PURPOSE:
Health-related quality of life (HRQOL) is an established prognostic factor for mortality; however, it is unclear if HRQOL is predictive of time to disease progression, a particularly meaningful outcome for patients. We examined the association between HRQOL and progression-free survival (PFS) in SWOG Cancer Research Network clinical trials.
METHODS:
For this secondary analysis, we reviewed all completed SWOG clinical trials to identify those for patients with advanced cancer that incorporated Functional Assessment of Cancer Therapy (FACT) questionnaires at baseline. FACT-Trial Outcome Index (FACT-TOI) was the primary independent variable. Associations between FACT-TOI and other FACT subscores with PFS and overall survival were evaluated via log-rank test and multivariable Cox regression analysis.
RESULTS:
Three clinical trials met our inclusion criteria: S0027 and S9509 for advanced non–small-cell lung cancer and S0421 for hormone-refractory prostate cancer. Of the 1,527 enrolled patients, 1,295 (85%) had both HRQOL and survival outcomes data available and were included in this analysis. In univariable analysis, we observed a statistically significant gradient effect in all three trials, with higher baseline FACT-TOI scores corresponding to better PFS (S0027, P < .001; S9509, P = .02; and S0421, P < .001). In multivariable analysis, FACT-TOI was significantly associated with PFS in S0027 (hazard ratio [HR] = 0.64; 95% CI, 0.42 to 1.00) but not in S9509 (HR = 0.77; 95% CI, 0.56 to 1.05) or S042 (HR = 0.86; 95% CI, 0.73 to 1.01). FACT-TOI was significantly associated with overall survival in multivariable analysis (P < .005 in all three trials).
CONCLUSION:
The association between baseline FACT-TOI scores and survival underscores their potential as a stratification factor in clinical trials.
INTRODUCTION
In the era of patient-centered care, patient-reported outcomes are essential for systematic screening, documenting and monitoring symptom burden, psychologic distress, satisfaction with care, and quality of care from the patients' perspective.1 One of the most commonly assessed patient-reported outcomes in the oncology setting is health-related quality of life (HRQOL), which typically encompasses physical, emotional, social, functional, and spiritual well-being.2 HRQOL is increasingly being incorporated into both cancer treatment and supportive care clinical trials and represents an important study outcome.3-6
HRQOL at baseline has been consistently found to be an independent prognostic indicator for overall survival (OS).7,8 A number of studies have also reported that patients' baseline HRQOL was associated with greater treatment response and less toxicity.9-13 However, only a handful of studies have examined the association between baseline HRQOL and progression-free survival (PFS).12,14-16 PFS is not only a surrogate end point for OS but also represents an important outcome on its own because it indicates the duration of disease response or stabilization, which is dependent on tumor biology, treatment efficacy, and patients' ability to remain on treatment while tolerating the adverse effects at therapeutic doses.17 For patients living with advanced cancer, PFS is a particularly meaningful outcome because it represents the period of time in which symptoms are generally well-controlled, quality of life (QOL) is relatively maintained, and the existential threat of cancer progression is delayed.18 For individuals with progressive advanced cancer who were well enough to consider a new line of treatment, baseline HRQOL may be helpful as a prognostic factor as the patient and oncology clinicians undertake treatment decision making.
Confirming the association between baseline HRQOL and PFS would (1) allow the use of the HRQOL score to indicate to both the patient and clinician whether a given patient might expect better outcomes and (2) provide more evidence to support the need to improve HRQOL in conjunction with initiating cancer treatments. A 2013 report by the Agency for Healthcare Research and Quality concluded that there was insufficient evidence to make any conclusion about the association between PFS and QOL and that further research is necessary.18 SWOG Cancer Research Network has completed several cancer treatment clinical trials that incorporated HRQOL as baseline measure. In this secondary analysis, we examined the association between baseline HRQOL and PFS in SWOG clinical trials involving patients with advanced cancer.19-21 We also examined the association between HRQOL and OS and selected grade 3 and 4 adverse events. We hypothesize that higher baseline HRQOL is associated with longer PFS, longer OS, and fewer grade 3 and 4 adverse effects.
METHODS
Design
This secondary analysis was designed to examine the association between HRQOL and PFS. We included all SWOG clinical trials that tested systemic cancer therapeutics in the palliative setting and included the Functional Assessment of Cancer Therapy (FACT) questionnaire at baseline. Studies that did not complete accrual were excluded. This proposal was approved by the SWOG Palliative and End of Life Care Committee. All participants provided signed informed consent.
Three clinical trials met the above criteria and were included in this analysis: S0027,20 S9509,19 and S0421.21 S0027 was a phase II trial that examined first-line treatment with vinorelbine and docetaxel in patients with advanced non–small-cell lung cancer.20 S9509 was a phase III randomized trial that compared first-line treatment with paclitaxel plus carboplatin versus vinorelbine plus cisplatin in patients with advanced non–small-cell lung cancer.19 S0421 was a phase III randomized trial that compared docetaxel with either atrasentan or placebo in patients with advanced castration-resistant prostate cancer.21
HRQOL Measures
FACT-General (FACT-G) is a psychometrically derived and extensively validated questionnaire commonly used in oncology clinical trials to assess HRQOL.22 It consists of 27 questions assessing the physical (seven questions), emotional (six questions), social (seven questions), and functional domains (seven questions). In addition to the core domains, tumor-specific domains have been developed. The FACT-Lung Cancer Symptom (LCS) Index includes seven additional questions related to lung cancer, and the FACT-Prostate Cancer Symptom (PCS) Index includes 12 more questions on prostate cancer.23,24 Each question was assessed using a 5-point Likert scale from not at all (0 points) to very much (4 points). A total FACT-G score is calculated from the sum of physical (0-28 points), emotional (0-24 points), social or family (0-28 points), and functional domains (0-28 points).
The total FACT-Lung score is represented by the sum of FACT-G and LCS, and the FACT-Prostate (FACT-P) total possible score is based on the sum of FACT-G score and PCS. In addition, Trial Outcome Index (TOI) is the summed total of three subscores that are most likely to change during cancer treatment: Physical, Functional, and Cancer-specific subscales (TOI-L = physical + functional + LCS = 0-84 points; TOI-P = physical + functional + PCS = 0-104 points). A higher score indicates better HRQOL. Both FACT-Lung and FACT-P have been found to be responsive to change.24,25
Covariates
Baseline demographics data included age, sex, race, height, and weight. Other variables included cancer stage (III v IV) and Zubrod performance status.
Outcomes
PFS was defined as the interval between date of trial registration and date of disease progression or death.19,20 Disease progression was defined a priori by the study protocols. For the lung cancer trials (S0027 and S9509), progression was based on radiologic and/or clinical deterioration.19,20 For the prostate cancer trial (S0421), progression was based on soft tissue, bone disease, or symptomatic pain criteria.21 OS was calculated as time from enrollment to death. For all time-to-event outcomes, censoring occurred at the date of last contact.
The Common Terminology Criteria for Adverse Events (CTCAE, version 2.0 in lung cancer trials and version 3.0 in prostate cancer trial) was used to document the adverse effects. For this secondary analysis, we examined selected hematologic (anemia, neutropenia, and thrombocytopenia) and nonhematologic adverse effects (nausea, vomiting, fatigue, sensory neuropathy, and motor neuropathy). These adverse effects were chosen because of their relative frequency and clinical relevance.
Statistical Analysis
For this secondary analysis, TOI was defined a priori to be the main independent variable. To facilitate comparison among the clinical trials, TOI scores were standardized to 0-100 by dividing the scores with the total score.26 Data from each clinical trial were analyzed separately because the patient populations (lung and prostate), study treatments, and TOI calculation differed. We plotted Kaplan-Meier survival curves by TOI divided into quartile groups to assess if there was a gradient. Multivariable Cox regression analysis was conducted to examine the association between TOI and PFS, adjusted for age, sex (except for prostate cancer trial S0421), disease stage, performance status, race (non-Hispanic White v Others), body mass index, study treatment assignment, and stratification factors (S9509: lactate dehydrogenase > upper limit of normal and weight loss > 5%; S0421: prior progression on the basis of measurable or nonmeasurable disease versus prostate-specific antigen only, use of bisphosphonates, worst pain by Brief Pain Inventory ≥ 4/10, and any extraskeletal metastases). In addition to TOI, we repeated this analysis for each FACT subscore (physical, emotional, social, and functional) and total FACT-G scores where available (FACT emotional, social, and total scores were not collected for S0027). Because of the lack of well-established cutoffs to define prognostic categories for FACT scores in the literature, the median was used as a cutoff in multivariable analysis. We also repeated the above analyses with OS as the dependent variable. Multivariable logistic regression was used to examine the association between FACT scores and incidence of any grade 3+ adverse effects, adjusting for the same covariates.
For our primary analysis of PFS, a P value of < .05 was considered to be statistically significant. All remaining analyses were considered to be hypothesis-generating in nature, and no corrections were made for multiple comparisons. The statistical program (SAS 9.4; SAS Institute Inc, Cary, NC) was used for computation.
RESULTS
Clinical Trials Included
This analysis included data from 1,295 patients who participated in three clinical trials. Specifically, 111 of 125 (89%) patients in S0027, 219 of 408 (54%) patients in S9509, and 965 of 994 (97%) patients in S0421 had both baseline HRQOL and outcomes data available and were included in this analysis. The patient characteristics are summarized in Table 1. Among the 1,295 patients included, the median age was 69 years (range 32-91 years), 1,174 (91%) were male, 1,019 (79%) were White, 1,253 (97%) had metastatic cancer, and 1,174 (91%) had performance status 0-1.
TABLE 1.
Baseline Demographics and Outcomes
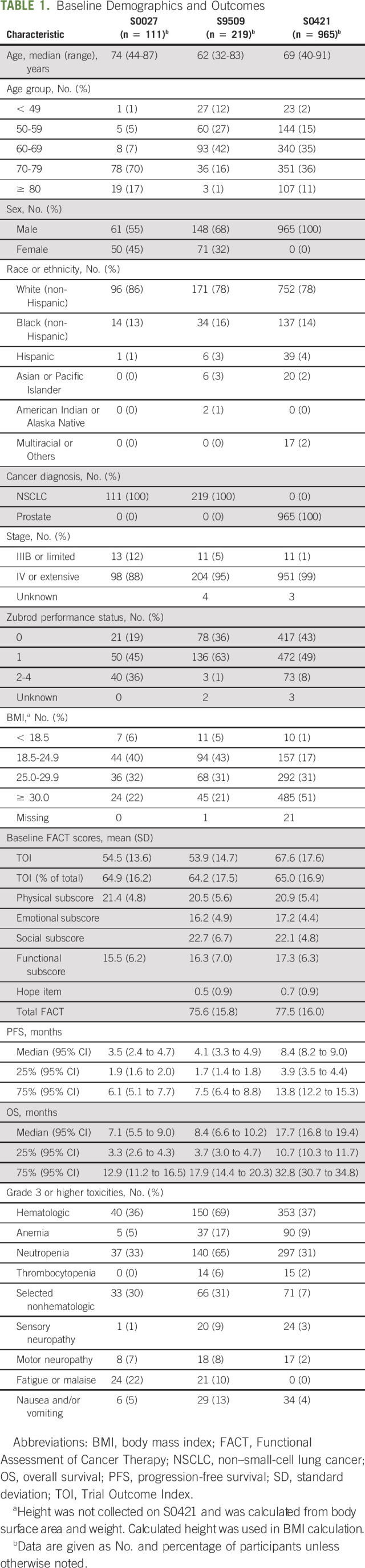
Treatment outcomes including PFS, OS, and adverse events are shown in Table 1. The median OS was 7.1 months (95% CI, 5.5 to 9.0 months) for S0027, 8.4 months (95% CI, 6.6 to 10.2 months) for S9509, and 17.7 months (95% CI, 16.8 to 19.4 months) for S0421.
Association Between FACT Scores and PFS
Figures 1A-1C show the association between TOI quartiles and PFS. We observed a statistically significant gradient effect in all three trials, with higher baseline TOI scores corresponding to better PFS (S0027, P < .001; S9509, P = .02; and S0421, P < .001).
FIG 1.
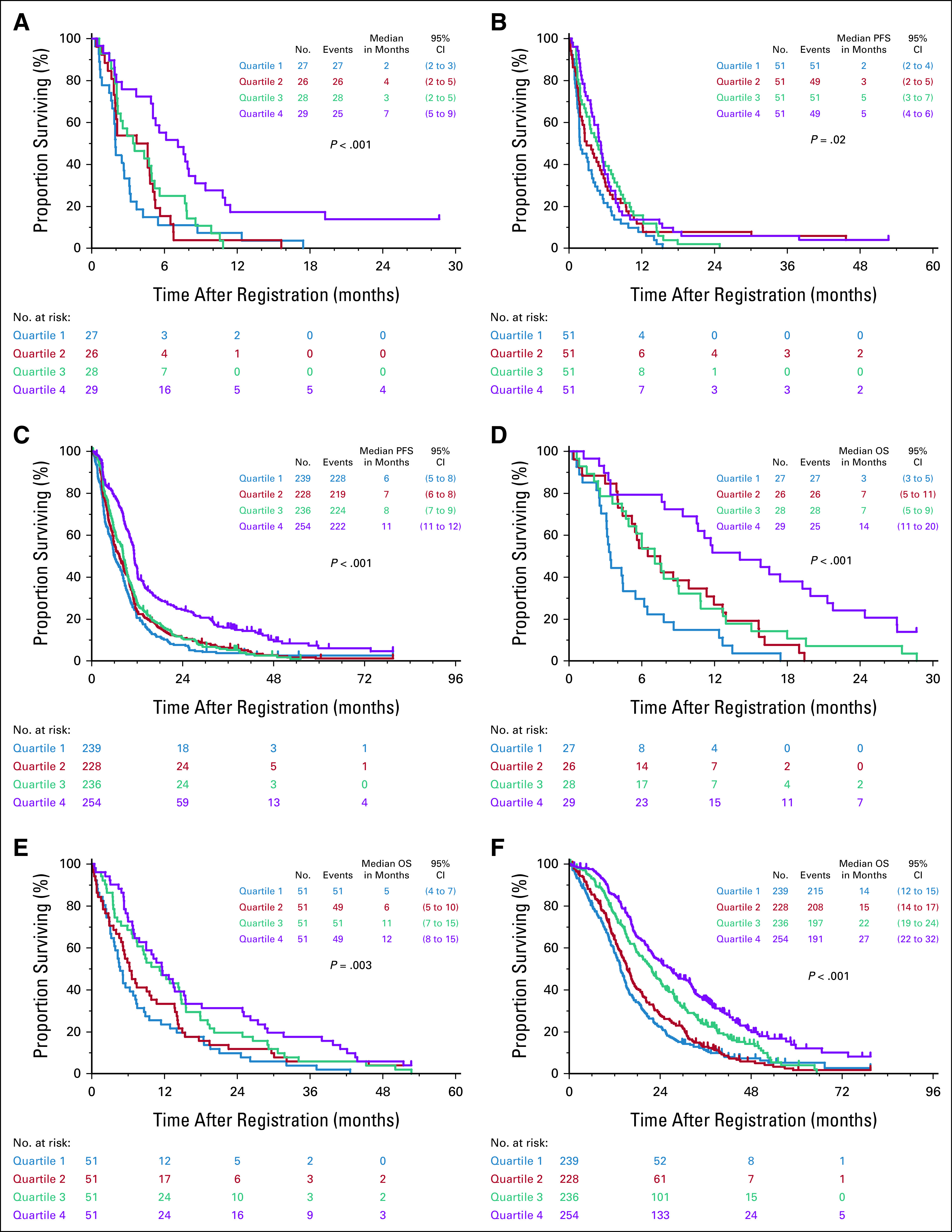
Gradient relationship between FACT-TOI and survival. We found a significant association between FACT-TOI scores and PFS in (A) S0027, (B) S9509, and (C) S0421. We also found a significant association between FACT-TOI scores and OS in (D) S0027, (E) S9509, and (F) S0421. We observed gradient effect in which better FACT-TOI scores corresponded to longer survival. FACT-TOI, Functional Assessment of Cancer Therapy Trial Outcome Index; OS, overall survival; PFS, progression-free survival.
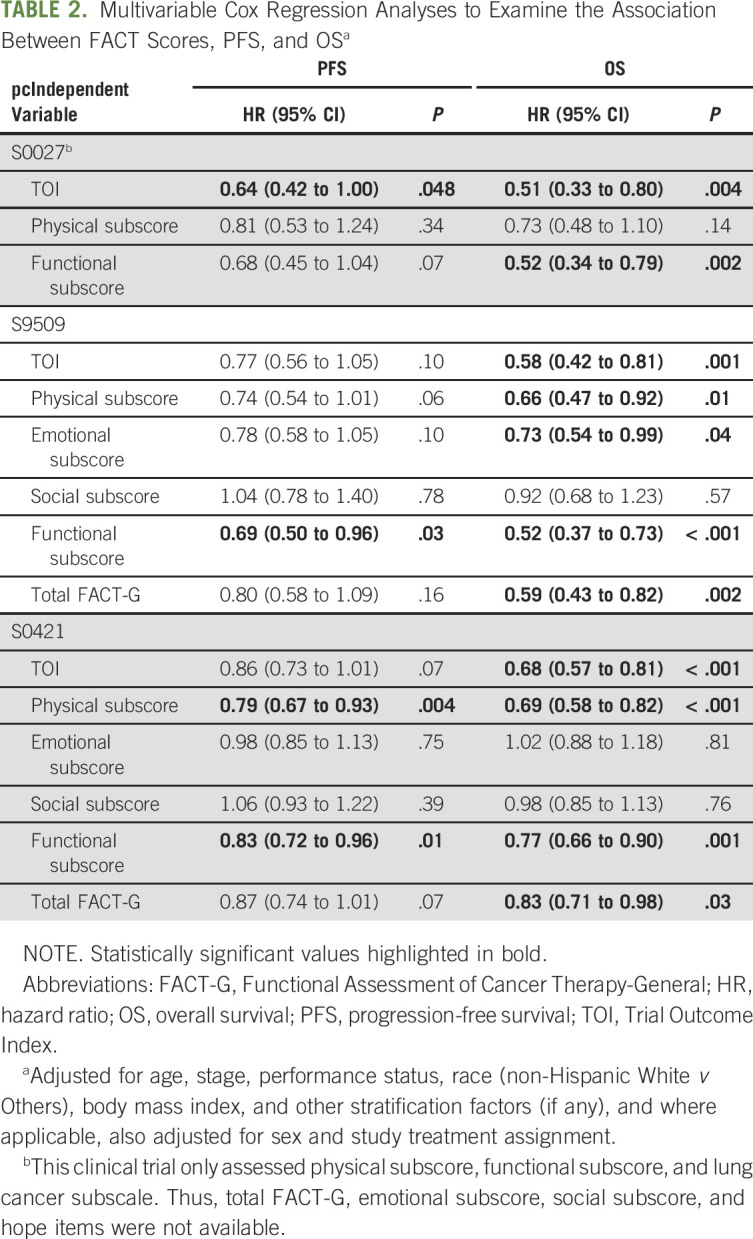
In multivariable analysis (Table 2), TOI was significantly associated with PFS in S0027 (hazard ratio [HR] = 0.64; 95% CI, 0.42 to 1.00) although it did not reach statistical significance for S9509 (HR = 0.77; 95% CI, 0.56 to 1.05) and S0421 (HR = 0.86; 95% CI, 0.73 to 1.01). Higher functional subscore values were significantly associated with longer PFS in S9509 (HR = 0.69; 95% CI, 0.50 to 0.96) and S0421 (HR = 0.83; 95% CI, 0.72 to 0.96).
TABLE 2.
Multivariable Cox Regression Analyses to Examine the Association Between FACT Scores, PFS, and OSa
Association Between FACT Scores and OS
The association between TOI and OS was consistent in all three clinical trials, with a gradient effect observed in Kaplan-Meier analyses (Figs 1D-1F), which remained statistically significant in multivariable analysis (S0027: HR = 0.51; 95% CI, 0.33 to 0.80; S9509: HR = 0.58; 95% CI, 0.42 to 0.81; S0421: HR = 0.68; 95% CI, 0.57 to 0.81). FACT physical and functional subscores were also significantly associated with OS in all three trials.
Association Between FACT Scores and Adverse Events
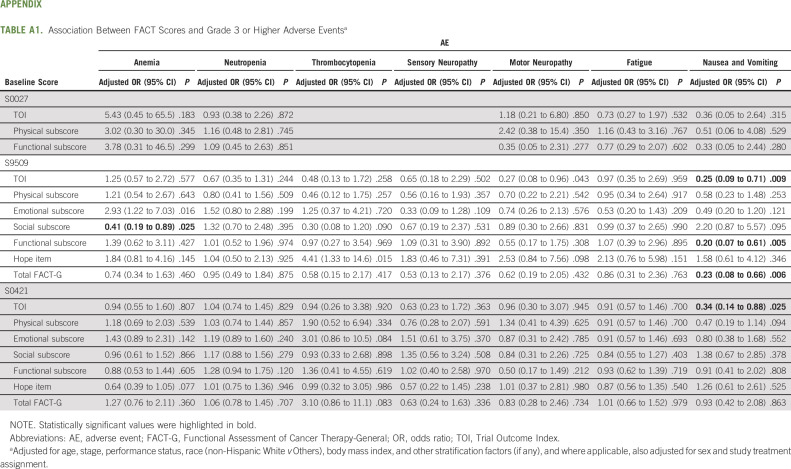
As shown in Appendix Table A1 (online only), patients with higher TOI scores were less likely to develop nausea and vomiting in S9509 (odds ratio [OR] = 0.25; 95% CI, 0.09 to 0.71) and S0421 (OR = 0.34; 95% CI, 0.14 to 0.88). No consistent association was found for other grade 3+ hematologic and nonhematologic adverse events.
DISCUSSION
In this secondary analysis of three SWOG clinical trials in patients with advanced lung and prostate cancer, we found that TOI was associated with better survival outcomes. In multivariable analysis adjusting for various prognostic factors, TOI was significantly associated with OS in all three trials although it was less consistent with PFS. We also found that higher HRQOL scores were associated with a lower likelihood of developing severe nausea and vomiting during treatment. Taken together, our findings highlight the potential prognostic role of HRQOL in patients undergoing cancer treatments.
Consistent with our hypothesis, we found that higher baseline TOI scores were associated with longer PFS. The gradient effect across all three studies supports the finding that TOI may be predictive of treatment outcomes. Conceptually, patients who reported better HRQOL at baseline may also have lower disease burden, fewer comorbidities, lower physical symptoms, higher function, and longer survival compared with patients with poorer HRQOL, which may allow them to better tolerate cancer treatments at therapeutic doses and stay on study longer, contributing to longer PFS. In this context, HRQOL could represent a marker for residual differences in disease stage or prognosis, even in a well-characterized and homogeneous clinical trial cohort. Indeed, a study examining 1,214 patients on 18 supportive care studies reported that higher baseline symptom burden was associated with greater risk of attrition.27 However, after adjusting for other variables such as demographics, performance status, and treatment assignment, the association was weakened and only remained statistically significant in one of the three trials. Interestingly, the FACT functional subscore, one of the three TOI domains, was independently associated with PFS in multivariable analysis; by contrast, no consistent association was observed with emotional and social subscores. Further studies are needed to examine the predictive role of functional subscore.
Our findings are consistent with a handful of studies investigating the relationship between HRQOL and PFS. In the National Cancer Institute of Canada OV10 study, the investigators found that European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30) global QOL score was associated with PFS (HR = 0.90; 95% CI, 0.84 to 0.96) and this was independent of performance status in patients with ovarian cancer.14 In another randomized trial comparing first-line sunitinib and interferon α in 750 patients with metastatic renal cell carcinoma, Cella et al15 found that baseline FACT-G scores were associated with longer PFS (HR = 0.93; 95% CI, 0.89 to 0.91 per 5-point change) in multivariable analysis. Similarly, in another study of 51 patients with metastatic renal cell carcinoma, baseline HRQOL assessed by EORTC QLQ-C30 was associated with treatment response and PFS (11 months v 5.9 months, P = .002) in univariate analysis.12 More recently, Beer et al16 reported that both baseline and changes in PFS were significantly associated with FACT-P in the AFFIRM and PREVAIL trials.
In addition to PFS, we found that FACT TOI and physical and functional subscores were significantly associated with OS, suggesting that pretreatment HRQOL is a good prognostic indicator. This finding has been well-documented in other oncology clinical trials.26,28,29 In a study that included 7,417 patients with various solid tumors from 30 randomized clinical trials, baseline EORTC QLQ-C30 scores were associated with OS after adjusting for age, sex, performance status, and distant metastasis.8,30
We did not find an association between FACT scores and adverse effects other than nausea. Specifically, patients with higher baseline HRQOL were less likely to report grade 3+ chemotherapy-induced nausea and vomiting (CINV). This observation may be explained in part by better functional reserve and lower risk of hospitalization, although other confounders such as anxiety, comorbidities, or other risk factors for nausea may also be contributory. A previous study of patients with advanced breast cancer found that lower physical well-being was associated with higher risk of nonhematologic grade 3 or 4 toxicity during the first four cycles of chemotherapy (OR 3.3; 95% CI, 1.5 to 7.2). However, physical well-being was assessed on the basis of a single linear analog scale and CINV was not specifically examined.13 It is unclear why adverse effects other than CINV were not significantly different.
Confirmation of the association between HRQOL and survival has important implications on cancer treatment decisions, clinical trial design, and optimizing care outcomes. The positive association between baseline HRQOL and PFS would enable the use of the HRQOL score to indicate to both the patient and clinician whether a given patient might expect to be better or worse than the median PFS and OS statistics. When the clinician, patient, and family discuss expectations and goals of care, the HRQOL score is a credible datum that might be particularly helpful for those at the extremes of high HRQOL (quartile 4, expect better outcomes) and low HRQOL (quartile 1, acknowledge low likelihood of even an average or median outcome). This ability of the HRQOL score to help set expectations is present in all three studies, but particularly so in 0027, which was the study of advanced lung cancer in persons age ≥ 70 years. Indeed, previous studies have found that patients with higher HRQOL score generally were more likely to want chemotherapy, to see treatment as beneficial, and to have a more optimistic outlook.31,32
In regard to trial design, our study adds to the growing literature on the prognostic and potentially predictive utility of baseline HRQOL data, raising the possibility that baseline HRQOL could be used as a stratifying factor in addition to other variables such as performance status. One challenge to routine application is the lack of well-established cutoffs for baseline TOI and many other HRQOL questionnaires. Further studies are needed to establish these cutoffs. Finally, given the importance of baseline HRQOL, it is important to optimize symptom control for patients with advanced cancer embarking on cancer therapeutics. There is a large body of literature to support the role of timely specialist palliative care referral and its impact on HRQOL.33,34 Involvement of palliative care early in the disease trajectory could help to optimize symptom control, nutrition, and function.
This study has several limitations. First, we were only able to include three clinical trials because many other SWOG trials either did not examine HRQOL at baseline or use other HRQOL measures. These trials were conducted between 2001 and 2013 and may not reflect contemporary practice. Second, the patient population was heterogeneous with cancer diagnosis and survival; however, we analyzed the three trials separately and the direction of association was consistent. Third, HRQOL was missing in a large proportion of patients in S9509, which may contribute to selection bias if sicker patients were less likely to complete the questionnaires. Fourth, we conducted multiple exploratory analyses, which may contribute to false positives because of multiple testing. This, coupled with the post hoc analysis, means that our findings should be considered as hypothesis-generating only. Fifth, there were limitations in adverse event analysis. Because we only focused on grade 3+ toxicities and these events were relatively rare, this study may not have adequate power to assess these events. Moreover, we were only able to assess adverse effects with older versions of CTCAE on the basis of clinician assessment, which may be less sensitive than patient-reported outcomes.35 Indeed, a patient-reported version of CTCAE has been developed and validated, which has been found to be associated with HRQOL.36
In summary, our findings underscore the prognostic and predictive utility of HRQOL assessment, with potential applications on cancer treatment decisions, clinical trial design, and optimizing care outcomes. Only three SWOG clinical trials have included FACT questionnaires at baseline, highlighting the need for HRQOL assessment to be more universally and more uniformly incorporated into contemporary oncology trials.
APPENDIX
TABLE A1.
Association Between FACT Scores and Grade 3 or Higher Adverse Eventsa
David Hui
Research Funding: Helsinn Healthcare
Ishwaria M. Subbiah
Consulting or Advisory Role: MedImmune (I)
Research Funding: Bayer (I), Novartis (I), GlaxoSmithKline (I), NanoCarrier (I), Celgene (I), Northwest Biotherapeutics (I), Incyte (I), Fujifilm (I), Pfizer (I), Amgen (I), AbbVie (I), Multivir (I), Exelixis (I), Loxo (I), Blueprint Medicines (I), Takeda (I)
Travel, Accommodations, Expenses: Bayer (I), PharmaMar (I), Novartis (I), MedImmune (I)
Dawn L. Hershman
Consulting or Advisory Role: AIM Specialty Health
No other potential conflicts of interest were reported.
SUPPORT
Supported in part by NIH/NCI/DCP Grant Award No. UG1CA189974 (D.L.H.) and by The Hope Foundation for Cancer Research (J.M.U.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. D.H. was supported in part by grants from the National Cancer Institute (Grant Nos. R01CA214960, R01CA225701, and R01CA231471).
AUTHOR CONTRIBUTIONS
Conception and design: David Hui, Amy K. Darke, Katherine A. Guthrie, Joseph M. Unger, Robert S. Krouse, Mark A. O'Rourke
Financial support: Joseph M. Unger
Administrative support: Dawn L. Hershman, Mark A. O'Rourke
Collection and assembly of data: Amy K. Darke
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Association Between Health-Related Quality of Life and Progression-Free Survival in Patients With Advanced Cancer: A Secondary Analysis of SWOG Clinical Trials
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
David Hui
Research Funding: Helsinn Healthcare
Ishwaria M. Subbiah
Consulting or Advisory Role: MedImmune (I)
Research Funding: Bayer (I), Novartis (I), GlaxoSmithKline (I), NanoCarrier (I), Celgene (I), Northwest Biotherapeutics (I), Incyte (I), Fujifilm (I), Pfizer (I), Amgen (I), AbbVie (I), Multivir (I), Exelixis (I), Loxo (I), Blueprint Medicines (I), Takeda (I)
Travel, Accommodations, Expenses: Bayer (I), PharmaMar (I), Novartis (I), MedImmune (I)
Dawn L. Hershman
Consulting or Advisory Role: AIM Specialty Health
No other potential conflicts of interest were reported.
REFERENCES
- 1.Lipscomb J, Gotay CC, Snyder CF: Patient-reported outcomes in cancer: A review of recent research and policy initiatives. CA Cancer J Clin 57:278-300, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Osoba D: Health-related quality of life and cancer clinical trials. Ther Adv Med Oncol 3:57-71, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Temel JS, Greer JA, Muzikansky A, et al. : Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med 363:733-742, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Basch E, Deal AM, Kris MG, et al. : Symptom monitoring with patient-reported outcomes during routine cancer treatment: A randomized controlled trial. J Clin Oncol 34:557-565, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cella D, Herbst RS, Lynch TJ, et al. : Clinically meaningful improvement in symptoms and quality of life for patients with non-small-cell lung cancer receiving gefitinib in a randomized controlled trial. J Clin Oncol 23:2946-2954, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Kretschmer A, Ploussard G, Heidegger I, et al. : Health-related quality of life in patients with advanced prostate cancer: A systematic review. Eur Urol Focus 7:742-751, 2020 [DOI] [PubMed] [Google Scholar]
- 7.Montazeri A: Quality of life data as prognostic indicators of survival in cancer patients: An overview of the literature from 1982 to 2008. Health Qual Life Outcomes 7:102, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quinten C, Coens C, Mauer M, et al. : Baseline quality of life as a prognostic indicator of survival: A meta-analysis of individual patient data from EORTC clinical trials. Lancet Oncol 10:865-871, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Eton DT, Fairclough DL, Cella D, et al. : Early change in patient-reported health during lung cancer chemotherapy predicts clinical outcomes beyond those predicted by baseline report: Results from Eastern Cooperative Oncology Group Study 5592. J Clin Oncol 21:1536-1543, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Kramer JA, Curran D, Piccart M, et al. : Identification and interpretation of clinical and quality of life prognostic factors for survival and response to treatment in first-line chemotherapy in advanced breast cancer. Eur J Cancer 36:1498-1506, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Perol M, Winfree KB, Cuyun Carter G, et al. : Association of baseline symptom burden with efficacy outcomes: Exploratory analysis from the randomized phase III REVEL study in advanced non-small-cell lung cancer. Lung Cancer 131:6-13, 2019 [DOI] [PubMed] [Google Scholar]
- 12.Herrmann E, Gerss J, Bierer S, et al. : Pre-treatment global quality of health predicts progression free survival in metastatic kidney cancer patients treated with sorafenib or sunitinib. J Cancer Res Clin Oncol 135:61-67, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee CK, Stockler MR, Coates AS, et al. : Self-reported health-related quality of life is an independent predictor of chemotherapy treatment benefit and toxicity in women with advanced breast cancer. Br J Cancer 102:1341-1347, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carey MS, Bacon M, Tu D, et al. : The prognostic effects of performance status and quality of life scores on progression-free survival and overall survival in advanced ovarian cancer. Gynecol Oncol 108:100-105, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Cella D, Cappelleri JC, Bushmakin A, et al. : Quality of life predicts progression-free survival in patients with metastatic renal cell carcinoma treated with sunitinib versus interferon alfa. J Oncol Pract 5:66-70, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beer TM, Miller K, Tombal B, et al. : The association between health-related quality-of-life scores and clinical outcomes in metastatic castration-resistant prostate cancer patients: Exploratory analyses of AFFIRM and PREVAIL studies. Eur J Cancer 87:21-29, 2017 [DOI] [PubMed] [Google Scholar]
- 17.Robinson AG, Booth CM, Eisenhauer EA: Progression-free survival as an end-point in solid tumours—Perspectives from clinical trials and clinical practice. Eur J Cancer 50:2303-2308, 2014 [DOI] [PubMed] [Google Scholar]
- 18.Gutman SI, Piper M, Grant MD, et al. : Progression-Free Survival: What Does It Mean for Psychological Well-Being or Quality of Life? Methods Research Report. Rockville, MD, Agency for Healthcare Research and Quality, 2013 [PubMed] [Google Scholar]
- 19.Kelly K, Crowley J, Bunn PA Jr, et al. : Randomized phase III trial of paclitaxel plus carboplatin versus vinorelbine plus cisplatin in the treatment of patients with advanced non–small-cell lung cancer: A Southwest Oncology Group trial. J Clin Oncol 19:3210-3218, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Hesketh PJ, Chansky K, Lau DH, et al. : Sequential vinorelbine and docetaxel in advanced non-small cell lung cancer patients age 70 and older and/or with a performance status of 2: A phase II trial of the Southwest Oncology Group (S0027). J Thorac Oncol 1:537-544, 2006 [PubMed] [Google Scholar]
- 21.Quinn DI, Tangen CM, Hussain M, et al. : Docetaxel and atrasentan versus docetaxel and placebo for men with advanced castration-resistant prostate cancer (SWOG S0421): A randomised phase 3 trial. Lancet Oncol 14:893-900, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Webster K, Cella D, Yost K: The Functional Assessment of Chronic Illness Therapy (FACIT) measurement system: Properties, applications, and interpretation. Health Qual Life Outcomes 1:79, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cella DF, Bonomi AE, Lloyd SR, et al. : Reliability and validity of the Functional Assessment of Cancer Therapy-Lung (FACT-L) quality of life instrument. Lung Cancer 12:199-220, 1995 [DOI] [PubMed] [Google Scholar]
- 24.Esper P, Mo F, Chodak G, et al. : Measuring quality of life in men with prostate cancer using the functional assessment of cancer therapy-prostate instrument. Urology 50:920-928, 1997 [DOI] [PubMed] [Google Scholar]
- 25.Cella D, Eton DT, Fairclough DL, et al. : What is a clinically meaningful change on the Functional Assessment of Cancer therapy-Lung (FACT-L) Questionnaire? Results from Eastern Cooperative Oncology Group (ECOG) Study 5592. J Clin Epidemiol 55:285-295, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Qi Y, Schild SE, Mandrekar SJ, et al. : Pretreatment quality of life is an independent prognostic factor for overall survival in patients with advanced stage non-small cell lung cancer. J Thorac Oncol 4:1075-1082, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hui D, Glitza I, Chisholm G, et al. : Attrition rates, reasons, and predictive factors in supportive care and palliative oncology clinical trials. Cancer 119:1098-1105, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Movsas B, Hu C, Sloan J, et al. : Quality of life analysis of a radiation dose-escalation study of patients with non-small-cell lung cancer: A secondary analysis of the radiation therapy oncology group 0617 randomized clinical trial. JAMA Oncol 2:359-367, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reyes ME, Ye Y, Zhou Y, et al. : Predictors of health-related quality of life and association with survival may identify colorectal cancer patients at high risk of poor prognosis. Qual Life Res 26:319-330, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quinten C, Martinelli F, Coens C, et al. : A global analysis of multitrial data investigating quality of life and symptoms as prognostic factors for survival in different tumor sites. Cancer 120:302-311, 2014 [DOI] [PubMed] [Google Scholar]
- 31.Cheng JD, Hitt J, Koczwara B, et al. : Impact of quality of life on patient expectations regarding phase I clinical trials. J Clin Oncol 18:421-428, 2000 [DOI] [PubMed] [Google Scholar]
- 32.Meropol NJ, Weinfurt KP, Burnett CB, et al. : Perceptions of patients and physicians regarding phase I cancer clinical trials: Implications for physician-patient communication. J Clin Oncol 21:2589-2596, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Hui D, Bruera E: Models of palliative care delivery for patients with cancer. J Clin Oncol 38:852-865, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hui D, Hannon BL, Zimmermann C, et al. : Improving patient and caregiver outcomes in oncology: Team-based, timely, and targeted palliative care. CA Cancer J Clin 68:356-376, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Basch E, Iasonos A, McDonough T, et al. : Patient versus clinician symptom reporting using the National Cancer Institute Common Terminology Criteria for Adverse Events: Results of a questionnaire-based study. Lancet Oncol 7:903-909, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Dueck AC, Mendoza TR, Mitchell SA, et al. : Validity and reliability of the US National Cancer Institute's patient-reported outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). JAMA Oncol 1:1051-1059, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]