Abstract
IRAK4 kinase plays a key role in TLR/IL-1R signaling pathways that regulate innate immune responses, and if uncontrolled, it is responsible for various inflammatory disorders. By high-throughput screening (HTS) and hit-to-lead optimization, compounds with a 5-aryl-2,4-diaminopyrimidine core structure have been identified as potent IRAK4 inhibitors. A cocrystal structure of IRAK4 protein with an early lead molecule helped with understanding the structure–activity relationship and the design of the new compounds. Initial HTS hits from this series of compounds were also found to inhibit TAK1 kinase, which would cause liver toxicity and potentially bone marrow failure. Optimization of this series resulted in improved selectivity over TAK1 kinase. The TAK1 selectivity was found to be closely associated with different sizes and types of substituents at the 5-position of the pyrimidine. The impact of other pyrimidine substituents on the potency and selectivity was also explored. A few representative compounds were evaluated in IL-1β-induced IL-6 inhibition animal model studies and showed modest efficacy.
Keywords: IRAK4, IRAK4 inhibitors, pyrimidine
Interleukin-1 receptor-associated kinase 4 (IRAK4), a serine/threonine kinase, is crucial for signaling downstream of most TLRs and IL-1Rs, which play important roles in innate immune responses, such as the production of pro-inflammatory cytokines.1 Inhibition of IRAK4 activity has been shown to be effective in animal models of diseases caused by inflammatory and cell proliferative disorders.2−9 Currently, multiple compounds are in clinical trials, including Pfizer’s zimlovisertib (PF-06650833) in multiple Phase II trials for the treatment of various immune-related diseases and conditions.10−12
Through high-throughput screening (HTS), a class of pyrimidine molecules were identified as IRAK4 inhibitors with moderate potency and slight selectivity over transforming growth factor β-activated kinase 1 (TAK1), as represented by compound 1 in Figure 1. The kinase TAK1 is a key mediator of prosurvival signals downstream of the TLR/IL-1R family, TNF receptor, and TGFβ receptor. Knockout studies in mice suggest that inhibition of TAK1 could lead to liver toxicity13,14 and bone marrow failure.15 To avoid any potential adverse effects derived from inhibition of TAK1 kinase and also to reduce the risk of over-immunosuppression by inhibition of both TLR and TNFR pathways, we decided to optimize IRAK4 inhibitors with high selectivity over TAK1. The potency of IRAK4 activity and selectivity over TAK1 were measured by biochemical assays, and a cell-based assay was used to further confirm on-target activities, i.e., inhibition of IL-23 production by THP cells after stimulation with LPS. Inhibition of IL-23 has been shown to be an effective treatment for various autoimmune diseases, including plaque psoriasis and psoriatic arthritis.16,17
Figure 1.
HTS hit 1: modest potency as an IRAK4 inhibitor.
As represented by compound 1, HTS hit molecules have a chiral (1S,2S,3R,4R)-bicyclo[2.2.1]hept-5-ene-3-amino-2-carboxamide moiety at the 4-position of the pyrimidine, an aryl group at the 5-position, and a substituted phenylaniline at the 2-position. We started structure–activity relationship (SAR) exploration by varying the substituent at the 2-position of the pyrimidine to further improve the IRAK4 potency and selectivity over TAK1. As shown in Table 1, removal of the methyl group on the phenyl ring (compound 2) did not have any significant impact on the potency and selectivity, while compound 3 with a shortened carbonyl linker showed great improvements in both potency and selectivity over TAK1. The effect was more pronounced with the amide substituent at the meta position of the phenyl group (compound 4).
Table 1. Variation of the Substituent at the Pyrimidine 2-Position.
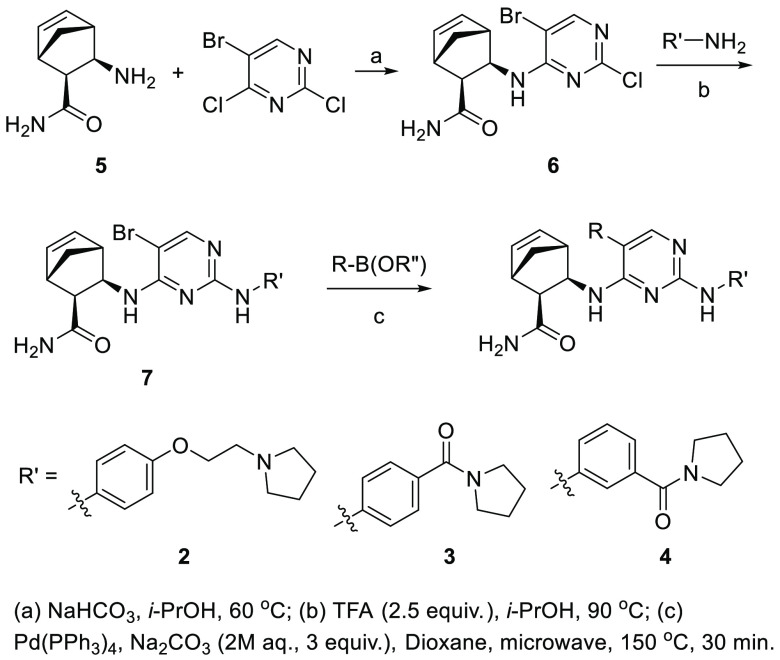
The synthesis of compounds 2–4 is outlined in Scheme 1. It starts with an SNAr reaction between (1S,2S,3R,4R)-bicyclo[2.2.1]hept-5-ene-3-amino-2-carboxamide (5)18 and 5-bromo-2,4-dichloropyrimidine in the presence of NaHCO3 to reduce the formation of the undesired 2-substituted byproduct. Various amino groups were introduced by subsequent SNAr reactions, which were facilitated by the addition of TFA (step (b) in Scheme 1). The final compounds were obtained by Suzuki coupling between intermediate 7 and the desired boronic acid or ester; for compounds 2–4, pyridin-3-ylboronic acid was used.
Scheme 1. General Synthesis Scheme.
Encouraged by the results for the first round of compounds, we then investigated the impact of varying the substituent at the pyrimidine 5-position on the IRAK4 potency and selectivity over TAK1. As shown in Table 2, compounds with smaller substituents (8 and 9) showed potent on-target activities but had lower TAK1 selectivities compared with compounds having larger aromatic groups. A similar trend was observed in a comparison of molecules with five-membered aromatic rings (10 and 11) as opposed to a six-membered phenyl group (12). While compound 12 has potency and selectivity over TAK1 similar to those of the 3-pyridine analogue 4, the 4-pyridine-substituted compound 13 lost TAK1 selectivity but had modestly improved IRAK4 and IL-23 cell-based potency. For compounds 14–16 with o-, m-, and p-fluorophenyl substitutions, compound 15 (m-fluorophenyl-substituted) showed better potency in both IRAK4 and IL-23 cell-based assays, despite moderate selectivity over TAK1. Unfortunately, although compound 14 has excellent TAK1 selectivity, it does not have adequate IL-23 cell-based potency. As for 3-fluoro-substituted pyridine analogues 17 and 18, 4-pyridyl compound 18 has better IRAK4 and IL-23 potency than 3-pyridyl analogue 17, but the selectivity over TAK1 was lower, which is the same trend as observed for compounds 4 and 13. Compounds 19 and 20 substituted with larger cyanophenyl groups retained the same potency as compound 12, but 4-cyano analogue 20 showed much-improved TAK1 selectivity.
Table 2. Different Substituents at the Pyrimidine 5-Position.
Compounds 10–20 were synthesized by the route outlined in Scheme 1. However, compound 8 was prepared by the use of the 5-methyl-2,4-dichloropyrimidine synthon in the first step, and for compound 9 an alternative catalytic system (Pd(OAc)2/(cHex)3P/K3PO4) was used to introduce the cyclopropyl group in the last step.
In order to understand the SAR and better design next-generation molecules, we obtained cocrystal structures of compound 15 with both IRAK4 and TAK1 proteins. As expected, and consistent with the docking results, hinge contact in the ATP binding pocket was formed with N1 of the pyrimidine ring and the NH at the pyrimidine 2-position, as shown in Figure 2. The chiral norbornenyl moiety was tucked into a hydrophobic pocket, and its carboxamide substituent forms a hydrogen bond with Ala-315. The aryl group substituted at the pyrimidine 5-position, i.e., 3-fluorophenyl in compound 15, forms a π–π stacking interaction with Tyr-262 in IRAK4 protein. Although TAK1 protein has a smaller Met-104 sitting at the back of the ATP binding pocket, in the same area as the larger Tyr-262 in the IRAK4 protein, because of the “pointing down” conformation of Met-104, which effectively reduces the size of the binding pocket, the 3-fluorophenyl group is forced to adopt a twisted orientation, making an almost 90° torsion angle with respect to the central pyrimidine ring, to be able to fit into the tighter space in TAK1 protein. This observation most likely explains why compounds with six-membered aromatic rings generally have better selectivity over TAK1 than the ones with five-membered rings (compounds 10 and 11) and much better selectivity than analogues with a much smaller cyclopropyl group (9) or methyl group (8). For compound 14 with an ortho substituent on the phenyl ring, the twisted conformation effect could be more profound and caused a significant loss of TAK1 potency. In IRAK4 protein, at the back of the ATP binding pocket, Lys-213, which is conserved in almost all protein kinases, also plays a very important role in the IRAK4 potency and selectivity over TAK1. Any groups/atoms that can potentially form hydrogen bonds with Lys-213 in IRAK4 or Lys-63 in TAK1 would have dramatic impact on the binding affinity, as observed in comparisons of direct analogues 4 versus 13 and 17 versus 18. Compounds 13 and 18, having 4-pyridine moieties, showed better potency than 3-pyridine analogues 4 and 17 in both IRAK4 and TAK1 biochemical assays. Because of the more significant increase in TAK1 potency, 4-pyridyl compounds 13 and 18 exhibited lower selectivities over TAK1 than the corresponding 3-pyridyl analogues 4 and 17. Also noticeable is the different orientation of the amino substituent at the pyrimidine 2-position. This conformational change resulted from the “extra” long loop αDE in IRAK4 protein, which is absent in TAK1 protein.
Figure 2.

X-ray cocrystal structures of compound 15 with IRAK4 and TAK1 proteins.
To further explore the SAR, additional compounds were synthesized by varying the substituent at the pyrimidine 2-position. In order to include amine moieties that would not undergo an acid-mediated second SNAr reaction and also to investigate a more efficient synthetic route for this purpose, we examined the alternative reaction sequence shown in Scheme 2. First the SNAr reaction between compound 5 and 2,6-dichloro-5-iodopyrimidine was carried out to provide intermediate 21, which was followed by Suzuki coupling to install an aromatic ring of choice. The final compounds were obtained by acid-mediated SNAr or palladium-catalyzed Buchwald reactions between synthon 22 and various amines. The use of 5-iodopyrimidine (step a in Scheme 2) instead of 5-bromopyrimidine (step a in Scheme 1) was to ensure a higher yield of intermediate 22 in the Suzuki reaction (step b in Scheme 2). When we initially tried bromo intermediate 6 for this reaction sequence, the Suzuki reaction was very slow, and additional impurities were formed under harsher conditions or with different catalytic systems (e.g., Pd(dppf)2Cl2/Na2CO3, Pd(OAc)2/KF/MeOH, or Pd(OAc)2/(cHex)3P/K3PO4), most likely because of the steric bulk of the neighboring norbornenyl group in combination with an unfavored electronic environment. As shown in Scheme 2, the optimized reaction conditions for generating the desired 4-substituted regioisomer 21 were found to be the use of sodium bicarbonate as the base at a lower temperature (0–30 °C). Under different reaction conditions, e.g., with DIPEA in CH3CN, compound 21 was obtained in a lower yield (47%), with the major byproduct being the 2-substituted isomer.
Scheme 2. Alternative Route for the Synthesis of 5-Ar-Substituted Pyrimidine Compounds.
Shown in Table 3 are representative compounds with a variety of substituents at the pyrimidine 2-position along with the 3-fluorophenyl group at the 5-position. For amide compounds 23 and 24 with the pyrrolidine in 15 replaced by morpholine or 4-methylpiperazine, respectively, not only was the IRAK4 and IL-23 potency not improved, but the selectivity over TAK1 was decreased as well. Pyrrolidine-substituted pyridyl compound 25 showed much improved selectivity over TAK1, but unfortunately, its on-target potency was lower than those of its close analogues 26 and 27 with meta-substituted morpholine and 4-methylpiperazine groups, respectively. Compounds 26 and 27 are more potent than compound 15 in the IL-23 cell-based assay, but the IRAK4 biochemical potency and the selectivity over TAK1 remained similar. The para-substituted pyridyl compounds 28 and 29 are much less potent than the meta-substituted analogues 26 and 27. Loss of potency was also observed for substituted phenyl analogues 30 and 31 compared with 15, 26, and 27, although 30 exhibited much-improved selectivity over TAK1. For compounds 32 and 33 having an oxazole substituent, decreased potency was observed with little difference between meta or para substitution. In Table 3, compounds 23–33 showed IRAK4 potencies very similar to that of compound 15, while their TAK1 potencies varied considerably from 0.1 to 2.4 μM. The wide range in TAK1 potencies is noteworthy, since substituents at the pyrimidine 2-position seem to occupy a relatively open space in the binding pocket on the basis of the TAK1 cocrystal structure with compound 15 (Figure 2).
Table 3. Effects of Substitution at the Pyrimidine 2-Position.
Selected compounds were further profiled in additional cell-based assays to assess the on-target potency, and an acute animal model was used to determine the in vivo efficacy. As shown in Figure 3, for inhibition of IL-619,20 in HUVEC, when stimulated by either LPS or IL-1β, compounds 17 and 18 showed good cell potency with IC50 values less than 0.2 μM. Similarly, these two compounds also showed adequate potency for IL-23 inhibition in human dendritic cells when stimulated by LPS and did not lose much potency for MIP-1β21−23 inhibition in a human whole-blood assay when stimulated by IL-1β.
Figure 3.

Further characterizations of 17 and 18.
In acute animal model studies, female Balb/c mice (8–12 weeks old) were dosed with a solution of the target compound orally (75 mg/kg) followed by intraperitoneal administration of recombinant murine IL-1β after 30 min. After another 1.5 h, serum IL-6 levels and compound plasma levels were measured and analyzed. Compounds 17 and 18 showed 54% and 64% inhibition of IL-6, respectively. The measured plasma concentrations of 17 and 18 were 2877 and 6817 ng/mL at 0.5 h and 496 and 700 ng/mL at 2 h, respectively. The better in vivo efficacy of compound 18 was most likely due to its higher plasma levels in mice.
In summary, from HTS hit to lead optimization, we have identified a series of 5-arylpyrimidine compounds with potent IRAK4 activities in both biochemical and cell-based assays. The selectivity over TAK1 kinase seems to be mainly determined by the size and hydrogen-bond-forming capability of the substituent at the pyrimidine 5-position. Both IRAK4 and TAK1 cocrystal structures with compound 15 were obtained to understand the SAR and guide rational drug design. Compounds 17 and 18 were further characterized by additional cell-based assays to assess the IRAK4 activity, and both molecules showed moderate efficacy in acute animal model studies for the inhibition of IL-6 production.
Acknowledgments
We thank Roy Frances, Sothy Yi, Stacey Siu, Chi Young, and Meagan Chan for running biological assays; Mark Irving, Duayne Tokushige, and Van Nguyen Ybarra for purifying some of the final compounds; Sarkiz Issakani, Caroline Sula, and Christina Coquilla for HTS screening; and Simon J. Shaw for helpful discussions during manuscript preparation.
Glossary
Abbreviations
- IRAK4
interleukin-1 receptor-associated kinase 4
- TLR
Toll-like receptor
- TAK1
transforming growth factor β-activated kinase 1
- TNFR
tumor necrosis factor receptor
- IL-1β
interleukin 1β
- LPS
lipopolysaccharide
- IL-23
interleukin 23
- THP
human acute monocytic leukemia cell line
- IL-6
interleukin 6
- HUVEC
human umbilical vein endothelial cells
- MIP-1β
macrophage inflammatory proteins-1β
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.2c00056.
Representative experimental procedures, 1H NMR and MS data for all compounds, biological assay methods, protocol for animal models, and additional in vitro and in vivo data for compounds 17 and 18 (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Flannery S.; Bowie A. G. The Interleukin-1 Receptor-Associated Kinases: Critical Regulators of Innate Immune Signalling. Biochem. Pharmacol. 2010, 80 (12), 1981–1991. 10.1016/j.bcp.2010.06.020. [DOI] [PubMed] [Google Scholar]
- Loiarro M.; Ruggiero V.; Sette C. Targeting TLR/IL-1R Signalling in Human Diseases. Mediators Inflammation 2010, 2010, 674363 10.1155/2010/674363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genung N. E.; Guckian K. M. Small Molecule Inhibition of Interleukin-1 Receptor-Associated Kinase 4 (IRAK4). Prog. Med. Chem. 2017, 56, 117–163. 10.1016/bs.pmch.2016.11.004. [DOI] [PubMed] [Google Scholar]
- Patra M. C.; Choi S. Recent Progress in the Molecular Recognition and Therapeutic Importance of Interleukin-1 Receptor-Associated Kinase 4. Molecules 2016, 21, 1529 10.3390/molecules21111529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z.; Zhang Y.; Ma Y.; Sun T. Research Progress of Small Molecule IRAK-4 Inhibitors. Zhongnan Yaoxue 2015, 13, 1017–1024. [Google Scholar]
- Nair S.; Kumar S. R.; Paidi V. R.; Sistla R.; Kantheti D.; Polimera S. R.; Thangavel S.; Mukherjee A. J.; Das M.; Bhide R. S.; Pitts W. J.; Murugesan N.; Dudhgoankar S.; Nagar J.; Subramani S.; Mazumder D.; Carman J. A.; Holloway D. A.; Li X.; Fereshteh M. P.; Ruepp S.; Palanisamy K.; Mariappan T. T.; Maddi S.; Saxena A.; Elzinga P.; Chimalakonda A.; Ruan Q.; Ghosh K.; Bose S.; Sack J.; Yan C.; Kiefer S. E.; Xie D.; Newitt J. A.; Saravanakumar S. P.; Rampulla R. A.; Barrish J. C.; Carter P. H.; Hynes J. Optimization of Nicotinamides as Potent and Selective IRAK4 Inhibitors with Efficacy in a Murine Model of Psoriasis. ACS Med. Chem. Lett. 2020, 11 (7), 1402–1409. 10.1021/acsmedchemlett.0c00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajapaksa N. S.; Gobbi A.; Drobnick J.; Do S.; Kolesnikov A.; Liang J.; Chen Y.; Sujatha-Bhaskar S.; Huang Z.; Brightbill H.; Francis R.; Yu C.; Choo E. F.; Dement K.; Ran Y.; An L.; Emson C.; Maher J.; Wai J.; McKenzie B. S.; Lupardus P. J.; Zarrin A. A.; Kiefer J. R.; Bryan M. C. Discovery of Potent Benzolactam IRAK4 Inhibitors with Robust in Vivo Activity. ACS Med. Chem. Lett. 2020, 11 (3), 327–333. 10.1021/acsmedchemlett.9b00380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gummadi V. R.; Boruah A.; Ainan B. R.; Vare B. R.; Manda S.; Gondle H. P.; Kumar S. N.; Mukherjee S.; Gore S. T.; Krishnamurthy N. R.; Marappan S.; Nayak S. S.; Nellore K.; Balasubramanian W. R.; Bhumireddy A.; Giri S.; Gopinath S.; Samiulla D. S.; Daginakatte G.; Basavaraju A.; Chelur S.; Eswarappa R.; Belliappa C.; Subramanya H. S.; Booher R. N.; Ramachandra M.; Samajdar S. Discovery of CA-4948, an Orally Bioavailable IRAK4 Inhibitor for Treatment of Hematologic Malignancies. ACS Med. Chem. Lett. 2020, 11 (12), 2374–2381. 10.1021/acsmedchemlett.0c00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seganish W. M.; Fischmann T. O.; Sherborne B.; Matasi J.; Lavey B.; McElroy W. T.; Tulshian D.; Tata J.; Sondey C.; Garlisi C. G.; Devito K.; Fossetta J.; Lundell D.; Niu X. Discovery and Structure Enabled Synthesis of 2,6-Diaminopyrimidin-4-one IRAK4 Inhibitors. ACS Med. Chem. Lett. 2015, 6 (8), 942–947. 10.1021/acsmedchemlett.5b00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottell J.; Wright N. E.; Warr M.; Taylor J. G. Recent Advances in the Discovery and Development of Interleukin Receptor Associated Kinase 4 (IRAK4) Inhibitors. Med. Chem. Rev. 2019, 54, 97–125. 10.29200/acsmedchemrev-v54.ch5. [DOI] [Google Scholar]
- Lee K. L.; Ambler C. M.; Anderson D. R.; Boscoe B. P.; Bree A. G.; Brodfuehrer J. I.; Chang J. S.; Choi C.; Chung S.; Curran K. J.; Day J. E.; Dehnhardt C. M.; Dower K.; Drozda S. E.; Frisbie R. K.; Gavrin L. K.; Goldberg J. A.; Han S.; Hegen M.; Hepworth D.; Hope H. R.; Kamtekar S.; Kilty I. C.; Lee A.; Lin L. L.; Lovering F. E.; Lowe M. D.; Mathias J. P.; Morgan H. M.; Murphy E. A.; Papaioannou N.; Patny A.; Pierce B. S.; Rao V. R.; Saiah E.; Samardjiev I. J.; Samas B. M.; Shen M. W. H.; Shin J. H.; Soutter H. H.; Strohbach J. W.; Symanowicz P. T.; Thomason J. R.; Trzupek J. D.; Vargas R.; Vincent F.; Yan J.; Zapf C. W.; Wright S. W. Discovery of Clinical Candidate 1-{[(2S,3S,4S)-3-Ethyl-4-fluoro-5-oxopyrrolidin-2-yl]methoxy}-7-methoxyisoquinoline-6-carboxamide (PF-06650833), a Potent, Selective Inhibitor of Interleukin-1 Receptor Associated Kinase 4 (IRAK4), by Fragment-Based Drug Design. J. Med. Chem. 2017, 60 (13), 5521–5542. 10.1021/acs.jmedchem.7b00231. [DOI] [PubMed] [Google Scholar]
- Info: https://clinicaltrials.gov/.
- Inokuchi S.; Aoyama T.; Miura K.; Österreicher C. H.; Kodama Y.; Miyai K.; Akira S.; Brenner D. A.; Seki E. Disruption of TAK1 in Hepatocytes Causes Hepatic Injury, Inflammation, Fibrosis, and Carcinogenesis. Proc. Natl. Acad. Sci. U. S. A. 2010, 107 (2), 844–849. 10.1073/pnas.0909781107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang M.; Wei X.; Guo Y.; Breslin P.; Zhang S.; Zhang S.; Wei W.; Xia Z.; Diaz M.; Akira S.; Zhang J. TAK1 Is Required for the Survival of Hematopoietic Cells and Hepatocytes in Mice. J. Exp. Med. 2008, 205 (7), 1611–1619. 10.1084/jem.20080297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink P. M.; Smout W. M.; Driessen-Engels L. J.; de Bruin A. M.; Delsing D.; Krajnc-Franken M. A.; Jansen A. J.; Rovers E. F.; van Puijenbroek A. A.; Kaptein A.; Nolte M. A.; Garritsen A.; van Eenennaam H. In Vivo Knockdown of TAK1 Accelerates Bone Marrow Proliferation/Differentiation and Induces Systemic Inflammation. PLoS One 2013, 8 (3), e57348 10.1371/journal.pone.0057348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang C.; Chen S.; Qian H.; Huang W. Interleukin-23: As a Drug Target for Autoimmune Inflammatory Diseases. Immunology 2012, 135 (2), 112–124. 10.1111/j.1365-2567.2011.03522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K.; Oak A. S. W.; Elewski B. E. Use of IL-23 Inhibitors for the Treatment of Plaque Psoriasis and Psoriatic Arthritis: A Comprehensive Review. Am. J. Clin. Dermatol. 2021, 22 (2), 173–192. 10.1007/s40257-020-00578-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argade A.Chemoenzymic synthesis of stereoisomerically enriched beta-lactams using lipase B. WO 2006055528 A2, 2006.
- McElroy W. T.; Tan Z.; Ho G.; Paliwal S.; Li G.; Seganish W. M.; Tulshian D.; Tata J.; Fischmann T. O.; Sondey C.; Bian H.; Bober L.; Jackson J.; Garlisi C. G.; Devito K.; Fossetta J.; Lundell D.; Niu X. Potent and Selective Amidopyrazole Inhibitors of IRAK4 That Are Efficacious in a Rodent Model of Inflammation. ACS Med. Chem. Lett. 2015, 6 (6), 677–682. 10.1021/acsmedchemlett.5b00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly P. N.; Romero D. L.; Yang Y.; Shaffer A. L.; Chaudhary D.; Robinson S.; Miao W.; Rui L.; Westlin W. F.; Kapeller R.; Staudt L. M. Selective Interleukin-1 Receptor–Associated Kinase 4 Inhibitors for the Treatment of Autoimmune Disorders and Lymphoid Malignancy. J. Exp. Med. 2015, 212 (13), 2189–2201. 10.1084/jem.20151074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaillon J. M. Cytokines and Macrophages. Biomed. Pharmacother. 1994, 48 (10), 445–453. 10.1016/0753-3322(94)90005-1. [DOI] [PubMed] [Google Scholar]
- O’Grady N. P.; Tropea M.; Preas H. L. II; Reda D.; Vandivier R. W.; Banks S. M.; Suffredini A. F. Detection of Macrophage Inflammatory Protein (MIP)-1α and MIP-β during Experimental Endotoxemia and Human Sepsis. J. Infect. Dis. 1999, 179 (1), 136–141. 10.1086/314559. [DOI] [PubMed] [Google Scholar]
- Zhang T.; Guo C. J.; Li Y.; Douglas S. D.; Qi X. X.; Song L.; Ho W. Z. Interleukin-1β Induces Macrophage Inflammatory Protein-1β Expression in Human Hepatocytes. Cell. Immunol. 2003, 226 (1), 45. 10.1016/j.cellimm.2003.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.