Abstract
Cancer treatments utilizing biologic or cytotoxic drugs compose the frontline of therapy, and though gains in treatment efficacy have been persistent in recent decades, much work remains in understanding cancer progression and treatment. Compounding this situation is the low rate of success when translating preclinical drug candidates to the clinic, which raises costs and development timelines. This underperformance is due in part to the poor recapitulation of the tumor microenvironment, a critical component of cancer biology, in cancer model systems. New technologies capable of both accurately observing and manipulating the tumor microenvironment are needed to effectively model cancer response to treatment. In this review, conventional cancer models are summarized, and a primer on emerging techniques for monitoring and modulating the tumor microenvironment is presented and discussed.
Keywords: Cancer, translational research, models, monitoring, tumor microenvironment, modulation
Impact Statement
The low success rate of prospective therapeutics to the clinic, with an average cost of ~$650 million dollars each, is a large barrier to cancer drug development. As high rates of failure occur at the end of clinical testing, the identification of effective and translatable candidates must be made more rigorous to mitigate the loss of time and capital. To accomplish this aim, integration of cancer tissue models with advanced tissue monitoring and control systems is needed.
Introduction
Cancer is characterized by the deregulation of cellular pathways that regulate several critical components of cell behavior, including growth and invasion into surrounding tissues. Particular types of cancer can differ in their cellular origins, acquired mutations, and microenvironmental conditions, which contributes to a broad range of phenotypes and frustrates the search for a silver bullet treatment. 1 Instead, cancer treatment utilizes a personalized approach, where adjustments to treatment are made in response to disease progression. Over the past 30 years, this strategy has shown some success as cancer death rates have fallen 31% due to improvements in prevention, detection, and treatment. 2 In spite of these gains, cancer remains the second leading cause of death in the United States, and more than one in three people are expected to develop cancer during their lifetime. 2 Even more concerning is the fact that novel cancer drugs entering clinical trials only have a 3.4% approval rate by the US Food and Drug Administration (FDA), and these drugs that do pass often have little to no effect on overall survival.3,4 These data suggest a disjunction in the drug development pipeline where clinical outcomes do not realize the same degree of therapeutic success observed in preclinical studies.
The low rate of clinical translation for novel cancer therapies is due in part to the complex and interconnected network of the tumor microenvironment (TME), where cancer cells reside.5,6 This background encompasses all of the participatory components of a tumor including local specialized cell types (immune cells, fibroblasts, etc.), the extracellular matrix (ECM), chemical gradients, and physical conditions, such as interstitial fluid pressure and shear stresses. Critically, these components vary at local and regional scales, and, when combined with the genomic instability of cancer cells, results in a highly heterogenous disease state. These local differences in the TME have been shown to affect clinically significant tumor properties, such as cancer development, progression, and therapeutic response.6,7 One particularly prominent process is the metastatic cascade, a hallmark of cancer that is long recognized as a significant cause of cancer-associated mortality, yet remains poorly characterized.8–14 The recent designation of metastasis-free survival as an emerging clinical trial endpoint by the FDA demonstrates both the importance of improving our understanding of metastasis and the influence of the TME in therapeutic development. 14
To study the role of the TME in cancer development and progression, cancer models are designed with varying degrees of experimental control, system complexity, and model accuracy. A particular challenge for cancer models is accounting for the heterogeneous nature of the TME while maintaining experimental reproducibility and practicality. The conventional pipeline for cancer research is to first screen for potential biological processes in vitro and then to validate therapeutics in vivo before moving to human testing. This approach is standard practice because the low cost and high-throughput capabilities of cell culturing techniques allow for robust candidate screening and pathway characterization, while in vivo models enhance the physiological relevance of therapeutic potential findings and test for systemic toxicities. Yet, the low success rate of this methodology in identifying clinically viable therapeutics suggests that there is room for improvement. Major concerns, namely, the poor recapitulation of physiological conditions in two-dimensional (2D) cell culture and the significant differences between human and murine drug response, encourage re-examination of conventions in translational cancer research.15,16 In the following section, we summarize conventional cancer models, discuss their advantages and disadvantages, and summarize common quantification techniques to motivate the need for emerging cancer research tools (Figure 1). In later sections, we review techniques and technologies that can address gaps present in conventional cancer model technology through TME monitoring and manipulation.
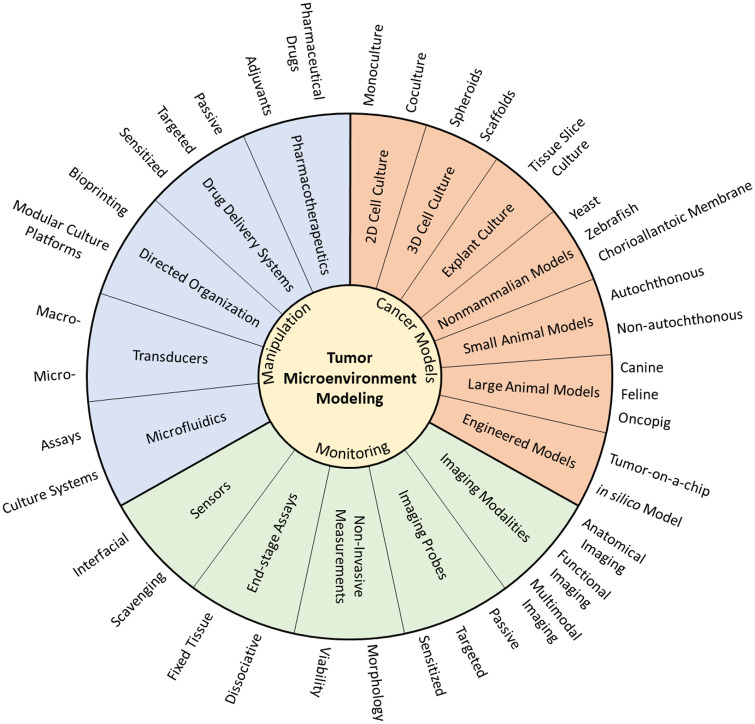
Figure 1.
Schematic of prominent technologies used for modeling the tumor microenvironment. Cancer models determine the biological complexity that is being studied. Monitoring technologies quantify the parameters of interest during a study, and manipulation improves experimental control and model relevance. Combinations of these technologies are needed for effective translation of findings to the clinic. (A color version of this figure is available in the online journal.)
Existing preclinical cancer models
In vitro and in vivo cancer models are critical tools in the investigation of cancer signaling pathways and development of novel diagnostic and therapeutic technologies. Recent advances in molecular characterization and genetic engineering have rapidly expanded both our grasp on cancer development and allowed for the rapid generation of new cancer models to better recapitulate particular aspects of cancer biology.17,18 In this section, a topical review of prominent in vitro and in vivo cancer modeling techniques is presented.
In vitro cancer models
Cell culture techniques are some of the most accessible methods for studying cancer biology. They enable a bottom-up approach where system complexity is constructed using well-characterized components to recreate specific niches in the cell microenvironment.17,18 This additive property of in vitro cell culture ensures a high degree of experimental control and target selectivity, which improves experimental reproducibility and enables high-throughput analysis. The bulk of in vitro cancer research is done using commercial cancer cell lines due to their straightforward validation between laboratories and ease of maintenance. 15 Cell line–based models are particularly useful in the evaluation of cancer cell–specific properties, such as oncogenes and drug sensitivity. However, cell lines also suffer from several limitations, including the selective pressures of monolayer culture, which can cause genetic alterations that are not found in vivo, and clonality, which loses the intra- and inter-tumoral heterogeneity found in the clinic.19–21 These changes can then contribute to misrepresentation of cell behavior and drug sensitivity. Primary cells are used to circumvent many of these concerns; however, sourcing and maintaining primary cells is significantly more challenging than commercial cell lines. Moreover, repeated passaging and expansion of primary cells depletes native ECM components and can lead to epigenetic drift and loss of tissue morphology. 20 Collectively, these shortcomings limit the scope of cell line monocultures to cancer cell–specific functions.
To compensate for these challenges, three-dimensional (3D) cell culture techniques have been used to more accurately model tumor architecture. Tumor spheroids are notable for their similarities to avascular tumors and are commonly used to recreate cell–cell interactions and differential exposure conditions 22 (see Table 1). These properties restrict the availability of oxygen and nutrients in the core of spheroids, resulting in a gradient of proliferative and metabolic cell behaviors that alter therapeutic response and correlate with tumor conditions found in vivo. 23 Spheroids can be generated from established cancer cell lines or derived from tumor tissue with varying degrees of cell dissociation and enrichment used to select for particular cell subpopulations.22,24,25 Importantly, the inclusion of multiple cellular constituents allows for the probing of specific interactions between cell types.22,23 Owing to their construction from selected cell lines, however, spheroids lack control over ECM characteristics and fail to recapitulate higher-order tissue behaviors such as vascularization. Precise control over spatiotemporal biophysical and biochemical factors is also not available with conventional techniques. In addition, not all cell types are amenable to spheroid formation, their small size makes handling difficult, and uniform spheroid formation is necessary for comparison. 39
Table 1.
Summary of 3D cancer cell culture techniques and animal models.
| Technology | Technique | Description | Advantages | Disadvantages |
|---|---|---|---|---|
| Spheroid models | Multicellular tumor spheroids | Aggregation and compaction of suspended cancer cell lines26,27 | Standardized cells; ideal for high-throughput screening (HTS); cell–cell interactions easily incorporated; and partial differentiation 24 | Immortalized cell lines and culture adapted |
| Tumorsphere (tumor organoids) | Clonal proliferation of cells suspended in stem-cell media 24 | Enriched for cancer stem cells | Clonal cell population; only cancer stem cells | |
| Tumor-derived spheroids | Partial dissociation and reorganization of tumor tissue | Recreates tumor properties/ microarchitecture | Not standardized cell lines and exclusively tumor cells | |
| Organotypic spheroids | Mechanically diced and rounding of tumor tissue | Preserves tumor heterogeneity and microarchitecture | Not standardized cell lines | |
| Scaffolds | Hydrogel-based scaffold | Cross-linked hydrophilic polymer network 28 | Control over ECM proteins and growth factors and cell encapsulation | Poor mechanical properties |
| Porous scaffolds | Various polymeric pore and fiber-forming techniques29–31 | Diverse material selection and engineered microstructures | Inefficient cell seeding and variable mechanical properties | |
| Decellularized scaffolds | Decellularized ECM from tumor tissues 32 | Mimics natural tissue properties and biocompatible | Inefficient cell seeding; immunogenic response; and technical preparation | |
| Explant model | Tissue slice | Sectioning of surgically extracted tumor tissue | Preserves tumor heterogeneity and tissue architecture | Low throughput and challenging to maintain long term |
| In vivo tumor models | Cell line–derived xenograft (CDX) | Transplantation of cultured cancer cells into immunocompromised mice20,33,34 | Easily established; synchronous growth; and low cost | Low genetic heterogeneity |
| Patient-derived xenograft (PDX) | Surgically derived tumor transplantation of samples into immunocompromised mice 34 | Retains human TME interactions at low passage numbers and serial transplantation avoids in vitro selection conditions | Human stroma loss in higher passages; high cost; time intensive; and engraftment variability20,34 | |
| Environmentally induced model (EIM) | Induction of carcinogenesis via exposure to environmental stimuli | Relevant for tumorigenesis; captures genetic; and phenotypic heterogeneity | Difficult to determine tumor burden and long latency.20,35 | |
| Genetically engineered mouse model (GEMM) | Induces cancer by cloning oncogenes or knocking out tumor suppressors in immunocompetent mice 34 | Native TME and intact immune system | Variable gene expression and potential for random integration 34 | |
| Humanized mouse (HM) | Engrafting human biological systems into immunocompromised mice | Incorporates aspects of the human immune system | Potential for graft rejection 33 | |
| Other mammalian models (companion animals) | Naturally occurring tumors in animals that are genetically closer to humans than mice 36 | Increased relevance compared to mouse models and more representative pharmacodynamics | Higher operational costs; longer lifespans; and specialized expertise | |
| Non-mammalian models | Tumor grafting on chorioallantoic membranes or zebrafish37,38 | Low-cost alternatives to mammalian models and fewer ethical concerns | Labor intensive and limited to specific facets of cancer progression |
ECM: extracellular matrix; TME: tumor microenvironment.
Scaffold-based culture techniques are another method of 3D tumor modeling when cell–ECM interactions are being investigated37,40 (see Table 1). Scaffolds can be hydrogels or porous substrates composed of natural or synthetic materials. Natural polymers (e.g. alginate, chitosan, gelatin, collagen, fibronectin, and Matrigel®) use prominent tumor ECM components that can contain native background factors and be reorganized by cells.37,41,42 This compositional complexity allows naturally derived polymers to mimic the structural heterogeneity found in vivo and promote organized cancer cell development. 37 Unfortunately, certain naturally derived polymers can be highly variable, overly complex, difficult to isolate, and lack human-specific markers.32,42,43 Basement membrane scaffolds also tend to be derived from tumor tissue and thus may contain unquantified background proteins and effectors. Alternatively, synthetic polymers, such as poly(ethylene glycol), poly(vinyl alcohol), and poly(lactic-co-glycolic acid), are used for their high reproducibility, tunable stiffness, and ease of modification. 44 Their commercial development provides a streamlined matrix selection process, but synthetic materials can also have cytotoxic components or unpredictable cell–polymer interactions. 42 Other challenges to scaffold-based cell culture include achieving sufficient cell distribution, infiltration, and recovery. 45 Cell–cell interactions are difficult to manage when seeding cell suspensions, but spheroid seeded scaffolds have shown higher drug resistance than spheroids on a flat surface and scaffolds seeded with dispersed cells. 46
Histoculture (explant cell culture) is another approach that captures much of the complexity of the TME by culturing sections of tumors grown in vivo (Table 1). While this setup is well suited for rapid testing and visualization, sample collection and long-term maintenance are difficult and only a fraction of the tumor’s overall heterogeneity is captured. 47
While discussion of all available in vitro assays for studying the TME is beyond the scope of this work, a broad summary is valuable for recognizing opportunities for advancement in quantification. Evaluation of in vitro cancer models is primarily accomplished through measurement of cell markers and cell behaviors. Drug sensitivity assays test concentrations of anticancer drugs in microtiter plates to evaluate therapeutic effect. 48 Cancer cell migration and invasion are observed using Transwell® migration assays, where the movement of cells (B16F10, HeLa, MCF-7, MDA-MB213, T-47D, etc.) across a membrane is used to evaluate chemoattractant response and malignancy. 15 Fluorescent reporter genes and colored dyes can be used to label markers, in particular, cancer pathways and track cell fate. 49 These assays have been integrated into hypoxia-response pathways to link hypoxia to increased fibrous tissue deposition. 50 For 3D tumor models, additional cell processing or alternative analysis techniques may be employed to preserve spatial or organizational information. Metrics commonly used for the characterization of spheroids include size, shape, and cellular organization, which are best visualized through optical microscopy techniques (e.g. bright field, dark field, differential interference contrast, and fluorescence imaging). 23 Flow cytometry can also be used for quantifying fluorescent markers in cells, but disaggregation is necessary for analysis, and therefore, steps must be taken to prevent the loss of spatial information.23,29 Other considerations for 3D in vitro cell culture techniques include the autofluorescence of certain scaffold materials, such as collagen, which can interfere with scaffold-based culture imaging, as well as background signal from out-of-plane fluorophores. 45 To counter these drawbacks, various sectioning techniques have been used to improve contrast and data collection, including light-sheet-based fluorescence microscopy, two-photon microscopy, and multiphoton microscopy.23,37,29 Finally, chemical gradients and active flow systems are difficult to establish and maintain in most conventional cancer cell culture formats, and some in vitro assays are restricted to endpoint analysis, limiting access to cellular dynamics. 51 As such, in vitro methods are best suited for high-throughput testing and screening studies with low model complexity for mechanism discovery and therapeutic candidate identification.
In vivo cancer models
In contrast to the low physiological relevance afforded by in vitro methods, in vivo models are used to capture a more complete picture of the biological complexity present in the TME by allowing cancer cells to grow in an environment that is similar to the human body (Table 1). This is a critical component for the translational research of novel therapeutics because it allows for systemic toxicity screens and provides more comprehensive data on the impact of clinical drug administration. However, the various autochthonous and non-autochthonous models that have been developed to study specific aspects of cancer progression are not universally applicable. For instance, immunotherapy, carcinogenesis, and early tumor growth are best studied with de novo techniques such as environmentally induced or genetically engineered mouse models, but, the rapid growth of multifocal tumors limits their application in studying late-stage cancer processes such as metastasis.20,52 On the contrary, transplant models using cell lines (4T1, B16, Lewis lung carcinoma, etc.) or patient-derived tumors are flexible platforms for observing therapeutic efficacy and tumor growth, as the location of tumor implantation can be chosen to simplify disease monitoring (ectopic), preserve TME interactions (orthotopic), or expedite metastatic dissemination and colonization (systemic).20,52 To model human cancers in animal models, cell line–derived xenografts (CDXs) are used for their lower costs and higher availability than patient-derived xenografts (PDXs), which have significantly higher clinical relevance but suffer from variable engraftment rates. 52 Incorporation of patient-derived cells in hollow fiber implants (mini-PDX) assays have also been demonstrated as an alternative approach to accelerate in vivo drug sensitivity testing. 53 Recently, the development of humanized mice has further increased the relevance of transplant models by integrating elements of the human immune system.33,54
Despite the many advantages to studying the TME in vivo, challenges, including cost, time, high model variance, and low throughput, limit its statistical power compared to in vitro systems. 41 Critically, concerns have also been raised that animal testing does not reliably translate to the clinic. 16 Tumor model differences including drug metabolism, immune system composition, tumorigenesis, and chimerism can all contribute to response divergence.33,37 Also, while inbred animal populations are desirable for experimental reproducibility, clinically relevant parameters such as population dynamics and inter-tumoral heterogeneity are absent. To combat some of these limitations, animal models with a higher genetic similarity to humans have been used, but their associated costs, development time, and handling expertise preclude widespread use. 36 Alternatively, non-mammalian tumor models, such as yeast, zebrafish, and chicken chorioallantoic membranes, can be used for applications including carcinogens, oncogenes, and angiogenesis, while mitigating the costs and ethical concerns associated with mammalian models37,55 (see Table 1).
When evaluating changes to the TME in animal models, macroscale indicators such as tumor size, weight, and metastatic spread, measured by necroscopy, histology, or cytology, are commonly used to gauge cancer growth and progression. 56 For observation of genetic and cellular changes occurring in the TME, molecular biology techniques (e.g. enzyme-linked immunosorbent assay (ELISA), quantitative polymerase chain reaction (qPCR), microarray, radioimmunoassay, flow cytometry, immunohistochemistry, western blot analysis, and proteomics) or optical imaging of labeled molecules (e.g. confocal, multiphoton, and wide-field fluorescence) are typical.56–58 These assays, however, are often end-stage, limiting data collection to single timepoints per animal, and can disrupt the TME’s spatial organization. In contrast, non-invasive quantification techniques can provide anatomical and functional data ranging from tumor structure, perfusion, and permeability to metabolic activity and drug distribution over time, allowing for higher statistical power with fewer animals and multimodal analysis. 59 Anatomical information is gathered using techniques with high spatial resolution, that is, computed tomography (CT), magnetic resonance imaging (MRI), photoacoustic imaging (PAI), and ultrasound (US), whereas, techniques with high sensitivity, such as bioluminescence imaging (BLI), fluorescence imaging (FI), intravital imaging (IVM), positron-emission spectroscopy (PET), Raman spectroscopy (RS), and single photon emission computed tomography (SPECT), are used for molecular imaging (Table 2). 60 Collectively, these techniques can illuminate tissue-level processes to gauge overall tumor behavior and therapeutic performance. Confocal and multiphoton microscopy, in particular, are advantageous for obtaining non-destructive optical sections of intact tissue. 57 Implementation of these technologies with intravital imaging techniques further enhances the study of TME dynamics by allowing observation without tumor excision.57,61–64 Other considerations include imaging agent requirements, penetration depth, temporal resolution, and exposure to ionizing radiation, when deciding on a technique. Thus, in vivo models are optimum for performing crosstalk studies with high model complexity (angiogenesis and metastasis), analyzing drug biodistribution, and assessing systemic toxicity.
Table 2.
Characteristics of current imaging modalities.
| Sensitivity (M) | Spatial resolution | Depth of penetration | Temporal resolution | Cost | Multiplexing capability | ||
|---|---|---|---|---|---|---|---|
| Magnetic resonance | Magnetic resonance imaging (MRI) | 10−3 to 10−5 | 25 to 100 μm | No limit | Min to h | $$$ | No |
| Nuclear | Positron emission tomography (PET) | 10−11 to 10−12 | 1 to 2 mm | No limit | 10 s to min | $$$ | No |
| Single-photon emission computed tomography (SPECT) | 10−10 to 10−11 | 1 to 2 mm | No limit | Min | $$ | Yes | |
| Optical | Bioluminescence imaging (BLI) | 10−15 to 10−17 | 3 to 5 mm | 1 to 2 cm | Sec to min | $ | Yes |
| Fluorescence imaging (FI) | 10−9 to 10−12 | 2 to 3 mm | <1 cm | Sec to min | $ | Yes | |
| Intravital microscopy (IVM) | 10−15 to 10−17 | ~1 to 10 μm | 700 μm | Sec to days | $$ | Yes | |
| Photoacoustic imaging (PAI) | N/A | 10 μm to 1 mm | ~6 mm to 5 cm | Sec to min | $ | Yes | |
| Surface-enhanced Raman spectroscopy (SERS) | 10−12 to 10−15 | mm | ~5 mm | Min to days | $ | Yes | |
| Ultrasound | Ultrasound (US) | >10−12 | 10–500 μm to 1–2 mm | mm to cm | Sec to min | $ | Yes |
| X-rays | Computed tomography (CT) | 10−2 to 10−3 | 50 to 200 μm | No limit | Min | $$ | N/A |
Source: Adapted from James and Gambhir. 60
Improvements to preclinical cancer models via enhanced imaging and localized modulation
While conventional in vitro and in vivo cancer models have provided numerous insights into the TME, the continued low success rate of clinical translation indicates room for improvement. It stands to reason then that one of two changes needs to occur: either in vivo models must become satisfactory predictors of clinical success, or in vitro models should achieve sufficient clinical relevance that animal models are replaced. Thus, the following section surveys the use of novel engineering strategies to improve translational cancer model relevance and accessibility for more accurate findings. This is discussed in the context of technological improvements to monitoring and manipulation of the TME.
Monitoring and biomarker detection in the TME
As the high level of heterogeneity in the TME makes it difficult to trace crucial signaling pathways, minimally invasive study at high resolution is needed for observation and detection of cell populations and molecular concentrations to track changes in diseased tissue and gauge therapeutic response. Improvements in data collection from 3D tissue structures are expected to preserve signaling and transduction pathways, reduce the number of animals needed for statistical significance, and improve the development time of potential therapeutics. To accomplish this need, an array of molecular imaging techniques and biosensor systems are used for biomarker recognition and visualization.
Imaging probes for TME characterization
The multitude of changes in the TME, such as ECM remodeling, neovascularization, proteolysis, metabolic changes, and levels of reactive oxygen species (ROS) provide ample opportunities for biomarker recognition and imaging. Still, detection and quantification require sufficient target labeling with minimal background signal. Imaging probes are combinations of ligands, linkers, and reporters used to identify targets through biomarker recognition. They can include genetically encoded reporters, exogenous fluorophores, and contrast agents. Their ability to bind with high specificity and selectivity to target moieties at sufficient concentrations and durations to detect above background is essential for TME characterization. 60 Currently, conventional histological techniques have limited biomarker labeling capabilities and are restricted to 2D tissue slices (Table 3). Recent advances in multiplexed immunohistochemistry/immunofluorescence platforms (mIHC/mIF), however, have achieved high-throughput labeling of tissues using iterative staining, imaging and inactivation with dye-labeled antibodies.65,66 This approach allows the assessment of biomarker colocalization, distribution, and cell/tissue composition. 66 Similarly, tissue clearing for section-free volumetric microscopy and histo-cytometry is an emerging field for the collection of quantitative information from intact 3D tissue samples that has the potential to reduce sampling errors associated with heterogenous tissues and preserve anatomical relationships.67–69 Despite the impressive gains in TME detection and quantification, these techniques are still limited to end-stage analysis, and preparation times are significant (days).
Table 3.
Summary of detection methods for studying the tumor microenvironment.
| Category | Detection method | Techniques | Advantages | Disadvantages | Invasiveness | Sensitivity |
|---|---|---|---|---|---|---|
| Imaging probes | Passive | Enhanced permeability and retention, blood cell membrane coated70–73 | Simple, cheap | Non-specific, transient, size, surface charge, and TME dependent | Low | Low |
| Targeted | Ligand functionalization, cell-mediated74–83 | Significantly enhanced delivery, active internalization | Advanced synthesis, increased clearance rate | Low | Moderate-high | |
| Sensitized | Pro-drugs, stimuli-responsive systems, and copolymer nanoparticles 84 | Enhanced delivery and several stimuli options | Advanced synthesis, stability, and premature drug release | Low | Moderate-high | |
| Non-invasive measurements | Observational | Tumor size and tumor count | Simple and direct | Subjective, bulk measurement, and superficial | Low | Low |
| Survival | Simple, population dynamics, and clinically relatable | Larger sample size and slow | Low | Low | ||
| Extracted | Lateral flow assay 85 | Rapid, portable, user-friendly, and moderate specificity | Subjective, low signal intensity, batch variability, and limit of detection | Low | Moderate | |
| End-stage assays | Fixed tissue | Histology and immunohistochemistry 86 | Simple, relatively inexpensive, and tissue-level detail | Time-consuming preparation and semi-quantitative, subjective | High | Moderate |
| Dissociative | Immunoassays, nucleic amplification assays, chromatography, flow cytometry mass spectroscopy, and filter binding assay 87 | Standardized technology and signal amplification opportunities | Batch processing, time consuming, technical, and temperature sensitive | High | High | |
| Optical sensors | Fluorescence | Grating coupled-fluorescence plasmonics 88 | Stable and multiplexable | Specialized equipment and needs readout standardization | Moderate | High |
| Interferometry | Optical backscatter reflectometry 89 | Cheap, label free, simple, real time, endoscopy compatible | Long-term stability and temperature dependent | Moderate | High | |
| Surface plasmon resonance (SPR) | SPR, SPR imaging, localized SPR, and ring resonator90–92 | Label free and real time | Complex instrumentation and technical operation | Moderate | High | |
| Electrochemical sensors | Amperometric | Voltammetry and chronoamperometry 93 | Simple operation, miniaturization, cheap, real time, and reproducible | Needs redox amplification, temperature sensitive, poor selectivity without membranes or enzymes, and small dynamic range | Moderate | High |
| Impedimetric | Conductometry and electrochemical impedance spectroscopy 94 | Label free, low cost, simple, real time, stable, low detection limit, wide linear range, and accurate | Low specificity, bulky, low selectivity, and temperature sensitive | Moderate | High | |
| Potentiometric | Ion-selective electrodes and field-effect transistors 95 | Label free, high specificity, real time, inexpensive, and wide detection range | Complex, sensitive to temperature, sensor drift, and pH sensitive | Moderate | High | |
| Gravimetric sensors | Piezoelectric | Quartz crystal microbalance and surface acoustic wave 96 | Real time, simple, label free, short analysis, and low cost | Temperature and stress sensitive, poor stability, low repeatability, low liquid sensitivity, and prone to non-specific binding | Moderate | High |
| Electromechanical | Cantilever 97 | Real time and multiplexable | Temperature sensitive and large readout instrumentation | Moderate | High | |
| Magnetoelastic | Magnetoelastic ribbon 98 | Independent of temperature and pH, wireless, low cost, and stable | Requires external driving and sensing coils | Moderate | High |
TME: tumor microenvironment.
For dynamic labeling of the TME, high-efficiency delivery methods are necessary due to the dilution and elimination of imaging probes in living systems. Current methods utilize genetic reporters and injectable imaging agents to achieve sufficient contrast, which has enabled the quantification of a vast array of TME properties, including cell populations, tumor fluid perfusion, signal transduction, therapeutic action and biomarker expression70–73 (see Table 3). To further improve detection, targeted nano-carriers are being designed with moieties such as antibodies, small molecules, aptamers, and dendrimers to prioritize localization in desired tissues. 74 Their larger size allows for the incorporation of multiple targeting probes and reporter molecules to fine-tune signal strength as well as the option for multimodal imaging to overcome limitations of specific imaging techniques. 75 Pharmacokinetic characterization of these particles is necessary to account for their biodistribution profiles using techniques like tumor perfusion imaging. 76 Other targeting methods utilize sensitized carriers or activation sites that respond to unique conditions in the TME, such as pH, O2, and protease concentrations.77–83 For instance, fluorescence imaging of denatured collagen with photo-triggerable folding of collagen mimetic peptides circumvents limitations associated with targeting unstructured proteins. 99 Similar strategies have also been demonstrated with fibrin imaging using MRI and SPECT.100,101 Finally, cell-mediated labeling strategies, where cell-penetrating fluorophores are used to mark neighboring cells can be used to identify local cell–cell interactions. 102 Analysis of cells marked by this approach demonstrates a strategy for nonspecific functional characterization of the metastatic niche.
Sensors for study of the TME
In addition to the novel molecular labeling approaches discussed in the previous section, TME sensors are promising alternatives to monitor both analyte concentrations (e.g. proteins, nucleic acids) and biophysical properties (e.g. pressure, stiffness) of the TME. 58 Their wide range of readouts, including electrochemical, optical, and gravimetric (piezoelectric), provide additional sensing opportunities for biomarker detection and TME characterization that are not available with imaging probes103–105 (see Table 3). They can also find application in lieu of cost-prohibitive analytical techniques. As dedicated reviews on biosensors and their various classifications have been recently covered, we instead emphasize two key TME sensor characteristics: invasiveness and sensitivity.106,107
Invasiveness is a crucial consideration for TME investigation as significant disruption of the tissue can alter cell behavior and distort experimental results. This property is especially important in vivo, where animal stress, tissue damage, and biocompatibility issues underly long-term sensor use. Consequently, highly invasive techniques such as atomic force microscopy (AFM) and electron microscopy, where major surgery or tissue processing is required, are most appropriate when quantifying cell and TME tissue properties that are difficult to collect otherwise.108,109 For example, Mao et al. 110 used AFM to characterize the nanomechanical properties of aortic intima in response to pharmaceutical stimulation in vivo. Similarly, molecular analysis with micro-dialysis and in vivo mass spectroscopy have immense potential in characterizing the concentration of analytes in the TME but require surgery and analyte extraction.111–113 Efforts to minimize sensor invasiveness, such as the incorporation of wireless readout capabilities or miniaturization of implants, improve sensor usability over larger timescales but typically involve more complicated fabrication procedures.114,115 Biochemical sensing strategies have been widely explored for their straightforward production methods (e.g. electrochemical sensors, evanescent wave sensors). Label-free detection methods are minimally invasive and allow for real-time monitoring but their signal-to-noise ratio can be insufficient for reliable detection of small molecules. 116
The ability to accurately detect low concentrations of analytes is another critical property in sensor application. Due to the diverse and heterogenous nature of the TME, high sensitivity and a low detection limit are needed for reliable data collection. For biomarker detection, sensing is typically done through functionalization with affinity-based recognition elements, such as antibodies, antigens, enzymes, nucleic acids, receptors, or whole cells.97,106,117 Using direct detection methods, label-free biosensors rely on physical interactions between the biomarker and sensor interface but are limited by analyte availability and generally weak interface sensitivity. In cases where sufficient sensitivity is not achieved, amplification of the biomarker or signal can improve device performance at the expense of more complicated sample processing (longer assay time) and increased risk of distorting binding characteristics. 116 Common applications of this strategy include PCR for nucleic acid amplification and signal amplification via sandwich immunoassays or enzyme conjugation. 118 Other strategies for improving sensor performance include increasing the detection region, improving mass transport effects near the sensor, and using magnetic nanoparticles for analyte scavenging.95,118
Future considerations of monitoring the TME
While these strategies used for TME observation and biomarker detection are not new, challenges in achieving acceptable signal-to-noise ratios, biodistribution profiles, and target identification are still under intense investigation. For imaging probes, physicochemical optimization of the size, surface charge, circulation half-life, and biocompatibility are all factors that must be considered during development, particularly for in vivo applications. Limitations of particular imaging modalities are also areas of concern. Currently optical modalities provide the best option for high temporal resolution, but tradeoffs in working distance and spatial resolution, as well as optical scattering in biological tissues and out-of-plane photobleaching, hinder deep tissue study. In addition, high-resolution imaging of large tissue regions is time consuming and has high computational requirements for processing and analysis. 67 On the contrary, biosensors are anticipated to reduce dependence on expensive equipment and expertise required for advanced detection systems. To accomplish this function, sensors need to be practical, robust, reproducible, and miniaturizable. Identification of optimal sensor modalities for the target applications will also require reliable performance metrics for comparison, and characterization of binding kinetics will be necessary for dynamic measurements. Due to the heterogenous nature of the TME, extension of the sensing interface could be advantageous for sampling larger tissue regions. Finally, while improvements to detection and quantification in the TME are certainly important, the integration of advanced biosensing technology into conventional testing formats should also be considered to maximize technology uptake by the scientific community (Figure 2(a)–(c)). 119
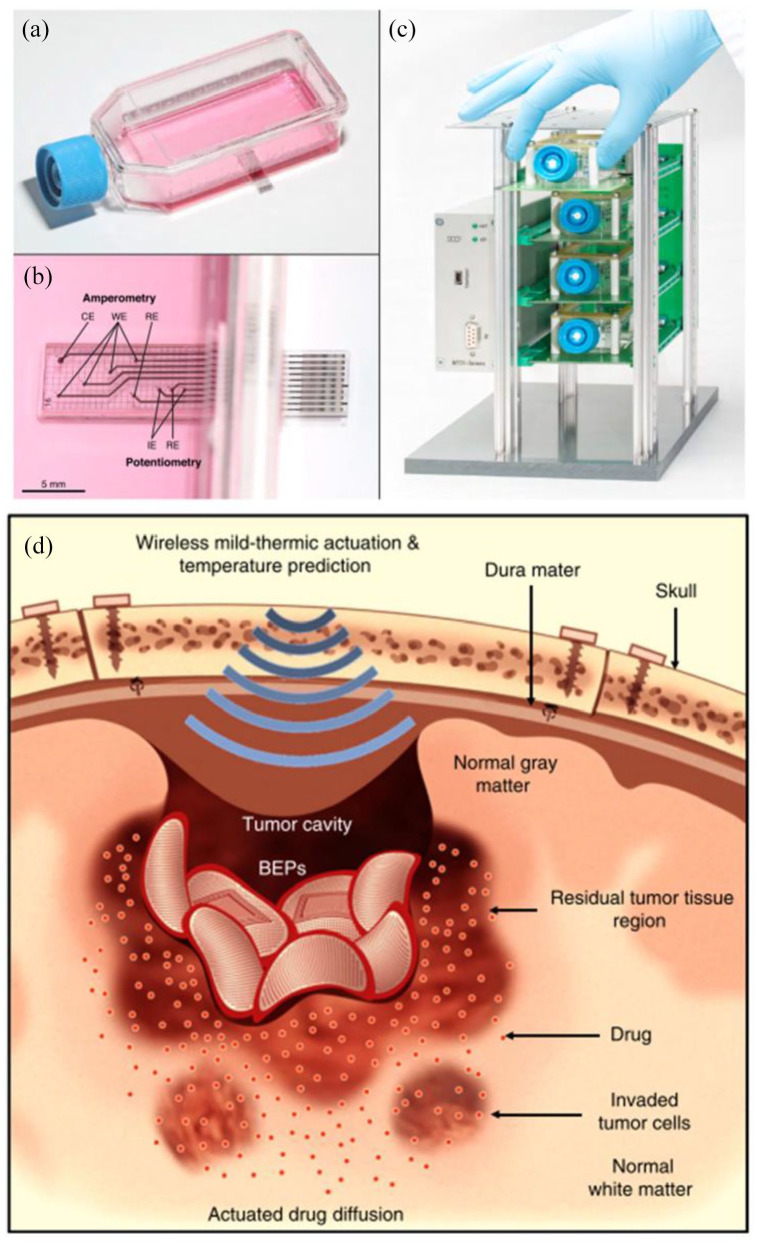
Figure 2.
Engineered platforms for enhanced study of the tumor microenvironment: (a) sensing cell culture flask is designed around standard cell culture flasks to minimize protocol adjustment, (b) embedded electrodes allow for detection of a variety of biologically relevant chemicals; companion rack systems allow parallel real-time monitoring, and (d) the bioresorbable electronic patch uses wireless thermal actuation for enhanced drug delivery into glioblastomas. (A color version of this figure is available in the online journal.)
Source: Adapted from Kieninger et al. 119 and Lee et al. 120
Manipulation of the TME
To improve the success rate of therapeutic translation, more accurate modeling of clinically relevant cancers and treatment outcomes are needed. Still cancer characteristics, including systemic spread, multiscale heterogeneity, and acquired resistance, present formidable complexity when recapitulating a comprehensive cancer model. Thus, the use of practical, application-specific cancer models is still warranted, particularly in basic research. However, the predictive efficacy of translational models needs work. To this end, greater experimental control in vivo and more representative in vitro cancer models would allow for effective modulation of the TME.
Pharmacotherapy targeting the TME
Pharmacotherapy in combination with radiation and surgery is the primary treatment strategy used in the treatment of cancer. It is comprised of cytotoxic chemotherapy and radiation therapy, which non-specifically interfere with cell division, immunotherapy, which promotes the immune system’s anticancer activity, and hormonal therapy and targeted therapy, which interfere with cancer growth signaling pathways. These drugs encompass the majority of clinical effectors used to treat cancer yet challenges such as drug resistance and nonspecific toxicity limit their clinical effectiveness.121,122 To alleviate these issues and improve the efficacy of pharmacotherapeutics, various drug delivery methodologies have been demonstrated.
The most prolific method of selective pharmacotherapy is via biomolecular recognition of target sites. Surface targeting ligands, as mentioned previously in the discussion on analyte recognition, allow for targeting of differentially expressed receptors on specific cell types and the capture of signaling molecules. This directed behavior is used to selectively eliminate cancer cells, promote non-cancerous cell behavior, and tune aspects of the TME for therapeutic benefit.123–125 For instance, radiopharmaceuticals, which serve as calcium analogs or chelators, are used for their preferential accumulation in bones to target bone metastases with localized radiation. 126 Oftentimes, however, a single anticancer therapeutic is not potent enough to eliminate cancer on its own. In these cases, combinations of anticancer drugs and adjuvant TME therapies can have synergistic effects. TME modification with antiangiogenic therapy can transiently improve nanotherapeutic delivery by reducing the interstitial fluid pressure of solid tumors to restore convective transport. 127 The result is higher drug bioavailability in the tumor, which impacts drug efficacy and therapeutic response.
Cell-mediated therapy is another targeting approach that has explored the loading of tumor-homing cells with therapeutics or receptors to circumvent typical barriers to nanoparticle delivery and immunosurveillance.128–130 Implantable cell encapsulation technology can be used to isolate populations of cells for genetically directed secretion of therapeutics (e.g. prodrug activators, cytotoxic agents, and immunostimulants) into the TME. 131 Immunotherapy uses checkpoint inhibitors and T-cells that are genetically modified with chimeric antigen receptors (CAR T-cells) or T-cell receptors (TCR T-cells) to overcome the immunosuppressive TME and tumor evasion mechanisms. 132 This therapeutic strategy is not universally applicable, however, as complications including T-cell production, specificity, and exhaustion, as well as side effects (e.g. cytokine release syndrome and neurotoxicity) limit patient compatibility. 133 Efforts to alleviate these shortcomings and improve target selectivity include combinatorial antigen recognition to reduce bystander cell recognition and exhaustion-resistant phenotypes.134–136
Alternatively, TME-specific release can also be achieved by sensitizing drug delivery systems (DDSs) to endogenous physiochemical conditions, such as hypoxia and pH.137–140 In these cases, localized therapeutic release can be realized without altering the drug itself. This targeting is possible due to the significant remodeling that occurs during tumor progression, producing abnormal vasculature, nutrient gradients, and metabolic states. 141 The accumulation and retention of many large drugs and nanomedicines in solid tumors due to leaky vasculature and non-functioning lymphatics, called the enhanced permeability and retention (EPR) effect, is one such method of tumor targeting that has been widely explored.142,143 Recent work has shown that this effect is not universal, however, and that intratumoral drug distributions are heterogenous. To overcome these limitations, Li et al.144,145 used size-switching nanoparticle superstructures to accumulate in tumor tissue via the EPR effect, and dissociate into small molecule particles to diffuse more readily through the TME. For extended delivery applications, controlled degradation of drug-loaded hydrogels has been used to minimize systemic exposure. 146 Here, tuning the composition of the hydrogel and the degradation rate of the individual components, allows for variable control over the release of multiple drugs. Exogenous triggers (e.g. light, radiofrequencies, and ultrasound) can also be used to control drug kinetics or perform photothermal therapy, but target accessibility can limit viable signaling modalities.147,148
Engineered tissue models for recapitulating the TME
Like biosensors, transducers are capable of operating at the cellular scale, improving in vitro modeling and affording direct control over the neighboring TME. These devices can bridge the gap between experimentally robust in vitro models and physiologically relevant in vivo models through the incorporation of pertinent biophysical and biochemical conditions. This approach allows the study of crucial aspects of tumor biology that are not easily observed otherwise, such as metastasis and angiogenesis. Methods to accomplish this feat include functional and structural improvements to existing cancer model technology. 149 One such example uses a magnetic actuating platform to mimic respiration-induced tissue stretching in vitro. 150 Results show actuation of breast cancer cells decreases metabolic activity and inhibits matrix degradation, indicating a potential role in dormancy and reactivation. 150 To improve the structural composition of tumor models, microscale organization of cells, and bioactive materials can be achieved using bioprinting and scaffold technologies. This strategy has enabled the management of local cell–cell interactions as well as cell confinement and ECM stiffness through variable cross-linking.151–153 Other engineering solutions seek to improve the analysis of 3D tissue models without the use of sectioning or isolation methods. TRACER (tissue roll for analysis of cellular environment and response) uses a stackable cell culture design to enable rapid disassembly and layer-by-layer analysis of 3D tissue constructs.154,155 The miniaturization of sensors and fluid handling technology to the cellular scale and beyond has empowered the development of microfluidic systems for novel cell culture platforms and drug delivery applications. These organ-on-a-chip (OOC) devices are capable of dynamically managing mechanical signals, biochemical gradients, and cellular interactions to improve cell differentiation and tissue organization over conventional 3D culture techniques. 156 OOCs have been used to model angiogenesis, tumor progression, drug exposure, and crosstalk between cells, as well as visualize spatial heterogeneity.156–159 In one implementation, metastatic and intravasation potential were evaluated by producing dynamic oxygen gradients across a collagen barrier to observe matrix breakdown. 160 This OOC thus provides functional control over physiologically relevant conditions that are not manageable in conventional cancer model formats. OOCs also provide an opportunity for the development of personalized cancer models using patient cells for treatment screening and predicting therapeutic response. 161 More advanced microphysiological systems are also being explored for modeling multiple cellular compartments (body-on-a-chip technology) to experimentally validate pharmacokinetic models as an intermediate to clinical testing.162,163
Microfabricated system for manipulation and study of the TME
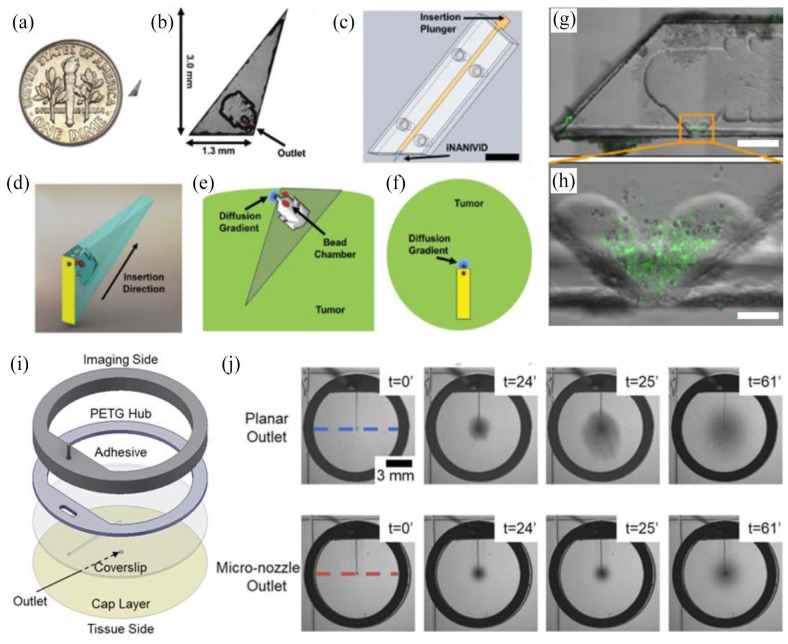
For in vivo applications, improvements to therapy administration and control factors are of broad interest. Implantable drug reservoirs, used to extend therapeutic release profiles, circumvent many issues associated with repeated injection regimens and prevent the rapid clearance of therapeutics from the target site.146,164–167 Another strategy to limit systemic exposure incorporates collagen-binding moieties to limit drug transport out of injection sites by anchoring to generalizable target sites in tumors.168,169 For situations where drug distribution is insufficient, actuated drug delivery has been used to improve cellular uptake. 120 This approach allows dynamic control over therapeutic concentrations in the TME. The use of biodegradable materials can also be selected to obviate the need for retrieval surgery and complications associated with chronic implants (Figure 2(d)). 120 Improvements to tumor grafting methods using cell sheet transplants are a novel way of subcutaneously engrafting tumor cells in biologically intact structures with high efficiency compared to enzymatically treated cell cultures. 170 Presumably, this technology could be extended to graft tumoroids and bioprinted tissues for directed TME formation in vivo. The application of micro-control systems in vivo presents many benefits as localized manipulation can enable internal controls, reducing the number of animals need for a study, and improve data collection. 171 The nano-intravital device (NANIVID) is one implementation that has been used for an array of studies in vitro and in vivo including hydrogel-mediated release of the chemotaxis agents (epidermal growth factor), hypoxia mimetics (deferoxamine and cobalt chloride), and ROS inductors (H2O2), as well as for cell collection (Figure 3(a)–(h)).166,172–175 Further work in this direction has aimed at extending experiment duration through the integration of fluidic control with intravital imaging windows (ported mammary imaging window) (Figure 3(i)).63,176 Recently, the development of integrated micro-nozzles for enhanced control over localized delivery has provided additional impetus for high resolution study in vivo (Figure 3(j)). 177
Figure 3.
Implantable devices for simultaneous imaging and drug delivery in vivo: (a) the induction nano-intravital device (NANIVID) next to a US dime, (b) the NANIVID is designed to penetrate solid tumor tissue for passive delivery, (c) insertion is facilitated by an applicator which aligns the device with the tumor surface, (d) a 3D render demonstrates the device orientation during insertion, (e) a cross-sectional view of an implanted NANIVID depicts the location of the outlet and generated diffusion gradient, (f) top–down view of the insertion site for imaging, (g) alternative NANIVID design for cell collection, scale bar = 500 μm, (h) magnified view of the device outlet where green fluorescing cells were collected, scale bar = 100 μm, (i) exploded view of the microfluidic imaging window for active reagent delivery, (j) demonstration of improved dye localization in hydrogel tissue mimics with a micro-nozzle outlet. (A color version of this figure is available in the online journal.)
Source: Adapted from Williams et al.166,174 and Head et al. 177
Genome editing to manipulate the TME
For functional analysis of genetic alterations in cancer, CRISPR/Cas9 (clustered regularly interspaced short palindromic repeats) has enabled specific, efficient, and affordable genome editing. Building off of earlier genome editing techniques (e.g. RNA interference, transcription activator-like effector nucleases, and zinc finger nucleases), pooled screening of novel therapeutic targets with CRISPR/Cas9 can identify essential driver genes and genotype-specific vulnerabilities. 178 This technology has been used to identify potential tumor suppressor genes and a gene involved in tumor metastasis in colorectal cancer organoid models. 179 Genome editing with CRISPR/Cas9 systems has also expedited the creation of novel genetic cancer models.180,181 Somatic gene editing in live animals provides a scalable alternative for model generation but has limited targeting capabilities currently. 182 Rapid progress in this field, however, already promises additional genomic editing tools and applications, such as point mutations and epigenetic editing.183–186 Finally, targeted delivery of CRISPR/Cas9 systems in the TME has the potential to identify critical intercellular interactions by disrupting communication with cancer-associated cells.
Future considerations of TME manipulation
Improvements to TME model controls for increased physiological relevance are imperative for improving the translative success of preclinical data. Molecular and microphysiological manipulation allow probing of all aspects of cell signaling which feed into development and therapeutic response. Ongoing research in pharmacotherapeutics is centered on drug screens and formulation technology. Synthesis of novel drugs face issues in maintaining activity, stability, toxicity, and delivery while balancing regulatory and manufacturing hurdles. Larger macromolecular and nanoparticle formulations currently must contend with poor biodistribution profiles due to high drug clearance and poor interstitial diffusion. Still, novel biopharmaceuticals, including RNA and recombinant proteins, have tremendous potential for revolutionizing medicine due to their flexible design and sequence recognition properties. Efficient delivery of miRNAs, siRNAs, and antisense oligonucleotides also have gene silencing capabilities that would allow for targeting of previously undruggable non-coding RNAs. 187
Although engineered cancer models encompass a wide range of techniques, general design principles for comparing methodologies exist. As a model’s main objective is to faithfully convey the behavior of a target system, model–user interactions must be weighed against model accuracy. With increasing model complexity, a concomitant increase in specialized knowledge and loss in throughput are typical. These factors, as well as the use of intensive fabrication techniques and equipment, can limit utilization by the scientific community. 188 To avoid this limitation, integrated compatibility with standard data collection techniques, including those technologies mentioned previously, can mitigate specialized training and startup costs.45,158,189 Application-oriented design can also reduce peripheral systems that have little or no role in the process of interest. 190 Standardization of components and protocols is also necessary to perform meaningful comparisons across multiple modeling techniques and improve reproducibility.
Conclusions
Despite the availability of a diverse field of cancer models, systems with reliable indicators of clinical success are still lacking. The low predictive power of current preclinical cancer models stems from their ineffective recapitulation of the human TME. To resolve this situation, advancements in preclinical model relevance and accessibility through technological innovation are necessary. This review surveys conventional model systems and advancements in both monitoring and manipulation techniques for enhanced experimental control. Balancing biological complexity and model practicality are critical for optimal model identification and selection. Still, the largest obstacles to the adoption of new technologies are regulatory barriers and accessibility. For regulatory approval, the safety and stability of novel drugs and medical devices must be established, often without clear guidance for evaluation. Accessibility comes in both the scalability of the manufacturing process, companion equipment cost, and the ease of operation. Accessibility also plays a significant role in uptake by the scientific community, which is driven largely by performance comparisons to equivalent systems and standardization of protocols and analysis. Nevertheless, the number of cancer modeling technologies will undoubtably continue to grow, and the development of sophisticated cancer models in novel preclinical workflows will require collaboration across institutions and disciplines to pool resources and expertise.
Acknowledgments
The authors thank Yubing Xie for reviewing the manuscript.
Footnotes
Authors’ Contributions: The first draft of the manuscript was written by TH and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the RNA Institute at the University at Albany, SUNY (grant no. 5T32GM132066-03); and the National Institutes of Health/National Cancer Institute in collaboration with Albert Einstein College of Medicine (AECOM) (grant no. 5R01CA216248-06).
ORCID iD: Tristen Head  https://orcid.org/0000-0002-0117-5393
https://orcid.org/0000-0002-0117-5393
References
- 1. Lambert AW, Pattabiraman DR, Weinberg RA. Emerging biological principles of metastasis. Cell 2017;168:670–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. American Cancer Society. Cancer facts & figures 2021, https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2021/cancer-facts-and-figures-2021.pdf
- 3. Wong CH, Siah KW, Lo AW. Estimation of clinical trial success rates and related parameters. Biostatistics 2019;20:273–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Prasad V. Do cancer drugs improve survival or quality of life? BMJ 2017;359:4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Balkwill FR, Capasso M, Hagemann T. The tumor microenvironment at a glance. J Cell Sci 2012;125:5591–6 [DOI] [PubMed] [Google Scholar]
- 6. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646–74 [DOI] [PubMed] [Google Scholar]
- 7. Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 2012;21:309–22 [DOI] [PubMed] [Google Scholar]
- 8. Sleeman J, Steeg PS. Cancer metastasis as a therapeutic target. Eur J Cancer 2010;46:1177–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gandalovičová A, Rosel D, Fernandes M, Veselý P, Heneberg P, Čermák V, Petruželka L, Kumar S, Sanz-Moreno V, Brábek J. Migrastatics—anti-metastatic and anti-invasion drugs: promises and challenges. Trends Cancer 2017;3:391–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rosel D, Fernandes M, Sanz-Moreno V, Brábek J. Migrastatics: redirecting R&D in solid cancer towards metastasis? Trends Cancer 2019;5:755–6 [DOI] [PubMed] [Google Scholar]
- 11. Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science 2011;331:1559–64 [DOI] [PubMed] [Google Scholar]
- 12. Dillekås H, Rogers MS, Straume O. Are 90% of deaths from cancer caused by metastases? Cancer Med 2019;8:5574–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Seyfried TN, Huysentruyt LC. On the origin of cancer metastasis. Crit Rev Oncog 2013;18:43–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Beaver JA, Kluetz PG, Pazdur R. Metastasis-free survival—a new end point in prostate cancer trials. N Engl J Med 2018;378:2458–60 [DOI] [PubMed] [Google Scholar]
- 15. Katt ME, Placone AL, Wong AD, Xu ZS, Searson PC. In vitro tumor models: advantages, disadvantages, variables, and selecting the right platform. Front Bioeng Biotechnol 2016;4:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mak IWY, Evaniew N, Ghert M. Lost in translation: animal models and clinical trials in cancer treatment. Am J Transl Res 2014;6:114–8 [PMC free article] [PubMed] [Google Scholar]
- 17. Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SFT, Csiszar K, Giaccia A, Weninger W, Yamauchi M, Gasser DL, Weaver VM. Matrix crosslinking forces tumor progression by enhancing integrin crosslinking. Cell 2009;139:891–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pijuan J, Barceló C, Moreno DF, Maiques O, Sisó P, Marti RM, Macià A, Panosa A. In vitro cell migration, invasion, and adhesion assays: from cell imaging to data analysis. Front Cell Dev Biol 2019;7:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gillet JP, Calcagno AM, Varma S, Marino M, Green LJ, Vora MI, Patel C, Orina JN, Eliseeva TA, Singal V, Padmanabhan R, Davidson B, Ganapathi R, Sood AK, Rueda BR, Ambudkar SV, Gottesman MM. Redefining the relevance of established cancer cell lines to the study of mechanisms of clinical anti-cancer drug resistance. Proc Natl Acad Sci USA 2011;108:18708–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gengenbacher N, Singhal M, Augustin HG. Preclinical mouse solid tumour models: status quo, challenges and perspectives. Nat Rev Cancer 2017;17:751–65 [DOI] [PubMed] [Google Scholar]
- 21. Kunnumakkara AB, Bordoloi D, Sailo BL, Roy NK, Thakur KK, Banik K, Shakibaei M, Gupta SC, Aggarwal BB. Cancer drug development: the missing links. Exp Biol Med 2019;244:663–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ham SL, Joshi R, Thakuri PS, Tavana H. Liquid-based three-dimensional tumor models for cancer research and drug discovery. Exp Biol Med 2016;241:939–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Costa EC, Moreira AF, de Melo-Diogo D, Gaspar VM, Carvalho MP, Correia IJ. 3D tumor spheroids: an overview on the tools and techniques used for their analysis. Biotechnol Adv 2016;34:1427–41 [DOI] [PubMed] [Google Scholar]
- 24. Weiswald LB, Bellet D, Dangles-Marie V. Spherical cancer models in tumor biology. Neoplasia 2015;17:1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ishiguro T, Ohata H, Sato A, Yamawaki K, Enomoto T, Okamoto K. Tumor-derived spheroids: relevance to cancer stem cells and clinical applications. Cancer Sci 2017;108:283–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sutherland RM. Cell and environment interactions in tumor microregions: the multicell spheroid model. Science 1988;240:177–84 [DOI] [PubMed] [Google Scholar]
- 27. Kelm JM, Timmins NE, Brown CJ, Fussenegger M, Nielsen LK. Method for generation of homogeneous multicellular tumor spheroids applicable to a wide variety of cell types. Biotechnol Bioeng 2003;83:173–80 [DOI] [PubMed] [Google Scholar]
- 28. Unnikrishnan K, Thomas LV, Ram Kumar RM. Advancement of scaffold-based 3D cellular models in cancer tissue engineering: an update. Front Oncol 2021;11:733652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jensen C, Teng Y. Is it time to start transitioning from 2D to 3D cell culture? Front Mol Biosci 2020;7:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Belgodere JA, King CT, Bursavich JB, Burow ME, Martin EC, Jung JP. Engineering breast cancer microenvironments and 3D bioprinting. Front Bioeng Biotechnol 2018;6:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Annabi N, Nichol JW, Zhong X, Ji C, Koshy S, Khademhosseini A, Dehghani F. Controlling the porosity and microarchitecture of hydrogels for tissue engineering. Tissue Eng Part B Rev 2010;16:371–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bahcecioglu G, Basara G, Ellis BW, Ren X, Zorlutuna P. Breast cancer models: engineering the tumor microenvironment. Acta Biomater 2020;106:1–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Morton JJ, Bird G, Refaeli Y, Jimeno A. Humanized mouse xenograft models: narrowing the tumor-microenvironment gap. Cancer Res 2016;76:6153–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Day CP, Merlino G, Van Dyke T. Preclinical mouse cancer models: a maze of opportunities and challenges. Cell 2015;163:39–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu Y, Yin T, Feng Y, Cona MM, Huang G, Liu J, Song S, Jiang Y, Xia Q, Swinnen JV, Bormans G, Himmelreich U, Oyen R, Ni Y. Mammalian models of chemically induced primary malignancies exploitable for imaging-based preclinical theragnostic research. Quant Imaging Med Surg 2015;5:708–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cekanova M, Rathore K. Animal models and therapeutic molecular targets of cancer: utility and limitations. Drug Des Devel Ther 2014;8:1911–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mapanao AK, Voliani V. Three-dimensional tumor models: promoting breakthroughs in nanotheranostics translational research. Appl Mater Today 2020;19:100552 [Google Scholar]
- 38. Barriuso J, Nagaraju R, Hurlstone A. Zebrafish: a new companion for translational research in oncology. Clin Cancer Res 2015;21:969–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Han SJ, Kwon S, Kim KS. Challenges of applying multicellular tumor spheroids in preclinical phase. Cancer Cell Int 2021;21:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Astashkina A, Grainger DW. Critical analysis of 3-D organoid in vitro cell culture models for high-throughput drug candidate toxicity assessments. Adv Drug Deliv Rev 2014;69–70:1–18 [DOI] [PubMed] [Google Scholar]
- 41. Shang M, Soon RH, Lim CT, Khoo BL, Han J. Microfluidic modelling of the tumor microenvironment for anti-cancer drug development. Lab Chip 2019;19:369–86 [DOI] [PubMed] [Google Scholar]
- 42. Rijal G, Li W. 3D scaffolds in breast cancer research. Biomaterials 2016;81:135–56 [DOI] [PubMed] [Google Scholar]
- 43. Breslin S, O’Driscoll L. Three-dimensional cell culture: the missing link in drug discovery. Drug Discov Today 2013;18:240–9 [DOI] [PubMed] [Google Scholar]
- 44. Booij TH, Price LS, Danen EHJ. 3D cell-based assays for drug screens: challenges in imaging, image analysis, and high-content analysis. SLAS Discov 2019;24:615–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Asghar W, El Assal R, Shafiee H, Pitteri S, Paulmurugan R, Demirci U. Engineering cancer microenvironments for in vitro 3-D tumor models. Mater Today 2015;18:539–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ho WJ, Pham EA, Kim JW, Ng CW, Kim JH, Kamei DT, Wu BM. Incorporation of multicellular spheroids into 3-D polymeric scaffolds provides an improved tumor model for screening anticancer drugs. Cancer Sci 2010;101:2637–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shamir ER, Ewald AJ. Three-dimensional organotypic culture: experimental models of mammalian biology and disease. Nat Rev Mol Cell Biol 2014;15:647–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Girda E, Huang EC, Leiserowitz GS, Smith LH. The use of endometrial cancer patient-derived organoid culture for drug sensitivity testing is feasible. Int J Gynecol Cancer 2017;27:1701–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Thoma CR, Zimmermann M, Agarkova I, Kelm JM, Krek W. 3D cell culture systems modeling tumor growth determinants in cancer target discovery. Adv Drug Deliv Rev 2014;69–70:29–41 [DOI] [PubMed] [Google Scholar]
- 50. Spivak-Kroizman TR, Hostetter G, Posner R, Aziz M, Hu C, Demeure MJ, Von Hoff D, Hingorani SR, Palculict TB, Izzo J, Kiriakova GM, Abdelmelek M, Bartholomeusz G, James BP, Powis G. Hypoxia triggers hedgehog-mediated tumor-stromal interactions in pancreatic cancer. Cancer Res 2013;73:3235–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Halldorsson S, Lucumi E, Gómez-Sjöberg R, Fleming RMT. Advantages and challenges of microfluidic cell culture in polydimethylsiloxane devices. Biosens Bioelectron 2015;63:218–31 [DOI] [PubMed] [Google Scholar]
- 52. Gomez-Cuadrado L, Tracey N, Ma R, Qian B, Brunton VG. Mouse models of metastasis: progress and prospects. Dis Model Mech 2017;10:1061–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhang F, Wang W, Long Y, Liu H, Cheng J, Guo L, Li R, Meng C, Yu S, Zhao Q, Lu S, Wang L, Wang H, Wen D. Characterization of drug responses of mini patient-derived xenografts in mice for predicting cancer patient clinical therapeutic response. Cancer Commun 2018;38:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Morton JJ, Alzofon N, Jimeno A. The humanized mouse: emerging translational potential. Mol Carcinog 2020;59:830–8 [DOI] [PubMed] [Google Scholar]
- 55. Guaragnella N, Palermo V, Galli A, Moro L, Mazzoni C, Giannattasio S. The expanding role of yeast in cancer research and diagnosis: insights into the function of the oncosuppressors p53 and BRCA1/2. FEMS Yeast Res 2014;14:2–16 [DOI] [PubMed] [Google Scholar]
- 56. Puaux A-L, Ong LC, Jin Y, Teh I, Hong M, Chow PKH, Golay X, Abastado J-P. A comparison of imaging techniques to monitor tumor growth and cancer progression in living animals. Int J Mol Imaging 2011;2011:321538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Coste A, Oktay MH, Condeelis JS, Entenberg D. Intravital imaging techniques for biomedical and clinical research. Cytometry A 2020;97:448–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cui F, Zhou Z, Zhou HS. Review—measurement and analysis of cancer biomarkers based on electrochemical biosensors. J Electrochem Soc 2020;167:037525 [Google Scholar]
- 59. McHugh CI, Blocker SJ, Viola-Villegas N, Shields AF. Cancer imaging in preclinical models. In: Azmi A, Mohammad R. (eds) Animal models in cancer drug discovery. Amsterdam: Elsevier, 2019, pp.373–400 [Google Scholar]
- 60. James ML, Gambhir SS. A molecular imaging primer: modalities, imaging agents, and applications. Physiol Rev 2012;92:897–965 [DOI] [PubMed] [Google Scholar]
- 61. Perrin L, Bayarmagnai B, Gligorijevic B. Frontiers in intravital multiphoton microscopy of cancer. Cancer Rep 2020;3:e1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wyckoff J, Gligorijevic B, Entenberg D, Segall J, Condeelis J. High-resolution multiphoton imaging of tumors in vivo. Cold Spring Harb Protoc 2011;6:1167–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Szulczewski JM, Inman DR, Entenberg D, Ponik SM, Aguirre-Ghiso J, Castracane J, Condeelis J, Eliceiri KW, Keely PJ. In vivo visualization of stromal macrophages via label-free FLIM-based metabolite imaging. Sci Rep 2016;6:25086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kedrin D, Gligorijevic B, Wyckoff J, Verkhusha VV, Condeelis J, Segall JE, van Rheenen J. Intravital imaging of metastatic behavior through a mammary imaging window. Nat Methods 2008;5:1019–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Gerdes MJ, Sevinsky CJ, Sood A, Adak S, Bello MO, Bordwell A, Can A, Corwin A, Dinn S, Filkins RJ, Hollman D, Kamath V, Kaanumalle S, Kenny K, Larsen M, Lazare M, Li Q, Lowes C, McCulloch CC, McDonough E, Montalto MC, Pang Z, Rittscher J, Santamaria-Pang A, Sarachan BD, Seel ML, Seppo A, Shaikh K, Sui Y, Zhang J, Ginty F. Highly multiplexed single-cell analysis of formalin-fixed, paraffin-embedded cancer tissue. Proc Natl Acad Sci USA 2013;110:11982–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tan WCC, Nerurkar SN, Cai HY, Ng HHM, Wu D, Wee YTF, Lim JCT, Yeong J, Lim TKH. Overview of multiplex immunohistochemistry/immunofluorescence techniques in the era of cancer immunotherapy. Cancer Commun 2020;40:135–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Li W, Germain RN, Gerner MY. Multiplex, quantitative cellular analysis in large tissue volumes with clearing-enhanced 3D microscopy (Ce3D). Proc Natl Acad Sci USA 2017;114:E7321–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Li W, Germain RN, Gerner MY. High-dimensional cell-level analysis of tissues with Ce3D multiplex volume imaging. Nat Protoc 2019;14:1708–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Chen Y, Shen Q, White SL, Gokmen-Polar Y, Badve S, Goodman LJ. Three-dimensional imaging and quantitative analysis in CLARITY processed breast cancer tissues. Sci Rep 2019;9:5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Jain RK, Munn LL, Fukumura D. Measuring interstitial diffusion, convection, and binding parameters in mouse tumors. Cold Spring Harb Protoc 2013;8:678–80 [DOI] [PubMed] [Google Scholar]
- 71. Wu Y, Zhang W, Li J, Zhang Y. Optical imaging of tumor microenvironment. Am J Nucl Med Mol Imaging 2013;3:1–15 [PMC free article] [PubMed] [Google Scholar]
- 72. Gammon ST, Liu TW, Piwnica-Worms D. Interrogating cellular communication in cancer with genetically encoded imaging reporters. Radiol Imaging Cancer 2020;2:e190053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Condeelis J, Weissleder R. In vivo imaging in cancer. Cold Spring Harb Perspect Biol 2010;2:a003848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zhou Z, Lu Z. Molecular imaging of the tumor microenvironment. Adv Drug Deliv Rev 2017;113:24–48 [DOI] [PubMed] [Google Scholar]
- 75. Lee HW, Gangadaran P, Kalimuthu S, Ahn B. Advances in molecular imaging strategies for in vivo tracking of immune cells. Biomed Res Int 2016;2016:1946585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Stapleton S, Allen C, Pintilie M, Jaffray DA. Tumor perfusion imaging predicts the intra-tumoral accumulation of liposomes. J Control Release 2013;172:351–7 [DOI] [PubMed] [Google Scholar]
- 77. Anemone A, Consolino L, Arena F, Capozza M, Longo DL. Imaging tumor acidosis: a survey of the available techniques for mapping in vivo tumor pH. Cancer Metastasis Rev 2019;38:25–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Anemone A, Consolino L, Conti L, Irrera P, Hsu MY, Villano D, Dastrù W, Porporato PE, Cavallo F, Longo DL. Tumour acidosis evaluated in vivo by MRI-CEST pH imaging reveals breast cancer metastatic potential. Br J Cancer 2020;124:207–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Skala MC, Fontanella AN, Lan L, Izatt JA, Dewhirst MW. Longitudinal optical imaging of tumor metabolism and hemodynamics. J Biomed Opt 2010;15:011112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Mi P, Kokuryo D, Cabral H, Wu H, Terada Y, Saga T, Aoki I, Nishiyama N, Kataoka K. A pH-activatable nanoparticle with signal-amplification capabilities for non-invasive imaging of tumour malignancy. Nat Nanotechnol 2016;11:724–30 [DOI] [PubMed] [Google Scholar]
- 81. Zheng X, Wang X, Mao H, Wu W, Liu B, Jiang X. Hypoxia-specific ultrasensitive detection of tumours and cancer cells in vivo. Nat Commun 2015;6:5834. [DOI] [PubMed] [Google Scholar]
- 82. Anderson CF, Cui H. Protease-sensitive nanomaterials for cancer therapeutics and imaging. Ind Eng Chem Res 2017;56:5761–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ma T, Hou Y, Zeng J, Liu C, Zhang P, Jing L, Shangguan D, Gao M. Dual-ratiometric target-triggered fluorescent probe for simultaneous quantitative visualization of tumor microenvironment protease activity and pH in vivo. J Am Chem Soc 2018;140:211–8 [DOI] [PubMed] [Google Scholar]
- 84. Shabat D, Gnaim S, Scomparin A, Das S, Blau R, Satchi-Fainaro R. Direct real-time monitoring of prodrug activation by chemiluminescence. Angew Chem Int Ed 2018;57:9033–7 [DOI] [PubMed] [Google Scholar]
- 85. Di Nardo F, Chiarello M, Cavalera S, Baggiani C, Anfossi L. Ten years of lateral flow immunoassay technique applications: trends, challenges and future perspectives. Sensors 2021;21:5185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Gremel G, Grannas K, Sutton LA, Pontén F, Zieba A. In situ protein detection for companion diagnostics. Front Oncol 2013;3:271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Boellner S, Becker K-F. Reverse phase protein arrays—quantitative assessment of multiple biomarkers in biopsies for clinical use. Microarrays 2015;4:98–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Mendoza A, Torrisi DM, Sell S, Cady NC, Lawrence DA. Grating coupled SPR microarray analysis of proteins and cells in blood from mice with breast cancer. Analyst 2016;141:704–12 [DOI] [PubMed] [Google Scholar]
- 89. Bekmurzayeva A, Ashikbayeva Z, Myrkhiyeva Z, Nugmanova A, Shaimerdenova M, Ayupova T, Tosi D. Label-free fiber-optic spherical tip biosensor to enable picomolar-level detection of CD44 protein. Sci Rep 2021;11:19583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Zhao Q, Duan R, Yuan J, Quan Y, Yang H, Xi M. A reusable localized surface plasmon resonance biosensor for quantitative detection of serum squamous cell carcinoma antigen in cervical cancer patients based on silver nanoparticles array. Int J Nanomedicine 2014;9:1097–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Law WC, Yong KT, Baev A, Prasad PN. Sensitivity improved surface plasmon resonance biosensor for cancer biomarker detection based on plasmonic enhancement. ACS Nano 2011;5:4858–64 [DOI] [PubMed] [Google Scholar]
- 92. Damborsky P, Svitel J, Katrlik J. Optical biosensors. Essays Biochem 2016;60:91–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Ren QQ, Wu J, Zhang WC, Wang C, Qin X, Liu GC, Li ZX, Yu Y. Real-time in vitro detection of cellular H2O2 under camptothecin stress using horseradish peroxidase, ionic liquid, and carbon nanotube-modified carbon fiber ultramicroelectrode. Sens Actuators B Chem 2017;245:615–21 [Google Scholar]
- 94. Lei KF, Lin BY, Tsang NM. Real-time and label-free impedimetric analysis of the formation and drug testing of tumor spheroids formed via the liquid overlay technique. RSC Adv 2017;7:13939–46 [Google Scholar]
- 95. Shaibani PM, Etayash H, Naicker S, Kaur K. Metabolic study of cancer cells using a pH sensitive hydrogel nanofiber light addressable potentiometric sensor. ACS Sens 2017;2:151–6 [DOI] [PubMed] [Google Scholar]
- 96. Stratton D, Lange S, Kholia S, Jorfi S, Antwi-Baffour S, Inal J. Label-free real-time acoustic sensing of microvesicle release from prostate cancer (PC3) cells using a Quartz Crystal Microbalance. Biochem Biophys Res Commun 2014;453:619–24 [DOI] [PubMed] [Google Scholar]
- 97. Etayash H, Jiang K, Azmi S, Thundat T, Kaur K. Real-time detection of breast cancer cells using peptide-functionalized microcantilever arrays. Sci Rep 2015;5:13967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Zhang Y, Guo X, Fan L, Zhang Q, Sang S. A novel magnetoelastic immunosensor for ultrasensitively detecting carcinoembryonic antigen. Nanoscale Res Lett 2018;13:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Li Y, Foss CA, Summerfield DD, Doyle JJ, Torok CM, Dietz HC, Pomper MG, Yu SM. Targeting collagen strands by photo-triggered triple-helix hybridization. Proc Natl Acad Sci USA 2012;109:14767–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Uppal R, Medarova Z, Farrar CT, Dai G, Moore A, Caravan P. Molecular imaging of fibrin in a breast cancer xenograft mouse model. Invest Radiol 2012;47:553–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Starmans LWE, van Mourik T, Rossin R, Verelm I, Nicolay K, Gru H. Noninvasive visualization of tumoral fibrin deposition using a peptidic fibrin-binding single photon emission computed tomography tracer. Mol Pharmacerutics 2015;12:1921–8 [DOI] [PubMed] [Google Scholar]
- 102. Ombrato L, Nolan E, Kurelac I, Mavousian A, Heinze I, Chakravarty P, Horswell S, Gonzalez-Gualda E, Matacchione G, Weston A, Kirkpatrick J, Husain E, Collinson L, Ori A, Lee J, Malanchi I. Metastatic niche labelling reveals tissue parenchyma stem cell features. Nature 2019;572:603–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Mohammad S, Bari I, Reis LG, Nestorova GG. Calorimetric sandwich-type immunosensor for quantification of TNF-α. Biosens Bioelectron 2019;126:82–7 [DOI] [PubMed] [Google Scholar]
- 104. Misun PM, Rothe J, Schmid YRF, Hierlemann A, Frey O. Multi-analyte biosensor interface for real-time monitoring of 3D microtissue spheroids in hanging-drop networks. Microsyst Nanoeng 2016;2:16022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Su L, Zou L, Fong C, Wong W, Wei F, Wong K-Y, Wu RSS, Yang M. Detection of cancer biomarkers by piezoelectric biosensor using PZT ceramic resonator as the transducer. Biosens Bioelectron 2013;46:155–61 [DOI] [PubMed] [Google Scholar]
- 106. Naresh V, Lee N. A review on biosensors and recent development of nanostructured materials-enabled biosensors. Sensors 2021;21:1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Mehrotra P. Biosensors and their applications—a review. J Oral Biol Craniofac Res 2016;6:153–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Deng X, Xiong F, Li X, Xiang B, Li Z, Wu X, Guo C, Li X, Li Y, Li G, Xiong W, Zeng Z. Application of atomic force microscopy in cancer research. J Nanobiotechnology 2018;16:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Ogata G, Ishii Y, Asai K, Sano Y, Nin F, Yoshida T, Higuchi T, Sawamura S, Ota T, Hori K, Maeda K, Komune S, Doi K, Takai M, Findlay I, Kusuhara H, Einaga Y, Hibino H. A microsensing system for the in vivo real-time detection of local drug kinetics. Nat Biomed Eng 2017;1:654–66 [DOI] [PubMed] [Google Scholar]
- 110. Mao Y, Sun Q, Wang X, Ouyang Q, Han L, Jiang L, Han D. In vivo nanomechanical imaging of blood-vessel tissues directly in living mammals using atomic force microscopy. Appl Phys Lett 2009;95:013704 [Google Scholar]
- 111. Bartlett DW, Wu A, Li X, Kraus M, Wang H, Kindt E. Development of an in vivo retrodialysis calibration method using stable isotope labeling to monitor metabolic pathways in the tumor microenvironment via microdialysis. J Pharm Sci 2019;108:3124–9 [DOI] [PubMed] [Google Scholar]
- 112. Fatou B, Saudemont P, Leblanc E, Vinatier D, Mesdag V, Wisztorski M, Focsa C, Salzet M, Ziskind M, Fournier I. In vivo real-time mass spectrometry for guided surgery application. Sci Rep 2016;6:25919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Zhang J, Rector J, Lin JQ, Young JH, Sans M, Katta N, Giese N, Yu W, Nagi C, Suliburk J, Liu J, Bensussan A, DeHoog RJ, Garza KY, Ludolph B, Sorace AG, Syed A, Zahedivash A, Milner TE, Eberlin LS. Nondestructive tissue analysis for ex vivo and in vivo cancer diagnosis using a handheld mass spectrometry system. Sci Transl Med 2017;9:eaan3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Song SH, Kim A, Brown M, Jung C, Ko S, Ziaie B. An implantable wireless interstitial pressure sensor with integrated guyton chamber: in vivo study in solid tumors. IEEE Trans Biomed Eng 2016;63:2273–7 [DOI] [PubMed] [Google Scholar]
- 115. Vassiliou CC, Liu VH, Cima MJ. Miniaturized, biopsy-implantable chemical sensor with wireless, magnetic resonance readout. Lab Chip 2015;15:3465–72 [DOI] [PubMed] [Google Scholar]
- 116. Peltomaa R, Glahn-Martinez B, Benito-Peña E, Moreno-Bondi MC. Optical biosensors for label-free detection of small molecules. Sensor 2018;18:4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Iverson NM, Hofferber EM, Stapleton JA. Nitric oxide sensors for biological applications. Chemosensors 2018;6:8 [Google Scholar]
- 118. Wu Y, Tilley RD, Gooding JJ. Challenges and solutions in developing ultrasensitive biosensors. J Am Chem Soc 2019;141:1162–70 [DOI] [PubMed] [Google Scholar]
- 119. Kieninger J, Tamari Y, Enderle B, Jobst G, Sandvik JA, Pettersen EO, Urban GA. Sensor access to the cellular microenvironment using the sensing cell culture flask. Biosensors 2018;8:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Lee J, Cho HR, Cha GD, Seo H, Lee S, Park C, Kim JW, Qiao S, Wang L, Kang D, Kang T, Ichikawa T, Kim J, Lee H, Lee W, Kim S, Lee S, Lu N, Hyeon T, Choi SH, Kim D. Flexible, sticky, and biodegradable wireless device for drug delivery to brain tumors. Nat Commun 2019;10:5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Zugazagoitia J, Guedes C, Ponce S, Ferrer I, Molina-pinelo S, Paz-Ares L. Current challenges in cancer treatment. Clin Ther 2016;38:1551–66 [DOI] [PubMed] [Google Scholar]
- 122. Padma VV. An overview of targeted cancer therapy. BioMedicine 2015;5:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Yang M, Li J, Gu P, Fan X. The application of nanoparticles in cancer immunotherapy: targeting tumor microenvironment. Bioact Mater 2021;6:1973–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Saeed M, Gao J, Shi Y, Lammers T, Yu H. Engineering nanoparticles to reprogram the tumor immune microenvironment for improved cancer immunotherapy. Theranostics 2019;9:7981–8000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Geretti E, Leonard SC, Dumont N, Lee H, Zheng J, De Souza R, Gaddy DF, Espelin CW, Jaffray DA, Moyo V, Nielsen UB, Wickham TJ, Hendriks BS. Cyclophosphamide-mediated tumor priming for enhanced delivery and antitumor activity of HER2-targeted liposomal doxorubicin (MM-302). Mol Cancer Ther 2015;14:2060–71 [DOI] [PubMed] [Google Scholar]
- 126. Choi JY. Treatment of bone metastasis with bone-targeting radiopharmaceuticals. Nucl Med Mol Imaging 2018;52:200–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Jain RK, Stylianopoulos T. Delivering nanomedicine to solid tumors. Nat Rev Clin Oncol 2010;7:653–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Choi MR, Stanton-Maxey KJ, Stanley JK, Levin CS, Bardhan R, Akin D, Badve S, Sturgis J, Robinson JP, Bashir R, Halas NJ, Clare SE. A cellular trojan horse for delivery of therapeutic nanoparticles into tumors. Nano Lett 2007;7:3759–65 [DOI] [PubMed] [Google Scholar]
- 129. Choi MR, Bardhan R, Stanton-Maxey KJ, Badve S, Nakshatri H, Stantz KM, Cao N, Halas NJ, Clare SE. Delivery of nanoparticles to brain metastases of breast cancer using a cellular Trojan horse. Cancer Nanotechnol 2012;3:47–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Qi Y, Yan X, Xia T, Liu S. Use of macrophage as a Trojan horse for cancer nanotheranostics. Mater Des 2021;198:109388 [Google Scholar]
- 131. Shah K. Encapsulated stem cells for cancer therapy. Biomatter 2013;3:e24278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Liu X, Zhao Y. CRISPR / Cas9 genome editing: fueling the revolution in cancer immunotherapy. Curr Res Transl Med 2018;66:39–42 [DOI] [PubMed] [Google Scholar]
- 133. Ghobadi A. Chimeric antigen receptor T cell therapy for non-Hodgkin lymphoma. Curr Res Transl Med 2018;66:43–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Roybal KT, Rupp LJ, Morsut L, Walker WJ, McNally KA, Park JS, Lim WA. Precision tumor recognition by T cells with combinatorial antigen-sensing circuits. Cell 2016;164:770–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Stadtmauer EA, Fraietta JA, Davis MM, Cohen AD, Weber KL, Lancaster E, Mangan PA, Kulikovskaya I, Gupta M, Chen F, Tian L, Gonzalez VE, Xu J, Jung I-Y, Melenhorst JJ, Plesa G, Shea J, Matlawski T, Cervini A, Gaymon AL, Desjardins S, Lamontagne A, Salas-Mckee J, Fesnak A, Siegel DL, Levine BL, Jadlowsky JK, Young RM, Chew A, Hwang W-T, Hexner EO, Carreno BM, Nobles CL, Bushman FD, Parker KR, Qi Y, Satpathy AT, Chang HY, Zhao Y, Lacey SF, June CH. CRISPR-engineered T cells in patients with refractory cancer. Science 2020;367:eaba7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Poorebrahim M, Melief J, Pico de, Coaña Y, Wickström SL, Cid-Arregui A, Kiessling R. Counteracting CAR T cell dysfunction. Oncogene 2021;40:421–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Papadopoulos KP, Goel S, Beeram M, Wong A, Desai K, Haigentz M, Mani S, Lalani AS, Sarantopoulos J, Milia MIL. A phase 1 open-label, accelerated dose-escalation study of the hypoxia-activated prodrug AQ4N in patients with advanced malignancies. Clin Cancer Res 2008;14:7110–5 [DOI] [PubMed] [Google Scholar]
- 138. Li Y, Zhao L, Li X. Targeting hypoxia: hypoxia-activated prodrugs in cancer therapy. Front Oncol 2021;11:700407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Zhang Y, Dang M, Tian Y, Zhu Y, Liu W, Tian W, Su Y, Ni Q, Xu C, Lu N, Tao J, Li Y, Zhao S, Zhao Y, Yang Z, Sun L, Teng Z, Lu G. Tumor acidic microenvironment targeted drug delivery based on pHLIP-modified mesoporous organosilica nanoparticles. ACS Appl Mater Interfaces 2017;9:30543–52 [DOI] [PubMed] [Google Scholar]
- 140. Wang B, Zhao Q, Zhang Y, Liu Z, Zheng Z, Liu S, Meng L, Xin Y, Jiang X. Targeting hypoxia in the tumor microenvironment: a potential strategy to improve cancer immunotherapy. J Exp Clin Cancer Res 2021;40:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Danhier F, Feron O, Préat V. To exploit the tumor microenvironment: passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J Control Release 2010;148:135–46 [DOI] [PubMed] [Google Scholar]
- 142. Hansen AE, Petersen AL, Henriksen JR, Boerresen B, Rasmussen P, Elema DR, Rosenschoeld PM, Kristensen AT, Kjær A, Andresen TL. Positron emission tomography based elucidation of the enhanced permeability and retention effect in dogs with cancer using copper-64 liposomes. ACS Nano 2015;9:6985–95 [DOI] [PubMed] [Google Scholar]
- 143. Lee H, Hoang B, Fonge H, Reilly RM, Allen C. In vivo distribution of polymeric nanoparticles at the whole-body, tumor, and cellular levels. Pharm Res 2010;27:2343–55 [DOI] [PubMed] [Google Scholar]
- 144. Li H, Du J, Liu J, Du X, Shen S, Zhu Y, Wang X, Ye X, Nie S, Wang J. Smart superstructures with ultrahigh pH-sensitivity for targeting acidic tumor microenvironment: instantaneous size switching and improved tumor penetration. ACS Nano 2016;10:6753–61 [DOI] [PubMed] [Google Scholar]
- 145. Li H, Du J, Du X, Xu C, Sun C, Wang H, Cao Z, Yang X-Z, Zhu Y-H, Nie S, Wang J. Stimuli-responsive clustered nanoparticles for improved tumor penetration and therapeutic efficacy. Proc Natl Acad Sci USA 2016;113:4164–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. He Y, Li X, Ma J, Ni G, Yang G, Zhou S. Programmable codelivery of doxorubicin and apatinib using an implantable hierarchical-structured fiber device for overcoming cancer multidrug resistance. Small 2019;15:e1804397. [DOI] [PubMed] [Google Scholar]
- 147. Karagiannis GS, Pastoriza JM, Wang Y, Allison S, Entenberg D, Pignatelli J, Sharma VP, Xue EA, Cheng E, Alfonso TMD, Jones JG, Anampa J, Thomas E, Sparano JA, Condeelis JS, Oktay MH. Neoadjuvant chemotherapy induces breast cancer metastasis through a TMEM-mediated mechanism. Sci Transl Med 2017;9:eaan0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Overchuk M, Zheng G. Biomaterials overcoming obstacles in the tumor microenvironment: recent advancements in nanoparticle delivery for cancer theranostics. Biomaterials 2018;156:217–37 [DOI] [PubMed] [Google Scholar]
- 149. Cassereau L, Miroshnikova YA, Ou G, Lakins J, Weaver VM. A 3D tension bioreactor platform to study the interplay between ECM stiffness and tumor phenotype. J Biotechnol 2015;193:66–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Enríquez Á, Libring S, Field TC, Jimenez J, Lee T, Park H, Satoski D, Wendt MK, Calve S, Tepole AB, Solorio L, Lee H. High-throughput magnetic actuation platform for evaluating the effect of mechanical force on 3D tumor microenvironment. Adv Funct Mater 2020;31:2005021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Reid JA, Mollica PA, Bruno RD, Sachs PC. Consistent and reproducible cultures of large-scale 3D mammary epithelial structures using an accessible bioprinting platform. Breast Cancer Res 2018;20:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Pathak A, Kumar S. Independent regulation of tumor cell migration by matrix stiffness and confinement. Proc Natl Acad Sci USA 2012;109: 10334–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Datta P, Dey M, Ataie Z, Unutmaz D, Ozbolat IT. 3D bioprinting for reconstituting the cancer microenvironment. NPJ Precis Oncol 2020;4:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Rodenhizer D, Dean T, Xu B, Cojocari D, McGuigan AP. A three-dimensional engineered heterogeneous tumor model for assessing cellular environment and response. Nat Protoc 2018;13:1917–57 [DOI] [PubMed] [Google Scholar]
- 155. Rodenhizer D, Cojocari D, Wouters BG, McGuigan AP. Development of TRACER: tissue roll for analysis of cellular environment and response. Biofabrication 2016;8:045008. [DOI] [PubMed] [Google Scholar]
- 156. Esch MB, King TL, Shuler ML. The role of body-on-a-chip devices in drug and toxicity studies. Annu Rev Biomed Eng 2011;13:55–72 [DOI] [PubMed] [Google Scholar]
- 157. Sung KE, Yang N, Pehlke C, Keely PJ, Eliceiri KW, Friedl A, Beebe DJ. Transition to invasion in breast cancer: a microfluidic in vitro model enables examination of spatial and temporal effects. Integr Biol 2011;3:439–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Shirure VS, Bi Y, Curtis MB, Lezia A, Goedegebuure MM, Goedegebuure SP, Aft R, Fields RC, George SC. Tumor-on-a-chip platform to investigate progression and drug sensitivity in cell lines and patient-derived organoids. Lab Chip 2018;18:3687–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Choi Y, Hyun E, Seo J, Blundell C, Kim HC, Lee E, Lee SH, Moon A, Moon WK, Huh D. A microengineered pathophysiological model of early-stage breast cancer. Lab Chip 2015;15:3350–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Acosta MA, Jiang X, Huang P-K, Cutler KB, Grant CS, Walker GM, Gamcsik MP. A microfluidic device to study cancer metastasis under chronic and intermittent hypoxia. Biomicrofluidics 2014;8:054117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Sontheimer-Phelps A, Hassell BA, Ingber DE. Modelling cancer in microfluidic human organs-on-chips. Nat Rev Cancer 2019;19:65–81 [DOI] [PubMed] [Google Scholar]
- 162. Sung JH, Srinivasan B, Esch MB, McLamb WT, Bernabini C, Shuler ML, Hickman JJ. Using PBPK guided “body-on-a-chip” systems to predict mammalian response to drug and chemical exposure. Exp Biol Med 2014;239:1225–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Sung JH, Wang YI, Sriram NN, Jackson M, Long C, Hickman JJ, Shuler ML. Recent advances in body-on-a-chip systems. Anal Chem 2019;91:330–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Jonas O, Landry HM, Fuller JE, Santini Jr, JT, Baselga J, Tepper RI, Cima MJ, Langer R. An implantable microdevice to perform high-throughput in vivo drug sensitivity testing in tumors. Sci Transl Med 2015;7:284ra57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Sasikala ARK, Unnithan AR, Yun Y, Park CH, Kim CS. An implantable smart magnetic nanofiber device for endoscopic hyperthermia treatment and tumor-triggered controlled drug release. Acta Biomater 2016;31:122–33 [DOI] [PubMed] [Google Scholar]
- 166. Williams JK, Entenberg D, Wang Y, Avivar-Valderas A, Padgen M, Clark A, Aguirre-Ghiso JA, Castracane J, Condeelis JS. Validation of a device for the active manipulation of the tumor microenvironment during intravital imaging. Intravital 2016;5:e1182271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Xing WK, Shao C, Qi ZY, Yang C, Wang Z. The role of Gliadel wafers in the treatment of newly diagnosed GBM: a meta-analysis. Drug Des Devel Ther 2015;9:3341–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Ishihara J, Ishihara A, Sasaki K, Lee SS-Y, Williford J-M, Yasui M, Abe H, Potin L, Hosseinchi P, Fukunaga K, Raczy MM, Gray LT, Mansurov A, Katsumata K, Fukayama M, Kron SJ, Swartz MA, Hubbell JA. Targeted antibody and cytokine cancer immunotherapies through collagen affinity. Sci Transl Med 2019;11:eaau3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Momin N, Mehta NK, Bennett NR, Ma L, Palermi JR, Chinn MM, Lutz EA, Kang B, Irvine DJ, Spranger S, Wittrup KD. Anchoring of intratumorally administered cytokines to collagen safely potentiates systemic cancer immunotherapy. Sci Transl Med 2019;11:eaaw2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Akimoto JUN, Nakayama M, Takagi S, Okano T. Improved in vivo subcutaneous tumor generation by cancer cell sheet transplantation. Anticancer Res 2018;38:671–6 [DOI] [PubMed] [Google Scholar]
- 171. Chin AL, Jiang S, Jang E, Niu L, Li L, Jia X, Tong R. Implantable optical fibers for immunotherapeutics delivery and tumor impedance measurement. Nat Commun 2021;12:5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Raja WK, Gligorijevic B, Wyckoff J, Condeelis JS, Castracane J. A new chemotaxis device for cell migration studies. Integr Biol 2010;2:696–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Raja WK, Padgen MR, Williams JK, Gertler FB, Wyckoff JB, Condeelis JS, Castracane J. Development path and current status of the NANIVID: a new device for cancer cell studies. J Micro Nanolithogr MEMS MOEMS 2012;11:013013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Williams JK, Padgen MR, Wang Y, Entenberg D, Gertler F, Condeelis JS, Castracane J. Probing the tumor microenvironment: collection and induction. Microfluid BioMEMS Med Microsyst X 2012;8251:41–7 [Google Scholar]
- 175. Butt L, Entenberg D, Hemachandra LPM, Strohmayer M, Keely P, Aguirre-Ghiso J, Condeelis JS, Castracane J. Development of microfluidic-based cell collection devices for in vitro and in vivo use. Prog Biomed Opt Imaging: Proc SPIE 2016;9705:1–6 [Google Scholar]
- 176. Jacquemin G, Benavente-Diaz M, Djaber S, Bore A, Dangles-Marie V, Surdez D, Tajbakhsh S, Fre S, Lloyd-Lewis B. Longitudinal high-resolution imaging through a flexible intravital imaging window. Sci Adv 2021;7:eabg7663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Head T, Tokranova N, Cady NC. Lithographically patterned micro-nozzles for controlling fluid flow profiles for drug delivery and in vitro imaging applications. MRS Commun 2021;11:584–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Zhan T, Rindtor N, Betge J, Ebert MP, Boutros M. CRISPR/Cas9 for cancer research and therapy. Semin Cancer Biol 2019;55:106–19 [DOI] [PubMed] [Google Scholar]
- 179. Takeda H, Kataoka S, Nakayama M, Ali MAE, Oshima H, Yamamoto D, Park JW, Takegami Y, An T, Jenkins NA, Copeland NG, Oshima M. CRISPR-Cas9-mediated gene knockout in intestinal tumor organoids provides functional validation for colorectal cancer driver genes. Proc Natl Acad Sci USA 2019;116:15635–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Klinghammer K, Walther W, Hoffmann J. Choosing wisely—preclinical test models in the era of precision medicine. Cancer Treat Rev 2017;55:36–45 [DOI] [PubMed] [Google Scholar]
- 181. Ng SR, Rideout WM, Akama-Garren EH, Bhutkar A, Mercer KL, Schenkel JM, Bronson RT, Jacks T. CRISPR-mediated modeling and functional validation of candidate tumor suppressor genes in small cell lung cancer. Proc Natl Acad Sci USA 2020;117:513–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Weber J, Rad R. Engineering CRISPR mouse models of cancer. Curr Opin Genet Dev 2019;54:88–96 [DOI] [PubMed] [Google Scholar]
- 183. Gaudelli NM, Komor AC, Rees HA, Packer MS, Badran AH, Bryson DI, Liu DR. Programmable base editing of T to G C in genomic DNA without DNA cleavage. Nature 2017;551:464–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Anzalone AV, Randolph PB, Davis JR, Sousa AA, Koblan LW, Levy JM, Chen PJ, Wilson C, Newby GA, Raguram A, Liu DR. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature 2019;576:149–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Liu XS, Wu H, Ji X, Stelzer Y, Wu X, Czauderna S, Shu J, Dadon D, Young RA, Jaenisch R. Editing DNA methylation in the mammalian genome. Cell 2016;167:233–47.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Gjaltema RAF, Rots MG. Advances of epigenetic editing. Curr Opin Chem Biol 2020;57:75–81 [DOI] [PubMed] [Google Scholar]
- 187. Barata P, Sood AK, Hong DS. RNA-targeted therapeutics in cancer clinical trials: current status and future directions. Cancer Treat Rev 2016;50:35–47 [DOI] [PubMed] [Google Scholar]
- 188. LaVan DA, McGuire T, Langer R. Small-scale systems for in vivo drug delivery. Nat Biotechnol 2003;21:1184–91 [DOI] [PubMed] [Google Scholar]
- 189. Lin Z, Luo G, Du W, Kong T, Liu C, Liu Z. Recent advances in microfluidic platforms applied in cancer metastasis: circulating tumor cells’ (CTCs) isolation and tumor-on-a-chip. Small 2020;16:e1903899. [DOI] [PubMed] [Google Scholar]
- 190. Chiu DT, DeMello AJ, Carlo D, Di Doyle PS, Hansen C, Maceiczyk RM, Wootton RCR. Small but perfectly formed? Successes, challenges, and opportunities for microfluidics in the chemical and biological sciences. Chem 2017;2:201–23 [Google Scholar]