Abstract
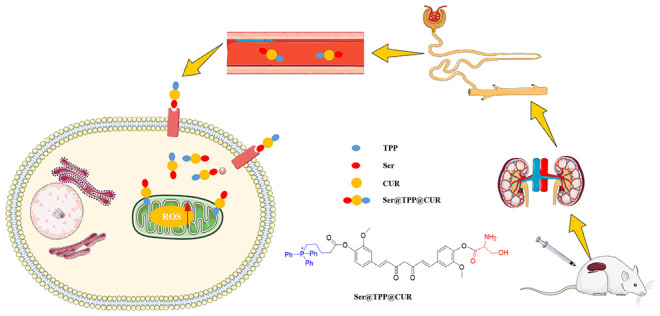
Based on the pathological mechanisms of acute kidney injury (AKI), a stepwise targeting curcumin derivative, Ser@TPP@CUR, was developed in this study. Ser@TPP@CUR can be specifically internalized by renal tubular epithelial cells via KIM-1 receptor-mediated endocytosis and then actively distributed in mitochondria under the effect of TPP, a mitochondrial targeting molecule. Both in vitro and in vivo results showed that Ser@TPP@CUR effectively ameliorated injured renal tubular epithelial cells and improved renal functions of AKI mice.
Keywords: Acute kidney injury, oxidative stress, KIM-1 receptor, mitochondria, renal tubular epithelial cells, curcumin
Acute kidney injury (AKI) is usually characterized by a rapid decline in renal excretion function, a decrease in urine, and the accumulation of nitrogen-containing metabolic wastes.1 It has been reported that more than 12.3 million cases and more than 1.7 million deaths worldwide are associated with acute kidney injury each year.2 AKI is caused by excessive infiltration of reactive oxygen species (ROS) into the kidney. It usually occurs in cases of overdose of nephrotoxins (including chemotherapeutic agents such as cisplatin), ischemia–reperfusion, and sepsis. These excessive ROS can upset the redox balance and directly destroy lipids, nucleic acids, and proteins in the kidney.3 With the serious injury and loss of function of renal tubules, nitrogen-containing metabolic wastes, especially serum creatinine (Scr) and blood urea nitrogen (BUN), will accumulate excessively in the body. However, there is no specific drug for AKI except expensive and difficult kidney transplantation and some supplementary treatments such as rehydration and hemodialysis. In clinical therapy, although N-acetyl-l-cysteine provides some relief from AKI induced by contrast agents or nephrotoxins,4 its high-dose use and low bioavailability make it inadequate for AKI remissions.
The excessive ROS in the lesions are highly invasive and oxidative, which may not only cause the disease but also hinder the self-repair of the lesions and cause serious damage to the surrounding normal tissues. Endogenous reducing glutathione (GSH) is not sufficient to counter such excess oxygen free radicals and restore the original redox balance, which has led to the development of highly effective exogenous antioxidant drugs.5 In recent years, natural antioxidants with the ability to scavenge reactive oxygen free radicals have been widely investigated for the treatment of various diseases caused by reactive oxygen free radicals and the alleviation of oxidative stress in the focus of disease. Natural antioxidants are usually found in everyday vegetables, fruits, and plants, such as curcumin (CUR), gallic acid, and procyanidins.6−8 Because of their antioxidant activity, low cost, and low side effects, these antioxidants have received extensive attention in recent years. CUR is a small-molecule polyphenolic compound extracted from the rhizomes of some plants of the Zingiberaceae and Araceae families. It has anti-inflammatory, antioxidant, antiradiation, and immune-regulation functions. It was demonstrated that CUR can effectively protect renal tubular epithelial cells from oxidative stress induced by H2O2 and plays an important role in antioxidation.9 However, CUR has no selective specificity for renal tissues, and thus, its efficacy is often unsatisfactory. That is, the drug concentration in the target area is too low to reach the effective therapeutic concentration. Therefore, the increased distribution of antioxidant drugs in renal tissue, renal tubular epithelial cells, and intracellular mitochondria is the key to antioxidant therapy for AKI.
The KIM-1 receptor on renal tubular epithelial cells is significantly upregulated in ischemia–reperfusion-induced AKI.10 KIM-1 is a significantly upregulated transmembrane protein that recognizes phospholipid serine and oxidized lipids on the surfaces of dead cells, induces the internalization of dead cells, and thereby eliminates dead cells.11 Therefore, the curcumin derivative Ser@CUR was first synthesized in order to increase the distribution of curcumin in injured renal tubular epithelial cells via the interaction between serine and the KIM-1 receptor. On this basis, (5-hydroxy-5-oxopentyl)triphenylphosphonium bromide (TPP), a mitochondrial targeting molecule, was further attached to synthesize Ser@TPP@CUR. TPP contains three phenyl rings that can increase the molecular surface area and form a delocalized positive charge, effectively penetrating the mitochondrial double-layer hydrophobic membrane.9 Under the action of TPP, CUR actively enters the injured renal tubular epithelial cells and is further transported to the mitochondria to eliminate excessive ROS.
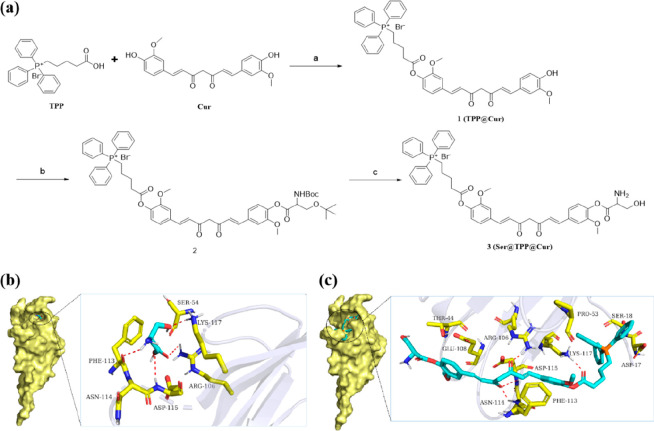
Herein we report the design and evaluation of the stepwise targeting curcumin derivative Ser@TPP@CUR. The synthetic route for Ser@TPP@CUR (compound 3) is illustrated in Figure 1a. The esterification reaction of curcumin with TPP yielded TPP@CUR (1). Then compound 1 and N-(tert-butoxycarbonyl)-O-(tert-butyl)serine was coupled efficiently to give compound 2. The final product 3 was conveniently obtained by removing the tert-butoxycarbonyl (Boc) and tert-butyl protecting groups from 2 in trifluoroacetic acid and dichloromethane. The detailed process for the synthesis and the synthetic yields are presented in the Supporting Information. Compounds 1–3 were characterized by 1H, 13C, and 31P NMR spectroscopy and electrospray ionization high-resolution mass spectrometry (ESI-HRMS) (Figures S1–S12).
Figure 1.
Synthesis and characterization of Ser@TPP@CUR. (a) Synthetic route for Ser@TPP@CUR. (b, c) Binding models for (b) l-serine and (c) Ser@TPP@CUR on the molecular surface of KIM-1.
Molecular docking of l-serine and Ser@TPP@CUR with KIM-1 was performed first. The docking scores of l-serine and Ser@TPP@CUR with KIM-1 were −6.1 and −3.4 kcal/mol, respectively. The binding models for l-serine and Ser@TPP@CUR with KIM-1 are shown in Figure 1b,c. l-Serine and Ser@TPP@CUR exhibit suitable steric complementarity with the binding site of KIM-1. In addition, hydrogen-bonding interactions were formed between TIM1 and l-serine as well as Ser@TPP@CUR. Oxygen atoms O3 of l-serine and O6 of Ser@TPP@CUR, regarded as hydrogen-bond acceptors, form hydrogen bonds with nitrogen atom Nε of Lys117. Oxygen atoms O2 of l-serine and O2 of Ser@TPP@CUR, regarded as hydrogen-bond acceptors, form hydrogen bonds with the nitrogen atom (NH2) of Arg106. The nitrogen atom and oxygen atom O1 of l-serine, regarded as hydrogen-bond donors, form hydrogen bonds with the oxygen atom of Phe113 and the nitrogen atom of Asp115, respectively. Oxygen atom O3 of Ser@TPP@CUR, regarded as a hydrogen-bond acceptor, forms two hydrogen bonds with the nitrogen atoms of Asp115 and Asn114, respectively. Van der Waals interactions are also formed among l-serine or Ser@TPP@CUR and the surrounding residues. These interactions mainly contribute to the binding energies of KIM-1 with l-serine and Ser@TPP@CUR.
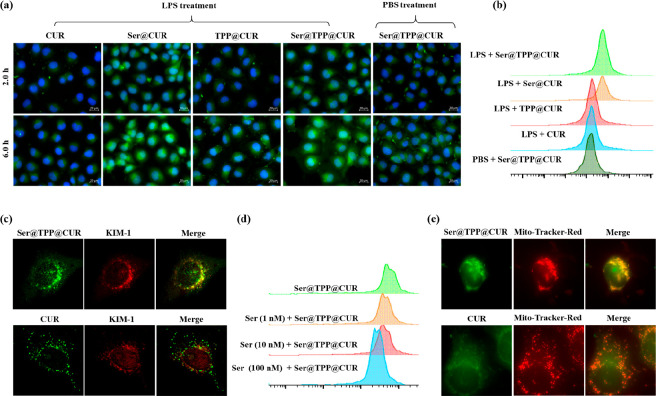
KIM-1 receptors were upregulated in lipopolysaccharide (LPS)-induced HK-2 cells (Figure S15), which was a prerequisite for KIM-1-receptor-mediated endocytosis of Ser@TPP@CUR by inflammatory cells in renal tissues. The internalization of Ser@TPP@CUR into cells with LPS-induced injury was investigated using renal tubular epithelial (HK-2) cells as model cells. As shown in Figure 2a, the distributions of Ser@TPP@CUR and Ser@CUR in LPS-injured HK-2 cells were obviously higher than those of CUR and TPP@CUR after incubation for 2.0 and 6.0 h. It was speculated that the increased distribution of CUR in the injured cells was closely related to the modification of Ser. In addition, the distribution of Ser@TPP@CUR in normal cells is lower than its distribution in injured cells, showing that the targeting effect of Ser was associated with the KIM-1 receptor specifically expressed by injured cells. Flow cytometry (Figure 2b) also confirmed that the modification of Ser could increase the distribution of CUR in injured cells. Next, immunofluorescence technology was used to clarify that the specific distribution of Ser@TPP@CUR in injured cells was related to the KIM-1 receptors. As shown in Figure 2c, the green fluorescence of Ser@TPP@CUR had a better coincidence with the red fluorescence of the KIM-1 receptor than that of CUR (Pearson’s correlation coefficient ρ = 0.33 vs ρ = 0.97; Figure S16). Free Ser could reduce the internalization of Ser@TPP@CUR by injured cells in a concentration-dependent manner (Figure 2d), demonstrating that the targeted transport of Ser@TPP@CUR in injured tubular epithelial cells is closely related to the KIM-1 receptors.
Figure 2.
Time-dependent cellular uptake and mitochondrial colocalization of Ser@TPP@CUR on HK-2 cells in vitro. (a) Representative fluorescence images of time-dependent cellular uptake of Ser@TPP@CUR by HK-2 cells with or without LPS treatment. (b) Flow cytometry results for cellular uptake (2.0 h) of Ser@TPP@CUR by HK-2 cells with or without LPS treatment. (c) Representative fluorescence images of Ser@TPP@CUR (green) with KIM-1 receptors (red) on HK-2 cells. Free CUR was used as the control. (d) Flow cytometry results for cellular uptake of Ser@TPP@CUR by HK-2 cells treated with different concentrations of free Ser. (e) Representative fluorescence images of Ser@TPP@CUR with MitoTracker Red on HK-2 cells. Free CUR was used as the control.
On the basis of the increased distribution of Ser@TPP@CUR in injured cells, we further investigated whether TPP modification enhanced the distribution of CUR in mitochondria. The mitochondria were labeled with MitoTracker Red. As shown in Figure 2e, the coincidence of the Ser@TPP@CUR green fluorescence signal and mitochondrial red fluorescence signal was significantly higher than that of CUR (ρ = 0.95 vs ρ = −0.2; Figure S17), showing that Ser@TPP@CUR had good mitochondrial targeting ability.
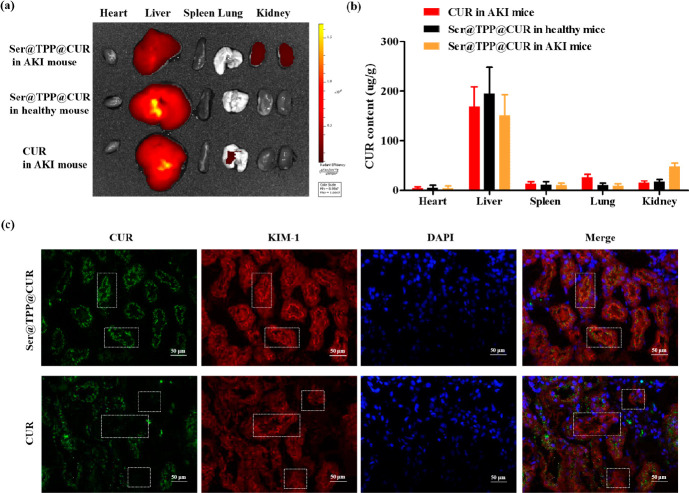
The renal distribution of Ser@TPP@CUR in AKI mice was investigated using an IVIS Lumina III in vivo imaging system. As shown in Figure 3a, the distribution of Ser@TPP@CUR (CUR, 4 mg/kg) in the renal tissues of AKI mouse was significantly better than that of CUR at 2 h after intravenous injection, but its distribution was not improved in the renal tissues of normal mouse. The distribution of CUR was further determined in the isolated tissue sections (Figure 3b), which showed an increased CUR level in renal tissues of AKI treated with Ser@TPP@CUR. In vivo colocalization analysis was also performed to determine whether the endocytosis of Ser@TPP@CUR was associated with the KIM-1 receptor. As shown in Figure 3c, the overlap of Ser@TPP@CUR (green) and KIM-1 receptor (red) was better than that of CUR (the white box), suggesting that KIM-1-receptor-mediated endocytosis in renal tubule epithelial cells improved the renal distribution of Ser@TPP@CUR.
Figure 3.
Renal distribution and renal tubule accumulation of Ser@TPP@CUR. (a) Representative fluorescence images of the main organs (heart, lung, liver, spleen, and kidney) in AKI mice treated with Ser@TPP@CUR or free CUR. (b) Tissue distributions of Ser@TPP@CUR in AKI mice at 2.0 h after intravenous administration of Ser@TPP@CUR or free CUR as a control. Data are presented as mean ± SD (n = 6). (c) Representative fluorescence images of colocalization, noted by the white box, of Ser@TPP@CUR (green) with KIM-1 receptors (red) expressed in renal tissues of AKI mice. Free CUR was used as the control.
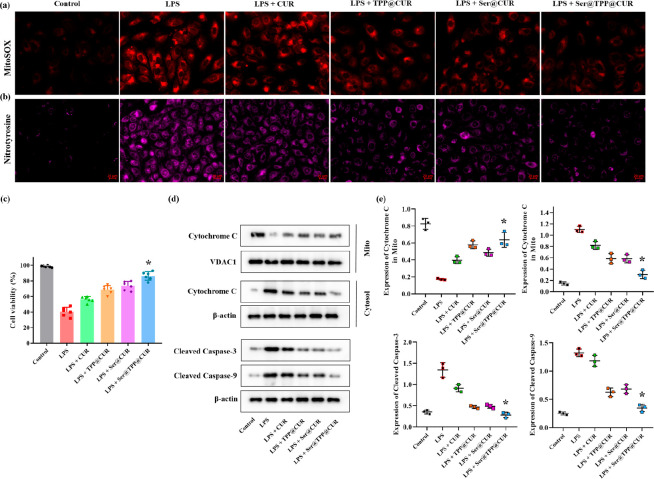
MitoSOX Red mitochondrial superoxide indicator was used to reflect mitochondrial ROS production in HK-2 cells treated with Ser@TPP@CUR (7.16 μg/mL), with CUR, TPP@CUR, and Ser@CUR as controls (Figures 4a and S18). Once in the mitochondria, MitoSOX Red reagent is oxidized by superoxide and exhibits red fluorescence in HK-2 cells treated with LPS. The intracellular fluorescence intensity of the ROS probe was reduced differentially after CUR-based treatment, and Ser@TPP@CUR was superior to CUR, TPP@CUR, and Ser@CUR. Stepwise targeting Ser@TPP@CUR was not only easily internalized by LPS-induced HK-2 cells but also tended toward mitochondria to scavenge ROS. Next, intracellular nitrotyrosine, a sensitive marker for oxidative stress, was examined.12 ROS reacts with nitric oxide to form peroxynitrite, which binds to protein residues such as tyrosine to produce highly cytotoxic nitrotyrosine. As shown in Figures 4b and S19, nitrotyrosine was observed in LPS-induced HK-2 cells, as reflected by the positive fluorescence intensity. After the CUR-based treatment, the nitrotyrosine level was reduced to varying degrees, and Ser@TPP@CUR showed the best antioxidant activity.
Figure 4.
Effects of Ser@TPP@CUR on oxidative stress and cell viability in vitro. (a) Representative fluorescence images of mitochondrial ROS analysis with MitoSOX in LPS-induced HK-2 cells. (b) Representative fluorescence images of nitrotyrosine expression in LPS-induced HK-2 cells. Scale bars: 20 μm. (c) Cell viability of LPS-induced HK-2 cells (n = 6). (d) Immunoblot images of cytochrome c (Mito and Cytosol), cleaved caspase-9, and cleaved caspase-3 expression in HK-2 cells. (e) Corresponding protein expression amounts of cytochrome c (Mito and Cytosol), cleaved caspase-9, and cleaved caspase-3 normalized by β-actin. Data are presented as mean ± SD (n = 3).
The MTT assay was performed to assess the effect of Ser@TPP@CUR on cell viability.13 As shown in the Figure 4c, the cell viability was approximately 40% after incubation with 15 μg/mL LPS for 24 h. The cell viability was significantly improved in LPS-induced HK-2 cells treated with Ser@TPP@CUR in comparison with other CUR-based drugs. The effects of Ser@TPP@CUR on mitochondria-mediated apoptotic protein expression were investigated, and the protein levels of cytochrome c in mitochondria and cytosol, cleaved caspase-3, and cleaved caspase-9 were examined. As shown in Figure 4d,e, compared with other CUR-based drugs, the increased expression levels of cytosolic cytochrome c, cleaved caspase-3, and cleaved caspase-9 were significantly inhibited in LPS-treated HK-2 cells incubated with Ser@TPP@CUR. In addition, the reduced expression of mitochondrial cytochrome c was reversed by Ser@TPP@CUR.
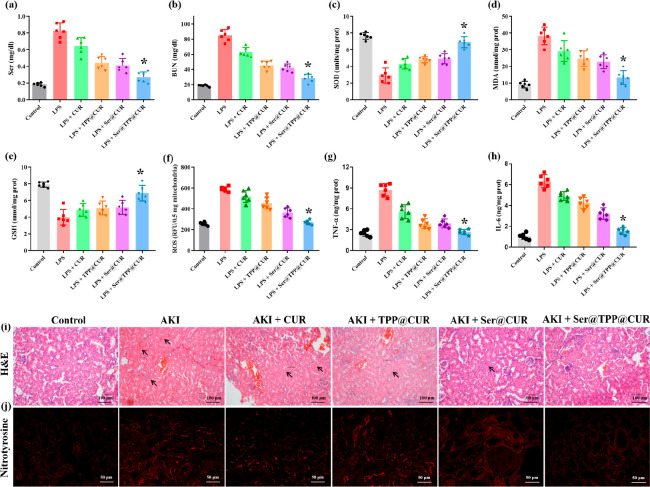
Supported by the good antioxidative stress activity of Ser@TPP@CUR, its in vivo efficacy was further investigated in LPS-induced AKI mice. The renal function indexes Scr and BUN were measured using an automatic biochemical analyzer 24 h after administration. As shown in Figure 5a,b, abnormally increased Scr and BUN values were observed in AKI mice, which indicated the presence of injured renal functions in AKI mice. After CUR-based treatment (CUR, 4 mg/kg), the levels of Scr and BUN were alleviated to different degrees, and Ser@TPP@CUR treatment showed the most significant attenuation of Scr and BUN values, demonstrating that Ser@TPP@CUR effectively restored renal function of AKI mice.
Figure 5.
Effects of Ser@TPP@CUR on renal functions in AKI mice. (a, b) Changes of Scr and BUN in AKI mice (n = 6). (c–e) Levels of SOD, MDA, and GSH in renal tissues of AKI mice (n = 6). (f, g) Levels of TNF-α and IL-6 in renal tissues of AKI mice (n = 6). (h) Mitochondrial ROS production in renal tissues of AKI mice (n = 6). (i) Histological evaluations of renal tissue stained with H&E. Scale bar: 100 μm. (j) Expression of nitrotyrosine in renal tissue stained with immunohistochemistry. Scale bar: 50 μm.
The levels of superoxide dismutase (SOD), glutathione (GSH), and malondialdehyde (MDA), indicators of the degree of kidney damage caused by ROS, in renal tissues were examined to evaluate the antioxidant activity of Ser@TPP@CUR. As shown in Figure 5c,e, SOD and GSH in renal tissues of AKI mice were significantly depleted. Compared with CUR, TPP@CUR, and Ser@CUR, Ser@TPP@CUR treatment significantly improved the levels of SOD and GSH in renal tissues. In addition, Ser@TPP@CUR treatment also effectively reduced the abnormally increased MDA value in renal tissues of AKI mice. The level of ROS in isolated intact mitochondria was determined by the Amplex Red H2O2/peroxidase detection kit. As shown in Figure 5f, the level of H2O2 was significantly increased in the mitochondrial fraction of renal tissues in AKI mice. Ser@TPP@CUR treatment effectively reduced the generation of H2O2 by the mitochondria, and its therapeutic effect was better than those of CUR, TPP@CUR, and Ser@CUR (*, P < 0.05).
Oxidative stress increases inflammatory cytokine production, and in turn, the release of cytokines can further stimulate ROS production.14,15 The inflammatory cytokines tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) were measured by enzyme-linked immunosorbent assay in renal tissues of AKI mice. As shown in Figure 5g,h, the levels of TNF-α and IL-6 were significantly increased in renal tissues of AKI mice. After CUR-based treatment, the levels of TNF-α and IL-6 were reduced to different degrees, and treatment with Ser@TPP@CUR gave the lowest cytokine levels, suggesting that Ser@TPP@CUR had the strongest anti-inflammatory activity. Renal histopathology examination of AKI mice was performed by hematoxylin and eosin (H&E) staining and observation with an optical microscope. As show in Figure 5i, the renal tissues of AKI mice showed swollen tubular epithelial cells, serious vacuolar degeneration, and disappeared and expanded tubular brush border. This severe renal damage was attenuated by CUR-based treatment, and the best therapeutic outcome was observed in AKI mice treated with Ser@TPP@CUR. The expression of nitrotyrosine was significantly upregulated in renal tubules of AKI mice, suggesting serious oxidative-stress damage in renal tissues in AKI mice. Ser@TPP@CUR treatment had lowest positive expression of nitrotyrosine in comparison with CUR, TPP@CUR, and Ser@CUR.
In this study, we successfully constructed a stepwise targeting curcumin derivative, Ser@TPP@CUR. It was demonstrated that Ser@TPP@CUR has good targeted distribution characteristics for damaged renal tubular epithelial cells and intracellular mitochondria. It effectively reduces the level of ROS in the renal tissue of AKI model animals to achieve efficient and precise targeted therapy.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (81801832), the Medical and Health Science and Technology Project of Zhejiang Province (2019KY6370), the Traditional Chinese Medicine Science and Technology Project of Zhejiang Province (2019ZB117), the Natural Science Foundation of Ningbo (2019A610231, 2019A610207, 2019A610366, 202003N4303), the Special Scientific Research Project of Hospital Pharmacy of the Zhejiang Pharmaceutical Society (2019ZYY08), the Fundamental Research Funds for the Provincial Universities of Zhejiang (SJLY2021008), and the Research Fund of Ningbo University (XYL20024).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.1c00585.
Additional experimental procedures; analytical data; 1H, 13C, and 31P NMR spectra; and ESI-HRMS spectra (PDF)
Author Contributions
∇ X.Y. and X.-Y.T. contributed equally to this paper
The authors declare no competing financial interest.
Supplementary Material
References
- Bellomo R.; Kellum J. A.; Ronco C. Acute kidney injury. Lancet 2012, 380, 756–766. 10.1016/S0140-6736(11)61454-2. [DOI] [PubMed] [Google Scholar]
- Zhu H.; Ren A.; Zhou K.; Chen Q.; Zhang M.; Liu J. Impact of Dexmedetomidine Infusion on Postoperative Acute Kidney Injury in Elderly Patients Undergoing Major Joint Replacement: A Retrospective Cohort Study. Drug. Des. Dev. Ther. 2020, 14, 4695–4701. 10.2147/DDDT.S278342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W.; Li D. Reactive Oxygen Species (ROS)-Responsive Nanomedicine for Solving Ischemia-Reperfusion Injury. Front. Chem. 2020, 8, 732. 10.3389/fchem.2020.00732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedre B.; Barayeu U.; Ezeriņa D.; Dick T. P. The mechanism of action of N-acetylcysteine (NAC): The emerging role of H2S and sulfane sulfur species. Pharmacol. Ther. 2021, 228, 107916. 10.1016/j.pharmthera.2021.107916. [DOI] [PubMed] [Google Scholar]
- Pisoschi A. M.; Pop A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. 10.1016/j.ejmech.2015.04.040. [DOI] [PubMed] [Google Scholar]
- Fernandes-Negreiros M. M.; Batista L. A. N. C.; Silva Viana R. L.; Araujo Sabry D.; Paiva A. A. O.; Paiva W. S.; Machado R. I. A.; de Sousa Junior F. L.; de Lima Pontes D.; Vitoriano J. d. O.; Alves Junior C.; Lanzi Sassaki G.; Rocha H. A. O. Gallic Acid-Laminarin Conjugate Is a Better Antioxidant than Sulfated or Carboxylated Laminarin. Antioxidants (Basel) 2020, 9, 1192 10.3390/antiox9121192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasanzadeh S.; Read M. I.; Bland A. R.; Majeed M.; Jamialahmadi T.; Sahebkar A. Curcumin: an inflammasome silencer. Pharmacol. Res. 2020, 159, 104921. 10.1016/j.phrs.2020.104921. [DOI] [PubMed] [Google Scholar]
- Duan Y.; Wang Z.; Zhang H.; He Y.; Lu R.; Zhang R.; Sun G.; Sun X. The preventive effect of lotus seedpod procyanidins on cognitive impairment and oxidative damage induced by extremely low frequency electromagnetic field exposure. Food Funct. 2013, 4, 1252–1262. 10.1039/c3fo60116a. [DOI] [PubMed] [Google Scholar]
- Wang D. W.; Li S. J.; Tan X. Y.; Wang J. H.; Hu Y.; Tan Z.; Liang J.; Hu J. B.; Li Y. G.; Zhao Y. F. Engineering of stepwise-targeting chitosan oligosaccharide conjugate for the treatment of acute kidney injury. Carbohydr. Polym. 2021, 256, 117556. 10.1016/j.carbpol.2020.117556. [DOI] [PubMed] [Google Scholar]
- Zhao X.; Chen X.; Zhang Y.; George J.; Cobbs A.; Wang G.; Li L.; Emmett N. Kidney Injury Molecule-1 Is Upregulated in Renal Lipotoxicity and Mediates Palmitate-Induced Tubular Cell Injury and Inflammatory Response. Int. J. Mol. Sci. 2019, 20, 3406 10.3390/ijms20143406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimura T.; Asseldonk E. J.; Humphreys B. D.; Gunaratnam L.; Duffield J. S.; Bonventre J. V. Kidney injury molecule-1 is a phosphatidylserine receptor that confers a phagocytic phenotype on epithelial cells. J. Clin. Invest. 2008, 118, 1657–1668. 10.1172/JCI34487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Harbi N. O.; Nadeem A.; Ahmad S. F.; Alanazi M. M.; Aldossari A. A.; Alasmari F. Amelioration of sepsis-induced acute kidney injury through inhibition of inflammatory cytokines and oxidative stress in dendritic cells and neutrophils respectively in mice: Role of spleen tyrosine kinase signaling. Biochimie 2019, 158, 102–110. 10.1016/j.biochi.2018.12.014. [DOI] [PubMed] [Google Scholar]
- Wei P.; Xu Y.; Gu Y.; Yao Q.; Li J.; Wang L. IGF-1-releasing PLGA nanoparticles modified 3D printed PCL scaffolds for cartilage tissue engineering. Drug Delivery 2020, 27, 1106–1114. 10.1080/10717544.2020.1797239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziyadeh F. N.; Wolf G. Pathogenesis of the podocytopathy and proteinuria in diabetic glomerulopathy. Curr. Diabetes Rev. 2008, 4, 39–45. 10.2174/157339908783502370. [DOI] [PubMed] [Google Scholar]
- Yoshida L. S.; Tsunawaki S. Expression of NADPH oxidases and enhanced H2O2-generating activity in human coronary artery endothelial cells upon induction with tumor necrosis factor-alpha. Int. Immunopharmacol. 2008, 8, 1377–1385. 10.1016/j.intimp.2008.05.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.