Abstract
Intermediate filaments were first described in muscle in 1968, and desmin was biochemically identified about 10 years afterwards. Its importance grew after the identification of desminopathies and desmin mutations that cause mostly cardiopathies. Since its characterization until recently, different functions have been attributed to desmin. Here, we use bibliometric tools to evaluate the articles published about desmin and to assess its several putative functions. We identified the most productive authors and the relationships between research groups. We studied the more frequent words among 9734 articles (September 2021) containing “desmin” on the title and abstract, to identify the major research focus. We generated an interactive spreadsheet with the 934 papers that contain “desmin” only on the title that can be used to search and quantify terms in the abstract. We further selected the articles that contained the terms “function” or “role” from the spreadsheet, which we then classified according to type of function, organelle, or tissue involved. Based on the bibliographic analysis, we assess comparatively the putative functions, and we propose an alternative explanation for the desmin function.
Keywords: Desmin, intermediate filaments, cytoskeleton
Impact Statement
More than 50 years after the identification of intermediate filaments, there is still a debate about their functions. While on one hand, several knockouts and genetic variants have been studied, on the other hand, there is still a tendency to mix together functions that are related to all intermediate filaments, dismissing the fact that desmin is specific to muscle cells. The present review uses a bibliometric approach to assess the research on desmin, and to compare its alleged functions. Based on the interpretation of the comparative list of functions we organized, we propose a new approach to interpret the function of desmin. This review should be useful to those interested in cytoskeleton, particularly in intermediate filaments, and in myogenesis and muscle structure and function, by providing a comprehensive view of the research on desmin.
Introduction
The cytoskeletal component “intermediate filament” (IF) has been characterized by Holtzer in 1968 1 because of its diameter of 10 nm, based on his observation of chick muscle cells in culture. He pointed out that other similarly sized filaments have been described in other cell types, and he assumed that these structures were related. Later it was shown that, while IFs form the nuclear lamina in all cell types, not all cell types have cytoplasmic IFs. Furthermore, IFs are composed of different proteins in different tissues. 2 Here, we will focus on the muscle- (and endothelial) specific filament protein desmin. The first purification of muscle IF protein has been reported by Cooke in 1976, 3 followed by Lazarides, 4 who suggested the name “desmin” for its ability to join structures, and Small, who suggested the name “skeletin.” 5 This sequence of events, from structure to biochemistry, is the opposite of what happened to microfilaments, where the biochemical identification of actin and myosin took place around 50 years before the visualization of contractile filaments, and similar to microtubules, that were first identified by electron microscopy and only biochemically purified 20 years afterwards. Yet the fundamental role of actin and myosin in contraction and tubulin in chromosome movement during mitosis, in all eukaryotic cells, appeared quite early in research, explaining why all cell types express actin and tubulin. The most obvious function assigned to IF is vaguely categorized as “mechanical integration.” 6 While this mechanical function can be undoubtedly observed in epithelial cells, the concept that all cell types have the same IF function is somewhat contradictory with the fact that each cell type has a different set of IF proteins.
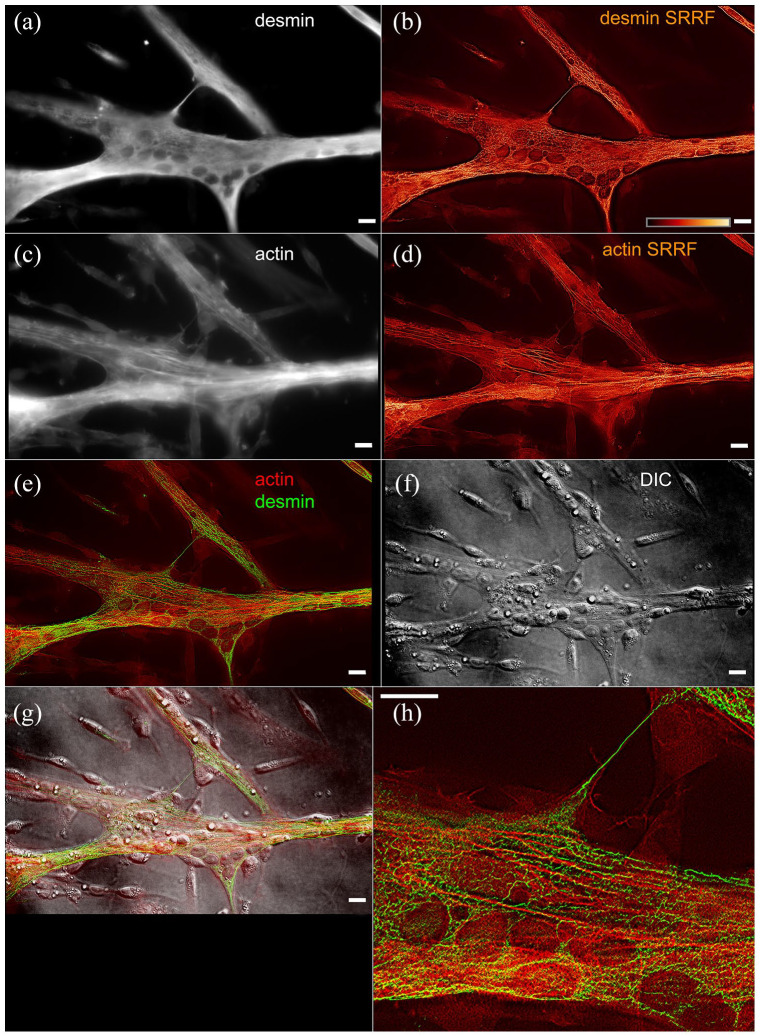
Contrary to actin and tubulin, which are compact globular proteins that have to undergo an extra chaperone processing step before they assume their final form, 7 IF proteins are elongated molecules, with two varying globular domains and a conserved central rod domain. Polymerization of actin and tubulin involves a smaller region of the subunit than IFs, which first assemble as antiparallel dimers throughout their central domain. 2 While the polymerization of actin and tubulin involves nTP hydrolysis, the polymerization of IF is regulated by phosphorylation. In the immunofluorescence image of cultured cells (Figure 1), desmin filaments spread in every direction of the muscle cells, contrary to actin filaments which are mostly oriented in the direction of contraction along the major axis of the cell. We used the radiality-based processing (SRRF 8 ) to better visualize both desmin and actin filaments. This method is based on the direction of changes in brightness among several images of the same field, much like the stochastic position determining microscopies such as STORM. Actin in these embryonic chicken primary cultures of skeletal myotubes 9 is still distributed in non-periodical stress fibers, while desmin is clearly distributed around the nuclei (visible in the interference contrast figure).
Figure 1.
Immunofluorescence labeling of chick primary culture myoblasts for desmin (a) and actin (c), and images processed with SRRF for desmin (b) and actin (d). The merged image (e) shows desmin labeled in green and actin labeled in red, compared with the interference contrast image (f) and (g)—the superposition of immuno and contrast. In the inset (h), it is interesting to note how the distribution of the processed image of actin and desmin mostly exclude each other. Scale bars 10 µm. (A color version of this figure is available in the online journal.)
Many diseases have been related to mutations in IFs. 10 The hereditary disease epidermolysis bullosa simplex (EBS) is caused by alterations on the genes of cytokeratins. 11 The pathophysiology of EBS has been clearly established, with the symptoms of skin fragility combining to the loss of structural continuity between the cytokeratin network of adjacent skin cells. The disease is well modeled by cytokeratins K5 and K14 knockout mice, and a similar phenotype can be observed in alterations in desmosomes (IF-related cell–cell adhesion structures), such as pemphigus. Desminopathy has been characterized as a disease caused by alterations in the desmin gene or alterations that lead to the abnormal distribution and/or accumulation of desmin inside the cells. 12 As it happens to many other diseases, there is a gap between medical and basic cell biology knowledge, 13 and the desmin-related physiopathological mechanisms are not clearly characterized: it is still not clear whether the disease is caused by the lack of function of altered desmin or to the accumulation of unprocessed molecular aggregates.
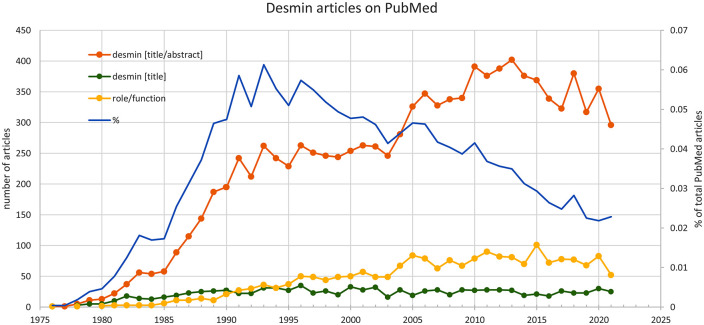
Here, we used bibliometrics, the quantitative study of publications, to review desmin function. Until September 2021, 10,570 articles have been published in PubMed citing desmin on the title or abstract, and 970 articles cite desmin on the title (Figure 2). Among the first category, 797 articles were classified as “review,” and about 7.5% of the total also mentioned the words “role” or “function.” The annual number of articles on desmin has been growing sharply, and there is a change in the total number of articles concerning “function” since 2005, at about the same time when the field of desminopathies became better investigated. The growth in the number of articles, however, should be kept in perspective because when we compared with the whole PubMed production, the proportion of articles about desmin actually became smaller over time (blue line on graph). Since there are numerous excellent reviews on several aspects of desmin, we decided to concentrate on the putative function(s) of desmin.
Figure 2.
Number of articles with desmin on title or desmin on title and abstract, compared to the total number of articles on PubMed. (A color version of this figure is available in the online journal.)
Patients
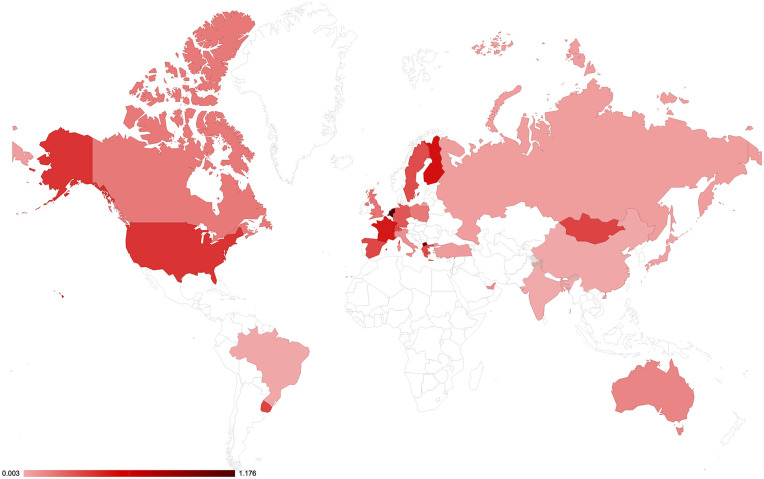
The quest for the function of desmin is ever more important because the identification of new cases of desminopathies that are being frequently identified and can have severe consequences for patients. In these cases, the literature either focuses on medical case-descriptions or on trying to establish details of each mutation at the cellular and molecular levels. From the medical point of view, the lack of awareness regarding desminopathies may represent a serious bias in determining the prevalence of desminopathies in different countries. Indeed, there is a huge difference in the number of diagnosed cases in different countries, which suggests an under-notification of cases and that the number of cases reported per country is more dependent on the local medical expertise than on the country’s human development index (data not shown) or possibly the population’s genetic background. Assuming that all the desmin mutation cases in the world should be registered in the Leiden Open Variation Database (LOVD, https://databases.lovd.nl/shared/genes/DES, maintained by the University of Leiden at https://www.dmd.nl/), we analyzed the worldwide incidence of desmin mutations (Figure 3). There is a large variation in the incidence from country to country, even considering the differences in population size. For instance, we could find only two descriptions of desminopathy in Brazil, which are not listed on the Leiden database nor on PubMed. Based on the average incidence of 0.25 cases per million habitants (calculated from the sum of cases in the LOVD database), we should expect about 53 cases in Brazil.
Figure 3.
Worldwide distribution of desmin cases in each country relative to the size of the population, based on the LOVD database, with additional information for Brazil. Several countries do not have any cases reported and are left without color. (A color version of this figure is available in the online journal.)
Researchers
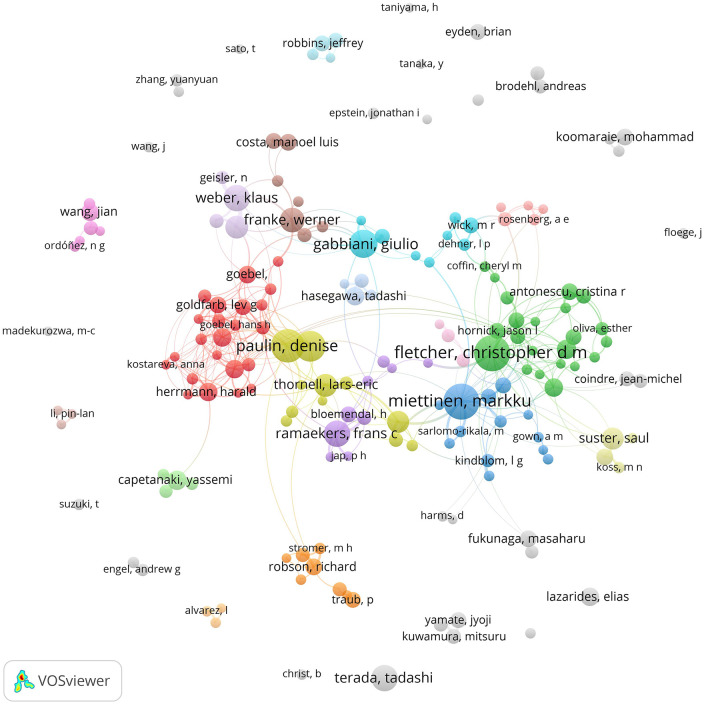
One of the questions that can be analyzed bibliometrically is who the main researchers in the desmin field are, and how the research groups are related. We built from PubMed (September 2021) a database of 9731 articles with the word “desmin” in the title or abstract (including “skeletin” and excluding the dermatan sulfate “Desmin 370”). Using the software Vosviewer, we assembled a diagram showing the number of publications per authors and their relationship on Figure 4 (the files that allow interactive visualization with Vosviewer app: https://app.vosviewer.com can be provide upon request). While more than 72% of the 39,450 authors published less than five articles about desmin, 175 authors published more than 10 articles. The five authors with most papers are: Christopher Fletcher (Brigham and Women’s Hospital, US), Markku Miettinen (Nat Cancer Inst, US), Denise Paulin (Université Pierre et Marie Curie Paris 6, FR), Zhenlin Li (Université Pierre et Marie Curie Paris 6, FR), and Giulio Gabbiani (Université de Genève, SW), with more than 50 articles published each. While Fletcher and Miettinen publish mostly on tumor pathology, the others usually publish on basic cell biology science: the major focus of the articles will be discussed in the next section.
Figure 4.
Diagram showing the number of publications per author and the relationship between authors in the database of articles with “desmin” in title/abstract in PubMed. The size of each author (circle) is proportional to the number of articles from the database we retrieved from PubMed (September 2021) with 9731 articles containing the word “desmin” in the title or abstract (including “skeletin” and excluding the dermatan sulfate “Desmin 370”), and the thickness of the connecting lines to the number of articles in co-authorship. (A color version of this figure is available in the online journal.)
Research themes
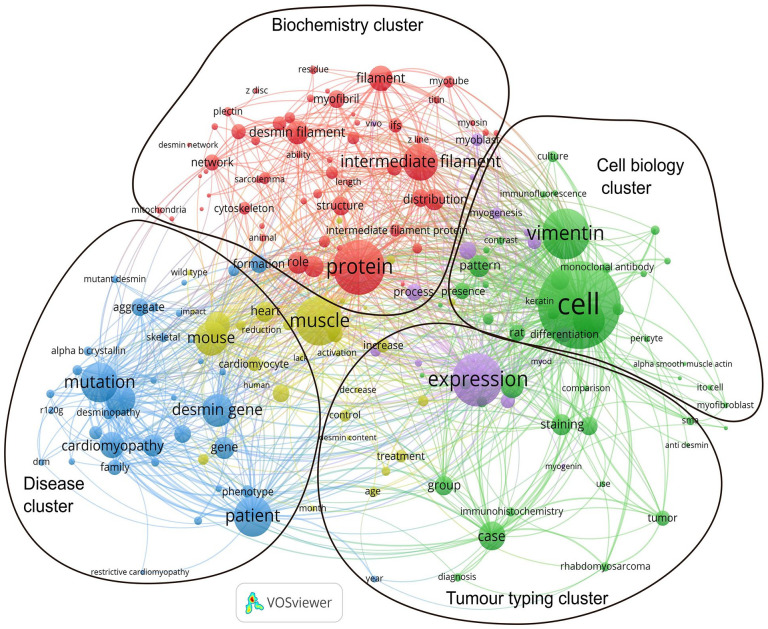
Bibliometric analysis also allows the visualization of the most frequent words, which can be assumed to represent the main subjects in a field. The software Vosviewer identified the most frequent words in the desmin PubMed database, organized them according to their relationship (co-occurrence), and displayed them in a map (Figure 5, the files that allow interactive visualization with Vosviewer app: https://app.vosviewer.com can be provide upon request). From the association between these words, we can try to identify the major research areas (marked in the diagram): (1) articles that use desmin as a tumor marker, (2) articles on desmin-related diseases, (3) articles on the cell biology of desmin, and (4) articles on desmin biochemistry. The first group includes several medical cases and other articles in which desmin is used as a marker for the identification of a given cell type. Frequent words in this group are “expression,” “immunohistochemistry,” “staining” and “marker,” and medical terms such as “diagnosis,” “case,” and “treatment.” The second group (mostly colored in blue) includes articles on desmin-related diseases, with words such as “myopathy,” “cardiomyopathy,” “patient,” “mutation,” and “desmin gene” highly frequent. The third group (mostly colored in green) has been tentatively composed of words related to cell biology, with high frequency of words such as “cell,” “differentiation,” “vimentin,” and “keratin.” Interestingly, words related to immunostain (“monoclonal antibody,” “antibody,” and “immunofluorescence”) are also frequently used, but distinct from the first cluster. Finally, the fourth cluster (mostly colored in red) is tentatively associated with biochemistry, centered in the word “protein,” and including as frequent words “intermediate filament,” “filament” but also “myotube” and “myoblast.” In the center of the figure there are some words colored in yellow, which are linked to all the groups, such as “muscle,” “heart,” and “function.” Since these words link to words in all clusters, we considered them to be in an intersection between all groups and to not constitute an independent cluster.
Figure 5.
Diagram showing the most frequent words in the database of articles with “desmin” in title/abstract in PubMed and their relationship. The size of each circle is proportional to the frequency of word from the database we retrieved from PubMed (September 2021) with 9731 articles containing the word “desmin” in the title or abstract (including “skeletin” and excluding the dermatan sulfate “Desmin 370”), and the thickness of the connecting lines to their co-occurrence. Clusters are automatically colored. The overlay represents our interpretation of each cluster characteristic. The yellow words in the center interact with words in all other clusters, and therefore are not classified in a separate group. (A color version of this figure is available in the online journal.)
Functions
Since the classification of all 10-nm filaments previously observed in several tissues as intermediate filaments, there has been a tendency to assume that they all have similar functions. The central core of all the IFs proteins, through which they polymerize, is conserved and they can be considered quite similar. However, it should be kept in mind that the head and tail of each of the tissue-specific IFs proteins are different, which must confer different properties to the filaments themselves, which in turn enable them to have different functions. 14 There should be a reason for the cell-type specificity of the IF proteins. Here we used a bibliographic protocol to select articles on desmin to comparatively assess the putative functions. First, based on the EuropePMC search for articles with “desmin” on the Title, we manually assembled a spreadsheet including the abstract of each article that can be made available upon request. The spreadsheet can be used to search and quantify terms in the abstract or authors fields, and spreadsheet cells can be classified according to the desired criteria, such as number of citations or publication year. Using this spreadsheet, we could observe that the impact of articles with “function” or “role” on the abstract is significantly higher than the rest of the papers: the average number of citations of “desmin” + “function” articles is 34.4, while the average number of “desmin” articles is 26.4. This difference could be justified because the scientific community has been emphasizing research focused on mechanisms of action rather than description of cases or observations. 13
We then used the spreadsheet to further analyze the articles that contained “function” or “role” in their abstract and that were not Reviews. We extracted the papers listed on Table 1 among those 164 articles. We classified each article according to: (1) alleged function, (2) conceptual approach (pathological, physiological, or structural), (3) level (subcellular, cellular, or tecidual), (4) model had a genetic modification such as knockouts, mutant (naturally occurring), and variant (experimentally constructed), (5) biological model, and (6) tissue type.
Table 1.
List of desmin functions, with the respective articles, classified according to alleged function, conceptual approach, structural level, genetic modification, biological model, and tissue type.
| # | Authors | Function | Type | Level | Genetic modification | Biological model | Tissue |
|---|---|---|---|---|---|---|---|
| 1 | Alam et al. 15 | Regulates mitochondrial fission | Pathological | Mitochondria | Mutant | Mouse | Cardiac muscle |
| 2 | Balogh et al. 16 | Regulates sarcomere alignment | Structural | Myofibril | KO | Mouse | Cardiac muscle |
| 3 | Boriek et al. 17 | Regulates stiffness and force production | Structural | Myofibril | KO | Mouse | Skeletal muscle |
| 4 | Bouvet et al. 18 | Induce toxic aggregates formation | Physiological | Degradation systems | Variant molecule | Rat | Cardiac muscle |
| 5 | Cao et al. 19 | Regulates intercellular electrical coupling | Physiological | Gap junctions | – | Human/rat | Cardiac muscle |
| 6 | Capetanaki et al. 20 | Regulates cellular structural and functional integrity | Physiological/structural | Myofibril | Variant molecule | Mouse | Skeletal, cardiac and smooth muscle |
| 7 | Charrier et al. 21 | Regulates myoblast stiffness | Physiological | Cell stiffness | – | Mouse | Skeletal muscle |
| 8 | Chaurasia et al. 22 | Remodeling of chorneal myofibroblasts | Physiological | Myofibroblast | – | Rabbit | Other |
| 9 | Chen et al. 23 | Regulates mechanoelectric feedback | Physiological | Cell signaling | Variant molecule | Rat | Cardiac muscle |
| 10 | Conover et al. 24 | Regulates actin microfilament and nebulin size | Pathological | Myofibril | Mutant | Chicken/rat | Skeletal and cardiac muscle |
| 11 | Dagvadorj et al. 25 | Support respiration | Physiological | Respiratory system | Mutant | Human | Skeletal muscle |
| 12 | D’Amati et al. 26 | Allows contraction | Physiological | Myofibril | – | Human | Cardiac muscle |
| 13 | Datta et al. 27 | Prevent cytoskeletal remodeling | Pathological | Heart | – | Rat | Cardiac muscle |
| 14 | Di Somma et al. 28 | Regulates cardiac function | Physiological | Heart | – | Human | Cardiac muscle |
| 15 | Diermeier et al. 29 | Regulates stiffness | Pathological | Myofibril | Mutant | Mouse | Skeletal muscle |
| 16 | Diguet et al. 30 | Regulates mitochondrial function | Physiological | Mitochondria | KO | Mouse | Cardiac muscle |
| 17 | Eiber et al. 31 | Regulates cellular structural and functional integrity | Physiological/structural | Neuromuscular junction | KO | Mouse | Skeletal muscle |
| 18 | Galata et al. 32 | Interferes with laminopathy phenotype severity | Pathological | Nucleus | Variant molecule | Mouse | Cardiac muscle |
| 19 | Gard and Lazarides 33 | Regulates myofibril lateral organization | Structural | Myofibril | – | Chicken | Skeletal muscle |
| 20 | Gard et al. 34 | Prevents cardiac arrhythmogenesis | Physiological | Heart | Variant molecule | Mouse | Cardiac muscle |
| 21 | Heckmann et al. 35 | Allows contraction | Physiological | Myofibril | KO | Mouse | Cardiac muscle |
| 22 | Hijikata et al. 36 | Stabilizes the subsarcolemmal cytoskeleton | Structural | Cytoskeleton | – | Rat | Skeletal muscle |
| 23 | Hofner et al. 37 | Regulates mesodermal differentiation into cardiomyoblast | Physiological | Heart | Variant molecule | Mouse | Cardiac muscle |
| 24 | Höllrigl et al. 38 | Regulates myoblast fusion, but hampers cardiomyogenesis and blocks smooth muscle development | Physiological/pathological | Myoblast fusion | KO | Mouse | Cardiac and smooth muscle |
| 25 | Huang et al. 39 | Regulates myofibril alignment | Pathological | Myofibril | Mutant | Hamster | Cardiac muscle |
| 26 | Joanne et al. 40 | Interferes with myofibril, mitochondria and autophagy | Physiological | Myofibril | KO | Mouse | Skeletal muscle |
| 27 | Kayman et al. 41 | Regulates calcium flux in myofibers | Physiological | Calcium homeostasis | KO | Zebrafish | Skeletal muscle |
| 28 | Kostareva et al. 42 | Interferes with heart remodeling | Pathological | Heart | Mutant | Human | Cardiac muscle |
| 29 | Kouloumenta et al. 43 | Regulates lysosome positioning | Structural | Lysosome and endoplasmic reticulum | Variant molecule | Yeast/mouse | Cardiac muscle |
| 30 | Lapouge et al. 44 | Regulates cytoarchitecture in cardiomyocytes | Structural | Cell adhesion | Variant molecule | Yeast | Cardiac muscle |
| 31 | Lazarides 45 | Regulates nuclei positioning | Structural | Nucleus | – | Chicken | Cardiac muscle |
| 32 | Li et al. 46 | Prevent idiopathic dilated cardiomyopathy | Pathological | Heart | Mutant | Human | Cardiac muscle |
| 33 | Liu et al. 47 | Impairs proteolytic function if aggregated | Physiological | Degradation systems | Variant molecule | Rat | Cardiac muscle |
| 34 | Loufrani et al. 48 | Regulates vascular tone and blood flow | Physiological | Arteries | KO | Mouse | Other |
| 35 | Loufrani et al. 49 | Regulates arteries diameter and flow | Structural | Endothelial cells | KO | Mouse | Other |
| 36 | Lovering et al. 50 | Regulates muscle tension | Pathological | Myofibril | Mutant/KO | Mouse | Skeletal muscle |
| 37 | Mackiewicz et al. 51 | Regulates heart contractile function | Physiological | Heart | Variant molecule | Mouse | Cardiac muscle |
| 38 | Maloyan et al. 52 | Regulates mitochondrial function | Pathological | Mitochondria | Mutant | Mouse | Cardiac muscle |
| 39 | Mavroidis et al. 53 | Induces Z disk alterations | Pathological | Myofibril | Mutant | Mouse | Cardiac muscle |
| 40 | Mermelstein et al. 54 | Regulates myoblast cell shape | Physiological | Calcium homeostasis | – | Mouse | Skeletal muscle |
| 41 | Meyer et al. 55 | Regulates Z disk stabilization | Structural | Myofibril | – | Theoretical prediction | Skeletal muscle |
| 42 | Milner et al. 56 | Regulates cellular structural and functional integrity | Physiological/structural | Myofibril | Variant molecule | Mouse | Skeletal, cardiac and smooth muscle |
| 43 | Milner et al. 57 | Regulates mitochondrial function and positioning | Physiological/structural | Mitochondria | KO | Mouse | Skeletal and cardiac muscle |
| 44 | Mitsui et al. 58 | Regulates contraction | Physiological | Myofibril | – | Rabbit | Skeletal muscle |
| 45 | Mohamed et al. 59 | Regulates smooth muscle hypertrophy | Physiological | Bronchi | KO | Mouse | Smooth muscle |
| 46 | Nag et al. 60 | Regulates organelle positioning | Structural | Nucleus | – | Rat | Cardiac muscle |
| 47 | Oliveira et al. 61 | Regulates decidualization of endometrial cells | Physiological | Nucleus | – | Mouse | Other |
| 48 | Otten et al. 62 | Regulates intercalated disk maintenance | Pathological | Myofibril | Mutant | Human | Cardiac muscle |
| 49 | Panagopoulou et al. 63 | Regulates ventricular wall thickness | Physiological | Heart | Variant molecule | Mouse | Cardiac muscle |
| 50 | Ralston et al. 64 | Regulates nuclei positioning | Structural | Nucleus | KO | Rat | Skeletal muscle |
| 51 | Russ and Grandy 65 | Regulates muscular function | Physiological | Muscle | – | Rat | Skeletal muscle |
| 52 | Sam et al. 66 | Increases vulnerability to mechanical injury | Structural | Myofibril | Variant molecule | Rat | Cardiac muscle |
| 53 | Schultheiss et al. 67 | Not necessary for myofibril organization | Structural | Myofibril | Variant molecule | Chicken | Skeletal muscle |
| 54 | Shah et al. 68 | Prevents respiratory problems | Pathological | Respiratory system | Variant molecule | Human | Skeletal muscle |
| 55 | Shah et al. 69 | Regulates sarcomere arrangement | Structural | Myofibril | KO | Mouse | Skeletal muscle |
| 56 | Shah et al. 70 | Regulates myofibrillar mobility | Structural | Myofibril | KO | Mouse | Skeletal muscle |
| 57 | Shah et al. 71 | Regulates nuclei positioning | Structural | Nucleus | KO | Mouse | Skeletal muscle |
| 58 | Sheng et al. 72 | Regulates blood pressure | Physiological | Myofibril | Variant molecule | Mouse | Cardiac muscle |
| 59 | Singh et al.73,a | Protects muscle from damage | Physiological | Degradation systems | – | – | – |
| 60 | Smythe et al. 74 | Regulates myoblast proliferation and fusion | Physiological | Myoblast fusion | KO | Mouse | Skeletal muscle |
| 61 | Tao and Ip 75 | Regulates myoblast fusion | Physiological | Myoblast fusion | Variant molecule | Chicken/quail | Smooth muscle |
| 62 | Tassin et al. 76 | Not necessary for myofibril organization | Pathological | Myofibril | Mutant | Mouse | Skeletal muscle |
| 63 | Tokuyasu et al. 77 | Regulates myofibril organization | Structural | Myofibril | – | Chicken | Skeletal and cardiac muscle |
| 64 | Tolstonog et al. 78 | Contributes to genomic integrity | Physiological | Gene expression | – | Mouse | Other |
| 65 | Vermeer et al. 79 | Regulates cellular structural and functional integrity | Pathological | Mitochondria | Mutant | Human | Cardiac muscle |
| 66 | Wang et al. 80 | Regulates myofibril assembly | Structural | Myofibril | – | Chicken | Cardiac muscle |
| 67 | Wede et al. 81 | Regulates tension in micro arteries | Structural | Myofibril | KO | Mouse | Smooth muscle |
| 68 | Wieneke et al. 82 | Necessary for myofiber activation | Physiological | Myofibril | KO | Mouse | Skeletal muscle |
| 69 | Woolstenhulme et al. 83 | Regulates force generation | Physiological | Myofibril | – | Human | Skeletal muscle |
| 70 | Yamamoto et al. 84 | Regulates myosin maturation | Physiological | Myofibril | – | Human | Cardiac muscle |
OBS: Although this article is a review, it does propose a new function for desmin.
KO: knockout.
The long list of functions of Table 1 suggests some interpretations.
There are important differences among the cell types that express desmin, the three muscle types and endothelial cells. In the table, we can observe that most (61%) of the alleged functions are described only in a single muscle cell type: 45% on cardiac muscle, 34% on skeletal muscle, and only 3% on smooth muscle. Since the muscles are quite different, should we expect that the same desmin molecule performs the same function(s) in the different muscle types? It should be kept in mind that desmin can have several post-translational modifications, related to several circumstances in the cell, but there are no tissue-specific isoforms in the desmin molecule among the various muscle cell types. 85 It is worth mentioning that among several desmin partners, some are tissue-specific, such as cardiac muscle troponin I (TNNI3) and skeletal muscle nebulin (NEB), which could be an indication of tissue-specific desmin functions. Several desmin-interacting molecules have been previously discussed by us 14 and by others, such as Klymkowsky, 86 and can be identified with automated databases such as STRING (https://string-db.org/), and therefore will not be further discussed here. Furthermore, some of the functions that could be attributed to desmin, like mechanical resistance, are in fact attributed to IFs in general, sometimes from observations in other cell types such as epithelia. Our previous bibliographic search method did not analyze articles on IF, but a PubMed search for IF on title/abstract selected 11,052 papers, among them 3329 (30%) containing “function” or “role.” Comparatively, there are 9737 papers with desmin on the title/abstract and among them only 1803 papers (18%) containing “function” or “role.” Just to compare, there are 5512 papers with “GFAP” and “function” and 7,427 with “vimentin” and “function” on PubMed.
Papers investigate at different levels: some focus on human physiology such as respiration, others focus on a single organ, like the heart, others in general cell behavior, other at subcellular level for instance in myofibrils. It is therefore difficult to compare these results.
Several (68%) of the articles rely on genetic modifications of desmin, either engineered or naturally occurring (mutations), assuming that the comparison with normal will highlight the (lack of) functions. Two groups constructed desmin mice knockouts: Capetanaki 20 and Paulin. 87 Desmin KO are viable and have somewhat subtle phenotypes. More recently, a zebrafish knockout model has been created using CRISPR: fish are also viable but with a subtle phenotype of calcium sensitivity. 41 These results contrast the previous results obtained with zebrafish morpholino-based desmin knockdown 88 or with knockins, 89 which showed a highly damaged phenotype. Some of the alterations could be attributed to the morpholinos because they are known to disturb development. On the contrary, it is not possible to discard putative gene compensations in the CRISPR models because they are checked only for the affected genes: it is not unusual to have divergent results with morpholinos and CRISPR. Several papers study mutants, and many of them actually try to relate the affected part of the molecule with a specific function or phenotype.
Several (23%) of the alleged functions actually describe pathological situations, either using naturally occurring mutants or experimentally created modifications. As we mentioned before, in all these cases, the observed pathological alterations could be due to the loss of function of desmin, and their study can contribute to the identification of the desmin function. Alternatively, abnormal processing or mutant molecules could lead to the formation and accumulation of molecular aggregates which are pathological by themselves. Aggregates can be formed by changes in desmin sequence, in patients/mutants, by changes in desmin processing molecules, such as calpain and caspases or by changes in associated proteins, such as the heat-shock protein alphaBcrystallin. 90 It has been shown that some regions of the desmin molecules (which include regions where mutations have been reported) are prone to aggregation. Furthermore, there are already studies on the structural alterations caused by specific mutations. 12
One interesting type of alteration in muscle development are the electric organs of fish. These muscle-derived organs specialized to produce electric discharges, and they lose their contractile filaments during development. They are related to the Purkinje fibers in the heart of large mammals, which also are muscle-derived cells specialized to conduct electricity, and which also have several desmin isoforms. We could show that the intermediate filaments of electric organs of the electric eel Electrophorus electricus are composed of desmin. 91 Desmin was also identified in the electric organs of the ray Torpedo marmorata. 92 Recently, proteome analysis of the electric eel confirmed the biochemical identification and demonstrated that there are about 78 phosphorylated variants of desmin in the electric organs. 93 Phosphorylation of IFs has been linked to their disassembly, in the case of the nuclear lamina for instance. 85 Nevertheless, there are several phosphorus binding sites in desmin, and it is probable that phosphorylation could be involved in other activities such as signal transduction. Desmin is also linked to connexin 43, which is important for electrical coupling among cells. 19
5. Several (40%) of the functions focus on myofibrils. Since the visualization that desmin is distributed around the Z-lines and dense bodies in cultured cells in 1985, 94 it has been suggested that desmin has a role in the organization of myofibrils, particularly during myogenesis. Yet there are several other places where desmin is concentrated. This progressive change in desmin distribution is well illustrated in the zebrafish embryo myogenesis, 95 where it is possible to follow the initial distribution of desmin around the nuclei, then in striations, then accumulated at the myotendinous junction near the somite’s septa (a distribution that does not happen in cultured cells, probably because they do not make strong directional adhesions like actual muscle). Moreover, several muscle organelles, such as endoplasmic reticulum, due to the cytoplasm compression during contraction, not only have a periodical distribution but concentrate at regions that are not compressed, such as the Z line. Furthermore, myofibrillogenesis can happen in desmin KO or in cells transfected with truncated desmin. 67
6. Some of the alleged functions focus on mechanical forces, sometimes relating the IF network to the microfilaments. Boriek points out the desmin is oriented in all dimensions, while actin has only a single orientation (see Figure 1), and therefore, desmin can dissipate energy in all directions. 17 Besides interacting with myofibrils, desmin has been shown to interact with actin. Actually, actin was a contaminant in the initial desmin purification by Lazarides. More recently, the protein nebullete has been shown to link desmin with the actin cytoskeleton. 96 Another mechanical role is regulation of stiffness. Charrier shows that desmin mutations and the amount of functional desmin alters mechanical properties of the cell, 21 and Diermeier studied stiffness in mutant desmin, 29 besides the aforementioned work of Boriek. 17
7. Seventeen percent of the functions focus on mitochondria and nucleus. While it is quite clear that alterations in desmin changes the distribution and function of mitochondria,15,30,52,57,79 it should be noted that other cytoskeletal filaments participate on mitochondria positioning, and other organelles such as endoplasmic reticulum also interact with mitochondria. To further complicate the matter, mitochondria are involved in several cellular processes, including respiration, apoptosis, and calcium control. A few papers point to an involvement of desmin on calcium regulation. Again, calcium is involved in several pathways, and in muscle, the regulation of contraction and regulation of protein degradation (through calpain, for example). Desmin has been implicated in the positioning of other organelles: lysosomes and endoplasmic reticulum 43 and particularly nuclei.45,60,64,71 Desmin filaments are stably associated with the outer nuclear membrane of muscle cells. 97 Recently, the perinuclear region of muscle cells has been characterized as a membraneless organelle, rich in several cytoskeletal proteins, including desmin. 9 The perinuclear distribution of desmin could indicate a role in mechanotransduction.
8. Some of the listed functions correlate desmin to stress conditions. One could argue that the IF system in muscle is not necessary under normal conditions because muscle has a hypertrophied acto-myosin-based mechanical support, and only when the myofibrils are affected for some reason, like in electric organ differentiation, or in muscular dystrophies, the function of desmin becomes prominent. Several of the desmin KO features could be explained in this way: only when muscle is stressed, like after repeated contractions, does the difference between KO and wild type appear. Most of the human disease symptoms also appear in older persons, which could be related to the long-term stress of the myofibrillar system. The formation of desmin aggregates has been considered the cause of muscle diseases. However, a recent paper by Singh addressed aggregation as actually an adaptive response to stress. 73 It seems that desmin can involve other cytoskeleton components such as microtubules and gene regulation as stress response. 98 The gene regulatory role of desmin has been confirmed in heart formation through the regulation of the gene Nkx. 99
It is interesting to compare the alleged functions of desmin to the functions attributed to vimentin, the intermediate filament protein that is highly expressed in the beginning of myogenesis. Besides some functions already mentioned for intermediate filaments in general, vimentin participates in vascular remodeling 100 and epithelial-mesenchymal transition (EMT). 101 Through its linkage to adhesion structures and gene regulation, vimentin is one of the hallmarks of EMT and is related to cancer and cell differentiation.
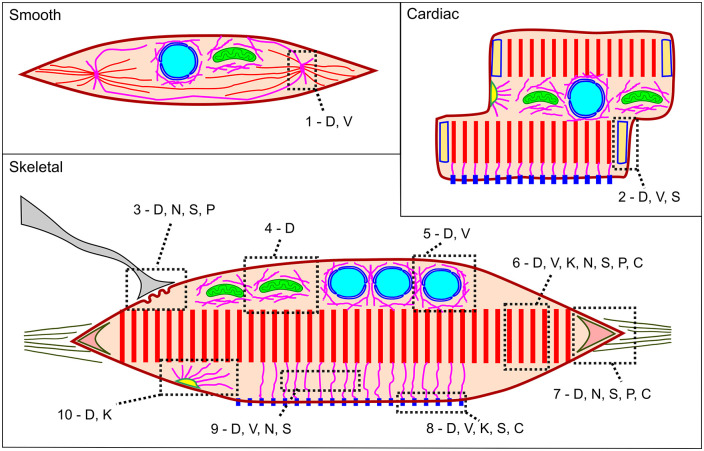
We show the distribution of intermediate filaments proteins in smooth, cardiac, and skeletal muscle in the scheme in Figure 6. We tried to depict the relative distribution of desmin, vimentin, cytokeratin, nestin, synemin, paranemin, and syncoilin, in previous characterized muscular structures: dense body, intercalated disk, neuromuscular junction, perimitochondrial, perinuclear, sarcomere, myotendinous junction, costamere, cytoplasm, and desmosome. While the only IF protein that has been described in all these structures is desmin, there are a few IF proteins that have been described only in specific cellular regions, such as nestin concentrated in neuromuscular and myotendinous junctions, and cytokeratin concentrated in desmosomes.
Figure 6.
Scheme showing the distribution of intermediate filaments in smooth, cardiac and skeletal muscle: D: desmin, V: vimentin, K: cytokeratin, N: nestin, S: synemin, P: paranemin, C: syncoilin, 1: dense body, 2: intercalated disk, 3: neuromuscular junction, 4: perimitochondrial, 5: perinuclear, 6: sarcomere, 7: myotendinous junction, 8: costamere, 9: cytoplasm, 10: desmosome. We do not depict other regions where IF proteins have been described, such as cytoplasm and sarcolemma because these categories are ambiguous. (A color version of this figure is available in the online journal.)
Conclusions
It is arguable that the muscular program is quite strong, in the sense that it prevails over other cell differentiation programs in experimental conditions. We showed that the forced expression of the MyoD gene in other cell types convert them to muscle. 102 Another sign of the robustness of the muscle gene regulation program is its redundancy, based on the compensatory expression of MyoD and Myf5 in mice knockouts. 103 It is tempting to include in this robustness concept the fact that muscle has an inhibitory differentiation and growth system, based on myostatin, rather than a stimulatory system. Would it be possible that desmin intermediate filaments are part of this “muscle back-up system”? Indeed, experiments such as with the double knockout of desmin and dystrophin 104 are pointing in this direction. They show that desmin can in some ways compensate for the damages of the lack of dystrophin. The function of desmin will probably be understood in the global picture of muscle structure and physiology.
Footnotes
Authors’ Contributions: M.L.C. conceived the work. M.L.C., A.D.J., C.M., G.G., K.A., M.S., and S.A. contributed to the acquisition of data and their analysis. M.L.C. wrote the first draft of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, funding no. 302115/2017-0 for CM, 301443/2018-1 for MLC) and Fundação de Apoio à Pesquisa do Estado do Rio de Janeiro (FAPERJ, funding no. E-26/202.920/2019 for CM and E26/210.220/2018 for MLC). A.D.J. is a fellow of the Estácio de Sá University (UNESA) Research Productivity Program and Institutos Nacionais de Ciência e Tecnologia (INCT/CAPES, grant no. 88887.568853/2020-00).
ORCID iDs: Sarah Azevedo  https://orcid.org/0000-0002-6435-5578
https://orcid.org/0000-0002-6435-5578
Manoel Luis Costa  https://orcid.org/0000-0002-9037-0085
https://orcid.org/0000-0002-9037-0085
References
- 1. Ishikawa H, Bischoff R, Holtzer H. Mitosis and intermediate-sized filaments in developing skeletal muscle. J Cell Biol 1968;38:538–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Herrmann H, Aebi U. Intermediate filaments: structure and assembly. Cold Spring Harb Perspect Biol 2016;8:a018242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cooke P. A filamentous cytoskeleton in vertebrate smooth muscle fibers. J Cell Biol 1976;68:539–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lazarides E, Hubbard BD. Immunological characterization of the subunit of the 100 A filaments from muscle cells. Proc Natl Acad Sci USA 1976;73:4344–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Small JV, Sobieszek A. Studies on the function and composition of the 10-NM(100-A) filaments of vertebrate smooth muscle. J Cell Sci 1977;23:243–68 [DOI] [PubMed] [Google Scholar]
- 6. Lazarides E. Intermediate filaments as mechanical integrators of cellular space. Nature 1980;283:249–56 [DOI] [PubMed] [Google Scholar]
- 7. Lewis SA, Tian G, Vainberg IE, Cowan NJ. Chaperonin-mediated folding of actin and tubulin. J Cell Biol 1996;132:1–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Culley S, Tosheva KL, Matos Pereira P, Henriques R. SRRF: universal live-cell super-resolution microscopy. Int J Biochem Cell Biol 2018;101: 74–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. do Amaral MJ, de Andrade Rosa I, Andrade SA, Fang X, Andrade LR, Costa ML, Mermelstein C. The perinuclear region concentrates disordered proteins with predicted phase separation distributed in a 3D network of cytoskeletal filaments and organelles. Biochim Biophys Acta Mol Cell Res 2022;1869:119161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Omary MB. “IF-pathies”: a broad spectrum of intermediate filament-associated diseases. J Clin Invest 2009;119:1756–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Homberg M, Magin TM. Beyond expectations: novel insights into epidermal keratin function and regulation. Int Rev Cell Mol Biol 2014;311:265–306 [DOI] [PubMed] [Google Scholar]
- 12. Clemen CS, Herrmann H, Strelkov SV, Schröder R. Desminopathies: pathology and mechanisms. Acta Neuropathol 2013;125:47–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Azevedo S, Seixas M, Jurberg A, Mermelstein C, Costa M. Do medicine and cell biology talk to each other? A study of vocabulary similarities between fields. Braz J Med Biol Res 2021;54:e11728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Costa ML, Escaleira R, Cataldo A, Oliveira F, Mermelstein CS. Desmin: molecular interactions and putative functions of the muscle intermediate filament protein. Braz J Med Biol Res 2004;37:1819–30 [DOI] [PubMed] [Google Scholar]
- 15. Alam S, Abdullah CS, Aishwarya R, Miriyala S, Panchatcharam M, Peretik JM, Orr AW, James J, Robbins J, Bhuiyan MS. Aberrant mitochondrial fission is maladaptive in desmin mutation-induced cardiac proteotoxicity. J Am Heart Assoc 2018;7:e009289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Balogh J, Merisckay M, Li Z, Paulin D, Arner A. Hearts from mice lacking desmin have a myopathy with impaired active force generation and unaltered wall compliance. Cardiovasc Res 2002;53:439–50 [DOI] [PubMed] [Google Scholar]
- 17. Boriek AM, Capetanaki Y, Hwang W, Officer T, Badshah M, Rodarte J, Tidball JG. Desmin integrates the three-dimensional mechanical properties of muscles. Am J Physiol Cell Physiol 2001;280:C46–52 [DOI] [PubMed] [Google Scholar]
- 18. Bouvet M, Dubois-Deruy E, Turkieh A, Mulder P, Peugnet V, Chwastyniak M, Beseme O, Dechaumes A, Amouyel P, Richard V, Lamblin N, Pinet F. Desmin aggrephagy in rat and human ischemic heart failure through PKCζ and GSK3β as upstream signaling pathways. Cell Death Discov 2021;7:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cao J, Gao Q, Chen H, Wang C, Zhang Q, Wang Z, Li Y. Desmin correlated with Cx43 may facilitate intercellular electrical coupling during chronic heart failure. Evid Based Complement Alternat Med 2021;2021:6621132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Capetanaki Y, Milner DJ, Weitzer G. Desmin in muscle formation and maintenance: knockouts and consequences. Cell Struct Funct 1997;22:103–16 [DOI] [PubMed] [Google Scholar]
- 21. Charrier EE, Montel L, Asnacios A, Delort F, Vicart P, Gallet F, Batonnet-Pichon S, Hénon S. The desmin network is a determinant of the cytoplasmic stiffness of myoblasts. Biol Cell 2018;110:77–90 [DOI] [PubMed] [Google Scholar]
- 22. Chaurasia SS, Kaur H, de Medeiros FW, Smith SD, Wilson SE. Dynamics of the expression of intermediate filaments vimentin and desmin during myofibroblast differentiation after corneal injury. Exp Eye Res 2009;89:133–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen L, Wang L, Li X, Wang C, Hong M, Li Y, Cao J, Fu L. The role of desmin alterations in mechanical electrical feedback in heart failure. Life Sci 2020;241:117119. [DOI] [PubMed] [Google Scholar]
- 24. Conover GM, Henderson SN, Gregorio CC. A myopathy-linked desmin mutation perturbs striated muscle actin filament architecture. Mol Biol Cell 2009;20:834–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dagvadorj A, Goudeau B, Hilton-Jones D, Blancato JK, Shatunov A, Simon-Casteras M, Squier W, Nagle JW, Goldfarb LG, Vicart P. Respiratory insufficiency in desminopathy patients caused by introduction of proline residues in desmin c-terminal alpha-helical segment. Muscle Nerve 2003;27:669–75 [DOI] [PubMed] [Google Scholar]
- 26. D’Amati G, Kahn HJ, Butany J, Silver MD. Altered distribution of desmin filaments in hypertrophic cardiomyopathy: an immunohistochemical study. Mod Pathol 1992;5:165–8 [PubMed] [Google Scholar]
- 27. Datta K, Basak T, Varshney S, Sengupta S, Sarkar S. Quantitative proteomic changes during post myocardial infarction remodeling reveals altered cardiac metabolism and Desmin aggregation in the infarct region. J Proteomics 2017;152:283–99 [DOI] [PubMed] [Google Scholar]
- 28. Di Somma S, Di Benedetto MP, Salvatore G, Agozzino L, Ferranti F, Esposito S, La Dogana P, Scarano MI, Caputo G, Cotrufo M, Santo LD, de Divitiis O. Desmin-free cardiomyocytes and myocardial dysfunction in end stage heart failure. Eur J Heart Fail 2004;6:389–98 [DOI] [PubMed] [Google Scholar]
- 29. Diermeier S, Iberl J, Vetter K, Haug M, Pollmann C, Reischl B, Buttgereit A, Schürmann S, Spörrer M, Goldmann WH, Fabry B, Elhamine F, Stehle R, Pfitzer G, Winter L, Clemen CS, Herrmann H, Schröder R, Friedrich O. Early signs of architectural and biomechanical failure in isolated myofibers and immortalized myoblasts from desmin-mutant knock-in mice. Sci Rep 2017;7:1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Diguet N, Mallat Y, Ladouce R, Clodic G, Prola A, Tritsch E, Blanc J, Larcher JC, Delcayre C, Samuel JL, Friguet B, Bolbach G, Li Z, Mericskay M. Muscle creatine kinase deficiency triggers both actin depolymerization and desmin disorganization by advanced glycation end products in dilated cardiomyopathy. J Biol Chem 2011;286:35007–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Eiber N, Fröb F, Schowalter M, Thiel C, Clemen CS, Schröder R, Hashemolhosseini S. Lack of desmin in mice causes structural and functional disorders of neuromuscular junctions. Front Mol Neurosci 2020;13:567084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Galata Z, Kloukina I, Kostavasili I, Varela A, Davos CH, Makridakis M, Bonne G, Capetanaki Y. Amelioration of desmin network defects by αB-crystallin overexpression confers cardioprotection in a mouse model of dilated cardiomyopathy caused by LMNA gene mutation. J Mol Cell Cardiol 2018;125:73–86 [DOI] [PubMed] [Google Scholar]
- 33. Gard DL, Lazarides E. The synthesis and distribution of desmin and vimentin during myogenesis in vitro. Cell 1980;19:263–75 [DOI] [PubMed] [Google Scholar]
- 34. Gard JJ, Yamada K, Green KG, Eloff BC, Rosenbaum DS, Wang X, Robbins J, Schuessler RB, Yamada KA, Saffitz JE. Remodeling of gap junctions and slow conduction in a mouse model of desmin-related cardiomyopathy. Cardiovasc Res 2005;67:539–47 [DOI] [PubMed] [Google Scholar]
- 35. Heckmann MB, Bauer R, Jungmann A, Winter L, Rapti K, Strucksberg KH, Clemen CS, Li Z, Schröder R, Katus HA, Müller OJ. AAV9-mediated gene transfer of desmin ameliorates cardiomyopathy in desmin-deficient mice. Gene Ther 2016;23:673–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hijikata T, Murakami T, Ishikawa H, Yorifuji H. Plectin tethers desmin intermediate filaments onto subsarcolemmal dense plaques containing dystrophin and vinculin. Histochem Cell Biol 2003;119:109–23 [DOI] [PubMed] [Google Scholar]
- 37. Hofner M, Höllrigl A, Puz S, Stary M, Weitzer G. Desmin stimulates differentiation of cardiomyocytes and up-regulation of brachyury and nkx2.5. Differentiation 2007;75:605–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Höllrigl A, Puz S, Al-Dubai H, Kim JU, Capetanaki Y, Weitzer G. Amino-terminally truncated desmin rescues fusion of des(-/-) myoblasts but negatively affects cardiomyogenesis and smooth muscle development. FEBS Lett 2002;523:229–33 [DOI] [PubMed] [Google Scholar]
- 39. Huang X, Li J, Foster D, Lemanski SL, Dube DK, Zhang C, Lemanski LF. Protein kinase C-mediated desmin phosphorylation is related to myofibril disarray in cardiomyopathic hamster heart. Exp Biol Med 2002;227:1039–46 [DOI] [PubMed] [Google Scholar]
- 40. Joanne P, Hovhannisyan Y, Bencze M, Daher MT, Parlakian A, Toutirais G, Gao-Li J, Lilienbaum A, Li Z, Kordeli E, Ferry A, Agbulut O. Absence of desmin results in impaired adaptive response to mechanical overloading of skeletal muscle. Front Cell Dev Biol 2021;9:662133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kayman Kürekçi G, Kural Mangit E, Koyunlar C, Unsal S, Saglam B, Ergin B, Gizer M, Uyanik I, Boustanabadimaralan Düz N, Korkusuz P, Talim B, Purali N, Hughes SM, Dincer PR. Knockout of zebrafish desmin genes does not cause skeletal muscle degeneration but alters calcium flux. Sci Rep 2021;11:7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kostareva A, Sjoberg G, Gudkova A, Smolina N, Semernin E, Shlyakhto E, Sejersen T. Desmin A213V substitution represents a rare polymorphism but not a mutation and is more prevalent in patients with heart dilation of various origins. Acta Myol 2011;30:42–5 [PMC free article] [PubMed] [Google Scholar]
- 43. Kouloumenta A, Mavroidis M, Capetanaki Y. Proper perinuclear localization of the TRIM-like protein myospryn requires its binding partner desmin. J Biol Chem 2007;282:35211–21 [DOI] [PubMed] [Google Scholar]
- 44. Lapouge K, Fontao L, Champliaud MF, Jaunin F, Frias MA, Favre B, Paulin D, Green KJ, Borradori L. New insights into the molecular basis of desmoplakin- and desmin-related cardiomyopathies. J Cell Sci 2006;119:4974–85 [DOI] [PubMed] [Google Scholar]
- 45. Lazarides E. The distribution of desmin (100 A) filaments in primary cultures of embryonic chick cardiac cells. Exp Cell Res 1978;112:265–73 [DOI] [PubMed] [Google Scholar]
- 46. Li D, Tapscoft T, Gonzalez O, Burch PE, Quiñones MA, Zoghbi WA, Hill R, Bachinski LL, Mann DL, Roberts R. Desmin mutation responsible for idiopathic dilated cardiomyopathy. Circulation 1999;100:461–4 [DOI] [PubMed] [Google Scholar]
- 47. Liu J, Tang M, Mestril R, Wang X. Aberrant protein aggregation is essential for a mutant desmin to impair the proteolytic function of the ubiquitin-proteasome system in cardiomyocytes. J Mol Cell Cardiol 2006;40:451–4 [DOI] [PubMed] [Google Scholar]
- 48. Loufrani L, Li Z, Lévy BI, Paulin D, Henrion D. Excessive microvascular adaptation to changes in blood flow in mice lacking gene encoding for desmin. Arterioscler Thromb Vasc Biol 2002;22:1579–84 [DOI] [PubMed] [Google Scholar]
- 49. Loufrani L, Matrougui K, Li Z, Levy BI, Lacolley P, Paulin D, Henrion D. Selective microvascular dysfunction in mice lacking the gene encoding for desmin. FASEB J 2002;16:117–9 [DOI] [PubMed] [Google Scholar]
- 50. Lovering RM, O’Neill A, Muriel JM, Prosser BL, Strong J, Bloch RJ. Physiology, structure, and susceptibility to injury of skeletal muscle in mice lacking keratin 19-based and desmin-based intermediate filaments. Am J Physiol Cell Physiol 2011;300:C803–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mackiewicz U, Czarnowska E, Brudek M, Pająk B, Duda M, Emanuel K, Csanyi G, Fedorowicz A, Grochal E, Tyrankiewicz U, Skórka T, Mende U, Lewartowski B, Chłopicki S. Preserved cardiomyocyte function and altered desmin pattern in transgenic mouse model of dilated cardiomyopathy. J Mol Cell Cardiol 2012;52:978–87 [DOI] [PubMed] [Google Scholar]
- 52. Maloyan A, Sanbe A, Osinska H, Westfall M, Robinson D, Imahashi K, Murphy E, Robbins J. Mitochondrial dysfunction and apoptosis underlie the pathogenic process in alpha-B-crystallin desmin-related cardiomyopathy. Circulation 2005;112:3451–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mavroidis M, Panagopoulou P, Kostavasili I, Weisleder N, Capetanaki Y. A missense mutation in desmin tail domain linked to human dilated cardiomyopathy promotes cleavage of the head domain and abolishes its Z-disc localization. FASEB J 2008;22:3318–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mermelstein CS, Amaral LM, Rebello MI, Reis JS, Borojevic R, Costa ML. Changes in cell shape and desmin intermediate filament distribution are associated with down-regulation of desmin expression in C2C12 myoblasts grown in the absence of extracellular Ca2+. Braz J Med Biol Res 2005;38:1025–32 [DOI] [PubMed] [Google Scholar]
- 55. Meyer GA, Kiss B, Ward SR, Morgan DL, Kellermayer MS, Lieber RL. Theoretical predictions of the effects of force transmission by desmin on intersarcomere dynamics. Biophys J 2010;98:258–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Milner DJ, Weitzer G, Tran D, Bradley A, Capetanaki Y. Disruption of muscle architecture and myocardial degeneration in mice lacking desmin. J Cell Biol 1996;134:1255–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Milner DJ, Mavroidis M, Weisleder N, Capetanaki Y. Desmin cytoskeleton linked to muscle mitochondrial distribution and respiratory function. J Cell Biol 2000;150:1283–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mitsui T, Kawajiri M, Kunishige M, Endo T, Akaike M, Aki K, Matsumoto T. Functional association between nicotinic acetylcholine receptor and sarcomeric proteins via actin and desmin filaments. J Cell Biochem 2000;77:584–95 [DOI] [PubMed] [Google Scholar]
- 59. Mohamed JS, Hajira A, Li Z, Paulin D, Boriek AM. Desmin regulates airway smooth muscle hypertrophy through early growth-responsive protein-1 and microRNA-26a. J Biol Chem 2011;286:43394–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Nag AC, Krehel W, Cheng M. Distributions of vimentin and desmin filaments in embryonic cardiac muscle cells in culture. Cytobios 1986;45:195–209 [PubMed] [Google Scholar]
- 61. Oliveira SF, Greca CP, Abrahamsohn PA, Reis MG, Zorn TM. Organization of desmin-containing intermediate filaments during differentiation of mouse decidual cells. Histochem Cell Biol 2000;113:319–27 [DOI] [PubMed] [Google Scholar]
- 62. Otten E, Asimaki A, Maass A, van Langen IM, van der Wal A, de Jonge N, van den Berg MP, Saffitz JE, Wilde AA, Jongbloed JD, van Tintelen JP. Desmin mutations as a cause of right ventricular heart failure affect the intercalated disks. Heart Rhythm 2010;7:1058–64 [DOI] [PubMed] [Google Scholar]
- 63. Panagopoulou P, Davos CH, Milner DJ, Varela E, Cameron J, Mann DL, Capetanaki Y. Desmin mediates TNF-alpha-induced aggregate formation and intercalated disk reorganization in heart failure. J Cell Biol 2008;181:761–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ralston E, Lu Z, Biscocho N, Soumaka E, Mavroidis M, Prats C, Lømo T, Capetanaki Y, Ploug T. Blood vessels and desmin control the positioning of nuclei in skeletal muscle fibers. J Cell Physiol 2006;209:874–82 [DOI] [PubMed] [Google Scholar]
- 65. Russ DW, Grandy JS. Increased desmin expression in hindlimb muscles of aging rats. J Cachexia Sarcopenia Muscle 2011;2:175–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sam M, Shah S, Fridén J, Milner DJ, Capetanaki Y, Lieber RL. Desmin knockout muscles generate lower stress and are less vulnerable to injury compared with wild-type muscles. Am J Physiol Cell Physiol 2000;279:C1116–22 [DOI] [PubMed] [Google Scholar]
- 67. Schultheiss T, Lin ZX, Ishikawa H, Zamir I, Stoeckert CJ, Holtzer H. Desmin/vimentin intermediate filaments are dispensable for many aspects of myogenesis. J Cell Biol 1991;114:953–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Shah F, Franklin KA, Holmlund T, Levring Jäghagen E, Berggren D, Forsgren S, Stål P. Desmin and dystrophin abnormalities in upper airway muscles of snorers and patients with sleep apnea. Respir Res 2019;20:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Shah SB, Peters D, Jordan KA, Milner DJ, Fridén J, Capetanaki Y, Lieber RL. Sarcomere number regulation maintained after immobilization in desmin-null mouse skeletal muscle. J Exp Biol 2001;204:1703–10 [DOI] [PubMed] [Google Scholar]
- 70. Shah SB, Su FC, Jordan K, Milner DJ, Fridén J, Capetanaki Y, Lieber RL. Evidence for increased myofibrillar mobility in desmin-null mouse skeletal muscle. J Exp Biol 2002;205:321–5 [DOI] [PubMed] [Google Scholar]
- 71. Shah SB, Davis J, Weisleder N, Kostavassili I, McCulloch AD, Ralston E, Capetanaki Y, Lieber RL. Structural and functional roles of desmin in mouse skeletal muscle during passive deformation. Biophys J 2004;86:2993–3008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sheng JJ, Feng HZ, Pinto JR, Wei H, Jin JP. Increases of desmin and α-actinin in mouse cardiac myofibrils as a response to diastolic dysfunction. J Mol Cell Cardiol 2016;99:218–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Singh SR, Kadioglu H, Patel K, Carrier L, Agnetti G. Is desmin propensity to aggregate part of its protective function? Cells 2020;9:491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Smythe GM, Davies MJ, Paulin D, Grounds MD. Absence of desmin slightly prolongs myoblast proliferation and delays fusion in vivo in regenerating grafts of skeletal muscle. Cell Tissue Res 2001;304:287–94 [DOI] [PubMed] [Google Scholar]
- 75. Tao JX, Ip W. Site-specific antibodies block kinase A phosphorylation of desmin in vitro and inhibit incorporation of myoblasts into myotubes. Cell Motil Cytoskeleton 1991;19:109–20 [DOI] [PubMed] [Google Scholar]
- 76. Tassin AM, Pinçon-Raymond M, Paulin D, Rieger F. Unusual organization of desmin intermediate filaments in muscular dysgenesis and TTX-treated myotubes. Dev Biol 1988;129:37–47 [DOI] [PubMed] [Google Scholar]
- 77. Tokuyasu KT, Dutton AH, Singer SJ. Immunoelectron microscopic studies of desmin (skeletin) localization and intermediate filament organization in chicken cardiac muscle. J Cell Biol 1983;96:1736–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Tolstonog GV, Wang X, Shoeman R, Traub P. Intermediate filaments reconstituted from vimentin, desmin, and glial fibrillary acidic protein selectively bind repetitive and mobile DNA sequences from a mixture of mouse genomic DNA fragments. DNA Cell Biol 2000;19:647–77 [DOI] [PubMed] [Google Scholar]
- 79. Vermeer MC, Bolling MC, Bliley JM, Arevalo Gomez KF, Pavez-Giani MG, Kramer D, Romero-Herrera PH, Westenbrink BD, Diercks GF, van den Berg MP, Feinberg AW, Silljé HHW, van der Meer P. Gain-of-function mutation in ubiquitin-ligase KLHL24 causes desmin degradation and dilatation in hiPSC-derived engineered heart tissues. J Clin Invest 2021;131:e140615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wang SM, Huang YS, Wu JC, Tseng YZ. Role of desmin filaments in chicken cardiac myofibrillogenesis. J Cell Biochem 2000;77:635–44 [DOI] [PubMed] [Google Scholar]
- 81. Wede OK, Löfgren M, Li Z, Paulin D, Arner A. Mechanical function of intermediate filaments in arteries of different size examined using desmin deficient mice. J Physiol 2002;540:941–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Wieneke S, Stehle R, Li Z, Jockusch H. Generation of tension by skinned fibers and intact skeletal muscles from desmin-deficient mice. Biochem Biophys Res Commun 2000;278:419–25 [DOI] [PubMed] [Google Scholar]
- 83. Woolstenhulme MT, Jutte LS, Drummond MJ, Parcell AC. Desmin increases with high-intensity concentric contractions in humans. Muscle Nerve 2005;31:20–4 [DOI] [PubMed] [Google Scholar]
- 84. Yamamoto M, Abe S, Rodríguez-Vázquez JF, Fujimiya M, Murakami G, Ide Y. Immunohistochemical distribution of desmin in the human fetal heart. J Anat 2011;219:253–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Winter DL, Paulin D, Mericskay M, Li Z. Posttranslational modifications of desmin and their implication in biological processes and pathologies. Histochem Cell Biol 2014;141:1–16 [DOI] [PubMed] [Google Scholar]
- 86. Klymkowsky MW. Filaments and phenotypes: cellular roles and orphan effects associated with mutations in cytoplasmic intermediate filament proteins. F1000Res 2019;8:F1000 Faculty Rev-1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Li Z, Mericskay M, Agbulut O, Butler-Browne G, Carlsson L, Thornell LE, Babinet C, Paulin D. Desmin is essential for the tensile strength and integrity of myofibrils but not for myogenic commitment, differentiation, and fusion of skeletal muscle. J Cell Biol 1997;139:129–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Li M, Andersson-Lendahl M, Sejersen T, Arner A. Knockdown of desmin in zebrafish larvae affects interfilament spacing and mechanical properties of skeletal muscle. J Gen Physiol 2013;141:335–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ramspacher C, Steed E, Boselli F, Ferreira R, Faggianelli N, Roth S, Spiegelhalter C, Messaddeq N, Trinh L, Liebling M, Chacko N, Tessadori F, Bakkers J, Laporte J, Hnia K, Vermot J. Developmental alterations in heart biomechanics and skeletal muscle function in desmin mutants suggest an early pathological root for desminopathies. Cell Rep 2015;11:1564–76 [DOI] [PubMed] [Google Scholar]
- 90. Kedia N, Arhzaouy K, Pittman SK, Sun Y, Batchelor M, Weihl CC, Bieschke J. Desmin forms toxic, seeding-competent amyloid aggregates that persist in muscle fibers. Proc Natl Acad Sci USA 2019;116:16835–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Costa ML, Moura Neto V, Chagas C. Desmin heterogeneity in the main electric organ of Electrophorus electricus. Biochimie 1988;70:783–9 [DOI] [PubMed] [Google Scholar]
- 92. Walker JH, Boustead CM, Witzemann V, Shaw G, Weber K, Osborn M. Cytoskeletal proteins at the cholinergic synapse: distribution of desmin, actin, fodrin, neurofilaments, and tubulin in Torpedo electric organ. Eur J Cell Biol 1985;38:123–33 [PubMed] [Google Scholar]
- 93. Traeger LL, Sabat G, Barrett-Wilt GA, Wells GB, Sussman MR. A tail of two voltages: proteomic comparison of the three electric organs of the electric eel. Sci Adv 2017;3:e1700523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Tokuyasu KT, Maher PA, Singer SJ. Distributions of vimentin and desmin in developing chick myotubes in vivo. II. Immunoelectron microscopic study. J Cell Biol 1985;100:1157–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Costa ML, Escaleira RC, Rodrigues VB, Manasfi M, Mermelstein CS. Some distinctive features of zebrafish myogenesis based on unexpected distributions of the muscle cytoskeletal proteins actin, myosin, desmin, alpha-actinin, troponin and titin. Mech Dev 2002;116:95–104 [DOI] [PubMed] [Google Scholar]
- 96. Hernandez DA, Bennett CM, Dunina-Barkovskaya L, Wedig T, Capetanaki Y, Herrmann H, Conover GM. Nebulette is a powerful cytolinker organizing desmin and actin in mouse hearts. Mol Biol Cell 2016;27:3869–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Mermelstein CS, Andrade LR, Portilho DM, Costa ML. Desmin filaments are stably associated with the outer nuclear surface in chick myoblasts. Cell Tissue Res 2006;323:351–7 [DOI] [PubMed] [Google Scholar]
- 98. Giovarelli M, Zecchini S, Martini E, Garrè M, Barozzi S, Ripolone M, Napoli L, Coazzoli M, Vantaggiato C, Roux-Biejat P, Cervia D, Moscheni C, Perrotta C, Parazzoli D, Clementi E, De Palma C. Drp1 overexpression induces desmin disassembling and drives kinesin-1 activation promoting mitochondrial trafficking in skeletal muscle. Cell Death Differ 2020;27:2383–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Fuchs C, Gawlas S, Heher P, Nikouli S, Paar H, Ivankovic M, Schultheis M, Klammer J, Gottschamel T, Capetanaki Y, Weitzer G. Desmin enters the nucleus of cardiac stem cells and modulates Nkx2.5 expression by participating in transcription factor complexes that interact with the nkx2.5 gene. Biol Open 2016;5:140–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Tang L, Dai F, Liu Y, Yu X, Huang C, Wang Y, Yao W. RhoA/ROCK signaling regulates smooth muscle phenotypic modulation and vascular remodeling via the JNK pathway and vimentin cytoskeleton. Pharmacol Res 2018;133:201–12 [DOI] [PubMed] [Google Scholar]
- 101. Usman S, Waseem NH, Nguyen TKN, Mohsin S, Jamal A, Teh MT, Waseem A. Vimentin is at the heart of epithelial mesenchymal transition (EMT) mediated metastasis. Cancers 2021;13:4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Choi J, Costa ML, Mermelstein CS, Chagas C, Holtzer S, Holtzer H. MyoD converts primary dermal fibroblasts, chondroblasts, smooth muscle, and retinal pigmented epithelial cells into striated mononucleated myoblasts and multinucleated myotubes. Proc Natl Acad Sci USA 1990;87:7988–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Rudnicki MA, Schnegelsberg PN, Stead RH, Braun T, Arnold HH, Jaenisch R. MyoD or Myf-5 is required for the formation of skeletal muscle. Cell 1993;75:1351–9 [DOI] [PubMed] [Google Scholar]
- 104. Ferry A, Messéant J, Parlakian A, Lemaitre M, Roy P, Delacroix C, Lilienbaum A, Hovhannisyan Y, Furling D, Klein A, Li Z, Agbulut O. Desmin prevents muscle wasting, exaggerated weakness and fragility, and fatigue in dystrophic mdx mouse. J Physiol 2020;598:3667–89 [DOI] [PubMed] [Google Scholar]