Abstract

Wiskott–Aldrich Syndrome Protein Family (WASF) members regulate actin cytoskeletal dynamics, and WASF3 is directly associated with breast cancer metastasis and invasion. WASF3 forms a heteropentameric complex with CYFIP, NCKAP, ABI, and BRK1, called the WASF Regulatory Complex (WRC), which cooperatively regulates actin nucleation by WASF3. Since aberrant deployment of the WRC is observed in cancer metastasis and invasion, its disruption provides a novel avenue for targeting motility in breast cancer cells. Here, we report the development of a second generation WASF3 mimetic peptide, WAHMIS-2, which was designed using a combination of structure-guided design, homology modeling, and in silico optimization to disrupt binding of WASF3 to the WRC. WAHMIS-2 was found to permeate cells and inhibit cell motility, invasion, and MMP9 expression with greater potency than its predecessor, WAHM1. Targeted disruption of WASF3 from the WRC may serve as a useful strategy for suppression of breast cancer metastasis.
Keywords: Breast cancer, Metastasis, Stapled peptide, Rational optimization, WASF, Constrained peptide
Cancer metastasis includes the hijacking of intrinsic signaling pathways involved in cell migration that is required for many routine biological processes including embryonic morphogenesis, immune surveillance, tissue repair, and regeneration.1 The aberrant employment of migratory signaling pathways allows cancer cells to invade surrounding tissues and vasculature, which can result in distant metastases.2−4 Cell migration and invasion is initiated through the formation of membrane protrusions that are driven by localized polymerization of submembrane actin filaments. To facilitate invasion, cancer cells use specialized F-actin rich protrusions called invadopodia. Invadopodia secrete matrix metalloproteinases (MMPs) to degrade the surrounding extracellular matrix (ECM) and allow cancer cells to migrate and invade though their microenvironment.5,6 Rearrangement of the actin cytoskeleton is critical for the formation of F-actin-rich invadopodia. It is mediated by actin related protein 2/3 (Arp2/3) and tightly controlled by its interactions with nucleation factors such as the Wiskott–Aldrich syndrome protein (WASP) family, which includes WASP and neuronal-WASP (N-WASP/WASL); WASP family protein members (WASF1/WAVE1/SCAR1, WASF2/WAVE2/SCAR2, WASF3/WAVE3/SCAR3); WASP homologue associated with actin, membranes, and microtubules (WHAMM); WASP and SCAR homologue (WASH/WASHC1); and junction-mediating regulatory protein (JMY).7−10 The WASP family of proteins is responsible for the spatiotemporal control of F-actin assembly in a wide range of cellular processes including polarization, adhesion, vesicle trafficking, migration, and invasion.10
In resting cells, the three WASF members (WASF1–3) of the WASP family are constitutively sequestered in a heteropentametric complex dubbed the WASF regulatory complex (WRC). The WRC is comprised of WASF proteins along with CYFIP, NCKAP, ABI, and BRK1.11,12 WASF1–3 all play important and, in some cases, overlapping roles in cell protrusion formation; however, only WASF3 is directly associated with cancer cell invasion and metastasis.13 The role of WASF3 in breast cancer invasion and metastasis was shown in model organisms14,15 and through the clinical observation that high WASF3 expression is associated with more aggressive, high grade cancer subtypes.14−16 Additionally, forced re-expression of WASF3 in nonmetastatic breast cancer cells was sufficient to promote the development of an aggressive metastatic phenotype.17
The pivotal role played by WASF3 in breast cancer invasion and metastasis makes it an attractive target for therapeutic intervention; however, it has not yet been effectively targeted using a small molecule approach. Previously, all-hydrocarbon stapled peptides were designed to disrupt the helical bundle interaction between WASF3 and WRC as a strategy to disrupt protein–protein interactions (PPIs) within the WRC to suppress cancer cell invasion and metastasis in vitro and in vivo.13,18 Although important as a proof-of-principle that the complex serves as a viable target for suppression of metastasis, these compounds had several limitations including limited cell permeation and moderate potency with ∼50–70% inhibition observed in various assays at 10 μM dosing. In addition, identification of a more potent compound may enhance its translational potential in animal models for metastasis inhibition.
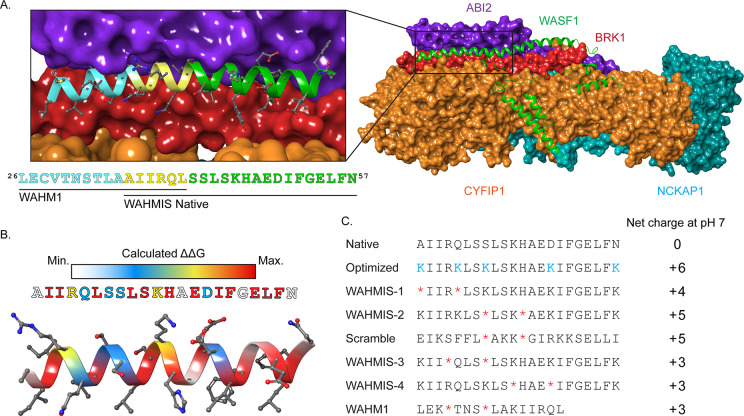
These first-generation stapled peptides targeting the interaction between WASF3 and the WRC, referred to as WASF Helix Mimics (WAHM1/2), were based on the crystal structure (PDB ID: 3P8C) of WASF1 in complex with the WRC (Figure 1A). This structure revealed a trimeric helical bundle comprised of WASF1–BRK1–ABI2 and a dimer consisting of NCKAP1–CYFIP1. The sequence of WASF1 within the trimeric helical bundle is conserved within the WASF subfamily and is referred to as the WASF homology domain (WHD; Figure 1A). In order to design an optimized stapled peptide inhibitor of the WASF3–WRC interaction, a series of homology models were generated using Modeler for WASF3 based on the structure of the WASF1–BRK1–ABI2 helical bundle isolated from the previous WRC crystal structure (PDB ID: 3P8C; Figure S1).19 These models were then optimized and scored using the Rosetta Relax protocol.20 The optimized homology model containing WASF3 residues 21 to 83 in complex with ABI2 and BRK1 was then used as the input for the Rosetta PeptiDerive protocol21 to identify a nonmodified, linear peptide sequence between 18 and 22 residues, derived from WASF3 that partly overlaps with the WAHM1 sequence but extends beyond the original sequence and was predicted to provide greater energetic contributions than WAHM1 when bound to ABI2 and BRK1.
Figure 1.
Rationale and design of WASF mimetic stapled peptides. (A) Crystal structure of the WASF Regulatory Complex (WRC) with CYFIP1 (orange), NCKAP1 (cyan), ABI2 (purple), and BRK1 (red) in surface view and WASF1 (green) in ribbon representation (PDB ID: 3P8C). The inset shows the WASF3 segment corresponding to the prior lead peptide WAHM1 (cyan, yellow) as well as the segment identified by Rosetta PeptiDerive based on its predicted energetic contribution to binding, WAHMIS native (yellow, green). (B) Heatmap displaying the binding energy contribution of individual residues within the WASF3-derived peptide as determined by in silico alanine scanning using Rosetta. (C) The information yielded from the structural WASF3 model and in silico alanine scan were used to design a library of stapled peptides by placing synthetic olefinic amino acid residues (*) at i, i + 4 positions and lysine substitutions (K) to achieve a net positive charge at positions that were not predicted to contribute to the protein-binding interface. Peptide net charges were calculated using PepCalc.
Using this native sequence, we performed an in-silico alanine scan within Rosetta22,23 to identify individual residues that were predicted to energetically contribute to the binding interface (Figures 1B, S2, and S3 and Table S1). This information, coupled with the structural model, were used to identify positions where the olefinic amino acids could be introduced in positions along the nonbinding interface to ultimately form the hydrocarbon staple. We then developed synthetic libraries to experimentally test the compounds where the staple position was varied. The non-natural amino acid (s)-2-(4-pentenyl)alanine (S5) was incorporated at i, i + 4 positions along the nonbinding interface and subsequently cyclized using a first generation Grubbs catalyst to form the hydrocarbon staple to constrain the secondary structure of the peptide. In addition, several residues that were predicted to comprise the solvent-exposed interface of the peptide were substituted to positively charged Lys (K) residues in an effort to enhance water solubility and cellular permeation (Figure S3, Table S1).24−26 The newly designed stapled peptide library was named WAHMInSilico, WAHMIS (Figure 1C).
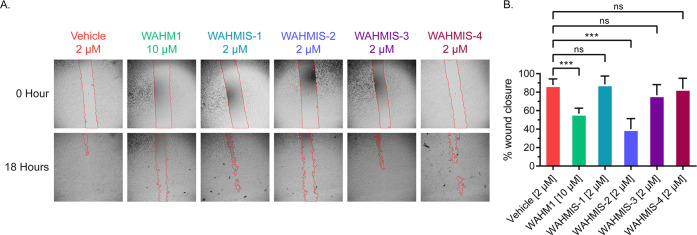
As a strategy to screen whether the newly designed peptides could effectively disrupt the WASF3-containing WRC, wound healing assays were performed in the triple-negative breast cancer cell line MDA-MB-231 to assess their effect on cell motility (Figure 2A). WAHMIS-2 was found to cause an overall reduction of ∼48% in wound healing as compared to the DMSO control (Figure 2B). Further, a lower dose of WAHMIS-2 caused a comparable level of inhibition in wound healing as compared to the previously developed inhibitor, WAHM1 (2 μM versus 10 μM), demonstrating increased potency by WAHMIS-2. All other WAHMIS peptides demonstrate minimal inhibitory effects on cell motility when compared to the DMSO control.
Figure 2.
WAHMIS-2 reduces cell motility. (A) Representative images of wound healing assays (n = 3) performed with MDA-MB-231 cells in the presence of WAHMIS1–4, WAHM1, or DMSO. The wound area is marked by red lines generated using the MRI Wound Healing Tool for ImageJ. (B) Quantification of cell motility as measured by percent wound healing over an 18-h time period across at least three independent wound healing assays. Wound area was calculated using the MRI Wound Healing Tool for ImageJ. ***p < 0.01; n.s., not significant, as assessed by one-way ANOVA and Bonferroni’s multiple comparisons test. Error bars represent standard deviation.
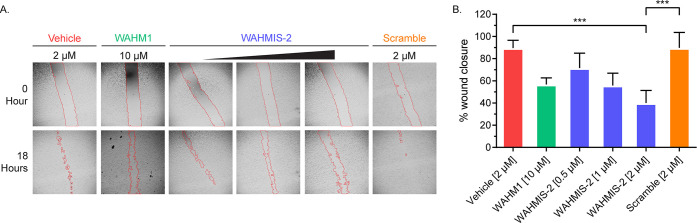
To determine whether WAHMIS-2 could inhibit cell motility in a dose-dependent manner, wound healing assays were performed over a concentration gradient of 0.5–2 μM and compared against its scramble control (2 μM), WAHM1 (10 μM), or vehicle (DMSO; Figure 3A). Wound area analysis revealed a dose-dependent reduction in overall cell motility with WAHMIS-2 treatment while the scramble control had no effect on cell motility (Figure 3B). To verify that the motility effects seen by WAHMIS-2 were not due to cytotoxic effects, MTS and LDH release assays were performed to measure cell viability and membrane lysis. Since Lys residues were substituted in the WAHMIS-2 sequence to improve cell permeability and it has been previously reported that strongly basic peptides can induce cell lysis, we wanted to explore whether they could cause membrane rupture.27 Cells were treated with either 2 μM WAHMIS-2 or its scramble control. No significant reduction in cell viability or increase in LDH release was detected under all conditions as compared to the DMSO-treated control (Figures S4, S5). Thus, although WAHMIS-2 is not inducing lysis at the concentration tested, it is possible that the addition of Lys residues to other peptides in the WAHMIS library may impact their activity.
Figure 3.
WAHMIS-2 reduces cell motility in a dose-dependent manner. (A) Representative images of wound healing assays (n = 3) performed over a concentration range of WAHMIS-2 (0.5, 1, or 2 μM), its scramble control, WAHM1, or DMSO. The wound area is marked by red lines generated using the MRI Wound Healing Tool for ImageJ. (B) Quantification of cell motility as measured by percent wound healing over an 18-h time period across at least three independent wound healing assays. WAHMIS-2-treated cells show a statistically significant, dose-dependent reduction in wound healing. Wound area was calculated using the MRI Wound Healing Tool for ImageJ. ***p < 0.01 as assessed by one-way ANOVA and Bonferroni’s multiple comparisons test. Error bars represent standard deviation.
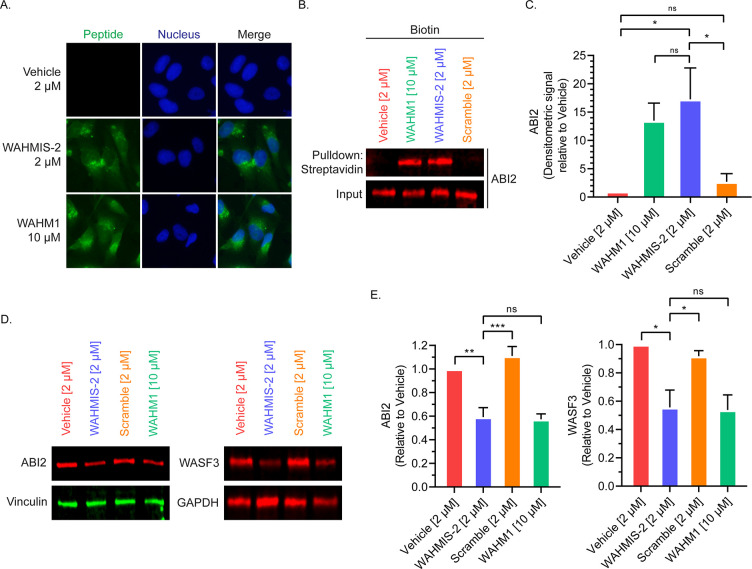
WAHMIS-2 was chosen to be further characterized for cellular uptake and target binding. Cell permeation was assessed by treating cells with either 2 μM 5(6)-carboxyfluorescein (FAM)-labeled WAHMIS-2, 10 μM FAM-labeled WAHM1, or vehicle (DMSO) and imaged by fluorescence microscopy. At a 4 h time point, a lower dosage of WAHMIS-2 demonstrated cellular uptake that was on par with WAHM1 with fluorescence intensity distributed throughout the cytoplasm (Figure 4A, Figure S3). Of note, an unstapled control for WAHMIS-2 showed poor cell permeability and was thus not used in subsequent cell-based assays (Figure S7). To verify that WAHMIS-2 bound to the WRC, pulldowns were then performed using biotin-labeled WAHMIS-2. Cell lysates were treated with 2 μM biotin-labeled WAHMIS-2, its scramble control, 10 μM WAHM1, or vehicle (DMSO) for 4 h followed by pulldowns using streptavidin-agarose resin. Pulldowns were analyzed by immunoblotting for ABI2 (Figure 4B,C), a member of the WRC that forms a binding interface with WASF3. ABI2 was found to interact with WAHMIS-2 while the scramble control did not, indicating that WAHMIS-2 binds its intended target in the WRC.
Figure 4.
WAHMIS-2 permeates cells, binds to ABI2, and reduces WRC protein levels. (A) Fluorescence microscopy images of cells treated with fluorescein-labeled version of the indicated peptides for 4 h (n = 4). WAHMIS-2 demonstrates cytoplasmic fluorescence that is comparable to WAHM1 despite being added to cells at a lower dosage. (B) Representative Western blot (n = 4) demonstrating that WAHMIS-2, but not the scramble control, binds its target, ABI2. Cell lysates were treated with biotin-labeled peptides for 4 h followed by streptavidin pulldowns and detection of ABI2 by immunoblotting. (C) Densitometric quantification of four independent pulldown assays using LI-COR Image Studio showing WAHMIS-2 binds to ABI2 while the scramble control does not. *p < 0.05; n.s., not significant, as assessed by one-way ANOVA and Bonferroni’s multiple comparisons test. Error bars represent standard deviation. (D) Representative Western blot of cells treated with the indicated peptides for 72 h (n = 3). WAHMIS-2- and WAHM1-treated cells show reduced protein levels of ABI2 and WASF3. (E) Densitometric quantification of three independent Western blots demonstrates a statistically significant reduction in ABI2 and WASF3 protein levels in WAHMIS-2- and WAHM1-treated cells. Quantification was performed using LI-COR Image Studio. *p < 0.05; **p < 0.01; ***p < 0.001; ns, not significant as assessed by one-way ANOVA and Bonferroni’s multiple comparisons test. Error bars represent standard deviation.
Previous studies utilizing both RNAi as well as stapled peptides have revealed that targeted disruption of individual members of the WRC can lead to a measurable reduction in protein levels for other members of the complex.4,13,28 To measure whether WAHMIS-2-mediated disruption of WASF3 from the WRC would result in altered levels of other members of the WRC, Western blots were performed after 72 h peptide treatments (Figure 4D,E). Both WAHMIS-2 and WAHM1 were found to promote a statistically significant reduction in expression levels of both ABI2 and WASF3. This reduction in the protein levels caused by the peptides could be rescued by MG132 treatment, demonstrating that the proteasomal degradation pathway is somehow linked to this peptide-mediated loss of proteins in the WRC (Figure S8).
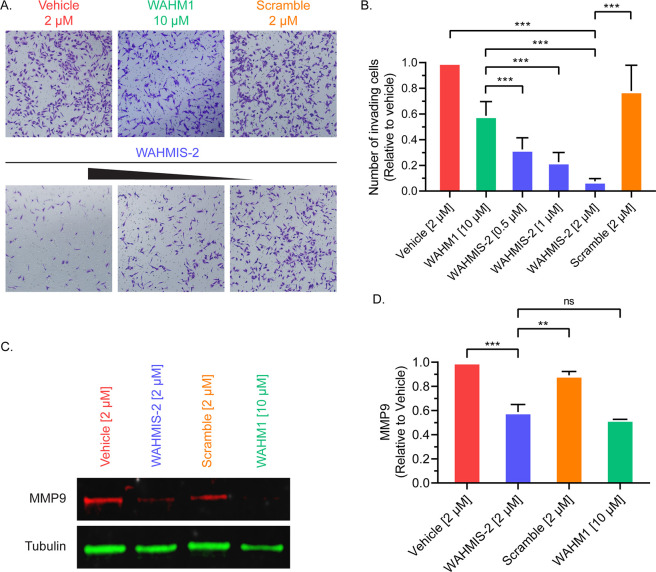
Since activation of WRC has been implicated in promoting degradation of the surrounding extracellular matrix and invasion of neighboring tissue in metastasis,4,13,18 we next sought to evaluate the effects of WAHMIS-2 on cell invasion. The effect of WAHMIS-2 on cell invasion was assessed using transwell migration assays (Figure 5A). Cells treated with WAHMIS-2 showed a dose-dependent reduction in the number of invading cells. Further, all concentrations tested for WAHMIS-2 showed a statistically significant reduction in the number of invading cells as compared to WAHM1 (10 μM) or the scramble control (2 μM; Figure 5B), demonstrating that WAHMIS-2 potently inhibits cell migration and invasion.
Figure 5.
WAHMIS-2 suppresses cell invasion in a dose dependent manner and reduces MMP9 expression. (A) Representative images from Matrigel invasion assays (n = 4) of cells treated with a concentration range of WAHMIS-2 (0.5–2 μM), its scramble control, WAHM1 (10 μM), or DMSO. Invading cells were fixed using paraformaldehyde and stained with crystal violet for imaging and quantification. (B) Invading cells were quantified from at least three independent Matrigel invasion assays as measured using ImageJ. ***p < 0.01 as assessed by one-way ANOVA and Bonferroni’s multiple comparisons test. Error bars represent standard deviation. (C) Representative Western blot from MDA-MB-231 cells treated with indicated peptides for 72 h (n = 3). WAHMIS-2- and WAHM1-treated cells show reduced levels of MMP9 expression. (D) Densitometric quantification of three independent Western blots indicated a significant reduction in MMP9 protein levels in WAHMIS-2 and WAHM1 treated cells. Quantification was performed using LI-COR Image Studio. **p < 0.01; ***p < 0.001; n.s., not significant, as assessed by one-way ANOVA and Bonferroni’s multiple comparisons test. Error bars represent standard deviation.
Additionally, formation of cell protrusions driven by WRC was previously shown to be accompanied by secretion of Matrix Metalloproteinase 9 (MMP9) to facilitate cell invasion.4,13,18 Following this line, we next assessed whether WAHMIS-2 could reduce MMP9 expression (Figure 5C). Cells were treated with WAHMIS-2, WAHM1, or controls for 72 h prior to measuring MMP9 levels by immunoblotting. WAHMIS-2 caused a statistically significant reduction in MMP9 levels (approximately 40%) as compared to the scramble control or vehicle (Figure 5D). Thus, WAHMIS-2 appears to inhibit MMP9 expression as a consequence of disruption of the WRC.
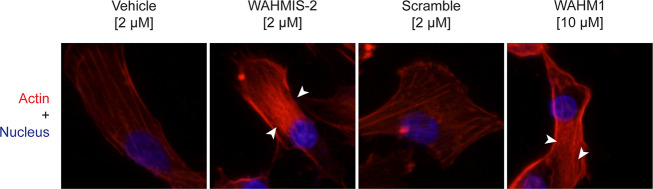
Since activation of WRC and its regulation of Arp2/3 is essential in maintaining actin cytoskeletal dynamics, extraneous disruption of the WRC results in increased stress fiber formation and decreased cell motility.13 To determine whether WAHMIS-2 could promote increased actin stress fiber formation due to disruption of the WRC, cells were treated with either WAHMIS-2, its scramble control, WAHM1, or vehicle (DMSO) followed by phalloidin staining to visualize polymeric actin (Figure 6). Cells treated with either WAHM1 or WAHMIS-2 showed considerably more intense staining for actin stress fiber bundles as compared to cells treated with either the scramble control or vehicle (DMSO), suggesting that WAHMIS-2 increased stress fiber formation as a consequence of WRC disruption (Figure 6, Figure S4).
Figure 6.
WAHMIS-2 treatment increases the density of actin stress fibers. Representative fluorescence images of cells treated with indicated biotin labeled peptides for 4 h followed by fixation, permeabilization, and staining with phalloidin-iFluor 594 (n = 4). Cells treated with WAHMIS-2 and WAHM1 display dense bundles of actin stress fibers (white arrows) while vehicle and scramble control do not.
In summary, here we report the design and development of a computationally optimized, second generation WASF3 mimetic stapled peptide, WAHMIS-2. WAHMIS-2, like its predecessor, WAHM1, was designed to disrupt the binding of WASF3 into the WRC, thereby disrupting and destabilizing formation of the WRC. WAHMIS-2 was found to directly interact with ABI2, one of the primary binding partners of WASF3. In addition to permeating cells, WAHMIS-2 was found to inhibit both cell motility and invasion at comparable levels to WAHM1 but at considerably lower concentrations (5-fold difference). Further, WAHMIS-2 was found to suppress cell motility through increased actin stress fiber formation, reduced MMP9 expression, and reduced protein levels for other members that comprise the WRC complex, ABI2, and WASF3.
Complications arising from the distant metastases remains one of the most lethal aspects of breast cancer.29 Despite the advances in early stage detection and treatment, the 5-year survival rate for metastatic breast cancer remains staggeringly low at 28% compared to over 90% for localized malignancies.30 This highlights the need for targeted intervention of metastasis in breast cancer and the need for the development of therapeutics aimed at impeding cell migration and invasion. Given that WASF3 expression strongly correlates with a poor prognosis for a variety of diverse cancers,15,31−35 its targeted disruption from WRC may provide a novel avenue for therapeutic intervention. The development of a more potent inhibitor of the WRC such as WAHMIS-2 may provide new translational opportunities for potential therapeutic applications in WASF3-driven metastatic cancers.
Glossary
Abbreviations
- WASP
Wiskott–Aldrich Syndrome Protein
- WASF
WASP Family Protein Member
- CYFIP
Cytoplasmic FMRP Interacting Protein
- NCKAP
Nck Associated Protein
- ABI
Abelson Interactor
- BRK1
BRICK1 Subunit of SCAR/WAVE Actin Nucleating Complex
- WRC
WASF Regulatory Complex
- WAHM
WASF Helix Mimics
- WAHMIS
WAHM In-Silico
- MMP
Matrix Metalloproteinase
- ECM
Extracellular Matrix
- Arp2/3
Actin Related Protein 2/3
- PPI
Protein–Protein Interaction
- PDB
Protein Data Bank
- S5
(s)-2-(4-pentenyl)alanine
- DMSO
Dimethyl Sulfoxide
- LDH
Lactate Dehydrogenase
- FAM
Fluorescein Amidite
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.1c00627.
Materials and methods, homology model of WASF3, cell proliferation, cell imaging, quantification of stress fiber formation, and mass spectra of WAHMIS-2 and WAHMIS-2 scramble (PDF)
Author Contributions
‡ Authors contributed equally to this work
This work was supported by the National Institutes of Health (GM134168 to E.J.K.).
The authors declare no competing financial interest.
Supplementary Material
References
- De Craene B.; Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat. Rev. Cancer 2013, 13 (2), 97–110. 10.1038/nrc3447. [DOI] [PubMed] [Google Scholar]
- Mina L. A.; Sledge G. W. Jr. Rethinking the metastatic cascade as a therapeutic target. Nat. Rev. Clin Oncol 2011, 8 (6), 325–32. 10.1038/nrclinonc.2011.59. [DOI] [PubMed] [Google Scholar]
- Teng Y.; Ross J. L.; Cowell J. K. The involvement of JAK-STAT3 in cell motility, invasion, and metastasis. JAKSTAT 2014, 3 (1), e28086. 10.4161/jkst.28086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng Y.; Qin H.; Bahassan A.; Bendzunas N. G.; Kennedy E. J.; Cowell J. K. The WASF3-NCKAP1-CYFIP1 Complex Is Essential for Breast Cancer Metastasis. Cancer Res. 2016, 76 (17), 5133–42. 10.1158/0008-5472.CAN-16-0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy D. A.; Courtneidge S. A. The ’ins’ and ’outs’ of podosomes and invadopodia: characteristics, formation and function. Nat. Rev. Mol. Cell Biol. 2011, 12 (7), 413–26. 10.1038/nrm3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H. Pathological roles of invadopodia in cancer invasion and metastasis. Eur. J. Cell Biol. 2012, 91 (11–12), 902–7. 10.1016/j.ejcb.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Insall R. H.; Machesky L. M. Actin dynamics at the leading edge: from simple machinery to complex networks. Dev Cell 2009, 17 (3), 310–22. 10.1016/j.devcel.2009.08.012. [DOI] [PubMed] [Google Scholar]
- Yilmaz M.; Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009, 28 (1–2), 15–33. 10.1007/s10555-008-9169-0. [DOI] [PubMed] [Google Scholar]
- Krause M.; Gautreau A. Steering cell migration: lamellipodium dynamics and the regulation of directional persistence. Nat. Rev. Mol. Cell Biol. 2014, 15 (9), 577–90. 10.1038/nrm3861. [DOI] [PubMed] [Google Scholar]
- Alekhina O.; Burstein E.; Billadeau D. D. Cellular functions of WASP family proteins at a glance. J. Cell Sci. 2017, 130 (14), 2235–2241. 10.1242/jcs.199570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z.; Borek D.; Padrick S. B.; Gomez T. S.; Metlagel Z.; Ismail A. M.; Umetani J.; Billadeau D. D.; Otwinowski Z.; Rosen M. K. Structure and control of the actin regulatory WAVE complex. Nature 2010, 468 (7323), 533–8. 10.1038/nature09623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B.; Brinkmann K.; Chen Z.; Pak C. W.; Liao Y.; Shi S.; Henry L.; Grishin N. V.; Bogdan S.; Rosen M. K. The WAVE regulatory complex links diverse receptors to the actin cytoskeleton. Cell 2014, 156 (1–2), 195–207. 10.1016/j.cell.2013.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng Y.; Bahassan A.; Dong D.; Hanold L. E.; Ren X.; Kennedy E. J.; Cowell J. K. Targeting the WASF3-CYFIP1 Complex Using Stapled Peptides Suppresses Cancer Cell Invasion. Cancer Res. 2016, 76 (4), 965–73. 10.1158/0008-5472.CAN-15-1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sossey-Alaoui K.; Safina A.; Li X.; Vaughan M. M.; Hicks D. G.; Bakin A. V.; Cowell J. K. Down-regulation of WAVE3, a metastasis promoter gene, inhibits invasion and metastasis of breast cancer cells. Am. J. Pathol. 2007, 170 (6), 2112–21. 10.2353/ajpath.2007.060975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng Y.; Ren M. Q.; Cheney R.; Sharma S.; Cowell J. K. Inactivation of the WASF3 gene in prostate cancer cells leads to suppression of tumorigenicity and metastases. Br. J. Cancer 2010, 103 (7), 1066–75. 10.1038/sj.bjc.6605850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni S.; Augoff K.; Rivera L.; McCue B.; Khoury T.; Groman A.; Zhang L.; Tian L.; Sossey-Alaoui K. Increased expression levels of WAVE3 are associated with the progression and metastasis of triple negative breast cancer. PLoS One 2012, 7 (8), e42895. 10.1371/journal.pone.0042895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng Y.; Mei Y.; Hawthorn L.; Cowell J. K. WASF3 regulates miR-200 inactivation by ZEB1 through suppression of KISS1 leading to increased invasiveness in breast cancer cells. Oncogene 2014, 33 (2), 203–11. 10.1038/onc.2012.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowell J. K.; Teng Y.; Bendzunas N. G.; Ara R.; Arbab A. S.; Kennedy E. J. Suppression of Breast Cancer Metastasis Using Stapled Peptides Targeting the WASF Regulatory Complex. Cancer Growth Metastasis 2017, 10, 117906441771319. 10.1177/1179064417713197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb B.; Sali A. Comparative Protein Structure Modeling Using MODELLER. Curr. Protoc Bioinformatics 2016, 54, 5 6 1–5 6 37. 10.1002/cpbi.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohl C. A.; Strauss C. E.; Misura K. M.; Baker D. Protein structure prediction using Rosetta. Methods Enzymol 2004, 383, 66–93. 10.1016/S0076-6879(04)83004-0. [DOI] [PubMed] [Google Scholar]
- Sedan Y.; Marcu O.; Lyskov S.; Schueler-Furman O. Peptiderive server: derive peptide inhibitors from protein-protein interactions. Nucleic Acids Res. 2016, 44 (W1), W536–41. 10.1093/nar/gkw385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortemme T.; Kim D. E.; Baker D. Computational alanine scanning of protein-protein interfaces. Sci. STKE 2004, 2004 (219), pl2. 10.1126/stke.2192004pl2. [DOI] [PubMed] [Google Scholar]
- Kortemme T.; Baker D. A simple physical model for binding energy hot spots in protein-protein complexes. Proc. Natl. Acad. Sci. U. S. A. 2002, 99 (22), 14116–21. 10.1073/pnas.202485799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalafatovic D.; Giralt E. Cell-Penetrating Peptides: Design Strategies beyond Primary Structure and Amphipathicity. Molecules 2017, 22 (11), 1929. 10.3390/molecules22111929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Q.; Moellering R. E.; Hilinski G. J.; Kim Y.-W.; Grossmann T. N.; Yeh J. T.-H.; Verdine G. L. Towards understanding cell penetration by stapled peptides. MedChemComm 2015, 6 (1), 111–119. 10.1039/C4MD00131A. [DOI] [Google Scholar]
- Limaye A. J.; Bendzunas G. N.; Kennedy E. J. Targeted disruption of PKC from AKAP signaling complexes. RSC Chem. Biol. 2021, 2 (4), 1227–1231. 10.1039/D1CB00106J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird G. H.; Mazzola E.; Opoku-Nsiah K.; Lammert M. A.; Godes M.; Neuberg D. S.; Walensky L. D. Biophysical determinants for cellular uptake of hydrocarbon-stapled peptide helices. Nat. Chem. Biol. 2016, 12 (10), 845–52. 10.1038/nchembio.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derivery E.; Fink J.; Martin D.; Houdusse A.; Piel M.; Stradal T. E.; Louvard D.; Gautreau A. Free Brick1 is a trimeric precursor in the assembly of a functional wave complex. PLoS One 2008, 3 (6), e2462. 10.1371/journal.pone.0002462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung H.; Ferlay J.; Siegel R. L.; Laversanne M.; Soerjomataram I.; Jemal A.; Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin 2021, 71, 209. 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- Siegel R. L.; Miller K. D.; Fuchs H. E.; Jemal A. Cancer Statistics, 2021. CA Cancer J. Clin 2021, 71 (1), 7–33. 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- Teng Y.; Liu M.; Cowell J. K. Functional interrelationship between the WASF3 and KISS1 metastasis-associated genes in breast cancer cells. Int. J. Cancer 2011, 129 (12), 2825–35. 10.1002/ijc.25964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoshal P.; Teng Y.; Lesoon L. A.; Cowell J. K. HIF1A induces expression of the WASF3 metastasis-associated gene under hypoxic conditions. Int. J. Cancer 2012, 131 (6), E905–15. 10.1002/ijc.27631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J.; Wang G. C.; Chen X. J.; Xue Z. R. Expression of WASF3 in patients with non-small cell lung cancer: Correlation with clinicopathological features and prognosis. Oncol Lett. 2014, 8 (3), 1169–1174. 10.3892/ol.2014.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L.; Wang P.; Yang J.; Li X. MicroRNA-217 regulates WASF3 expression and suppresses tumor growth and metastasis in osteosarcoma. PLoS One 2014, 9 (10), e109138. 10.1371/journal.pone.0109138. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Wang G.; Fu Y.; Liu G.; Ye Y.; Zhang X. miR-218 Inhibits Proliferation, Migration, and EMT of Gastric Cancer Cells by Targeting WASF3. Oncol Res. 2017, 25 (3), 355–364. 10.3727/096504016X14738114257367. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.