungs. TSCT is most often performed on a multi-detector CT scanner using volumetric rather than axial incremental acquisition and covers the entire thorax in near isotropic resolution. With volumetric TSCT raw data, not only axially reconstructed thin-section images but also other planar images including sagittal and coronal images can be provided [18], [19]. TSCT is an important tool in the detection and characterization of lung pathology of ILDs in patients with CTD [10].
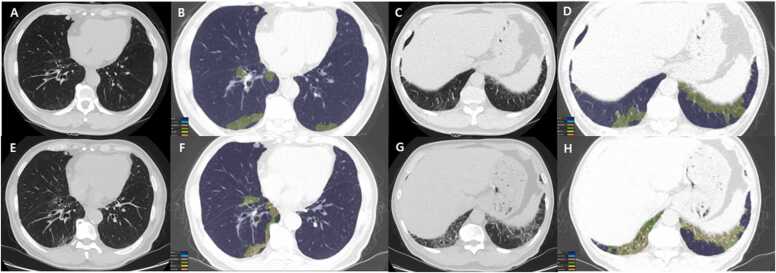
Volumetric analysis of TSCT is performed with pre-contrast images [20]. First, the whole-lung volume is calculated by the recognition of lung parenchyma, excluding pulmonary vessels or airways. After an overall analysis of the whole lung, each texture pattern analysis is completed separately. Texture patterns include emphysema, ground-glass opacity (GGO), reticulation, honeycombing, consolidation, and normal lungs; and not only the absolute volume but also the percentage of the total lung volume affected are calculated for each pattern. We can calculate the total abnormalities which are defined as the sum of the extent of honeycombing, reticulation, GGO, consolidation, and emphysema, and also calculate the fibrosis score defined as the sum of the extent of honeycombing and reticulation (Fig. 1).
Fig. 1.
Serial chest CT scans from a 68-year man with CTD (rheumatoid arthritis)-interstitial lung abnormality. (a-d) Baseline chest CT (top row) images obtained from lower lung zone show lung lesions composed of mild ground-glass opacity in subpleural region. The color-coded overlay (b & d) on CT images, by enabling automatic volume segmentation of lung parenchymal abnormalities, allows quantification of ground-glass opacity (3.14% involvement of total lung volume) and reticular (0.29% involvement of total lung volume) lesions.(e-f) Five-year follow-up CT (bottom row) images demonstrate changing pattern and distribution of lung abnormalities; decrease in ground-glass opacity (from 3.14% to 2.84%, green areas) and increase in reticulation (from 0.29% to 2.33%, yellow areas), when comparing (b & f) and (d & h) from each other.
2.1. Radiologic patterns of CTD-ILD
2.1.1. Imaging patterns in connective tissue disease
CTD demonstrating findings of ILD include SLE, RA, SSc, DM and PM, AS, SS, and MCTD. On histopathology, CTD-ILDs are diverse and include fibrosing nonspecific interstitial pneumonia (fNSIP), usual interstitial pneumonia (UIP) (Fig. 2), organizing pneumonia (OP), fibrosing OP, diffuse alveolar damage (DAD), as well as abnormalities in the pleura, airways, and blood vessels (Table 1). On TSCT, CTD-ILDs appear as abnormalities corresponding to their histopathologic features. Details on TSCT features will be discussed later.
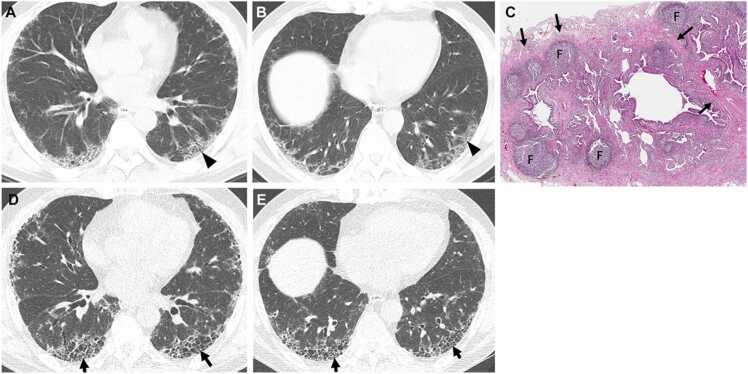
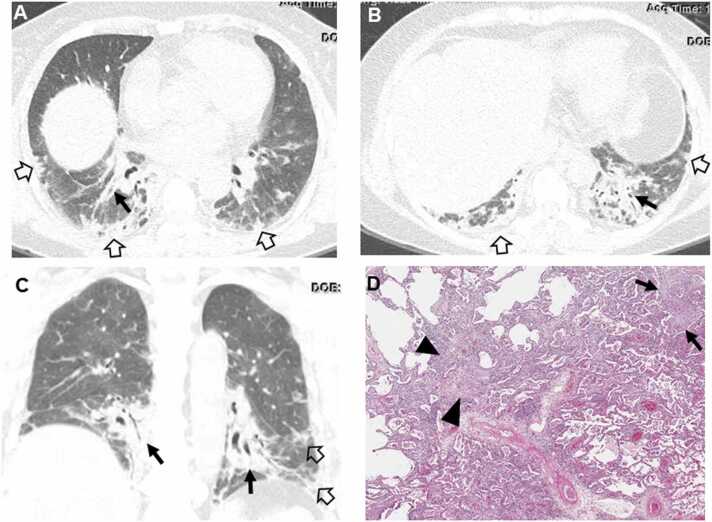
Fig. 2.
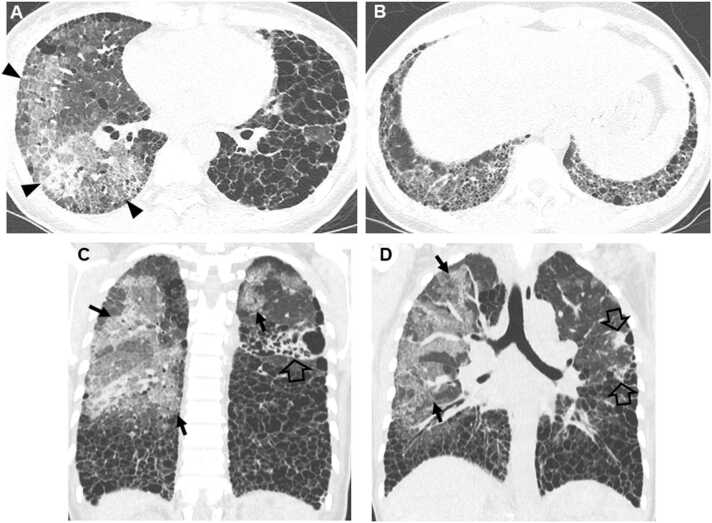
Interstitial pneumonia in autoimmune features in a 67-year-old man. (a, b) Lung window images of CT scans obtained at levels of right inferior pulmonary vein (a) and liver dome (b), respectively, show subpleural reticulation and traction bronchiolectasis (arrowheads) in both lungs. Patient had positive serologic tests; fluorescent antinuclear antibody (FANA, 1:320) and perinuclear antineutrophil cytoplasmic antibody (pANCA, 1:320). (c) Low-power magnification of lung demonstrates collapse of two secondary lobules (arrows) resulting in dilation of distal small airways (so-called honeycombing) with super imposed lymphoid follicles with reactive germinal centers (F). The histologic pattern is most consistent with UIP, and the superimposed lymphoid follicles with reactive germinal centers suggests CTD as the underlying cause of all the histopathologic findings. (d, e). Four-year follow-up CT scans obtained at similar levels to a & b, respectively, depict apparent areas of CT honeycombing (arrows) in posterior aspects of both lower lobes.
Table 1.
Common reactive histologic patterns in CTDs.
| Compartments | Histologic patterns | Histopathological findings |
|---|---|---|
| Alveolar parenchyma | Fibrotic NSIP | Diffuse temporally uniform fibrosis with little associated chronic inflammation |
| UIP | Non-uniform fibrosis with honeycomb change, fibroblast foci, mild inflammation | |
| OP | Plugs of loose connective tissue (Masson bodies) in distal airway lumens and alveolar spaces | |
| DAD | Alveolar wall edema and hyaline membranes in acute DAD, organization (OP) in airspaces and alveolar walls in organizing DAD | |
| LIP | Dense infiltrate of small lymphocytes, plasma cells, small clusters of epithelioid histiocytes, multinucleated giant cells that diffusely involves the distal parenchyma and markedly widens alveolar walls | |
| CIP | Mild diffuse interstitial infiltrate of chronic inflammatory cells that are considerably less dense than in LIP | |
| Lymphoid hyperplasia | Lymphoid aggregates and follicles with germinal centers throughout biopsy with/without follicular bronchiolitis | |
| Alveolar hemorrhage | Airspace red blood cells in acute hemorrhage, airspace macrophages with coarsely granular hemosiderin in chronic hemorrhage | |
| Pleura | Pleuritis | Acute and/or organizing fibrinous pleuritis, fibrous pleuritis, edema, variable chronic inflammation with/without germinal centers |
| Airways | Bronchitis/bronchiolitis | Prominent chronic and occasionally acute inflammatory cell infiltrate in walls of small airways |
| Follicular bronchiolitis | Lymphoid follicles containing prominent reactive germinal centers confined to the peribronchiolar interstitium | |
| Constrictive bronchiolitis | Subepithelial fibrosis causing luminal narrowing or luminal obliteration by fibrous tissue | |
| Vessels | Pulmonary hypertension | Spectrum from mild muscular hypertrophy and intimal thickening to severe concentric intimal fibrosis, luminal occlusion, plexiform and dilation lesions, and rarely fibrinoid necrosis and necrotizing arteritis |
| Vasculitis | Mural infiltrates of monocytes/histiocytes and neutrophils predominate | |
| Capillaritis | Necrotizing acute inflammation of alveolar wall capillaries |
NSIP: non-specific interstitial pneumonia; UIP: usual interstitial pneumonia; OP: organizing pneumonia; DAD: diffuse alveolar damage; LIP: lymphoid interstitial pneumonia; CIP: cellular interstitial pneumonia
Modified from pp 587–596, Colby [29].
2.1.2. Interstitial pneumonia with autoimmune features (IPAF) and its imaging features
Discussion will be dealt with in SUPPLEMENT TEXT because of its somewhat off the overall topic of this review.
2.1.3. Radiologic differences between CTD-ILD and IIP
CT findings of fNSIP, which is most frequent in CTD-ILDs, are bilateral, symmetric, lower lung zone-predominant reticular opacities with traction bronchiectasis (Fig. 3). Sometimes subpleural sparing of reticular involvement is seen (21%, 13/61). Peribronchial thickening (7%, 4/61) and HC (5%, 3/61) are occasional [21]. In an official ATS/ERS statement and its update of the international multidisciplinary classification of the IIPs, Travis et al. [22] commented on fibrosing variant of OP (fibrosing OP, even though not a formal classification category) (Fig. 4), in which category of the disease OP does not completely resolve despite prolonged treatment. In these cases, residual or progressive interstitial fibrosis is seen with or without recurrent episodes of OP. These fibrosing OP patients are found to have underlying PM or antisynthetase syndrome.
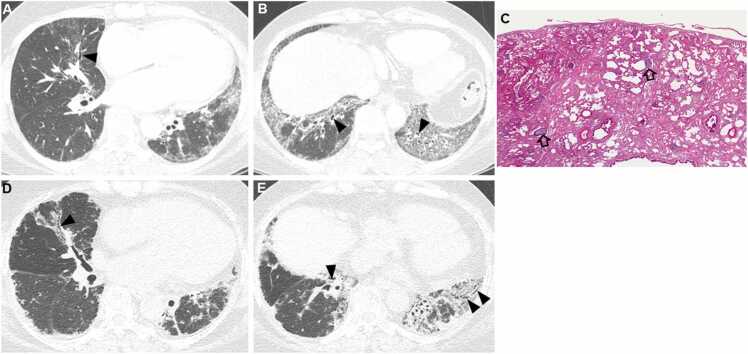
Fig. 3.
Fibrosing nonspecific interstitial pneumonia in a 48-year-old woman with dermatomyositis. (a, b) Lung window images of CT scans obtained at levels of right inferior pulmonary vein (a) and liver dome (b), respectively, show reticulation and traction bronchiectasis (arrowheads) in both lungs with lower lung zone predominance. (c) Medium-power magnification of lung specimen demonstrates temporally uniform diffuse parenchymal fibrosis typical of fibrosing NSIP. Superimposed are diffusely scattered lymphoid follicles (open arrows) containing reactive germinal centers, which suggest CTD as the underlying cause of the fibrosing NSIP. (d, e). Ten-year follow-up CT scans obtained at similar levels to a & b, respectively, depict progression of pulmonary fibrosis with apparent areas of traction bronchiectasis (arrowheads) in both lungs.
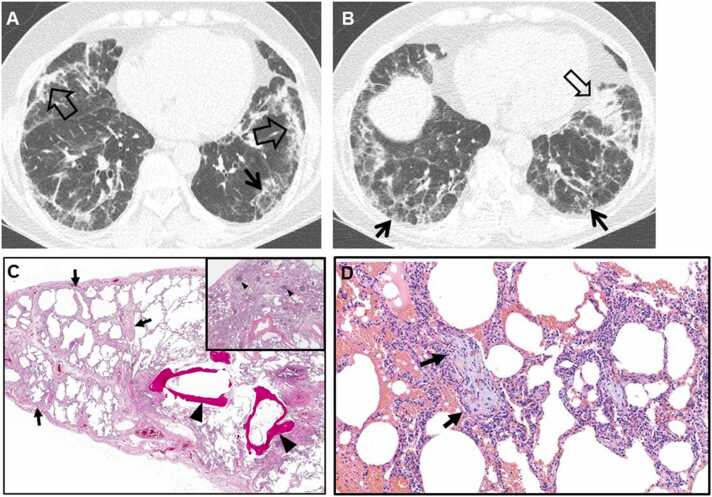
Fig. 4.
Fibrosing organizing pneumonia in a 58-year-old woman with interstitial pneumonia with autoimmune features (Interstitial pneumonia in autoimmune features; antineutrophil antibody [ANA], 1:160 and morning stiffness). (a, b) Lung window images of CT scans obtained at levels of cardiac ventricle (a) and liver dome (b), respectively, show patchy distribution of mixed areas of band-like consolidation (open arrows) and reticulation (arrows) in both lungs. (c) Low-power magnification of lung demonstrates temporally uniform diffuse lung fibrosis typical of fibrotic NSIP (arrows) associated with dendritic ossification (arrowheads), which is a reflection of chronicity of injury. Inset: lymphoid follicles containing reactive germinal centers (arrowheads) and suggesting CTD as the underlying cause of the fibrosing NSIP. (d) High-power magnification of lung (specimen obtained from right middle lobe) demonstrates organizing pneumonia (intra-alveolar loose myxoid polyps) (arrows).
Organizing pneumonia can be caused by CTD, and the findings of OP usually occur in the context of an already diagnosed CTD. OP with clinical features of infection can be the presenting findings of RA and SS [23] (Fig. 5).
Fig. 5.
Organizing pneumonia in a 56-year-old woman with dermatomyositis. (a, b) Lung window images of CT scans obtained at levels of liver dome (a) and 3 cm inferior to a (b), respectively, show patchy distribution of consolidation along bronchovascular bundles (arrows) and subpleural lungs (open arrows) in both lungs. (c) Coronal reformatted image demonstrates consolidation along bronchovascular bundles (arrows) and subpleural (open arrows) lungs. (d) Low power magnification of lung demonstrating organizing pneumonia (arrows) transitioning to fibrosing NSIP (arrowheads). CTD is in the etiologic differential of fibrosing NSIP, but there are no histologic findings in this images that suggest CTD as the underlying cause in contrast to the lymphoid follicles with reactive germinal centers in Fig. 1, Fig. 2, Fig. 3.
According to a study of 203 patients with CTD-ILD (31%), undifferentiated CTD (UCTD)-ILD (32%) and idiopathic pulmonary fibrosis (37%), the CT findings were not significantly different among three groups. Pulmonary symptoms were more common in IPF, while extrapulmonary symptoms were more common in the CTD-ILD and UCTD-ILD groups. Patients with CTD-ILD had more abnormal antibody tests than those of UCTD-ILD and IPF [24]. However, it has been asserted that in patients with CTD-ILD and UIP pattern on CT, straight edge sign (isolation of fibrosis to the lung bases without substantial extension along the lateral margins of the lungs on coronal images; Fig. 6), exuberant honeycombing sign (extensive honeycomb-like cyst formation within the lungs comprising greater than 70% of the fibrotic portions of lungs; Fig. 7) and anterior upper lobe sign (concentration of fibrosis within the anterior aspect of the upper lobes with relative sparing of the other aspects of the upper lobes and concomitant lower lobe involvement; Fig. 8) are significantly more common than IPF/UIP [25]. In another study aimed at determining whether specific CT findings can help differentiate CTDs manifesting as ILDs, the anterior upper lobe honeycomb-like lesion is a specific feature in RA-ILD with UIP or mixed UIP and NSIP pattern. In SSc- and PM/DM-ILD, fNSIP pattern was predominant without HC [26] (Fig. 2).
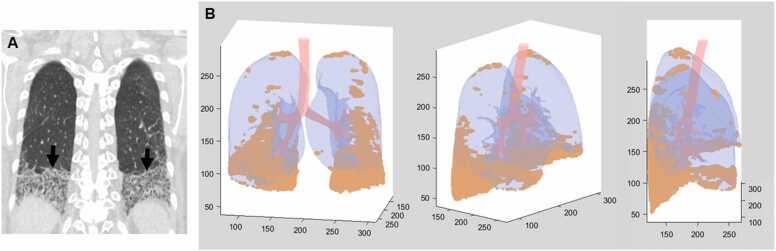
Fig. 6.
Connective tissue disease-related pulmonary fibrosis showing straight-edge sign in a 60-year-old woman with Sjogren’s syndrome. (a) Coronal reformatted image shows pulmonary fibrosis composed of reticulation and ground-glass opacity confined to lower lung zones with straight-edge (arrows) sign. (b) 3D rendering (from left to right; anterior, oblique, and lateral views) of lung parenchyma shows fibrotic lungs (orange color) composed mainly of reticulation and ground-glass opacity and normal lungs (blue). Fibrosis is more extensive in posterior lungs. Multiple coronal reformatted images are expected to show multiple different levels of straight-edge signs.
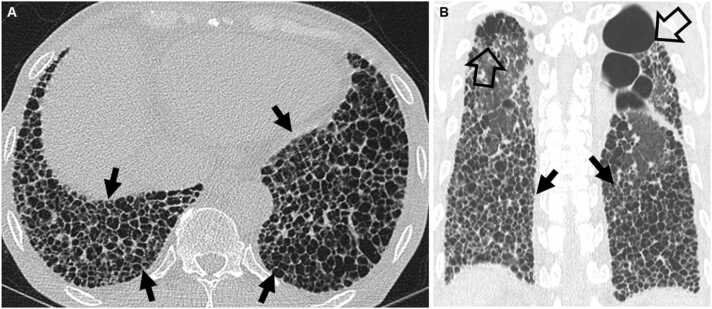
Fig. 7.
Connective tissue disease-related pulmonary fibrosis showing exuberant honeycombing sign in a 61-year-old woman with rheumatoid arthritis. (a) Lung window image of CT scan obtained at level of liver dome shows extensive honeycombing (arrows) in lower lung zones. (b) Coronal reformatted image demonstrates exuberant honeycombing in lower lung zones (arrows). Also note bullae (open arrows) in the upper lung zones.
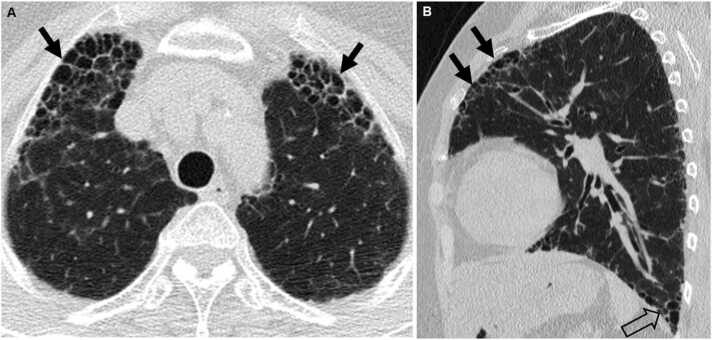
Fig. 8.
Connective tissue disease-related pulmonary fibrosis showing anterior upper lobe sign in a 59-year-old woman with rheumatoid arthritis. (a) Lung window image of CT scan obtained at level of left innominate vein demonstrates large area of honeycombing (arrows) in anterior aspects of both upper lobes. (b) Sagittal reformatted image discloses honeycombing and reticular lesions in anterior upper lobe (arrows) in left lung. Also note honeycombing lesions in subpleural portion (open arrow) of left lower lobe.
Acute exacerbation (AE) in idiopathic pulmonary fibrosis (IPF) is increasingly recognized as a relatively common clinical event with high morbidity. AE also occurs in patients with fNSIP and demonstrate better prognosis than that of IPF. In patients with CTD-ILD, AE occurs most commonly in RA-UIP and has a poor outcome [27] (Fig. 9).
Fig. 9.
Acute exacerbation of usual interstitial pneumonia in a 49-year-old man with rheumatoid arthritis. (a, b) Lung window images of CT scans obtained at levels of right inferior pulmonary vein (a) and liver dome (b), respectively, show exuberant honeycombing in middle and lower lung zones. Also note large areas of mixed consolidation and ground-glass opacity in right lung (arrowheads in a). (c, d) Coronal reformatted images demonstrate patchy and large areas of ground-glass opacity with crazy-paving appearance (arrows) and consolidation (open arrows). Also note exuberant honeycombing as underlying lung abnormality.
2.2. Pathologic findings of CTD-ILD
Pleuropulmonary involvement is common in CTD and includes a wide range of histopathologic findings that vary within each CTD and display overlap among CTDs [28]. In addition to the alveolar parenchyma (alveolar walls and airspaces), one or more of the remaining lung compartments including the pleura, airways, and pulmonary vessels, may be involved. Multi-compartment involvement is an important clue on lung biopsy and when present should bring CTD to the top of the etiologic differential as the underlying cause of findings. However, other than special findings such as necrobiotic nodules in RA, and hematoxylin bodies in SLE, histopathologic findings are rarely diagnostic in CTD [29]. Most often only nonspecific reactive patterns are identified [28], [29], the most common reactive histologic patterns by compartment are compiled in Table 1.
ILD is encountered in the majority of CTDs and is indistinguishable from the IIPs [30]. Fibrosing NSIP is the most common pattern identified across all CTDs [31], [32], with PM/DM demonstrating OP, RA demonstrating follicular bronchiolitis and occasionally OP, and SSc demonstrating cellular bronchiolitis either separately or in conjunction with fibrosing NSIP [31]. Approximately a third of patients with CTD-ILD have classifiable CTD when ILD is recognized [33], while in up to 25% of cases of CTD-ILD, clinical and serologic findings are not diagnostic of a classifiable CTD (UCTD) [34]. ILD can also precede extrathoracic manifestations of CTD by years making separation of CTD-ILD and IIP difficult [33], [34], [35].
2.3. ILA in CTD: radiologic perspective
In the recently published Fleischner Society Position Paper regarding ILA [17], the patients with CTD were not included, because the patients with CTD have known increased risk of developing ILD or ILA (Fig. 10) compared to the general population without CTD. Management of ILA in patient with CTD is different than ILA in patients without CTD. All subjects with CTD-ILA shall be actively monitored because of the known increased risk of progression with reassessment and repeated PFTs in 3–12 months.
Fig. 10.
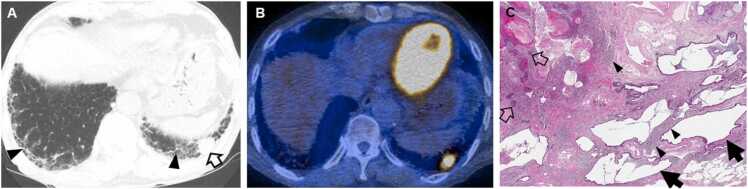
Interstitial lung abnormality and lung squamous cell carcinoma in an 80-year-old man with rheumatoid arthritis. (a) Lung window image of CT scan obtained at level of liver dome shows subpleural fibrotic interstitial lung abnormality composed of reticulation and traction bronchiolectasis (arrowheads). Also note a 22-mm-sized nodule (open arrow) in left lung base. (b) Fused CT/PET image demonstrates high FDG uptake within left lower lung zone tumor (proved to be squamous cell carcinoma). (c) Low power magnification of lung lesions obtained from left lower lobe demonstrates cystic dilation of distal small airways (arrows; microscopic honeycombing), which is consistent with UIP. But fibroblast foci are rare in the walls of the cysts. These findings are associated with patchy, moderate lymphoid infiltrates (arrowheads), but without definite lymphoid follicle. Open arrows indicate squamous cell carcinoma foci.
There are three fundamental questions on CTD-ILA: 1) How is CTD-ILA defined?; 2) When does CTD-ILA become CTD-ILD?; and 3) How is the management of CTD-ILA? The definition of CT findings for ILA in patients with CTD-ILA can be kept the same as ILA without CTD: Incidental identification of non-dependent abnormalities including ground-glass or reticular abnormality, architectural distortion, traction bronchiectasis and/or bronchiolectasis, honeycombing, and non-emphysematous cysts involving at 5% of a lung zone [15]. On the other hand, CTD-ILD is defined clinically by three criteria: 1) Respiratory symptoms or physical examination findings are possibly attributable to ILD; 2) Extensive disease on CT defined by non-trivial abnormalities present in three or more lung zones; or 3) Decrements in pulmonary function or gas exchange possibly attributable to ILD. Because there are known risks in patients with CTD, the optional management schema in patients with CTD-ILA is as follows: 1) All subjects with CTD-ILA shall be actively monitored because of the known increased risk of progression with reassessment and repeated PFTs in 3–12 months; and 2) Repeated CT at 12–24 months, or sooner if there is clinical or physiologic progression.
2.3.1. Thin-section CT findings of early ILD in CTD
In CTD-ILD, the extent of pulmonary fibrosis on initial CT is less and the progression of fibrosis is slower than IPF. In a serial CT study of SSc patients having interstitial lung disease (n = 40; mean follow-up period, 40 months), Kim et al. [36] found that the extent of disease, honeycombing and reticulation increased on follow-up CT. The increase of honeycombing correlated with decrease in diffusing capacity (DLco). The authors did not separate ILA from ILD. Wells et al. [37] (including 56 patients; 21 with IPF and 35 with SSc-UIP), found the extent of disease at presentation was 42.0% on CT in patients with IPF, whereas it was 20.8% in patients with SSc and interstitial pneumonia. Hartman et al. [38] (including 12 patients with biopsy-proved UIP) found the extent of honeycombing on initial CT occupied 12% of lung volume, which progressed to 18% of lung volume within 13 month follow-up study. However, in the study by Kim et al. [36] including 40 patients with SSc and interstitial pneumonia, the mean area of HC was 1.9% at initial CT and 5.0% at follow-up CT with a follow-up period of 39 months. The rate of progression of HC in patients with UIP is median of 0.4% of lung volume in a month, whereas the rate of HC was 0.07% per a month in the study by Kim et al. [36].
2.3.2. Interstitial lung abnormality in connective tissue disease
Clinically evident ILD occurs in 2–10% of patients with RA. ILA occurs in additional 20–60% and is associated with decrements in physiologic function. In RA patients, the detection of ILA and risk stratification shall provide a therapeutic window that could improve RA-ILD outcomes [39]. In addition, ILA in RA has been shown to be radiologically progressive in 57% of cases over a 1.5-year period [40]. The identification of progressive ILA may enable appropriate surveillance and the commencement of treatment with the goal of improving morbidity and mortality rates of established RA-ILD. Kawano-Dourado et al. [41] performed a longitudinal study in order to characterize risk factors associated with progression in RA-ILA and RA-ILD patients. Of 293 individuals with RA and clinically indicated CT studies, interstitial changes were observed in 64 (22%) of 293 individuals and the 64 were older male smokers; and one half of the 64 individuals had a respiratory complaint at the time of imaging. CT disease progression was seen in 38% over 4.4 years. Of patients with progressive ILA, one half had baseline CT scans performed for non-pulmonary indications. Subpleural distribution and higher baseline ILA and/or ILD extent were risk factors associated with progression.
2.3.3. Radiologic findings of ILA suggesting the presence of CTD
According to a study by Lucchino et al. [42], in 48 subjects (21 ACPA-positive subjects without arthritis, 10 early [disease duration < 6 months, treatment-naive] and 17 long-standing [disease duration < 36 months, on treatment]) with anti-citrullinated proteins antibodies (ACPA)-positive rheumatoid arthritis (RA), there is a subclinical, early lung involvement during the course of the disease RA, even before the onset of articular manifestation. Of the entire cohort of 48 ACPA-positive RA subjects, 30 (62.5%) had TSCT abnormalities [42]. The most frequently detected abnormality was nodules (24, 50%), followed by evidence of fibrosis (14, 29.1%). The less frequent abnormality was the presence of air trapping (4, 8.3%). There were no differences in the frequency of the various TSCT abnormalities according to smoking status; or, among long-standing RA patients, according to treatment with methotrexate. Even though detailed CT findings were not analyzed, the fibrosis represents the areas of reticulation and traction bronchiolectasis (subpleural fibrotic ILA) [42], [43]. In the study of Lucchino et al. [42], subgroup analysis revealed significantly higher rates of overall TSCT abnormalities, nodules, emphysema and fibrosis among longstanding RA patients compared with the other subgroups. Current and former smokers showed a significantly higher frequency of fibrosis compared with subjects who never smoked, with a relative risk of 2.77 (CI 95% 1.054–8.359). Of note, no difference in fibrosis prevalence was found based on methotrexate treatment. Thus, in subjects with (ACPA)-positive RA subjects, TSCT features of nodules and airway disease in addition to fibrotic ILA (reticular lesions and traction bronchiolectasis) may suggest the presence of underlying CTD (RA).
2.4. Evaluation and monitoring of ILD in CTD
2.4.1. General principles in evaluation of ILD (ATS/ERS IPF guidelines)
The treatment modality and choice of drugs as well as treatment response and survival for ILD is largely affected by the presence and types of CTD [11], [12]. Therefore, the current international guidelines recommend detailed history taking and physical examination to identify potential etiologies of ILD including environmental exposures and medication use as well as systemic diseases such as CTD as the key step in evaluation of newly detected ILD [13], [14], [44].
When the presence of CTD is suspicious, history taking and physical examination for symptoms and signs of CTD such as inflammatory arthritis, digital fissuring or tip ulceration, fixed rash on the digital extensor surfaces, or Raynaud’s phenomenon is necessary [45]. Regarding laboratory tests, the recent guideline on diagnosis of IPF by ATS/ERS/JRS/ALAT recommends serologic testing to identify or exclude CTD in newly detected ILD without apparent causes [13]. However, inclusion and exclusion of specific autoantibodies among various serological tests was not addressed in the guideline due to lack of evidence. The consensus research statement of ATS/ERS on IPAF may give us a clue as for including various autoantibodies in clinical practice which incorporate antinuclear antibody, rheumatoid factor, anti-CCP, anti-dsDNA, anti-SS-A, anti-SS-B, anti-ribonuclear protein, anti-smith, anti-topoisomerase, anti-tRNA synthetase, anti-PM-Scl, and anti-MDA-5 as serologic domain of diagnostic criteria [45]. Nonetheless, the utility of such serological testing as well as inclusion and exclusion of specific autoantibodies requires further validation.
Unlike IIP, the current guidelines do not recommend either for or against the lung biopsy in patients with CTD-ILD. Despite that histopathologic pattern is predictive of survival in CTD-ILD [46], [47], owing to the facts that both treatment and prognosis are strongly determined by other factors such as type of underlying CTD, extensiveness of the disease, or lung function and that TSCT pattern is well correlated with that of pathology we do not recommend strongly the surgical lung biopsy. And the potential risk of biopsy should be weighed before making a decision for obtaining lung pathologic specimens [11], [48], [49], [50].
2.4.2. ILA or ILD in pre-existing CTD: what should we do?
Due to the presence of certain predilections of radiologic and/or histopathologic patterns for specific CTD, it may be helpful to correlate the radiologic and/or histopathologic patterns with the patient’s underlying CTD to enhance the diagnostic certainty. Pulmonary function test should be performed, when possible, to evaluate the severity of the disease and to help decision for initiation of treatment and prediction of prognosis [49].
In clinical practice, physicians may encounter CTD patients with incidentally found abnormalities on chest TSCT scan without definite symptoms. These abnormalities have been frequently referred as “subclinical” or “preclinical” ILD (ILA). The prevalence and incidence of such cases is not precisely known owing to its vague definition; however, it is rather common. In a study by Gochuico et al. [40], 33% (21 of 64) of RA patients without pulmonary symptoms had preclinical ILD on TSCT scans. Among 21 patients, 12 (57%) patients progressed to RA-ILD. In another study, 61 (59%) out of 103 consecutive RA patients had RA-ILD among whom 57 (90%) lacked respiratory symptoms [51]. Of 52 patients with early (disease duration, < 36 months) SSc, at baseline visit, 40% (21 of 52) patients had TSCT abnormalities. Patients without CT abnormalities at baseline had a shorter disease duration (9 ± 7 months versus 14 ± 12 months). After 42 months, 8 (15%) of 52 patients, including 3 patients with normal TSCT findings at baseline, died due to SSc-related manifestations. Progression of lung fibrosis occurred in 16 (31%) patients at month 42, including 7 patients with normal CT at baseline. No clear predictors of progression could be identified [52]. When 37 patients with primary SS with normal chest radiographs were evaluated, 24 (65%) manifested abnormal TSCT findings [53]. TSCT abnormalities were found in seven patients with normal PFT. In anti-Jo-1 antibody (for making the diagnosis of antisynthetase syndrome)-positive patients, ILD was present in 86% (77 of 90) of cohort when analyzing clinical, radiologic, and/or pulmonary function data, suggesting that autoantibody may be a marker for the presence of ILD [54].
Optimal evaluation and management strategies for these patients are not yet established. Since treatment for CTD-ILD without symptoms or lung function abnormality is regarded as unnecessary, focus of evaluation should lie on identifying patients who would have disease progression eventually requiring treatment. Male gender, smoking, diffuse SSc compared to limited SSc, presence of circulating anti-SCL-70 antibody or anti-Jo-1 antibody, decrease in diffusing capacity, use of certain drug (e.g., methotrexate) have been reported as factors related to the presence or progression of preclinical or subclinical ILD (ILA) [40], [51], [53], [54]. However, additional studies are necessary to confirm the risk factors of disease progression, as well as the optimal intervals of follow-up studies and timing of treatment commencement. Furthermore, although it is not fully compatible with the definition of ILA, population-based studies have demonstrated that certain TSCT pattern like subpleural fibrotic ILA is prone to disease progression [17], [54]. Considering the fact that both concepts of preclinical or subclinical ILD and ILA are derived from CT definition, the significance of applying the definition of ILA and its proposed classification and management protocol on CTD-ILA might be meaningful and would need to be tested with further studies.
2.4.3. When do you suspect presence of CTD when encountering ILA/ILD features on TSCT?
It has been suggested that up to 20% of patients who are diagnosed with chronic ILD without overt CTD at the time of diagnosis may develop CTD during follow-up [35], [55]. However, there was no difference in clinical or laboratory characteristics between patients who developed CTD and who did not. Currently, there is no algorithm or protocol for predicting patients who will develop CTD. Given the fact that certain characteristics differ between IIP and CTD-ILD, it may be reasonable to examine patients based on such differences. Regarding demographic features, patients with CTD-ILD are younger, never smokers, and more likely to be women [11]. Thus, ILD patients presenting with such clinical characteristics should raise a suspicion for concomitantly having occult CTD, and thus comprehensive evaluation should be followed. Radiologically, fibrosing NSIP is the most frequently associated pattern with CTDs of SSc, SS, and PM/DM, necessitating assessment for the presence of CTD [10]. In contrast, UIP pattern is the most common pattern of RA-ILD. Moreover, although not confirmed in patients with ILD, the presence of autoantibodies before clinical manifestations of SLE or RA have been noted in CTD [56], [57]. This demographic, radiologic and/or histopathologic, and laboratory characteristics should be considered in patients with a newly diagnosed ILD.
2.5. Current management of CTD-related ILD
Clinical trial data on pharmacological treatment in CTD-ILDs focused on pulmonary outcome are tabulated in SUPPLEMENT Table 1 with SUPPLEMENT references.
It is difficult to establish the choice of drug for treating the whole types of CTD-ILDs. Because each CTD has different predilection for organ involvement and is unknown to share the pathophysiology of the related ILD in common, there have been studies focusing on each specific type of ILD-prone CTD such as SSc. Furthermore, the diagnosis of CTD-ILD does not always prompt treatment, given the fact that the disease may not progress rapidly, and treatment benefit and treatment-related complications should be balanced. Although the guidelines are not available, it is usually recommended that asymptomatic CTD-ILD patients with normal lung function and without evidence of disease progression may be followed-up without treatment. Immunosuppressive or antifibrotic agents for symptomatic and/or fibrosing CTD-ILD can be selected in patients who require treatment.
3. Conclusion
When encountering individuals with ILD or ILA features on CT, the presence of certain demographic (younger ages, never smokers, and women), histopathologic (fibrosing NSIP), or laboratory (autoantibodies) characteristics, namely the characteristics favoring CTD-ILD than idiopathic ILD or ILA, recalls suspicion of their having CTD. The incidence of ILDs related to CTD is greatest among patients with SSc followed by DM/PM, SS, RA and SLE. Even though proportions of ILDs vary histologically, NSIP pattern accounts for a large proportion, especially in SSc, DM/PM and MCTD. IPAF (alternatively called UCTD; please note SUPPLEMENT text) is defined as ILD in subjects with clinical, serologic and/or morphologic features of autoimmunity without characteristic CTD. In IPAF subjects, on TSCT or surgical lung biopsy, ILDs of similar patterns are identified as in CTD-ILD. In patients with CTD-ILD and UIP pattern, straight edge sign, exuberant HC sign and anterior upper lobe sign are significantly more common on CT than in those with IPF and UIP pattern. The presence of CTD-ILA can be determined with CT findings of ILA in CTD patients as is determined in subjects without CTD. However, it may be assumed that the potential management scheme of CTD-ILA is modified in consideration of the extent and stage of CTD per se. ILAs in RA patients are shown to be radiologically progressive in approximately 35–45% of them. Subpleural distribution and higher baseline ILA extent are risk factors associated with progression. It is usually recommended that asymptomatic CTD-ILD patients with normal lung function and without evidence of disease progression is followed-up without treatment. Immunosuppressive or antifibrotic agents for symptomatic and/or fibrosing CTD-ILD can be selected in patients who require treatment.
Ethical statement
None in all authors.
Funding statement
None.
Acknowledgments
We are grateful to Ms. Young Joo Moon, transcriptionist, Samsung Changwon Hospital, Changwon, Gyeongsangnam-do, South Korea, for her dedicated support formatting this manuscript.
Conflict of interest
None in all authors.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.ejro.2022.100419.
Appendix A. Supplementary material
Supplementary material
.
References
- 1.American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001, Am J Respir Crit Care Med 165 (2002) 277–304. https://doi.org/10.1164/ajrccm.165.2.ats01. [DOI] [PubMed]
- 2.Lederer D.J., Martinez F.J. Idiopathic pulmonary fibrosis. New Engl. J. Med. 2018;378:1811–1823. doi: 10.1056/NEJMra1705751. [DOI] [PubMed] [Google Scholar]
- 3.Ryerson C.J., Collard H.R. Update on the diagnosis and classification of ILD. Curr. Opin. Pulm. Med. 2013;19:453–459. doi: 10.1097/MCP.0b013e328363f48d. [DOI] [PubMed] [Google Scholar]
- 4.Lee C.T., Oldham J.M. Interstitial pneumonia with autoimmune features: overview of proposed criteria and recent cohort characterization. Clin. Pulm. Med. 2017;24:191–196. doi: 10.1097/CPM.0000000000000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fischer A., du Bois R. Interstitial lung disease in connective tissue disorders. Lancet. 2012;380:689–698. doi: 10.1016/S0140-6736(12)61079-4. [DOI] [PubMed] [Google Scholar]
- 6.Mira-Avendano I., Abril A., Burger C.D., Dellaripa P.F., Fischer A., Gotway M.B., Lee A.S., Lee J.S., Matteson E.L., Yi E.S., Ryu J.H. Interstitial lung disease and other pulmonary manifestations in connective tissue diseases. Mayo Clin. Proc. 2019;94:309–325. doi: 10.1016/j.mayocp.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Vacchi C., Sebastiani M., Cassone G., Cerri S., Della Casa G., Salvarani C., Manfredi A. Therapeutic options for the treatment of interstitial lung disease related to connective tissue diseases. A narrative review. J. Clin. Med. 2020;9 doi: 10.3390/jcm9020407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jeganathan N., Sathananthan M. Connective tissue disease-related interstitial lung disease: prevalence, patterns, predictors, prognosis, and treatment. Lung. 2020;198:735–759. doi: 10.1007/s00408-020-00383-w. [DOI] [PubMed] [Google Scholar]
- 9.Wijsenbeek M., Cottin V. Spectrum of fibrotic lung diseases. New Engl. J. Med. 2020;383:958–968. doi: 10.1056/NEJMra2005230. [DOI] [PubMed] [Google Scholar]
- 10.Kim E.A., Lee K.S., Johkoh T., Kim T.S., Suh G.Y., Kwon O.J., Han J. Interstitial lung diseases associated with collagen vascular diseases: radiologic and histopathologic findings. Radiographics. 2002:S151–S165. doi: 10.1148/radiographics.22.suppl_1.g02oc04s151. [DOI] [PubMed] [Google Scholar]
- 11.Park J.H., Kim D.S., Park I.N., Jang S.J., Kitaichi M., Nicholson A.G., Colby T.V. Prognosis of fibrotic interstitial pneumonia: idiopathic versus collagen vascular disease-related subtypes. Am. J. Respir. Crit. Care Med. 2007;175:705–711. doi: 10.1164/rccm.200607-912OC. [DOI] [PubMed] [Google Scholar]
- 12.Navaratnam V., Ali N., Smith C.J., McKeever T., Fogarty A., Hubbard R.B. Does the presence of connective tissue disease modify survival in patients with pulmonary fibrosis? Respir. Med. 2011;105:1925–1930. doi: 10.1016/j.rmed.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 13.Raghu G., Remy-Jardin M., Myers J.L., Richeldi L., Ryerson C.J., Lederer D.J., Behr J., Cottin V., Danoff S.K., Morell F., Flaherty K.R., Wells A., Martinez F.J., Azuma A., Bice T.J., Bouros D., Brown K.K., Collard H.R., Duggal A., Galvin L., Inoue Y., Jenkins R.G., Johkoh T., Kazerooni E.A., Kitaichi M., Knight S.L., Mansour G., Nicholson A.G., Pipavath S.N.J., Buendia-Roldan I., Selman M., Travis W.D., Walsh S., Wilson K.C., American Thoracic Society E.R.S.J.R.S., Latin American Thoracic S. Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am. J. Respir. Crit. Care Med. 2018;198:e44–e68. doi: 10.1164/rccm.201807-1255ST. [DOI] [PubMed] [Google Scholar]
- 14.Bradley B., Branley H.M., Egan J.J., Greaves M.S., Hansell D.M., Harrison N.K., Hirani N., Hubbard R., Lake F., Millar A.B., Wallace W.A., Wells A.U., Whyte M.K., Wilsher M.L., British Thoracic Society Interstitial Lung Disease Guideline Group B.T.S.So.C.C., Thoracic Society of A., New Zealand Thoracic S., Irish Thoracic S. Interstitial lung disease guideline: the British Thoracic Society in collaboration with the Thoracic Society of Australia and New Zealand and the Irish Thoracic Society. Thorax. 2008;63(Suppl 5):v1–v58. doi: 10.1136/thx.2008.101691. [DOI] [PubMed] [Google Scholar]
- 15.Hatabu H., Hunninghake G.M., Lynch D.A. Interstitial lung abnormality: recognition and perspectives. Radiology. 2019;291:1–3. doi: 10.1148/radiol.2018181684. [DOI] [PubMed] [Google Scholar]
- 16.Hata A., Schiebler M.L., Lynch D.A., Hatabu H. Interstitial lung abnormalities: state of the art. Radiology. 2021;301:19–34. doi: 10.1148/radiol.2021204367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hatabu H., Hunninghake G.M., Richeldi L., Brown K.K., Wells A.U., Remy-Jardin M., Verschakelen J., Nicholson A.G., Beasley M.B., Christiani D.C., San Jose Estepar R., Seo J.B., Johkoh T., Sverzellati N., Ryerson C.J., Graham Barr R., Goo J.M., Austin J.H.M., Powell C.A., Lee K.S., Inoue Y., Lynch D.A. Interstitial lung abnormalities detected incidentally on CT: a position paper from the Fleischner Society. Lancet Respir. Med. 2020;8:726–737. doi: 10.1016/S2213-2600(20)30168-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mayo J.R. CT evaluation of diffuse infiltrative lung disease: dose considerations and optimal technique. J. Thorac. Imaging. 2009;24:252–259. doi: 10.1097/RTI.0b013e3181c227b2. [DOI] [PubMed] [Google Scholar]
- 19.Verschakelen J.A. The role of high-resolution computed tomography in the work-up of interstitial lung disease. Curr. Opin. Pulm. Med. 2010;16:503–510. doi: 10.1097/MCP.0b013e32833cc997. [DOI] [PubMed] [Google Scholar]
- 20.Bartholmai B.J., Raghunath S., Karwoski R.A., Moua T., Rajagopalan S., Maldonado F., Decker P.A., Robb R.A. Quantitative computed tomography imaging of interstitial lung diseases. J. Thorac. Imaging. 2013;28:298–307. doi: 10.1097/RTI.0b013e3182a21969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Travis W.D., Hunninghake G., King T.E., Jr., Lynch D.A., Colby T.V., Galvin J.R., Brown K.K., Chung M.P., Cordier J.F., du Bois R.M., Flaherty K.R., Franks T.J., Hansell D.M., Hartman T.E., Kazerooni E.A., Kim D.S., Kitaichi M., Koyama T., Martinez F.J., Nagai S., Midthun D.E., Muller N.L., Nicholson A.G., Raghu G., Selman M., Wells A. Idiopathic nonspecific interstitial pneumonia: report of an American Thoracic Society project. Am. J. Respir. Crit. Care Med. 2008;177:1338–1347. doi: 10.1164/rccm.200611-1685OC. [DOI] [PubMed] [Google Scholar]
- 22.Travis W.D., Costabel U., Hansell D.M., King T.E., Jr., Lynch D.A., Nicholson A.G., Ryerson C.J., Ryu J.H., Selman M., Wells A.U., Behr J., Bouros D., Brown K.K., Colby T.V., Collard H.R., Cordeiro C.R., Cottin V., Crestani B., Drent M., Dudden R.F., Egan J., Flaherty K., Hogaboam C., Inoue Y., Johkoh T., Kim D.S., Kitaichi M., Loyd J., Martinez F.J., Myers J., Protzko S., Raghu G., Richeldi L., Sverzellati N., Swigris J., Valeyre D., Pneumonias A.E.Co.I.I. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am. J. Respir. Crit. Care Med. 2013;188:733–748. doi: 10.1164/rccm.201308-1483ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henriet A.C., Diot E., Marchand-Adam S., de Muret A., Favelle O., Crestani B., Diot P. Organising pneumonia can be the inaugural manifestation in connective tissue diseases, including Sjogren’s syndrome. Eur. Respir. Rev. 2010;19:161–163. doi: 10.1183/09059180.00002410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pan L., Liu Y., Sun R., Fan M., Shi G. Comparison of characteristics of connective tissue disease-associated interstitial lung diseases, undifferentiated connective tissue disease-associated interstitial lung diseases, and idiopathic pulmonary fibrosis in Chinese Han population: a retrospective study. Clin. Dev. Immunol. 2013;2013 doi: 10.1155/2013/121578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chung J.H., Cox C.W., Montner S.M., Adegunsoye A., Oldham J.M., Husain A.N., Vij R., Noth I., Lynch D.A., Strek M.E. CT features of the usual interstitial pneumonia pattern: differentiating connective tissue disease-associated interstitial lung disease from idiopathic pulmonary fibrosis. AJR Am. J. Roentgenol. 2018;210:307–313. doi: 10.2214/AJR.17.18384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamakawa H., Ogura T., Sato S., Nishizawa T., Kawabe R., Oba T., Kato A., Horikoshi M., Akasaka K., Amano M., Kuwano K., Sasaki H., Baba T., Matsushima H. The potential utility of anterior upper lobe honeycomb-like lesion in interstitial lung disease associated with connective tissue disease. Respir. Med. 2020;172 doi: 10.1016/j.rmed.2020.106125. [DOI] [PubMed] [Google Scholar]
- 27.Park I.N., Kim D.S., Shim T.S., Lim C.M., Lee S.D., Koh Y., Kim W.S., Kim W.D., Jang S.J., Colby T.V. Acute exacerbation of interstitial pneumonia other than idiopathic pulmonary fibrosis. Chest. 2007;132:214–220. doi: 10.1378/chest.07-0323. [DOI] [PubMed] [Google Scholar]
- 28.Schneider F., Gruden J., Tazelaar H.D., Leslie K.O. Pleuropulmonary pathology in patients with rheumatic disease. Arch. Pathol. Lab Med. 2012;136:1242–1252. doi: 10.5858/arpa.2012-0248-SA. [DOI] [PubMed] [Google Scholar]
- 29.Colby T.V. Pulmonary pathology in patients with systemic autoimmune diseases. Clin. Chest Med. 1998;19:587–612. doi: 10.1016/s0272-5231(05)70105-2. [DOI] [PubMed] [Google Scholar]
- 30.Vivero M., Padera R.F. Histopathology of lung disease in the connective tissue diseases. Rheum. Dis. Clin. North Am. 2015;41:197–211. doi: 10.1016/j.rdc.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 31.Tansey D., Wells A.U., Colby T.V., Ip S., Nikolakoupolou A., du Bois R.M., Hansell D.M., Nicholson A.G. Variations in histological patterns of interstitial pneumonia between connective tissue disorders and their relationship to prognosis. Histopathology. 2004;44:585–596. doi: 10.1111/j.1365-2559.2004.01896.x. [DOI] [PubMed] [Google Scholar]
- 32.Solomon J.J., Fischer A. Connective tissue disease-associated interstitial lung disease: a focused review. J. Intensive Care Med. 2015;30:392–400. doi: 10.1177/0885066613516579. [DOI] [PubMed] [Google Scholar]
- 33.Oliveira R.P., Ribeiro R., Melo L., Grima B., Oliveira S., Alves J.D. Connective tissue disease-associated interstitial lung disease. Pulmonology. 2020 doi: 10.1016/j.pulmoe.2020.01.004. [DOI] [PubMed] [Google Scholar]
- 34.Gutsche M., Rosen G.D., Swigris J.J. Connective tissue disease-associated interstitial lung disease: a review. Curr. Respir. Care Rep. 2012;1:224–232. doi: 10.1007/s13665-012-0028-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Homma Y., Ohtsuka Y., Tanimura K., Kusaka H., Munakata M., Kawakami Y., Ogasawara H. Can interstitial pneumonia as the sole presentation of collagen vascular diseases be differentiated from idiopathic interstitial pneumonia? Respiration. 1995;62:248–251. doi: 10.1159/000196457. [DOI] [PubMed] [Google Scholar]
- 36.Kim E.A., Johkoh T., Lee K.S., Ichikado K., Koh E.M., Kim T.S., Kim E.Y. Interstitial pneumonia in progressive systemic sclerosis: serial high-resolution CT findings with functional correlation. J. Comput. Assist Tomogr. 2001;25:757–763. doi: 10.1097/00004728-200109000-00015. [DOI] [PubMed] [Google Scholar]
- 37.Wells A.U., Rubens M.B., du Bois R.M., Hansell D.M. Serial CT in fibrosing alveolitis: prognostic significance of the initial pattern. AJR Am. J. Roentgenol. 1993;161:1159–1165. doi: 10.2214/ajr.161.6.8249719. [DOI] [PubMed] [Google Scholar]
- 38.Hartman T.E., Primack S.L., Kang E.Y., Swensen S.J., Hansell D.M., McGuinness G., Muller N.L. Disease progression in usual interstitial pneumonia compared with desquamative interstitial pneumonia. Assess. Ser. CT Chest. 1996;110:378–382. doi: 10.1378/chest.110.2.378. [DOI] [PubMed] [Google Scholar]
- 39.Doyle T.J., Dellaripa P.F., Batra K., Frits M.L., Iannaccone C.K., Hatabu H., Nishino M., Weinblatt M.E., Ascherman D.P., Washko G.R., Hunninghake G.M., Choi A.M.K., Shadick N.A., Rosas I.O. Functional impact of a spectrum of interstitial lung abnormalities in rheumatoid arthritis. Chest. 2014;146:41–50. doi: 10.1378/chest.13-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gochuico B.R., Avila N.A., Chow C.K., Novero L.J., Wu H.P., Ren P., MacDonald S.D., Travis W.D., Stylianou M.P., Rosas I.O. Progressive preclinical interstitial lung disease in rheumatoid arthritis. Arch. Intern Med. 2008;168:159–166. doi: 10.1001/archinternmed.2007.59. [DOI] [PubMed] [Google Scholar]
- 41.Kawano-Dourado L., Doyle T.J., Bonfiglioli K., Sawamura M.V.Y., Nakagawa R.H., Arimura F.E., Lee H.J., Rangel D.A.S., Bueno C., Carvalho C.R.R., Sabbag M.L., Molina C., Rosas I.O., Kairalla R.A. Baseline characteristics and progression of a spectrum of interstitial lung abnormalities and disease in rheumatoid arthritis. Chest. 2020;158:1546–1554. doi: 10.1016/j.chest.2020.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lucchino B., Di Paolo M., Gioia C., Vomero M., Diacinti D., Mollica C., Alessandri C., Diacinti D., Palange P., Di Franco M. Identification of subclinical lung involvement in ACPA-positive subjects through functional assessment and serum biomarkers. Int J. Mol. Sci. 2020;21 doi: 10.3390/ijms21145162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Doyle T.J., Hunninghake G.M., Rosas I.O. Subclinical interstitial lung disease: why you should care. Am. J. Respir. Crit. Care Med. 2012;185:1147–1153. doi: 10.1164/rccm.201108-1420PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cottin V. Significance of connective tissue diseases features in pulmonary fibrosis. Eur. Respir. Rev. 2013;22:273–280. doi: 10.1183/09059180.00003013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fischer A., Antoniou K.M., Brown K.K., Cadranel J., Corte T.J., du Bois R.M., Lee J.S., Leslie K.O., Lynch D.A., Matteson E.L., Mosca M., Noth I., Richeldi L., Strek M.E., Swigris J.J., Wells A.U., West S.G., Collard H.R., Cottin V., CTD-ILD E.A.T.Fo.U.Fo. An official European Respiratory Society/American Thoracic Society research statement: interstitial pneumonia with autoimmune features. Eur. Respir. J. 2015;46:976–987. doi: 10.1183/13993003.00150-2015. [DOI] [PubMed] [Google Scholar]
- 46.Fischer A., Swigris J.J., Groshong S.D., Cool C.D., Sahin H., Lynch D.A., Curran-Everett D., Gillis J.Z., Meehan R.T., Brown K.K. Clinically significant interstitial lung disease in limited scleroderma: histopathology, clinical features, and survival. Chest. 2008;134:601–605. doi: 10.1378/chest.08-0053. [DOI] [PubMed] [Google Scholar]
- 47.Nakamura Y., Suda T., Kaida Y., Kono M., Hozumi H., Hashimoto D., Enomoto N., Fujisawa T., Inui N., Imokawa S., Yasuda K., Shirai T., Suganuma H., Morita S., Hayakawa H., Takehara Y., Colby T.V., Chida K. Rheumatoid lung disease: prognostic analysis of 54 biopsy-proven cases. Respir. Med. 2012;106:1164–1169. doi: 10.1016/j.rmed.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 48.Bouros D., Wells A.U., Nicholson A.G., Colby T.V., Polychronopoulos V., Pantelidis P., Haslam P.L., Vassilakis D.A., Black C.M., du Bois R.M. Histopathologic subsets of fibrosing alveolitis in patients with systemic sclerosis and their relationship to outcome. Am. J. Respir. Crit. Care Med. 2002;165:1581–1586. doi: 10.1164/rccm.2106012. [DOI] [PubMed] [Google Scholar]
- 49.Winstone T.A., Assayag D., Wilcox P.G., Dunne J.V., Hague C.J., Leipsic J., Collard H.R., Ryerson C.J. Predictors of mortality and progression in scleroderma-associated interstitial lung disease: a systematic review. Chest. 2014;146:422–436. doi: 10.1378/chest.13-2626. [DOI] [PubMed] [Google Scholar]
- 50.Tsuchiya Y., Takayanagi N., Sugiura H., Miyahara Y., Tokunaga D., Kawabata Y., Sugita Y. Lung diseases directly associated with rheumatoid arthritis and their relationship to outcome. Eur. Respir. J. 2011;37:1411–1417. doi: 10.1183/09031936.00019210. [DOI] [PubMed] [Google Scholar]
- 51.Chen J., Shi Y., Wang X., Huang H., Ascherman D. Asymptomatic preclinical rheumatoid arthritis-associated interstitial lung disease. Clin. Dev. Immunol. 2013;2013 doi: 10.1155/2013/406927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vanaken L., Landini N., Lenaerts J., Claeys E., Lenaerts J., Wuyts W.A., Verschakelen J., De Langhe E. Progressive lung fibrosis and mortality can occur in early systemic sclerosis patients without pulmonary abnormalities at baseline assessment. Clin. Rheuma. 2020;39:3393–3400. doi: 10.1007/s10067-020-05105-4. [DOI] [PubMed] [Google Scholar]
- 53.Uffmann M., Kiener H.P., Bankier A.A., Baldt M.M., Zontsich T., Herold C.J. Lung manifestation in asymptomatic patients with primary Sjogren syndrome: assessment with high resolution CT and pulmonary function tests. J. Thorac. Imaging. 2001;16:282–289. doi: 10.1097/00005382-200110000-00009. [DOI] [PubMed] [Google Scholar]
- 54.Richards T.J., Eggebeen A., Gibson K., Yousem S., Fuhrman C., Gochuico B.R., Fertig N., Oddis C.V., Kaminski N., Rosas I.O., Ascherman D.P. Characterization and peripheral blood biomarker assessment of anti-Jo-1 antibody-positive interstitial lung disease. Arthritis Rheum. 2009;60:2183–2192. doi: 10.1002/art.24631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pereira D.A., Dias O.M., Almeida G.E., Araujo M.S., Kawano-Dourado L.B., Baldi B.G., Kairalla R.A., Carvalho C.R. Lung-dominant connective tissue disease among patients with interstitial lung disease: prevalence, functional stability, and common extrathoracic features. J. Bras. Pneumol. 2015;41:151–160. doi: 10.1590/S1806-37132015000004443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Arbuckle M.R., McClain M.T., Rubertone M.V., Scofield R.H., Dennis G.J., James J.A., Harley J.B. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. New Engl. J. Med. 2003;349:1526–1533. doi: 10.1056/NEJMoa021933. [DOI] [PubMed] [Google Scholar]
- 57.Nielen M.M., van Schaardenburg D., Reesink H.W., van de Stadt R.J., van der Horst-Bruinsma I.E., de Koning M.H., Habibuw M.R., Vandenbroucke J.P., Dijkmans B.A. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum. 2004;50:380–386. doi: 10.1002/art.20018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material