Abstract
It is unclear how oxidative stress triggered by smoking and vaping may alter specific immune cell subsets. In this study, we showed that tobacco cigarette smoking, but not electronic-cigarette vaping, is associated with increased expression of major proteins in the toll-like receptor 4 (TLR4) inflammasome-interleukin (IL)-6 signalling axis in monocyte subtypes and T cells. TLR4 senses oxidative stress in immune cells caspase-1 is a key protein of inflammasome activation, and IL-6R-α is the receptor for IL-6 that drives proatherogenic IL-6 signalling. These findings implicate the non-nicotine, pro-oxidant toxicants in tobacco cigarette smoke as instigators of increased expression of key proteins in the TLR4-inflammasome-IL-6 axis that contribute to atherogenesis.
RÉSUMÉ
On ignore comment le stress oxydatif, déclenché par le tabagisme et le vapotage, peut modifier des sous-ensembles particuliers de cellules immunitaires. Dans cette étude, nous avons montré que le tabagisme, mais pas le vapotage à l’aide de cigarettes électroniques, est associé à une augmentation de l’expression des principales protéines de l’axe de signalisation inflammasome-interleukine (IL)-6 du récepteur de type Toll 4 (TLR4) dans les sous-types de monocytes et les lymphocytes T. Le TLR4 détecte le stress oxydatif dans les cellules immunitaires; la caspase-1 est une protéine clé de l’activation de l’inflammasome, et l’IL-6R-α est le récepteur de l’IL-6 qui active la voie de signalisation proathérogène de l’IL-6. Ces résultats laissent entendre que les substances toxiques pro-oxydantes non nicotiniques de la fumée de cigarette provoqueraient l’augmentation de l’expression des protéines clés de l’axe TLR4-infilammasome-IL-6 qui contribuent à l’athérogénèse.
Atherosclerosis is a chronic inflammatory disease and immune cells such as monocytes, lymphocytes and natural killer (NK) cells perpetuate atherogenesis.1 The cross-talk between oxidative stress and immune cells as instigators of atherosclerosis has been widely studied.2 However, it is unclear how systemic oxidative stress triggered by environmental exposures, including smoking, may alter specific immune cell subsets such as monocyte subtypes, T cells, and NK cells that have a major role in initiation and perpetuation of atherosclerosis.3,4
The toll-like receptor (TLR) 4 (TLR4)-inflammasome-interleukin (IL)-6 signalling axis is critical for atherogenesis.1,5 Immune cells, specifically monocytes, T cells and NK cells may generate reactive oxygen species (ROS) that then contribute to pathogenesis of atherosclerosis. Oxidative stress is one of the “danger signals” sensed by TLR in immune cells, triggering expression of inflammasome proteins that activate the protease caspase-1.1 Caspase-1, in turn, cleaves pro-IL-1β to its active form, IL-1β, the proinflammatory cytokine that drives proatherosclerotic IL-6 signalling.1,5 Clinically, canakinumab, a monoclonal antibody against the caspase-1 substrate, IL-1β, has been shown to reduce recurrent cardiovascular events in survivors of myocardial infarction.5 IL-6R-α is the receptor for IL-6, and increased IL-6R-α expression would be expected to render immune cells more susceptible to activation by IL-6.1,5
We recently reported that monocytes from people who smoke tobacco cigarettes (TCIGs) have increased oxidative stress6 and increased expression of TLR4, caspase-1, and IL-6R-α compared with people who do not smoke or vape (nonsmokers). 7 However, it remains unknown within which of the various monocyte subtypes, including the classical (CD14++CD16−), nonclassical (CD14dimCD16+), and/or intermediate (CD14++CD16+) monocytes, increased expression of this axis is localized; further, it is unknown if smoking also triggers this axis in T cells and NK cells.
Although the rate of TCIG smoking is decreasing, the rate of electronic cigarette (ECIG) vaping is markedly increasing by smokers who are addicted to nicotine and by never-smoking adolescents and young adults.8 Emissions from TCIGs and ECIGs contain similar levels of addictive nicotine, but otherwise differ from each other in important ways.9 Smoke from TCIGs is generated by burning organic materials, and contains over 7000 toxicants, including almost 100 known carcinogens.10 In contrast, the aerosol from ECIGs is generated by heating, without combustion, a liquid containing solvents, flavorings and nicotine, and levels of toxicants including carcinogens are orders of magnitude lower.10 It is unclear whether vaping carries the same risk for atherosclerosis as smoking, a determination with significant public health implications.
To further elucidate the significance of the TLR4-inflammasome-IL-6 signalling axis in all of the major immune cell subsets (monocyte subtypes, T cells, and NK cells) that contribute to the relative risk of smoking and vaping for inflammatory atherosclerosis, we determined protein expression of caspase-1, IL-6R-α, and TLR4 in immune cell subtypes in otherwise healthy young people without other cardiac risk factors, who either smoke TCIGs or who vape ECIGs compared with healthy people who do not smoke nor vape (nonsmokers).
Methods
Study design
Healthy male and female volunteers between the ages of 21 and 45 years were eligible for enrollment if they were chronic (>1 year): (1) TCIG-smokers, or (2) ECIG vapers (no dual users), or (3) nonsmokers. Former TCIG-smokers were eligible if greater than 1 year had elapsed since quitting. End-tidal CO, elevated above 10 ppm in smokers, was measured in ECIG vapers and nonsmokers to confirm none were surreptitiously smoking TCIGs. All participants were required to meet the following criteria as previously described6: (1) non-obese (≤ 30 body mass index); (2) no known health problems; (3) alcoholic intake ≤ 2 drinks per day and no regular illicit drug use, including marijuana, determined through screening questionnaire and urine toxicology testing; (4) no prescription medications (oral contraceptives allowed); and (5) not exposed to second hand smoke, or using other tobacco products or licensed nicotine replacement therapies. Blood was drawn from fasting participants after abstaining from caffeine, tobacco product use and exercise for at least 12 hours, at the same time of day, approximately 8 AM. The experimental protocol was approved by the Institutional Review Board at the University of California, Los Angeles and written, informed consent was obtained from each participant.
Flow cytometry
We used a previously described6,7 flow cytometry method and thawed cryopreserved peripheral blood mononuclear cells (PBMCs) to determine expression (% of positive cells and median fluorescence intensity [MFI]) of key TLR4-inflammasome-IL-6 signalling axis proteins (TLR4, caspase-1, IL-6R-α). Gating strategies for viability dye and antibody staining for PBMCs have previously been described6,7 and are also shown in Figure 1A.
Figure 1.
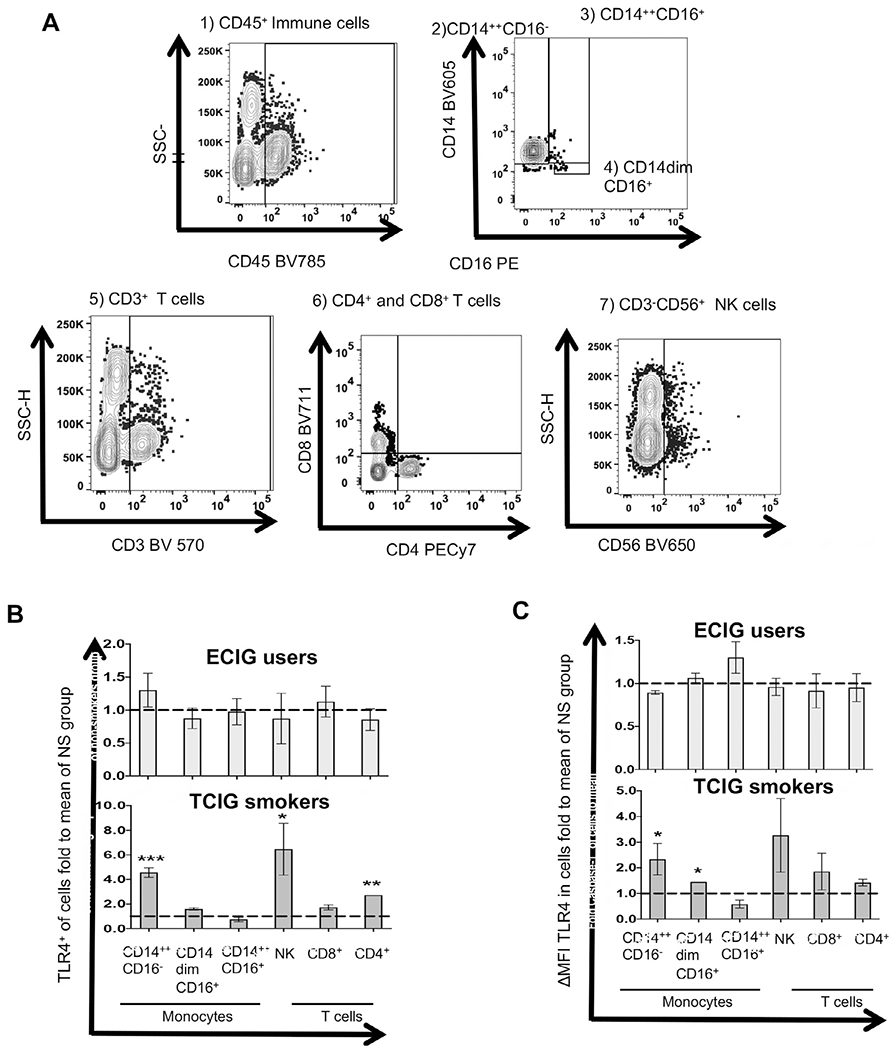
Protein levels of toll-like receptor 4 (TLR4) in monocyte subsets, natural killer (NK) cells, and T cells among smoker groups. (A-C) Flow cytometry was used to determine membrane protein levels of TLR4 among immune cell subtypes. (A) Gating strategy for immune cell subtypes. (B, C) Fluorescence intensity of a positive cell population was compared with a negative cell population (fluorescence minus 1 negative control for staining; ΔMFI). The compared groups were nonsmoker (NS), electronic cigarette (ECIG) vaper (light gray), and tobacco cigarette (TCIG) smokers (dark gray). The mean value of each measurement (percent of positive cells and ΔMFI of protein) was normalized by the mean value of each measurement in the NS group and expressed as fold to the mean of the NS group. Summary data are shown for the percent of positive cells for TLR4 (B) and ΔMFI of TLR4 (C) in immune cell subtypes. The Mann—Whitney test was used to compare individual 2 groups (compared with the NS group; * P < 0.05, ** P < 0.01, *** P < 0.001). MFI, median fluorescence intensity; SSC, side scatter; SSC-H, side scatter height.
Statistical analysis
Kruskal—Wallis analysis of variance (ANOVA) was used to compare the 3 groups and, if the results were statistically significant (P < 0.05), then the Mann—Whitney test was used to compare individual 2 groups. This study, largely exploratory, was not powered to detect effect sizes with adjustments for multiple comparisons.11
Results
Immune cells were collected from 33 (19 male) otherwise healthy young (mean age 24 years) people without lung disease, including 9 chronic (>1 year) TCIG-smokers, 12 chronic ECIG vapers, and 12 nonsmokers.6 Baseline characteristics of the 3 groups including age, sex, race, body mass index, and education have been published and did not differ among the groups.6 Importantly, smoking burden, as estimated by plasma cotinine levels, did not differ between smokers and vapers.6
TLR4 (Fig. 1B), caspase-1 (Fig. 2A), and IL-6R-α (Fig. 2C) protein levels (percent of cells positive for each protein) were increased in classical (CD14++CD16−) and CD4+ T cells of TCIG smokers but not in ECIG vapers compared with nonsmokers. The percent of cells positive for TLR4 and IL-6R-α were also increased in NK cells in TCIG smokers compared with nonsmokers (Fig. 1B, 2 C). The protein levels of IL-6R-α were also increased in CD8+ T cells in TCIG smokers but not in ECIG vapers compared with nonsmokers (Fig. 2C). Surprisingly, the percent of cells positive for caspase-1 was significantly and strikingly decreased in classical (CD14++CD16−) monocytes, and CD8+ and CD4+ T cells in ECIG vapers compared with nonsmokers (Fig. 2A). There were no other differences in protein levels (percent of cells positive for each protein) of TLR4, caspase-1 and IL-6R-α among other immune cell subtypes of TCIG smokers and ECIG vapers compared with nonsmokers (Figs. 1 and 2).
Figure 2.
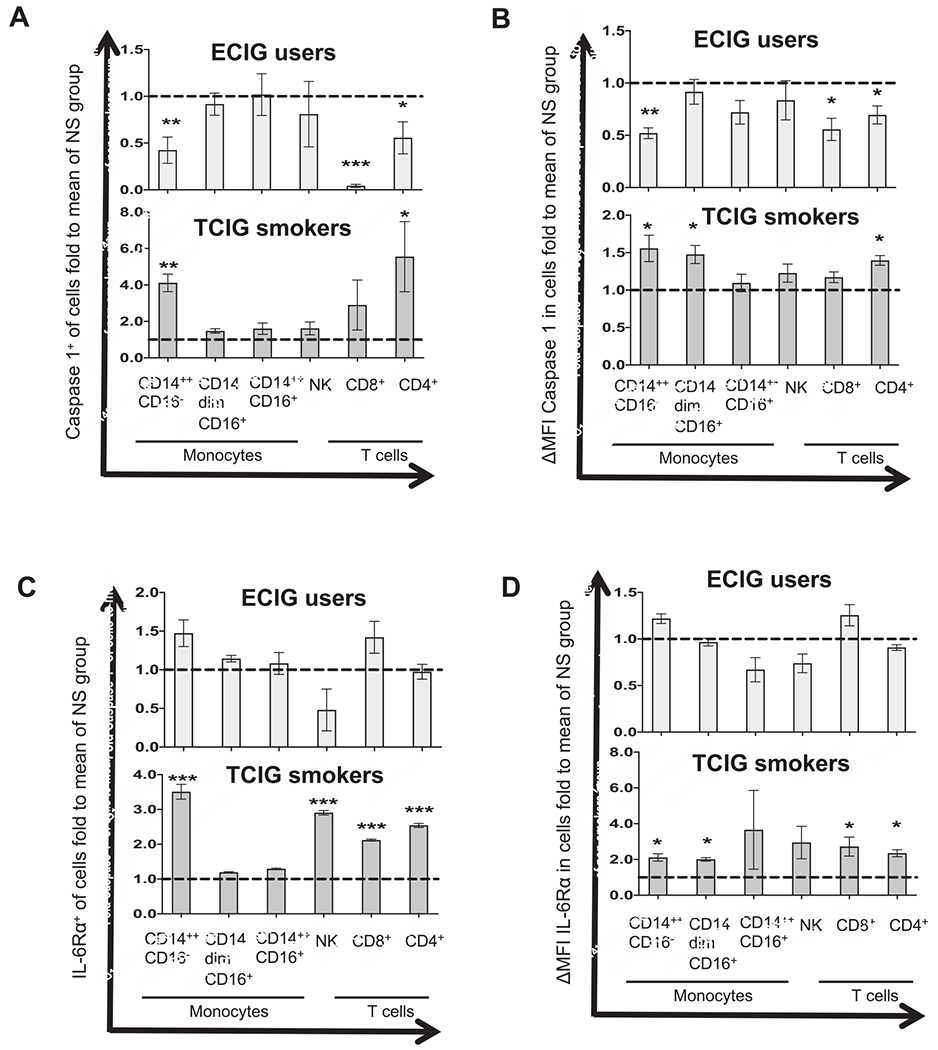
Protein levels of toll-like receptor 4 (TLR4) in monocyte subsets, natural killer (NK) cells, and T cells among smoker groups. (A-D) Flow cytometry was used to determine membrane protein levels of caspase-1 and interleukin (IL)-6R-α among immune cell subtypes. Fluorescence intensity of a positive cell population was compared with a negative cell population (fluorescence minus 1 negative control for staining; ΔMFI). The compared groups were nonsmokers (NS), electronic cigarette (ECIG) vapers (light gray), and tobacco cigarette (TCIG) smokers (dark gray). The mean value of each measurement (percent of positive cells and ΔMFI of protein) was normalized by the mean value of each measurement in the NS group and expressed as fold to the mean of the NS group. Summary data are shown for the percent of positive cells for caspase-1 (A), ΔMFI of caspase-1 (B), the percent of positive cells for interleukin (IL)-6R-α (C), and ΔMFI of IL-6R-α (D) in immune cell subtypes. The Mann—Whitney test was used to compare individual 2 groups (compared with the NS group; * P < 0.05, ** P < 0.01, *** P < 0.001). MFI, median fluorescence intensity.
The ΔMFI of TLR4 (Fig. 1C), caspase-1 (Fig. 2B) and IL-6R-α (Fig. 2D) were increased in CD14++CD16− and non-classical (CD14dimCD16+) monocytes in TCIG smokers but not in ECIG vapers compared with nonsmokers. The ΔMFI of caspase-1 and IL-6R-α were increased in CD4+ T cells of TCIG smokers but not in ECIG vapers compared with nonsmokers (Fig. 2B and D). The ΔMFI of IL-6R-α was increased in CD8+ T cells of TCIG-smokers but not in ECIG vapers compared with nonsmokers (Fig. 2 D). Surprisingly, once again the ΔMFI of caspase-1 was markedly decreased in CD14++CD16− monocytes, CD4+ T cells and CD8+ T cells in ECIG vapers compared with nonsmokers (Fig. 2B). There were no other differences in ΔMFI of TLR4, caspase-1 and IL-6R-α among other immune cell subtypes of TCIG smokers and ECIG vapers compared with nonsmokers (Figs. 1 and 2).
The most consistent and pronounced changes in both measures (% of positive cells and ΔMFI) of all 3 proteins (TLR4, caspase-1 and IL-6R-α) among smoker groups were seen in CD14++CD16− monocytes (representative data are shown in Fig. 3). Protein levels of TLR4, caspase-1 and IL-6R-α were also consistently (except for the ΔMFI of TLR4) increased in CD4+ T cells in TCIG smokers compared with ECIG vapers and nonsmokers.
Figure 3.
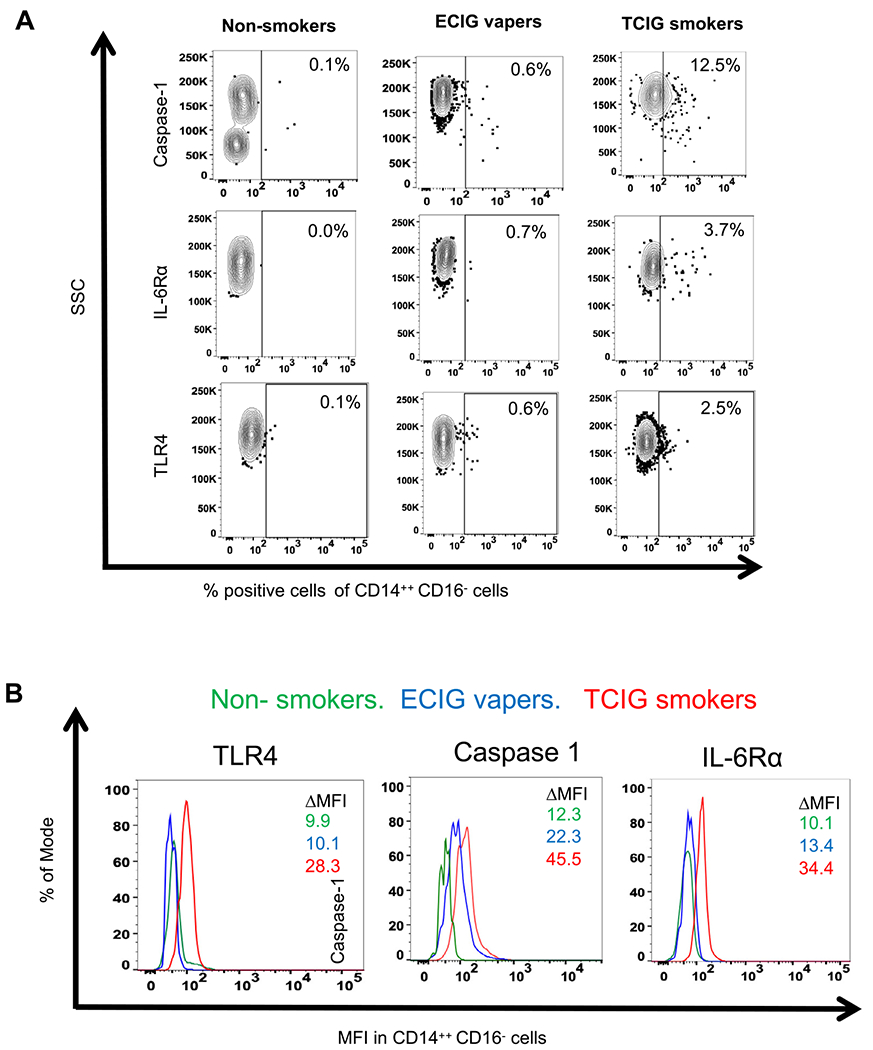
Representative data of percentage of CD14++CD16− monocytes that had positive staining for each protein (A) and for ΔMFI (B) of each protein in the compared groups are shown. ECIG, electronic cigarette; IL, interleukin; MFI, median fluorescence intensity; SSC, side scatter; TCIG, tobacco cigarette; TLR4, toll-like receptor 4.
Discussion
Each of the 3 monocyte subtypes has unique features; specifically, nonclassical (CD14dimCD16+) monocytes are known to exhibit the highest proinflammatory, senescent, and pro-oxidant (highest cellular content of ROS) phenotype followed by the intermediate (CD14++CD16+) monocytes, and then the classical (CD14++CD16−) monocyte subtypes.12 The CD14dimCD16+ monocytes secrete the highest levels of IL-6 and IL-1β cytokines and are the most proinflammatory in response to TLR stimulation in vitro.12 CD14++CD16− monocytes are the most abundant circulating monocytes that contribute to atherogenesis.12 To our knowledge, this is the first study in humans to show increases in key proteins of the TLR4-inflammasome-IL-6 signalling axis, including caspase-1 and TLR4, in relevant monocyte subtypes, T cells and NK cells in TCIG smokers but not in ECIG vapers compared with nonsmokers.
TLR4, caspase-1 and IL-6R-α were consistently increased in CD14++CD16− and CD14dimCD16+ monocytes and CD4+ T cells in TCIG smokers but not in ECIG vapers compared with nonsmokers. TLR4 and IL-6R-α were also increased in NK cells of TCIG smokers but not in ECIG vapers compared with nonsmokers. Our data suggest that not only monocytes but also CD4+ T cells and NK cells may respond through the TLR4-inflammasome-IL-6 axis to danger signals such as oxidative stress to contribute to inflammation and atherogenesis in TCIG smokers but not ECIG vapers.5 This novel finding is consistent with our previous report7 that ECIGs may be less likely to promote inflammation, including inflammatory atherosclerosis, compared with TCIGs. Importantly, levels of plasma cotinine, a metabolite of nicotine, were not different between ECIG vapers and TCIG smokers, consistent with similar nicotine exposure. This finding implicates the non-nicotine, pro-oxidant toxicants in TCIG smoke as instigators of increased expression of key proteins in the TLR4-inflammasome-IL-6 axis.
Notably, expression of TLR4, caspase-1, and IL-6R-α were also consistently increased in CD4+ T cells of TCIG-smokers compared with nonsmokers. Aberrant signalling of ROS, caspase-1, and IL-6 in CD4+ T cells drives pathogenesis of several diseases including autoimmune diseases, HIV, and cancer,13 but to our knowledge, the TLR4-inflammasome-IL-6 axis has not previously been studied in CD4+ T cells of TCIG-smokers. Because TLRs and T cell receptors share signalling pathways, it has been suggested that TLR signalling in effector CD4+ T cells in response to exogenous environmental stimuli regulate their subsequent T cell receptor activation.14 T helper (Th) cells regulate formation of atherosclerotic plaque but their role in atherogenesis is complex.3 The established close interaction of CD4+ T cells with monocytes in the arterial wall and the consistency of the increased protein levels of the TLR4-inflammasome-IL-6 axis in monocytes and CD4+ T cells of TCIG smokers in our study, suggests that this axis may have an important role in the proatherogenic cross-talk of these cells in TCIG smokers.
Similar to other immune cells, NK cells are also susceptible to ROS.15 Our findings that TLR4 and IL-6R-α, but not caspase-1, were increased in NK cells in TCIG smokers, suggests that TCIG-induced oxidative stress may impact TLR4 and IL-6 signalling but not inflammasome activation in NK cells. These data are consistent with prior studies that have shown that exogenous products of inflammasome activation, rather than intrinsic inflammasome signalling, may affect NK cell activation.16 Because NK cells are not the main source of proatherogenic IL-6 signalling,1 the effect of the upregulation of TLR4 and IL-6R-α protein levels in NK cells of TCIG smokers on long-term cardiovascular risk remains to be established.
Consistent with our previous findings, caspase-1 levels were significantly lower in classical (CD14++CD16−) monocytes and T cells (especially CD8+) in ECIG vapers compared with nonsmokers. This seemingly paradoxical finding is consistent with the notion that nicotine has an anti-inflammatory effect,17,18 including the suppression of activation of caspase-1 in response to danger signals such as oxidative stress in immune cells.1 Our results are also consistent with previous findings that nicotine has in vitro anti-inflammatory effects on antigen-presenting cells that closely interact with T cells.19 Differences in the content of nicotine receptors between T cells20 may explain the lower caspase-1 protein levels in CD8+ compared with CD4+ T cells in ECIG vapers.
Our study has limitations. Unknown confounders between the compared groups in our small study may explain our findings. Given the small size of our study, we did not adjust for multiple comparisons but the consistency of data between compared groups distinguished true findings.11 Further larger studies should validate our results.
In conclusion, our study is the first, to our knowledge, to report that TCIGs, but not ECIGs, are associated with increased expression of major proteins in the TLR4- inflammasome-IL-6 signalling axis in monocyte subtypes and T cells. These data support additional investigations into the role of ECIGs as part of a harm reduction strategy for adults addicted to TCIGs who are unwilling or unable to quit.
Funding Sources
This work was supported by the Tobacco-Related Disease Research Program under the contract number TRDRP 28IR-0065 (H.R.M.), by Univeristy of California Office of the President under the contract number R00RG2749 Emergency COVID-19 Research Seed Funding (H.R.M.), and by the National Institutes of Health National Center for Advancing Translational Science University of California Los Angeles Clinical and Translational Science Institute grant number L1TR001881. This work was also supported in part by National Institutes of Health grants R01AG059501, R03AG059462 (T.K.). The flow cytometry machine used in the study was purchased through the University of California Los Angeles Center for AIDS Research (P30AI28697) grant.
Footnotes
Disclosures
The authors have no conflicts of interest to disclose.
References
- 1.Karasawa T, Takahashi M. Role of NLRP3 inflammasomes in atherosclerosis. J Atheroscler Thromb 2017;24:443–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khosravi M, Poursaleh A, Ghasempour G, Farhad S, Najafi M. The effects of oxidative stress on the development of atherosclerosis. Biol Chem 2019;400:711–32. [DOI] [PubMed] [Google Scholar]
- 3.Saigusa R, Winkels H, Ley K. T cell subsets and functions in atherosclerosis. Nat Rev Cardiol 2020;17:387–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Getz GS, Reardon CA. Natural killer T cells in atherosclerosis. Nat Rev Cardiol 2017;14:304–14. [DOI] [PubMed] [Google Scholar]
- 5.Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017;377:1119–31. [DOI] [PubMed] [Google Scholar]
- 6.Kelesidis T, Tran E, Arastoo S, et al. Elevated cellular oxidative stress in circulating immune cells in otherwise healthy young people who use electronic cigarettes in a cross-sectional single-center study: implications for future cardiovascular risk. J Am Heart Assoc 2020;9: e016983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelesidis T, Zhang Y, Tran E, Sosa G, Middlekauff HR. Expression of key inflammatory proteins is increased in immune cells from tobacco cigarette smokers but not electronic cigarette vapers: implications for atherosclerosis. J Am Heart Assoc 2021;10: e019324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mayer M, Reyes-Guzman C, Grana R, Choi K, Freedman ND. Demographic characteristics, cigarette smoking, and e-cigarette use among US adults. JAMA Netw Open 2020;3: e2020694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shahab L, Goniewicz ML, Blount BC, et al. Nicotine, carcinogen, and toxin exposure in long-term e-cigarette and nicotine replacement therapy users: a cross-sectional study. Ann Intern Med 2017;166:390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goniewicz ML, Knysak J, Gawron M, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control 2014;23:133–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feise RJ. Do multiple outcome measures require p-value adjustment? BMC Med Res Methodol 2002;2:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ong SM, Hadadi E, Dang TM, et al. The pro-inflammatory phenotype of the human non-classical monocyte subset is attributed to senescence. Cell Death Dis 2018;9:266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yarosz EL, Chang CH. The role of reactive oxygen species in regulating T cell-mediated immunity and disease. Immune Netw 2018;18:e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzalez-Navajas JM, Fine S, Law J, et al. TLR4 signalling in effector CD4+ T cells regulates TCR activation and experimental colitis in mice. J Clin Invest 2010;120:570–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakamura K, Matsunaga K. Susceptibility of natural killer (NK) cells to reactive oxygen species (ROS) and their restoration by the mimics of superoxide dismutase (SOD). Cancer Biother Radiopharm 1998;13:275–90. [DOI] [PubMed] [Google Scholar]
- 16.Zitti B, Bryceson YT. Natural killer cells in inflammation and autoimmunity. Cytokine Growth Factor Rev 2018;42:37–46. [DOI] [PubMed] [Google Scholar]
- 17.Shields PG, Berman M, Brasky TM, et al. A review of pulmonary toxicity of electronic cigarettes in the context of smoking: a focus on inflammation. Cancer Epidemiol Biomarkers Prev 2017;26:1175–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang H, Yu M, Ochani M, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 2003;421:384–8. [DOI] [PubMed] [Google Scholar]
- 19.Nouri-Shirazi M, Guinet E. Evidence for the immunosuppressive role of nicotine on human dendritic cell functions. Immunology 2003;109:365–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fujii T, Mashimo M, Moriwaki Y, et al. Expression and function of the cholinergic system in immune cells. Front Immunol 2017;8:1085. [DOI] [PMC free article] [PubMed] [Google Scholar]


