Abstract
Plasmodium falciparum circumsporozoite protein (CSP) is a major sporozoite surface protein and a key target of pre-erythrocytic malaria subunit vaccines. A full-length recombinant CSP (rCSP) based strategy could be advantageous, as this antigen includes a region critical to sporozoite cell attachment and hepatocyte invasion. The adjuvant Glucopyranosyl Lipid A-liposome Quillaja saponaria 21 (GLA-LSQ) functions as a TLR4 agonist, promotes antigen-specific TH1 responses and stimulates cytotoxic T cell production. To date, one study has reported the clinical acceptability of GLA-LSQ. We present interim results of a phase 1 first-in-human dose-escalation clinical trial of full-length rCSP vaccine given with or without GLA-LSQ adjuvant. Participants experienced only mild to moderate related solicited adverse events. The lowest adjuvanted vaccine dose achieved >90-fold rise in geometric mean anti-CSP IgG antibody titer. These favorable safety and immunogenicity results confirm the immunostimulatory capacity of this relatively new adjuvant and support next steps in clinical product development.
Keywords: Malaria, Plasmodium falciparum, Malaria vaccine, Circumsporozoite protein
1. Introduction
A highly effective vaccine against Plasmodium falciparum, the most common and deadly cause of malaria, could save up to 400,000 lives annually [1]. A key target of pre-erythrocytic malaria vaccine efforts has been the circumsporozoite protein (CSP), the major surface protein densely coating the sporozoite as it traverses through the bloodstream to the liver. CSP contains an amino-terminal region critical to sporozoite host cell attachment and hepatocyte invasion (region I) [2], a central repeat region, and the carboxyl-terminus, comprised of Th2R and Th3R regions that each contain multiple T cell epitopes (Supplementary figure 1). RTS,S, the most advanced malaria vaccine to date, includes a truncated version of CSP that lacks the amino-terminus (Supplementary figure 1). In a Phase 3 RTS,S/AS01 clinical trial, vaccine efficacy in children 5–17 months of age was 46% (27% in infants 6–12 weeks of age) in the 18 months following the third dose, and efficacy waned to negligible levels by month 33 [3,4]. Immunogenicity analyses suggested a non-linear dose–response relationship between anti-CSP IgG antibody titers and vaccine-induced protection [5]. Given the malaria burden worldwide, widespread RTS,S/AS01 use could have significant effects on malaria morbidity. However, higher vaccine efficacy is needed for malaria eradication efforts.
A full-length recombinant CSP (rCSP) based strategy may improve the modest efficacy provided by RTS,S. Human antibodies that bind the junction between region I and the central repeat region were recently isolated from volunteers immunized with an irradiated sporozoite vaccine and protected from controlled human malaria infection (CHMI) [6,7]. These human antibodies protected mice from malaria infection in two different models: mice challenged with a transgenic P. berghei strain expressing P. falciparum CSP (Pb-PfCSP) and humanized mice (FRG-huHep) challenged with P. falciparum [6]. These findings highlight the potential advantage of including this junctional region in a CSP-based vaccine.
The CSP central repeat region previously hindered large-scale production of full-length rCSP using good manufacturing practice, but recently, a full-length, biologically active rCSP was successfully expressed in a Pseudomonas fluorescens platform [8]. In mice, rCSP immunization elicited a monoclonal antibody (mAb) that was the first identified with specificity against the junction between region I and the central repeat region and prevents the cleavage necessary for hepatocyte invasion. Indeed, this mAb inhibited hepatocyte infection when passively transferred to mice challenged with recombinant rodent malaria (P. berghei) containing a chimeric CSP with a P. falciparum amino-terminal region [9]. This rCSP vaccine also is the first full-length CSP protein vaccine to reach clinical trials and the first malaria antigen to be expressed by a P. fluorescens platform.
To elicit a vaccine response to CSP that mimics natural immunity acquired after years of repeated exposure [10], CSP-based vaccines are usually combined with an adjuvant. Glucopyranosyl Lipid A-liposome Quillaja saponaria 21 formulation (GLA-LSQ) was developed by the Infectious Disease Research Institute and chosen as an adjuvant to boost CSP-based immunogenicity. Glucopyranosyl lipid adjuvant (GLA) is a synthetic, homogeneous, nontoxic derivative of Gram-negative bacterial cell wall lipopolysaccharide [11]. It functions as a TLR4 agonist, promoting antigen-specific TH1-type responses [11]. Liposome Quillaja saponaria 21 formulation (LSQ) is a saponin extracted from Quillaja saponaria, a South American soapbark tree [12]. It promotes antigen-specific TH1 and antibody responses, and stimulates cytotoxic T cell production [12]. In a murine model, three doses of 20 μg of rCSP adjuvanted with GLA-LSQ demonstrated > 90% inhibition of liver stage development in mice challenged with Pb-PfCSP [8]. To date, only one published report exists describing GLA-LSQ clinical testing. The two cohorts that received the placental malaria vaccine, PAMVAC, with GLA-LSQ reported two grade 2 and one grade 3 related adverse event, but no serious adverse events [13]. We initiated a first-in-human Phase I clinical trial in malaria-naïve, adult volunteers to evaluate rCSP safety and immunogenicity with and without GLA-LSQ and now report interim safety and immunogenicity results.
2. Material and methods
2.1. Study design and participants
We began an open label, dose escalation study to determine the safety and immunogenicity of rCSP administered with or without GLA-LSQ in 30 healthy adult volunteers aged 18–45 years old. Participants underwent screening for acute and chronic illnesses through medical history, physical examination, and laboratory testing before enrollment. Females of childbearing potential and males agreed to use contraception from 30 days before enrollment until 30 days after third vaccination. Individuals with a history of malaria infection or significant previous exposure were excluded. Additional exclusion criteria included recent immunosuppressive agent use, history of anaphylaxis, severe adverse reaction to any vaccination, and recent receipt of blood products (complete listing of screening and inclusion/exclusion criteria in Supplementary Materials).
The University of Maryland, Baltimore, Institutional Review Board approved the study protocol. The National Institute of Allergy and Infectious Diseases (NIAID) provided onsite clinical monitoring and appointed a safety monitoring committee and an independent local safety monitor. The study complied with the International Conference on Harmonization of Good Clinical Practices and the Declaration of Helsinki. All volunteers provided written informed consent.
2.2. Study procedures
The study progressed in a telescoped design, with three groups of ten participants each. Two sentinel participants from Group 1 received the first dose of 10 μg rCSP + 5 μg GLA/LSQ (which contains 5ug GLA and 2ug of LSQ) and, after 24 h with no safety concerns, the other 8 volunteers received the immunization. At Day 29 with no safety concerns, the sentinels in Groups 2 and 3 received the first dose of 30 μg rCSP + 5 μg GLA-LSQ (Group 2) or 30 μg rCSP alone (Group 3). This unadjuvanted vaccine group was included due to limited safety, tolerability and immunogenicity data available regarding GLA-LSQ use in humans. Similarly, after 24 h with no safety concerns, the other 8 volunteers in each group received the immunization. Each group received a second and third vaccine 28 and 84 days after the first, respectively. See Supplementary Materials for details about clinical trial materials and administration.
To assess rCSP/GLA-LSQ safety and reactogenicity, primary endpoints included solicited local and systemic reactions and related severe laboratory adverse events for seven days following vaccination, related severe unsolicited adverse events and serious adverse events for 28 days following vaccination, and serious adverse events and adverse events of special interest during the entire participant follow-up period. Reactogenicity grading is detailed in Supplementary Tables 1 and 2. Participants completed a seven-day memory aid after each vaccination to document solicited adverse events. Complete blood count, alanine aminotransferase (ALT), and creatinine were assessed seven days after each vaccination. Study clinicians assessed adverse events, including laboratory abnormalities, for severity and relatedness to study product, and graded according to a protocol defined grading system (details in Supplementary Materials).
Secondary endpoints included anti-CSP antibody titer. Participants underwent venous blood collection to measure anti-CSP IgG by ELISA at baseline (day of first vaccination), 1 and 4 weeks after each vaccination, and 12 and 24 weeks after last vaccination. ELISA methods are detailed in Supplementary Materials. Laboratory personnel conducting immunogenicity studies were blinded to study group assignment, participant ID and visit number. We calculated geometric mean anti-CSP IgG titers with 95% confidence intervals and fold increases of anti-CSP IgG from baseline to post-vaccination. Seroconversion was defined as a greater than four-fold rise in anti-CSP IgG. All statistical analyses were performed using SAS software, Version 9.4 (Cary, NC).
3. Results
3.1. Study population
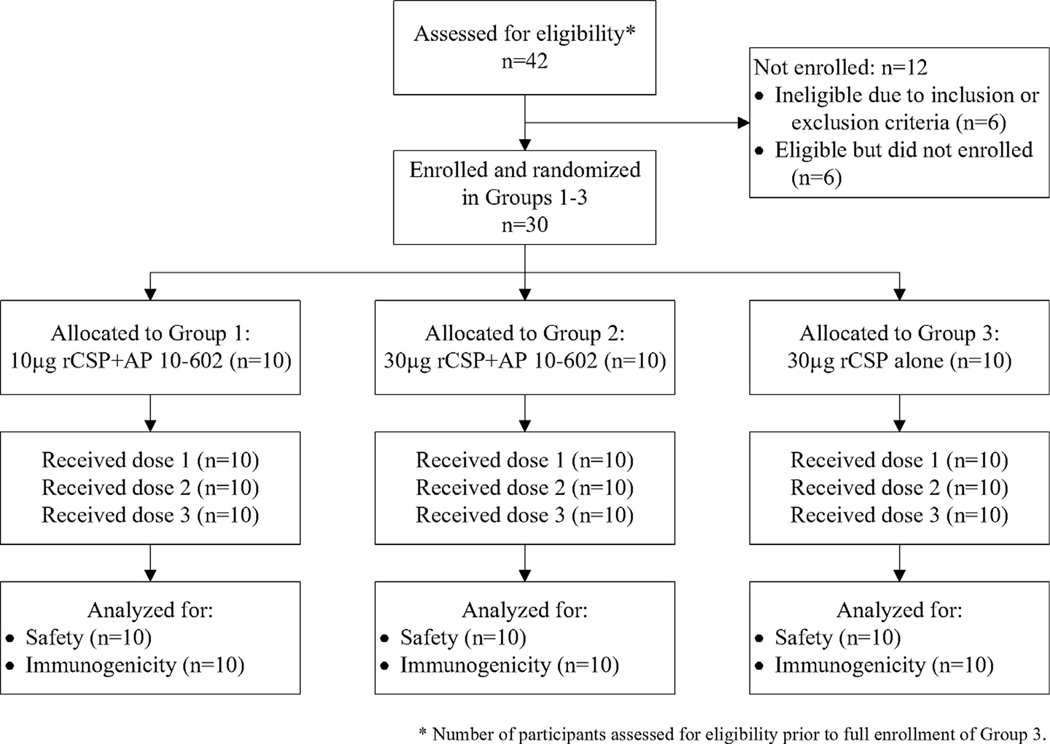
All 30 participants received three doses of rCSP with or without GLA-LSQ (CONSORT diagram in Fig. 1). Most participants (63.3%) were male, and mean participant age was 29 years (range 22 to 40). Additional participant characteristics are listed in Table 1 and Supplementary Table 3.
Fig. 1.
Screening, enrollment, vaccination, and follow-up of study participants.
Table 1.
Participant Characteristics.
| Demographic category | Characteristic | Group 1 (N = 10) n (%) | Group 2 (N = 10) n (%) | Group 3 (N = 10) n (%) | All participants (Na = 30) n (%) |
|---|---|---|---|---|---|
|
| |||||
| Sex | Male | 6 (60%) | 6 (60%) | 7 (70%) | 19 (63.3%) |
| Female | 4 (40%) | 4 (40%) | 3 (30%) | 11 (36.7%) | |
| Ethnicity | Not Hispanic or Latino | 9 (90%) | 10 (100%) | 9 (90%) | 28 (93.3%) |
| Hispanic or Latino | 1 (10%) | 0 (0%) | 1 (10%) | 2 (6.7%) | |
| Race | Asian | 1 (10%) | 0 (0%) | 1 (10%) | 2 (6.7%) |
| Black or African American | 3 (30%) | 3 (30%) | 3 (30%) | 9 (30%) | |
| White | 6 (60%) | 7 (70%) | 6 (60%) | 19 (63.3%) | |
| Age (years) | Mean (standard deviation) | 29.0 (5.6) | 30.5 (5.3) | 27.1 (3.8) | 28.9 (5.0) |
| [min, max] | [23, 37] | [26, 40] | [22, 35] | [22, 40] | |
| BMI (kg/m2) | Mean (standard deviation) | 26.7 (4.7) | 28.9 (4.7) | 26.2 (3.4) | 27.3 (4.3) |
| [min, max] | [20.5, 33.5] | [23.9, 37.4] | [19.2, 30.6] | [19.2, 37.4] | |
N = all enrolled participants
3.2. Safety
The vaccine demonstrated a favorable safety and tolerability profile. Six participants (20%) experienced at least one solicited systemic adverse event: one in Group 1, one in Group 2 and four in Group 3 (Table 2). One participant in Group 1 developed severe vomiting 5 days after the first vaccination due to viral gastroenteritis, one participant in Group 1 developed contact dermatitis after exposure to poison ivy and one participant in Group 3 developed severe nausea and vomiting five days after the second vaccination after consuming homemade mayonnaise left at ambient temperature. No other severe systemic adverse events occurred. Notably, no participants reported fever related to vaccination. No participant reported any related unsolicited adverse events.
Table 2.
Summary of Solicited Adverse Events, Maximum Intensity After Any Injection.
| Solicited Adverse Events - Locala | Solicited adverse event | Group 1 (N = 10) n (%) | Group 2 (N = 10) n (%) | Group 3 (N = 10) n (%) | All participants (Nb = 30) n (%) |
|---|---|---|---|---|---|
|
| |||||
| Any local symptom | None | 5 (50%) | 4 (40%) | 8 (80%) | 17 (56.7%) |
| Mild | 5 (50%) | 6 (60%) | 2 (20%) | 13 (43.3%) | |
| Moderate | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Severe | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Pain at injection site | None | 9 (90%) | 7 (70%) | 9 (90%) | 25 (83.3%) |
| Mild | 1 (10%) | 3 (30%) | 1 (10%) | 5 (16.7%) | |
| Moderate | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Severe | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Tenderness at injection site | None | 6 (60%) | 4 (40%) | 9 (90%) | 19 (63.3%) |
| Mild | 4 (40%) | 6 (60%) | 1 (10%) | 11 (36.7%) | |
| Moderate | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Severe | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Swelling at injection site | None | 10 (100%) | 8 (80%) | 9 (90%) | 27 (90%) |
| Mild | 0 (0%) | 2 (20%) | 1 (10%) | 3 (10%) | |
| Moderate | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Severe | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Erythema/Redness measurement | None | 10 (100%) | 9 (90%) | 10 (100%) | 29 (96.7%) |
| Mild | 0 (0%) | 1 (10%) | 0 (0%) | 1 (3.3%) | |
| Moderate | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Severe | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Induration/Swelling measurement | None | 10 (100%) | 10 (100%) | 10 (100%) | 30 (100%) |
| Mild | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Moderate | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Severe | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
|
| |||||
| Solicited Adverse Events - Systemica | Solicited adverse event | Group 1 (N = 10) n (%) | Group 2 (N = 10) n (%) | Group 3 (N = 10) n (%) | All participants (Nb = 30) n (%) |
|
| |||||
| Any systemic symptom | None | 9 (90%) | 9 (90%) | 6 (60%) | 24 (80%) |
| Mild | 0 (0%) | 1 (10%) | 2 (20%) | 3 (10%) | |
| Moderate | 0 (0%) | 0 (0%) | 1 (10%) | 1 (3.3%) | |
| Severe | 1 (10%) | 0 (0%) | 1 (10%) | 2 (6.7%) | |
| Arthralgia | None | 10 (100%) | 10 (100%) | 9 (90%) | 29 (96.7%) |
| Mild | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Moderate | 0 (0%) | 0 (0%) | 1 (10%) | 1 (3.3%) | |
| Severe | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Chills | None | 10 (100%) | 10 (100%) | 9 (90%) | 29 (96.7%) |
| Mild | 0 (0%) | 0 (0%) | 1 (10%) | 1 (3.3%) | |
| Moderate | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Severe | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Fatigue | None | 9 (90%) | 9 (90%) | 7 (70%) | 25 (83.3%) |
| Mild | 0 (0%) | 1 (10%) | 3 (30%) | 4 (13.3%) | |
| Moderate | 1 (10%) | 0 (0%) | 0 (0%) | 1 (3.3%) | |
| Severe | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Headache | None | 9 (90%) | 9 (90%) | 9 (90%) | 27 (90%) |
| Mild | 0 (0%) | 1 (10%) | 0 (0%) | 1 (3.3%) | |
| Moderate | 1 (10%) | 0 (0%) | 1 (10%) | 2 (6.7%) | |
| Severe | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Malaise | None | 9 (90%) | 9 (90%) | 8 (80%) | 26 (86.7%) |
| Mild | 0 (0%) | 1 (10%) | 2 (20%) | 3 (10%) | |
| Moderate | 1 (10%) | 0 (0%) | 0 (0%) | 1 (3.3%) | |
| Severe | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Myalgia | None | 10 (100%) | 9 (90%) | 9 (90%) | 28 (93.3%) |
| Mild | 0 (0%) | 1 (10%) | 1 (10%) | 2 (6.7%) | |
| Moderate | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Severe | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Nausea | None | 9 (90%) | 10 (100%) | 8 (80%) | 27 (90%) |
| Mild | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Moderate | 1 (10%) | 0 (0%) | 1 (10%) | 2 (6.7%) | |
| Severe | 0 (0%) | 0 (0%) | 1 (10%) | 1 (3.3%) | |
| Vomiting | None | 9 (90%) | 10 (100%) | 9 (90%) | 28 (93.3%) |
| Mild | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Moderate | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Severe | 1 (10%) | 0 (0%) | 1 (10%) | 2 (6.7%) | |
| Dizziness | None | 10 (100%) | 10 (100%) | 9 (90%) | 29 (96.7%) |
| Mild | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Moderate | 0 (0%) | 0 (0%) | 1 (10%) | 1 (3.3%) | |
| Severe | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Fever | None | 10 (100%) | 10 (100%) | 10 (100%) | 30 (100%) |
| Mild | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Moderate | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Severe | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
Participants are counted once at the highest severity reported.
N = all enrolled participants
Thirteen participants (43.3%) experienced solicited local adverse events: five in Group 1, six in Group 2 and two in Group 3 (Table 2). The most common solicited local events included pain, tenderness, and/or swelling at the injection site, all graded as mild. No participants had graded quantitative injection site induration or swelling. One participant experienced mild quantitative injection site erythema. Hematologic toxicities were rare: two participants in Group 1 developed mild anemia related to blood draws and two participants (one in Group 2 and one in Group 3) developed mild decrease in white blood cell counts related to intercurrent illness. No significant changes in platelets occurred in any group. Similarly, creatinine and ALT changes arose rarely: two participants in Group 2 and one participant in Group 3 developed a mild increase in creatinine, and one participant in group 2 had a mild increase in ALT, all unrelated to vaccination. No other measured biochemistry toxicities occurred.
3.3. Anti-CSP serum IgG responses
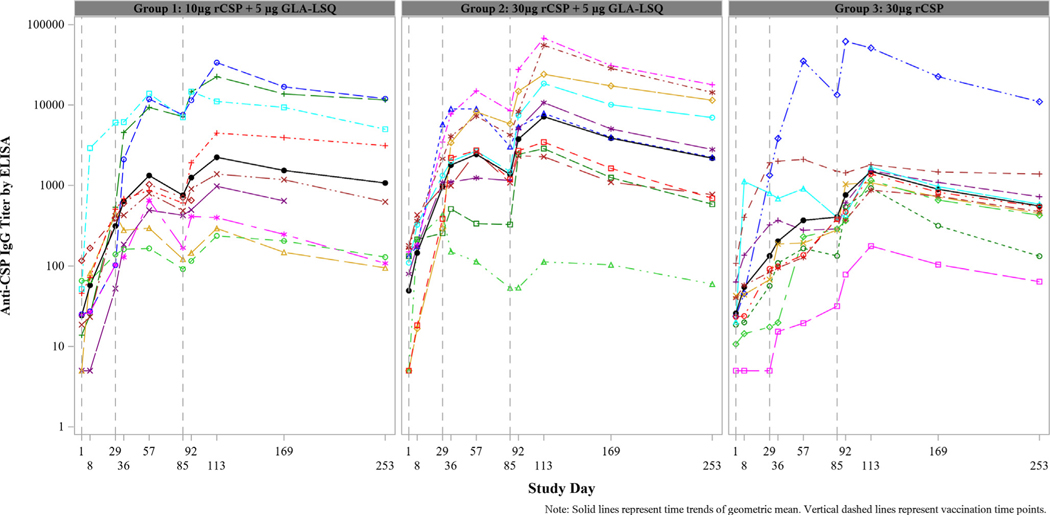
In all groups, the geometric mean anti-CSP IgG titer surpassed the seroconversion threshold (i.e. 4-fold rise over baseline) 28 days after first vaccination (Supplementary Table 4). Overall, the fold increases in geometric mean IgG titer 28 days after last vaccination were higher among participants who received rCSP with GLA-LSQ (Group 1: 92.4-fold and Group 2: 145.7-fold) compared to rCSP alone (Group 3: 58.4-fold). The median fold increases were slightly higher in Group 2 than Group 1 at each follow-up time point except for Days 57, 85, and 253, at which points the values are similar, despite the higher rCSP dose given to Group 2 (Supplementary Fig. 2 and Supplementary Table 4). Among the ten participants in Group 1, nine seroconverted; eight of them within 28 days after first vaccination. All participants in Groups 2 and 3 seroconverted after third vaccination. Individual kinetics of rCSP antibody responses are shown in Fig. 2.
Fig. 2.
Individual kinetic anti-CSP IgG titers in each treatment group. Datapoints represent serum IgG titers for individual volunteers. Vaccines were given on days 1, 29, and 85.
3.4. Discussion and conclusions
In this first-in-human clinical trial, the rCSP/GLA-LSQ vaccine demonstrated a favorable safety and tolerability profile. Most participants experienced no adverse events, and all adverse events related to vaccination were only mild to moderate in intensity. Similar numbers of participants experienced adverse events in each dose group tested, with or without adjuvant. The number and severity of adverse events did not increase with subsequent doses and no participant withdrew or was terminated.
The GLA-LSQ dose used in the current study was approximately double the dose used in a published study testing GLA-LSQ in healthy adults, but we report fewer adverse events [13]. The low rate of reported adverse events in our participants supports GLA-LSQ safety and tolerability in humans and may indicate low rCSP reactogenicity.
No reliable immunological correlate of protection exists for P. falciparum pre-erythrocytic vaccines, however, anti-CSP antibodies seem to play a role in preventing infection [5]. In our study, rCSP vaccination resulted in seroconversion in 97% of participants, regardless of dose or presence of adjuvant. However, GLA-LSQ increased the magnitude of CSP IgG titers; titers were also more homogeneous in groups that received the adjuvant. The tolerability and immunostimulatory capacity of GLA-LSQ make it an attractive adjuvant to stimulate TLR responses deemed critical for long-term protection against malaria.
Dose sparing regimens have not been shown to reduce immunogenicity in studies of CSP-based vaccines [14,15]. We observed similar anti-CSP IgG titers in groups that received the low and medium rCSP dose with GLA-LSQ. Therefore, as the study moves into the second phase, subsequent participants will be enrolled and assigned to receive low dose or high dose rCSP, both with the GLA-LSQ adjuvant. This next phase will measure preliminary efficacy against CHMI with homologous NF54 parasites. We will also complement the measurement of humoral immunity with high-throughput peptide microarrays to identify correlates of protection against CHMI. The low adverse event rate and promising immunogenicity results of rCSP/GLA-LSQ support advancement in clinical product development.
Supplementary Material
Acknowledgments
We would like to thank the volunteers from the Baltimore, Maryland, area for graciously consenting to be in this trial. We also thank the Division of Microbiology and Infectious Diseases at the NIAID, for their invaluable support and advice, and Dr. Karen Kotloff, the principal investigator of the VTEU contract, for her input and guidance during the trial and preparation of this manuscript. Our special thanks to Faith Pa’ahana-Brown and Lisa Chrisley for study coordination, Melissa Myers, Carey Martin, and Christine Schoenberger for project management, Mardi Reymann and the Applied Immunology Section for clinical specimen handling and analysis, Aly Kwon and Brenda Dorsey for regulatory oversight and quality assurance, and Panagiota Komninou for data entry. The Emmes Company, LLC, provided database and statistical support.
Funding
The clinical trial was supported by NIAID under the Vaccine and Treatment Evaluation Unit (VTEU) contract number HHSN272201300022I. DJF-K was supported by an NIH T32 Fellowship Training Program in Vaccinology to Dr. Kathleen Neuzil project T32 AI007524. The rCSP production and testing were supported by federal funds from the NIAID Malaria Vaccine Production Support Services contract number AI-N01–054210, and stability testing was continuously supported under NIAID contracts No. HHSN272201200005I and HHSN272201800009I. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Trial registration: ClinicalTrials.gov Identifier NCT03589794
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2020.12.023.
References
- [1].World Health Organization. World malaria report 2019. Geneva: World Health Organization; 2019. [Google Scholar]
- [2].Rathore D, Sacci JB, De La Vega P, McCutchan TF. Binding and invasion of liver cells by Plasmodium falciparum sporozoites. Essential involvement of the amino terminus of circumsporozoite protein. J Biol Chem 2002;277:7092–8. [DOI] [PubMed] [Google Scholar]
- [3].RTSS Clinical Trials Partnership. Efficacy and safety of the RTS, S/AS01 malaria vaccine during 18 months after vaccination: a phase 3 randomized, controlled trial in children and young infants at 11 African sites. PLoS Med 2014;11: e1001685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].RTSS Clinical Trials Partnership. Efficacy and safety of RTS, S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. The Lancet 2015;6736:31–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].White MT, Verity R, Griffin JT, et al. Immunogenicity of the RTS, S/AS01 malaria vaccine and implications for duration of vaccine efficacy: secondary analysis of data from a phase 3 randomised controlled trial. Lancet Infect Dis 2015;15:1450–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kisalu NK, Idris AH, Weidle C, et al. A human monoclonal antibody prevents malaria infection by targeting a new site of vulnerability on the parasite. Nat Med 2018;24:408–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Tan J, Sack BK, Oyen D, et al. A public antibody lineage that potently inhibits malaria infection through dual binding to the circumsporozoite protein. Nat Med 2018;24:401–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Noe AR, Espinosa D, Li X, et al. A full-length Plasmodium falciparum recombinant circumsporozoite protein expressed by Pseudomonas fluorescens platform as a malaria vaccine candidate. PLoS ONE 2014;9: e107764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Espinosa DA, Gutierrez GM, Rojas-Lopez M, et al. Proteolytic Cleavage of the Plasmodium falciparum Circumsporozoite Protein Is a Target of Protective Antibodies. J Infect Dis 2015;212:1111–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Noland GS, Hendel-Paterson B, Min XM, et al. Low prevalence of antibodies to preerythrocytic but not blood-stage Plasmodium falciparum antigens in an area of unstable malaria transmission compared to prevalence in an area of stable malaria transmission. Infect Immun 2008;76:5721–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Coler RN, Bertholet S, Moutaftsi M, et al. Development and characterization of synthetic glucopyranosyl lipid adjuvant system as a vaccine adjuvant. PLoS ONE 2011;6:e16333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kensil CR, Wu JY, Soltysik S. Structural and immunological characterization of the vaccine adjuvant QS-21. Pharm Biotechnol 1995;6:525–41. [DOI] [PubMed] [Google Scholar]
- [13].Mordmuller B, Sulyok M, Egger-Adam D, et al. First-in-human, Randomized, Double-blind Clinical Trial of Differentially Adjuvanted PAMVAC, A Vaccine Candidate to Prevent Pregnancy-associated Malaria. Clin Infect Dis 2019;69:1509–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Venkatraman N, Tiono A, Bowyer G, et al. Phase I assessments of first-in-human administration of a novel malaria anti-sporozoite vaccine candidate, R21 in matrix-M adjuvant, in UK and Burkinabe volunteers. In: American Society of Tropical Medicine and Hygiene Annual Meeting. (National Harbor, Maryland, USA: ). [Google Scholar]
- [15].Moon JE, Ockenhouse C, Regules JA, et al. A Phase IIa Controlled Human Malaria Infection and Immunogenicity Study of RTS, S/AS01E and RTS, S/AS01B Delayed Fractional Dose Regimens in Malaria-Naive Adults. J Infect Dis 2020;222:1681–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.