Abstract
Irinotecan is an anticancer agent widely used for the treatment of solid tumors, including colorectal and pancreatic cancers. Severe neutropenia and diarrhea are common dose-limiting toxicities of irinotecan-based therapy, and UGT1A1 polymorphisms are one of the major risk factors of these toxicities. In 2005, the US Food and Drug Administration revised the drug label to indicate that patients with UGT1A1*28 homozygous genotype should receive a decreased dose of irinotecan. However, UGT1A1*28 testing is not routinely used in the clinic, and specific reasons include lack of access to concise information on this wide issue as well as mixed recommendations by regulatory and professional entities. To assist oncologists in assessing whether and when to use UGT1A1 genetic testing in patients receiving irinotecan-based therapies, this article provided (1) essential knowledge of UGT1A1 polymorphisms; (2) an update on the impact of UGT1A1 polymorphisms on efficacy and toxicity of contemporary irinotecan-based regimens; (3) dosing adjustments based upon the UGT1A1 genotypes, and (4) recommendations from currently available guidelines from the US and international scientific consortia and major oncology societies.
INTRODUCTION
Irinotecan is an anticancer agent widely used to treat solid tumors, including colorectal and pancreatic cancers.1 The most common dose-limiting toxicities are severe neutropenia and late-onset diarrhea that may require treatment interruption or dose reduction.1,2 The irinotecan-induced toxicities are mainly because of the active metabolite, SN-38, with the potential contribution of irinotecan.2 The UGT1A1*28 polymorphism reduces the metabolism of SN-38, thereby affecting the risk of irinotecan-induced toxicities.3 In 2005, the US Food and Drug Administration (FDA) revised the irinotecan label to indicate that a reduced initial dose should be considered for the UGT1A1*28 homozygous patients,4 but this is not routinely implemented in the clinic.5 Over almost two decades, the clinical impact of UGT1A1 polymorphisms on toxicity and efficacy of irinotecan-based therapy has been extensively studied, yet the clinical context in which this test can be used remains to be established.
This review is focused on the clinical utility of the UGT1A1 genetic testing in irinotecan-based therapy. This document is not intended to proclaim the benefit of using UGT1A1 genetic information in irinotecan therapy but rather to provide oncologists concise, up-to-date information that will assist clinical decision making as to whether and when to use UGT1A1 genetic testing in patients receiving irinotecan therapy.
Drug Information
Irinotecan is a prodrug with topoisomerase I inhibitory activity.2 Irinotecan HCl (Camptosar, Pfizer, New York, NY) is a conventional form of irinotecan that is FDA-approved for advanced metastatic colorectal cancer in combination with fluorouracil (5-FU) and leucovorin (FOLFIRI).1 Irinotecan liposome (Onivyde, Merrimack Pharmaceuticals, Cambridge, MA) is irinotecan encapsulated in liposomes approved for the treatment of metastatic pancreatic cancer in combination with 5-FU and leucovorin.6 Sacituzumab govitecan-hziy (Trodelvy, Immunomedics, Morris Plains, NJ) is an antibody-SN-38 conjugate approved for metastatic triple-negative breast cancer.7
Prevalence of Irinotecan Toxicity and Patient Characteristics Increasing Risk
The most common irinotecan toxicities are severe neutropenia and diarrhea.1,8 Approximately 20%-54% of patients treated with irinotecan experience severe (Common Terminology Criteria for Adverse Events grade 3-4) neutropenia, and 11%-23% experience severe diarrhea.1,6,9 In addition, 8%-23% of patients are hospitalized because of these toxicities.1,10 Irinotecan toxicities are highly unpredictable and increase morbidity and mortality.11,12 Risk factors include age > 65 years,13,14 female sex,15 poor performance status (≥ 2),16,17 impaired liver function,16 coadministration of CYP3A418 and/or UGT1A1 inhibitors, and reduced UGT1A1 enzyme activity.1,19
The Prevalence of Toxicity-Related Mortality from Irinotecan in the United States
In 2021, 149,500 individuals in the United States are predicted to be diagnosed with colorectal cancer, and 32,890 (22%) of them are candidates for irinotecan therapy because they are predicted to present with advanced and/or metastatic disease.20 The prevalence of irinotecan toxicity-related mortality is < 1% across different irinotecan-based treatment regimens: 0.3% for FOLFIRI,21 0.44% for FOLFIRINOX (5-FU, leucovorin, irinotecan, and oxaliplatin),22 0.6% for XELIRI (capecitabine and irinotecan).21
Disposition of Irinotecan and SN-38 and Relationship with Toxicity
The most clinically relevant metabolic steps for irinotecan are as follows. Irinotecan is hydrolyzed to SN-38 in the liver.23 SN-38 is then subsequently metabolized to SN-38 glucuronide (SN-38G) in the liver via glucuronidation mainly by UGT1A1.24 SN-38G excreted into the bile can be deconjugated back into SN-38 via bacterial β-glucuronidases.2,25,26
Increasing concentrations of SN-38 in plasma are the main determinants of neutropenia, although the contribution of increased exposure to irinotecan cannot be entirely ruled out.27 Late-onset diarrhea is primarily because of excessive SN-38 accumulation in the intestine.26-28
UGT1A1 Deficiency: Symptoms, Laboratory Alterations, and Prevalence in the General Population
UGT1A1 deficiency is related to decreased UGT1A1 expression or enzymatic activity leading to reduced conversion of lipophilic molecules (eg, bilirubin, SN-38, etc) into water-soluble metabolites that can be eliminated through the bile and urine.29 Gilbert's syndrome is an inherited UGT1A1 deficiency (approximately 30% reduction in UGT1A1 function) caused by polymorphisms in the UGT1A1 gene (eg, UGT1A1*28/*28) and characterized by increased unconjugated bilirubin in the blood. Gilbert's syndrome is usually asymptomatic and occurs in 5%-10% of the US population.30
UGT1A1 Polymorphisms Conferring Increased Risk of Irinotecan Toxicity: Nomenclature and Population Frequency
The list of UGT1A1 polymorphisms is available at PharmGKB.31 The most studied and clinically relevant UGT1A1 polymorphisms are UGT1A1*28, UGT1A1*93, and UGT1A1*6 (Table 1). Patients with these polymorphisms have an increased risk of severe irinotecan toxicity partially because of impaired clearance of SN-38 by UGT1A1-mediated metabolism.3,15,32,33 Using the star nomenclature, UGT1A1*1 contains six thymine-adenine (TA) repeats in the gene promoter and is associated with normal UGT1A1 enzyme activity and expression.34
TABLE 1.
Common UGT1A1 Variants and Their Impact on UGT1A1 Function
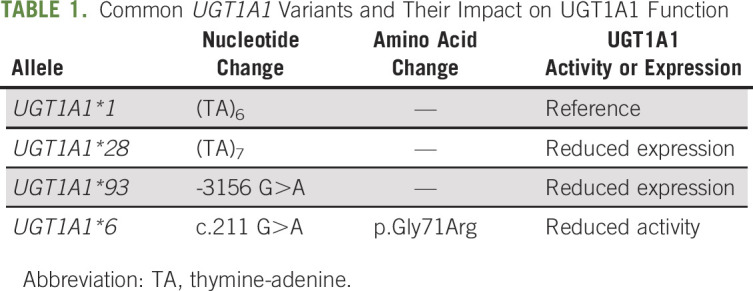
UGT1A1*28 contains seven TA repeats in the promoter, and this extra TA repeat results in decreased UGT1A1 transcription efficiency,35 leading to reduced UGT1A1 expression.34 It is commonly found in African (43%) and European ancestries (39%), but is less common in East Asian ancestry (16%).35
UGT1A1*93 is a polymorphism (G>A) about 3 Kbs upstream of the UGT1A1 exon 136 and is in linkage disequilibrium with UGT1A1*28 (r2 = 0.68).37 Although its functional significance is unknown, UGT1A1*93 is significantly associated with increased exposure to SN-38 and irinotecan toxicity, especially severe neutropenia.15 It is commonly found in African (34%) and European ancestries (27%), but is less common in East Asian ancestry (13%).38
UGT1A1*6 is a polymorphism (G>A) in the exon 1 region39 that is significantly associated with irinotecan toxicity.32,40 This polymorphism reduces UGT1A1 enzyme activity by substituting glycine into arginine (Gly71Arg). It is more commonly found in East Asian ancestry (15%) than African (0.1%) or European (1%) ancestry (Table 1).39
Association Between Alleles and Irinotecan Toxicity and Their Severity
The UGT1A1*28 and/or *6 polymorphisms were significantly associated with severe irinotecan toxicity. In a prospective study (N = 66) of single-agent irinotecan, patients (mainly White) carrying UGT1A1*28/*28 had a higher risk of grade 4 neutropenia than those carrying UGT1A1*1/*1 or *1/*28 (relative risk = 9.3; 95% CI, 2.4 to 36.4).3 In a retrospective study (N = 118) of irinotecan-based therapy, Japanese patients carrying UGT1A1*28/*28 or *1/*28 had a 5.2-fold risk of grade 4 leukopenia and/or grade 3 or 4 diarrhea (odds ratio = 5.21; 95% CI, 1.98 to 13.96; P < .001).33 In a prospective study (N = 107) of irinotecan and cisplatin, Korean patients carrying UGT1A1*6/*6 had a higher risk of grade 4 neutropenia than those carrying UGT1A1*1/*1 or *1/*6 (relative risk = 7.4; 95% CI, 1.2 to 44.2; P = .028).41
Interpretation of the Results of UGT1A1 Genetic Testing
The Clinical Pharmacogenetics Implementation Consortium (CPIC) assigned level A to the UGT1A1-irinotecan pair, indicating that genetic information should be used to change prescribing of the affected drug. However, a CPIC guideline for UGT1A1 and irinotecan is currently not available.42 The Dutch Pharmacogenetics Working Group (DPWG) of the Royal Dutch Association for the Advancement of Pharmacy (KNMP) and the French National Network of Pharmacogenetics (RNPGx) provide specific dose recommendations on the basis of the UGT1A1 genotype.43,44 For patients carrying UGT1A1*28/*28, KNMP-DPWG recommends starting with a 30% reduced irinotecan dose; RNPGx recommends starting with a 30% reduced dose for an irinotecan dose of 180-240 mg/m2 every three weeks as a single agent, whereas contraindication for an irinotecan dose of 240 mg/m2 or higher.
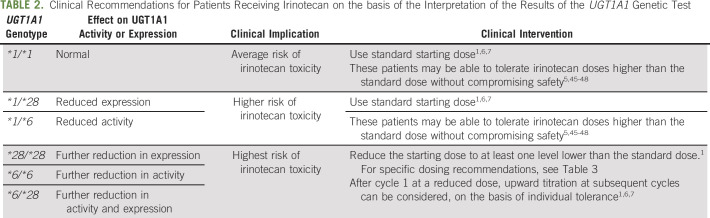
Based upon the information reported so far in this article, we provide the interpretation of the results of UGT1A1 genetic testing, its clinical implication, and clinical interventions in Table 2. Patients carrying two UGT1A1 alleles conferring decreased expression or function (ie, *28/*28, *6/*6, *28/*6) are at the highest risk for severe neutropenia and diarrhea, followed by those carrying one allele (ie, *1/*28, *1/*6) and no allele (ie, UGT1A1*1/*1).49,50 To manage the risk of toxicity, patients carrying *28/*28, *6/*6, or *28/*6 are recommended to receive a reduced dose of irinotecan.
TABLE 2.
Clinical Recommendations for Patients Receiving Irinotecan on the basis of the Interpretation of the Results of the UGT1A1 Genetic Test
Economic Impact of Irinotecan-Related Toxicity in Carriers of UGT1A1*28
There are no recent data available on financial costs of toxicity management in patients with UGT1A1*28/*28 in the US health care system. However, decision-tree modeling studies on the basis of treatment costs reported in 200651 and 200752 suggest that preemptive UGT1A1 genetic testing to guide irinotecan dosing (20%-25% dose reduction in patients with UGT1A1*28/*28) can reduce the cost of treatment with single-agent irinotecan51 or FOLFIRI52 in US patients with metastatic colorectal cancer compared with no UGT1A1 genetic testing. Consistent results were found in a more recent study using data from treated patients. A retrospective analysis of patients with colorectal cancer (N = 243) receiving FOLFIRI in Italy showed that the toxicity management cost was about six times higher in patients with UGT1A1*28/*28 (€4,886) than those with UGT1A1*1/*1 (€812; P < .001).53 The primary source of the cost was hospitalization because of irinotecan toxicity, which was much greater than the cost of UGT1A1 genetic testing.
Prevalence of Irinotecan-Related Toxic Deaths in Carriers of UGT1A1*28
According to a study of Israeli patients with colorectal cancer treated with irinotecan-based regimen,10 patients carrying UGT1A1*28/*28 showed higher short-term death within 2 months of treatment onset compared with those carrying UGT1A1*1/*1 or *1/*28 (13% v 3% and 5%, P = .027). However, since potential confounding factors (eg, coadministration of 5-FU) might have influenced this death, further studies are required to elucidate the effect of UGT1A1*28 polymorphism.
The Predictive Value of UGT1A1*28 Genetic Testing
UGT1A1*28 genetic testing for severe neutropenia had sensitivity of 11%, specificity of 94%, positive predictive value of 30%, and negative predictive value of 82%, considering *28/*28 as a positive test result.54 Similar test performance was observed for severe diarrhea: sensitivity of 13%, specificity of 92%, positive predictive value of 22%, and negative predictive value of 85%. Similar to other tests like DPYD testing for 5-FU toxicity,55 UGT1A1*28 genetic testing has low predictive power in patients who are not carriers of homozygous deficient alleles.
The number needed to genotype and the number needed to treat are54 79 and 9 for severe neutropenia, and 127 and 14 for severe diarrhea, respectively. For every 100 patients, 11 patients will have positive test results and may benefit from an intervention to reduce the risk of severe toxicity.
Effect of UGT1A1 Genetic Testing on Reduction of the Risk of Severe Irinotecan Toxicity
A model-based clinical simulation study45,56-59 demonstrated that the risk of severe irinotecan toxicity in patients carrying UGT1A1*28 could be reduced through UGT1A1 genetic testing. According to this study, if UGT1A1 genetic testing is performed and 25% reduced irinotecan dose is administered to patients carrying UGT1A1*28/*28, the prevalence of severe neutropenia and severe diarrhea will be reduced from 45% to 18% and from 19% to 9%, respectively. If, instead of a 25% dose reduction, prophylactic granulocyte colony-stimulating factors (eg, pegfilgrastim) with the standard dose of irinotecan is administered to these patients, the prevalence of severe neutropenia will be reduced to a similar extent (from 45% to 17%), but the prevalence of severe diarrhea will not be reduced. Prospective data on treated patients are not available yet. Whether a dose reduction based upon genotype can preserve antitumor efficacy is still an unanswered question.
Tolerability of Irinotecan Dosing on the basis of UGT1A1*28 and *6
Several phase I studies showed that patients with UGT1A1*1/*1, *1/*28, or *1/*6 genotypes could tolerate irinotecan doses substantially higher than standard doses. Some of these studies have also established the levels of dose reductions in patients with UGT1A1*28/*28, *6/*28, or *6/*6. For single-agent irinotecan (350 mg/m2 every 3 weeks), patients carrying UGT1A1*1/*1 and *1/*28 could tolerate irinotecan up to 850 mg and 700 mg (500 mg/m2 and 412 mg/m2, assuming average body surface area is 1.7 mg/m2), respectively, whereas those with *28/*28 could tolerate irinotecan up to 400 mg (235 mg/m2).46 The plasma exposure to SN-38 was simlar between these three genotype groups.
For FOLFIRI (irinotecan dose, 180 mg/m2 every 2 weeks), patients with European ancestry carrying UGT1A1*1/*1 and *1/*28 could tolerate irinotecan up to 370-390 mg/m2 and 310-340 mg/m2, respectively,45,47 whereas those with *28/*28 could tolerate irinotecan up to 130 mg/m2.47 Similarly, patients with Asian ancestry carrying UGT1A1*1/*1 and *1/*28 or *1/*6 could tolerate irinotecan up to 330 mg/m2 and 300 mg/m2, respectively, whereas those carrying *28/*28, *6/*28, or *6/*6 could tolerate irinotecan up to 150 mg/m2.48
For FOLFIRI plus bevacizumab, patients with European ancestry carrying *1/*1 and *1/*28 could tolerate irinotecan up to 310 mg/m2 and 260 mg/m2, respectively.5
For FOLFIRINOX, further dose reduction of irinotecan may be required for patients carrying UGT1A1*28. A study of modified FOLFIRINOX (N = 79)60 showed that, while patients carrying UGT1A1*1/*1 could tolerate a standard dose of irinotecan (180 mg/m2), those carrying *28 could not tolerate the regimen even with reduced doses of irinotecan (135 mg/m2 for *1/*28; 90 mg/m2 for *28/*28). Currently, the maximum tolerated dose of irinotecan on the basis of the UGT1A1 genotype has not been established yet for FOLFIRINOX.
Efficacy and Toxicity of Increased Irinotecan Dose on the basis of UGT1A1 Genotype
The study examined whether the administration of increased irinotecan dose is as safe and more effective than the standard dose in patients with metastatic colorectal cancer with UGT1A1*1/*1 or *1/*28. In a randomized phase II study (N = 79) of UGT1A1 genotype-guided increased dose (300 mg/m2 for *1/*1; 260 mg/m2 for *1/*28) or standard dose (180 mg/m2) of irinotecan of FOLFIRI in Spanish patients with metastatic colorectal cancer, the overall response rate was 67.5% in patients receiving increased dose of irinotecan versus 43.6% in patients receiving a standard dose (P = .001).61 Rates of severe toxicities were similar between the two groups, and no differences in survival were observed.
Combined Effect of UGT1A1 Polymorphism and Demographic or Clinical Risk Factors on Irinotecan Toxicity
A pharmacokinetic study62 suggests that potential demographic or clinical risk factors that interacted with UGT1A1 polymorphism may also need to be considered for UGT1A1 genotype-guided dosing. UGT1A1 polymorphism showed an additive effect with high pretreatment total bilirubin, but not with demographic risk factors (age > 65 years or female sex), on SN-38 clearance. Compared with UGT1A1 polymorphism alone, a combination with high (> 0.8 mg/dL) pretreatment total bilirubin further reduced SN-38 clearance, which may lead to increased irinotecan toxicity.
Oncology Guidelines on the UGT1A1*28 Polymorphism
The National Comprehensive Cancer Network Guidelines63 state that irinotecan should be used with caution in patients with Gilbert syndrome or elevated serum bilirubin. There is a commercially available test for UGT1A1. The guideline for its use in clinical practice has not been established. It also includes a caution that UGT1A1 testing on patients who experience irinotecan toxicity is not recommended because they will require a dose reduction regardless of UGT1A1 test result.
ASCO does not provide any guidelines on this matter.
The European Society for Medical Oncology consensus guidelines64 acknowledge the UGT1A1 polymorphism as a predictive biomarker of irinotecan toxicity. It states that UGT1A1 phenotyping remains an option and should be carried out in patients with a suspicion of UGT1A1 deficiency as reflected by low conjugated bilirubin (< 20% of total bilirubin) and in patients where an irinotecan dose of > 180 mg/m2 per administration is planned (recommendation grade C [insufficient evidence]).
The Japanese Society for Cancer of the Colon and Rectum guidelines65 state that because irinotecan toxicity cannot be predicted with certainty on the basis of the presence of a UGT1A1 genetic polymorphism alone, it is essential to monitor patients' general condition during treatment and to manage adverse drug reactions carefully, irrespective of whether a genetic polymorphism is detected.
Drug Label Information in the United States and Other Countries
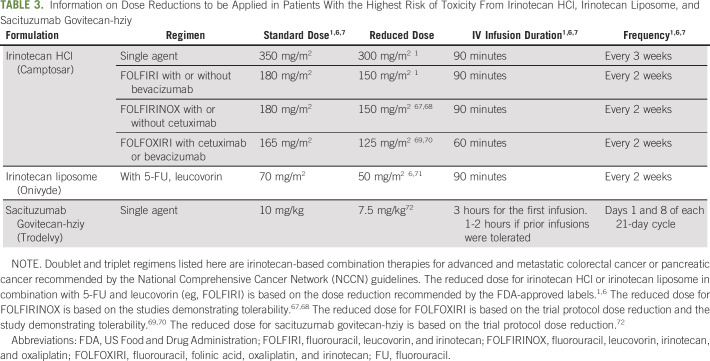
According to the Table of Pharmacogenetic Associations from the FDA,66 UGT1A1 *28/*28 results in higher systemic active metabolite concentrations and higher adverse reaction risk (severe neutropenia). Consider reducing the starting dosage by one level and modify the dosage on the basis of individual patient tolerance. Consistent with this, the FDA-approved drug labels for irinotecan and SN-38 (Camptosar, Onivyde, and Trodelvy) state that patients carrying UGT1A1*28/*28 are at increased risk of neutropenia.1,6,7 For these patients, the drug label for Camptosar (irinotecan HCl) recommends at least one level lower starting dose of irinotecan when administered in combination with other agents, or as a single agent1 (Table 3).1 However, the precise dose reduction in this patient population is not known, and subsequent dose modifications should be considered on the basis of individual patient tolerance to treatment. The FDA-approved drug label for Onivyde (irinotecan liposome) recommends a starting dose of 50 mg/m2 (approximately 30% reduction of the standard dose), with an increased dose to 70 mg/m2 as tolerated in subsequent cycles.2 The FDA-approved drug label for Trodelvy (sacituzumab govitecan-hziy) indicates that the dose should be adjusted on the basis of individual patient tolerance because the appropriate dose for these patients is unknown.6
TABLE 3.
Information on Dose Reductions to be Applied in Patients With the Highest Risk of Toxicity From Irinotecan HCl, Irinotecan Liposome, and Sacituzumab Govitecan-hziy
The Health Canada (Santé Canada)-approved drug label for irinotecan HCl recommends a reduction in the starting dose when administered in combination with other agents, or as a single agent but does not provide a specific dosing guideline.73 The Pharmaceuticals and Medical Devices Agency–approved drug labels for irinotecan HCl and irinotecan liposome in Japan indicate caution for increased risk of irinotecan toxicity in patients carrying UGT1A1*28/*28, *6/*6, or *28/*6.74,75 Although the drug label for irinotecan HCl does not provide a specific dosing guideline, the label for irinotecan liposome recommends a starting dose of 50 mg/m2 (70 mg/m2 in the subsequent cycle if tolerated) in these patients.75
Laboratories Providing UGT1A1 Genetic Testing
The NIH's Genetic Testing Registry76 lists 34 Clinical Laboratory Improvement Amendments–certified laboratories (29 in the United States and five in Europe) that provide UGT1A1 genetic testing.
DISCUSSION
This review presents a concise, albeit comprehensive, overview of clinical perspectives on using UGT1A1 genetic testing to obtain an evidence-based informed decision on irinotecan dosing. UGT1A1 genetic testing was initially proposed to reduce the risk of irinotecan toxicity in patients with high-risk UGT1A1 genotypes (UGT1A1*28/*28, *28/*6, and *6/*6).4 However, recent studies5,45,48,60,61 demonstrate a potential value of this testing as a means of maximizing treatment efficacy by increasing the dose in patients with low-risk UGT1A1 genotypes (UGT1A1*1/*1, *1/*28, and *1/*6). However, there are still barriers to implement this test in clinical practice. Although UGT1A1 genotype-based dosing has been extensively studied, neither the specific target exposure nor well-defined dosing recommendations have been established yet. Consequently, drug labels, oncology and pharmacology societies, and regulatory agencies in different countries vary significantly in their recommendations or lack thereof. The dose modification strategies using the UGT1A1 genotype are currently being actively investigated in patients treated with irinotecan-containing regimens. Once standardized dosing management strategies are established, we envision this approach as a strong framework for accelerating the implementation of precision dosing in patients with cancer receiving irinotecan-based chemotherapy.
Federico Innocenti
Honoraria: Tempus
Consulting or Advisory Role: Symberix, Emerald Lake Safety
Patents, Royalties, Other Intellectual Property: United States Patent: Flavopiridol drug combinations and methods with reduced side effects, Ratain MJ, Innocenti F, Iyer L. Filed on April 12, 2001, serial number 09/835,082. United States Patent: Optimization of cancer treatment with irinotecan, Ratain MJ, Innocenti F, Karabatsos P, Grimsley C, Di Rienzo A. Filed on February 12, 2003, serial number 60/446,942. United States Patent: Methods of identifying risk of bevacizumab-induced proteinuria and hypertension, Innocenti F, Quintanilha J, Lin D, Owzar K, Wang J. Filed on July 17, 2020, serial number 16/932,002. United States Provisional Patent Application: Plasma Levels of Angiopoietin-2, VEGF-A, And VCAM-1 as Markers of Bevacizumab Induced Hypertension, Innocenti F, Quintanilha J. Filed on April 1, 2021, serial number 63/169,301
No other potential conflicts of interest were reported.
SUPPORT
S.K. is funded by the National Institute of General Medical Sciences (NIGMS), grant number 5T32GM086330-10.
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
All You Need to Know About UGT1A1 Genetic Testing for Patients Treated With Irinotecan: A Practitioner-Friendly Guide
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Federico Innocenti
Honoraria: Tempus
Consulting or Advisory Role: Symberix, Emerald Lake Safety
Patents, Royalties, Other Intellectual Property: United States Patent: Flavopiridol drug combinations and methods with reduced side effects, Ratain MJ, Innocenti F, Iyer L. Filed on April 12, 2001, serial number 09/835,082. United States Patent: Optimization of cancer treatment with irinotecan, Ratain MJ, Innocenti F, Karabatsos P, Grimsley C, Di Rienzo A. Filed on February 12, 2003, serial number 60/446,942. United States Patent: Methods of identifying risk of bevacizumab-induced proteinuria and hypertension, Innocenti F, Quintanilha J, Lin D, Owzar K, Wang J. Filed on July 17, 2020, serial number 16/932,002. United States Provisional Patent Application: Plasma Levels of Angiopoietin-2, VEGF-A, And VCAM-1 as Markers of Bevacizumab Induced Hypertension, Innocenti F, Quintanilha J. Filed on April 1, 2021, serial number 63/169,301
No other potential conflicts of interest were reported.
REFERENCES
- 1.Pfizer : CAMPTOSAR® (irinotecan HCl) Prescribing Information, 2014. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/020571s048lbl.pdf [Google Scholar]
- 2.de Man FM, Goey AKL, van Schaik RHN, et al. : Individualization of irinotecan treatment: A review of pharmacokinetics, pharmacodynamics, and pharmacogenetics. Clin Pharmacokinet 57:1229-1254, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Innocenti F, Undevia SD, Iyer L, et al. : Genetic variants in the UDP-glucuronosyltransferase 1A1 gene predict the risk of severe neutropenia of irinotecan. J Clin Oncol 22:1382-1388, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Pfizer : Camptosar® (irinotecan HCl) Prescribing Information, 2005. https://www.accessdata.fda.gov/drugsatfda_docs/label/2005/020571s026lbl.pdf [Google Scholar]
- 5.Toffoli G, Sharma MR, Marangon E, et al. : Genotype-guided dosing study of FOLFIRI plus bevacizumab in patients with metastatic colorectal cancer. Clin Cancer Res 23:918-924, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ipsen : ONIVYDE® (irinotecan liposome) Prescribing Information, 2015. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/207793lbl.pdf [Google Scholar]
- 7.Immunomedics : TRODELVY®(sacituzumab govitecan-hziy) Prescribing Information, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761115s000lbl.pdf [Google Scholar]
- 8.Vanhoefer U, Harstrick A, Achterrath W, et al. : Irinotecan in the treatment of colorectal cancer: Clinical overview. J Clin Oncol 19:1501-1518, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Tam VC, Rask S, Koru-Sengul T, et al. : Generalizability of toxicity data from oncology clinical trials to clinical practice: Toxicity of irinotecan-based regimens in patients with metastatic colorectal cancer. Curr Oncol 16:13-20, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shulman K, Cohen I, Barnett-Griness O, et al. : Clinical implications of UGT1A1*28 genotype testing in colorectal cancer patients. Cancer 117:3156-3162, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ratain MJ: Irinotecan dosing: Does the CPT in CPT-11 stand for “can't predict toxicity”? J Clin Oncol 20:7-8, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Crawford J, Dale DC, Lyman GH: Chemotherapy-induced neutropenia. Cancer 100:228-237, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Rougier P, Bugat R, Douillard JY, et al. : Phase II study of irinotecan in the treatment of advanced colorectal cancer in chemotherapy-naive patients and patients pretreated with fluorouracil-based chemotherapy. J Clin Oncol 15:251-260, 1997 [DOI] [PubMed] [Google Scholar]
- 14.Rothenberg ML, Kuhn JG, Burris HA, et al. : Phase I and pharmacokinetic trial of weekly CPT-11. J Clin Oncol 11:2194-2204, 1993 [DOI] [PubMed] [Google Scholar]
- 15.Innocenti F, Kroetz DL, Schuetz E, et al. : Comprehensive pharmacogenetic analysis of irinotecan neutropenia and pharmacokinetics. J Clin Oncol 27:2604-2614, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freyer G, Rougier P, Bugat R, et al. : Prognostic factors for tumour response, progression-free survival and toxicity in metastatic colorectal cancer patients given irinotecan (CPT-11) as second-line chemotherapy after 5FU failure. CPT-11 F205, F220, F221 and V222 study groups. Br J Cancer 83:431-437, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sasaki Y, Hakusui H, Mizuno S, et al. : A pharmacokinetic and pharmacodynamic analysis of CPT-11 and its active metabolite SN-38. Jpn J Cancer Res 86:101-110, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kehrer DFS, Mathijssen RHJ, Verweij J, et al. : Modulation of irinotecan metabolism by ketoconazole. J Clin Oncol 20:3122-3129, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Kweekel D, Guchelaar H-J, Gelderblom H: Clinical and pharmacogenetic factors associated with irinotecan toxicity. Cancer Treat Rev 34:656-669, 2008 [DOI] [PubMed] [Google Scholar]
- 20.National Cancer Institute : Cancer Stat Facts: Colorectal Cancer. https://seer.cancer.gov/statfacts/html/colorect.html [Google Scholar]
- 21.Xu R-H, Muro K, Morita S, et al. : Modified XELIRI (capecitabine plus irinotecan) versus FOLFIRI (leucovorin, fluorouracil, and irinotecan), both either with or without bevacizumab, as second-line therapy for metastatic colorectal cancer (AXEPT): A multicentre, open-label, randomised, non-inferiority, phase 3 trial. Lancet Oncol 19:660-671, 2018 [DOI] [PubMed] [Google Scholar]
- 22.Conroy T, Bosset J-F, Etienne P-L, et al. : Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 22:702-715, 2021 [DOI] [PubMed] [Google Scholar]
- 23.Slatter JG, Su P, Sams JP, et al. : Bioactivation of the anticancer agent CPT-11 to SN-38 by human hepatic microsomal carboxylesterases and the in vitro assessment of potential drug interactions. Drug Metab Dispos 25:1157-1164, 1997 [PubMed] [Google Scholar]
- 24.Iyer L, King CD, Whitington PF, et al. : Genetic predisposition to the metabolism of irinotecan (CPT-11). Role of uridine diphosphate glucuronosyltransferase isoform 1A1 in the glucuronidation of its active metabolite (SN-38) in human liver microsomes. J Clin Invest 101:847-854, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.PHARMGKB: Irinotecan Pathway, Pharmacokinetics. https://www.pharmgkb.org/pathway/PA2001
- 26.Brandi G, Dabard J, Raibaud P, et al. : Intestinal microflora and digestive toxicity of irinotecan in mice. Clin Cancer Res 12:1299-1307, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Ratain MJ, Innocenti F: Individualizing dosing of irinotecan. Clin Cancer Res 16:371-372, 2010. [DOI] [PubMed] [Google Scholar]
- 28.Takasuna K, Hagiwara T, Hirohashi M, et al. : Involvement of beta-glucuronidase in intestinal microflora in the intestinal toxicity of the antitumor camptothecin derivative irinotecan hydrochloride (CPT-11) in rats. Cancer Res 56:3752-3757, 1996 [PubMed] [Google Scholar]
- 29.Steventon G: Uridine diphosphate glucuronosyltransferase 1A1. Xenobiotica 50:64-76, 2020 [DOI] [PubMed] [Google Scholar]
- 30.Ramakrishnan N, Bittar K, Jialal I: Impaired Bilirubin Conjugation. Treasure Island, FL, StatPearls, 2020 [PubMed] [Google Scholar]
- 31.PHARMGKB: UGT1A1. https://www.pharmgkb.org/gene/PA420/haplotype
- 32.Han J-Y, Lim H-S, Shin ES, et al. : Comprehensive analysis of UGT1A polymorphisms predictive for pharmacokinetics and treatment outcome in patients with non-small-cell lung cancer treated with irinotecan and cisplatin. J Clin Oncol 24:2237-2244, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Ando Y, Saka H, Ando M, et al. : Polymorphisms of UDP-glucuronosyltransferase gene and irinotecan toxicity: A pharmacogenetic analysis. Cancer Res 60:6921-6926, 2000 [PubMed] [Google Scholar]
- 34.Innocenti F, Vokes EE, Ratain MJ: Irinogenetics: What is the right star? J Clin Oncol 24:2221-2224, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Beutler E, Gelbart T, Demina A: Racial variability in the UDP- glucuronosyltransferase 1 (UGT1A1) promoter: A balanced polymorphism for regulation of bilirubin metabolism? Proc Natl Acad Sci USA 95:8170-8174, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Innocenti F, Grimsley C, Das S, et al. : Haplotype structure of the UDP-glucuronosyltransferase 1A1 promoter in different ethnic groups. Pharmacogenetics 12:725-733, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Cecchin E, Innocenti F, D’Andrea M, et al. : Predictive role of the UGT1A1, UGT1A7, and UGT1A9 genetic variants and their haplotypes on the outcome of metastatic colorectal cancer patients treated with fluorouracil, leucovorin, and irinotecan. J Clin Oncol 27:2457-2465, 2009 [DOI] [PubMed] [Google Scholar]
- 38.National Center for Biotechnology Information : dbSNP:rs10929302. https://www.ncbi.nlm.nih.gov/snp/rs10929302?vertical_tab=true#frequency_tab
- 39.National Center for Biotechnology Information : dbSNP:rs4148323. https://www.ncbi.nlm.nih.gov/snp/rs4148323#frequency_tab
- 40.Cheng L, Li M, Hu J, et al. : UGT1A1*6 polymorphisms are correlated with irinotecan-induced toxicity: A system review and meta-analysis in Asians. Cancer Chemother Pharmacol 73:551-560, 2014 [DOI] [PubMed] [Google Scholar]
- 41.Han J-Y, Lim H-S, Park YH, et al. : Integrated pharmacogenetic prediction of irinotecan pharmacokinetics and toxicity in patients with advanced non-small cell lung cancer. Lung Cancer 63:115-120, 2009 [DOI] [PubMed] [Google Scholar]
- 42.Clinical Pharamcogenetics Implementation Consortium : Genes-Drugs, 2021, https://cpicpgx.org/genes-drugs/ [Google Scholar]
- 43.KNMP : Pharmacogenetic Recommendations. https://www.knmp.nl/downloads/pharmacogenetic-recommendations-3mei2021.pdf [Google Scholar]
- 44.Etienne-Grimaldi M-C, Boyer J-C, Thomas F, et al. : UGT1A1 genotype and irinotecan therapy: General review and implementation in routine practice. Fundam Clin Pharmacol 29:219-237, 2015 [DOI] [PubMed] [Google Scholar]
- 45.Toffoli G, Cecchin E, Gasparini G, et al. : Genotype-driven phase I study of irinotecan administered in combination with fluorouracil/leucovorin in patients with metastatic colorectal cancer. J Clin Oncol 28:866-871, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Innocenti F, Schilsky RL, Ramírez J, et al. : Dose-finding and pharmacokinetic study to optimize the dosing of irinotecan according to the UGT1A1 genotype of patients with cancer. J Clin Oncol 32:2328-2334, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marcuello E, Páez D, Paré L, et al. : A genotype-directed phase I-IV dose-finding study of irinotecan in combination with fluorouracil/leucovorin as first-line treatment in advanced colorectal cancer. Br J Cancer 105:53-57, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim K-P, Hong YS, Lee J-L, et al. : A phase I study of UGT1A1 *28/*6 genotype-directed dosing of irinotecan (CPT- 11) in Korean patients with metastatic colorectal cancer receiving FOLFIRI. Oncology 88:164-172, 2015. [DOI] [PubMed] [Google Scholar]
- 49.Liu X, Cheng D, Kuang Q, et al. : Association of UGT1A1*28 polymorphisms with irinotecan-induced toxicities in colorectal cancer: A meta-analysis in Caucasians. Pharmacogenomics J 14:120-129, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Campbell JM, Stephenson MD, Bateman E, et al. : Irinotecan-induced toxicity pharmacogenetics: An umbrella review of systematic reviews and meta-analyses. Pharmacogenomics J 17:21-28, 2017 [DOI] [PubMed] [Google Scholar]
- 51.Obradovic M, Mrhar A, Kos M: Cost-effectiveness of UGT1A1 genotyping in second-line, high-dose, once every 3 weeks irinotecan monotherapy treatment of colorectal cancer. Pharmacogenomics 9:539-549, 2008 [DOI] [PubMed] [Google Scholar]
- 52.Gold HT, Hall MJ, Blinder V, et al. : Cost effectiveness of pharmacogenetic testing for uridine diphosphate glucuronosyltransferase 1A1 before irinotecan administration for metastatic colorectal cancer. Cancer 115:3858-3867, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roncato R, Cecchin E, Montico M, et al. : Cost evaluation of irinotecan-related toxicities associated with the UGT1A1*28 patient genotype. Clin Pharmacol Ther 102:123-130, 2017 [DOI] [PubMed] [Google Scholar]
- 54.Hulshof EC, Deenen MJ, Guchelaar H-J, et al. : Pre-therapeutic UGT1A1 genotyping to reduce the risk of irinotecan-induced severe toxicity: Ready for prime time. Eur J Cancer 141:9-20, 2020 [DOI] [PubMed] [Google Scholar]
- 55.Innocenti F, Mills SC, Sanoff H, et al. : All you need to know about DPYD genetic testing for patients treated with fluorouracil and capecitabine: A practitioner-friendly guide. JCO Oncol Pract 16:793-798, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Butzke B, Oduncu FS, Severin F, et al. : The cost-effectiveness of UGT1A1 genotyping before colorectal cancer treatment with irinotecan from the perspective of the German statutory health insurance. Acta Oncol 55:318-328, 2016 [DOI] [PubMed] [Google Scholar]
- 57.Côté J-F, Kirzin S, Kramar A, et al. : UGT1A1 polymorphism can predict hematologic toxicity in patients treated with irinotecan. Clin Cancer Res 13:3269-3275, 2007 [DOI] [PubMed] [Google Scholar]
- 58.Martinez-Balibrea E, Abad A, Martínez-Cardús A, et al. : UGT1A and TYMS genetic variants predict toxicity and response of colorectal cancer patients treated with first-line irinotecan and fluorouracil combination therapy. Br J Cancer 103:581-589, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hecht JR, Pillai M, Gollard R, et al. : A randomized, placebo-controlled phase II study evaluating the reduction of neutropenia and febrile neutropenia in patients with colorectal cancer receiving pegfilgrastim with every-2-week chemotherapy. Clin Colorectal Cancer 9:95-101, 2010 [DOI] [PubMed] [Google Scholar]
- 60.Sharma MR, Joshi SS, Karrison TG, et al. : A UGT1A1 genotype-guided dosing study of modified FOLFIRINOX in previously untreated patients with advanced gastrointestinal malignancies. Cancer 125:1629-1636, 2019 [DOI] [PubMed] [Google Scholar]
- 61.Páez D, Tobeña M, Fernández-Plana J, et al. : Pharmacogenetic clinical randomised phase II trial to evaluate the efficacy and safety of FOLFIRI with high-dose irinotecan (HD-FOLFIRI) in metastatic colorectal cancer patients according to their UGT1A 1 genotype. Br J Cancer 120:190-195, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Karas S, Etheridge AS, Nickerson DA, et al. : Integration of DNA sequencing with population pharmacokinetics to improve the prediction of irinotecan exposure in cancer patients. Br J Cancer 126:640-651, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.NCCN Guidelines Version 2.2021 Colon Cancer. National Comprehensive Cancer Network, 2021, https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf [Google Scholar]
- 64.Van Cutsem E, Cervantes A, Adam R, et al. : ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol 27:1386-1422, 2016 [DOI] [PubMed] [Google Scholar]
- 65.Watanabe T, Itabashi M, Shimada Y, et al. : Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2014 for treatment of colorectal cancer. Int J Clin Oncol 20:207-239, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.US Food and Drug Administration : Table of Pharmacogenetic Associations. https://www.fda.gov/medical-devices/precision-medicine/table-pharmacogenetic-associations [Google Scholar]
- 67.Conroy T, Hammel P, Hebbar M, et al. : FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med 379:2395-2406, 2018. [DOI] [PubMed] [Google Scholar]
- 68.Assenat E, Desseigne F, Thezenas S, et al. : Cetuximab plus FOLFIRINOX (ERBIRINOX) as first-line treatment for unresectable metastatic colorectal cancer: A phase II trial. Oncologist 16:1557-1564, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Loupakis F, Cremolini C, Masi G, et al. : Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med 371:1609-1618, 2014 [DOI] [PubMed] [Google Scholar]
- 70.Folprecht G, Hamann S, Schütte K, et al. : Dose escalating study of cetuximab and 5-FU/folinic acid (FA)/oxaliplatin/irinotecan (FOLFOXIRI) in first line therapy of patients with metastatic colorectal cancer. BMC Cancer 14:521, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang-Gillam A, Li C-P, Bodoky G, et al. : Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): A global, randomised, open-label, phase 3 trial. Lancet 387:545-557, 2016 [DOI] [PubMed] [Google Scholar]
- 72.Bardia A, Mayer IA, Vahdat LT, et al. : Sacituzumab govitecan-hziy in refractory metastatic triple-negative breast cancer. N Engl J Med 380:741-751, 2019. [DOI] [PubMed] [Google Scholar]
- 73.HCSC-approved drug label. Irinotecan Hydrochloride. https://pdf.hres.ca/dpd_pm/00056704.PDF [Google Scholar]
- 74.PMDA-approved drug label. Irinotecan Hydrochloride. https://www.pmda.go.jp/PmdaSearch/iyakuDetail/ResultDataSetPDF/672212_4240404A1 105_3_03 [Google Scholar]
- 75.PMDA-approved drug label. Onivyde® (irinotecan liposome). https://www.pmda.go.jp/PmdaSearch/iyakuDetail/ResultDataSetPDF/530457_4240404A3 027_1_01 [Google Scholar]
- 76.National Center for Biotechnology Information : NIH Genetic Testing Registry. https://www.ncbi.nlm.nih.gov/gtr/ [Google Scholar]