Abstract
The risks associated with diabetes in pregnancy include congenital anomalies, stillbirth and miscarriage, and correlate with glycaemia. The optimisation of diabetes during pregnancy is therefore both challenging and essential. Technology has revolutionised how clinicians and patients manage diabetes. This review article focuses on the role of continuous glucose monitoring (CGM) in pregnancy, assessing the evidence available and providing an update on current guidance.
Keywords: Continuous subcutaneous insulin infusion, insulin pump, continuous glucose monitoring, flash glucose monitoring, pregnancy, macrosomia, diabetes mellitus
Introduction
Diabetes in pregnancy carries multiple risks, in particular fetal macrosomia, congenital anomalies, stillbirth and miscarriage. These risks correlate with glycaemia; 1 thus, national guidelines emphasise the importance of optimising care in the pre-conception period and during pregnancy. 2 In 2018, Parson’s et al. (2018) highlighted in parallel to the physiological risks, the impact of diabetes on the psychological health of pregnant women. The report revealed that women with gestational diabetes (GDM) felt stigmatised and had a sense of reduced autonomy throughout the course of their pregnancy. 3
The emergence of continuous glucose monitoring (CGM) has revolutionised the management of diabetes mellitus (DM); its use has demonstrated improvements in glycaemic control with respect to both hyperglycaemic and hypoglycaemic events,4,5 as well as reported benefits to quality-of-life, thus exemplifying how technology can positively impact on the management of diabetes. 6 Given these promising findings, CGM is likely to significantly affect the way we manage diabetes in pregnancy and this article will examine this in greater detail.
What is CGM?
CGM devices utilise waterproof, self-administered subcutaneous sensors to measure interstitial fluid glucose levels continuously. The commonest sites of implantation are the upper arm, as seen in Figure 1 or the abdomen. 7 Sensors can last up to a fortnight before they should be changed. Because the sensors read interstitial fluid glucose, there is usually a lag of 5 to 10 min compared with capillary blood glucose (CBG) readings. 8
Figure 1.
An example of an implanted flash glucose monitoring sensor.
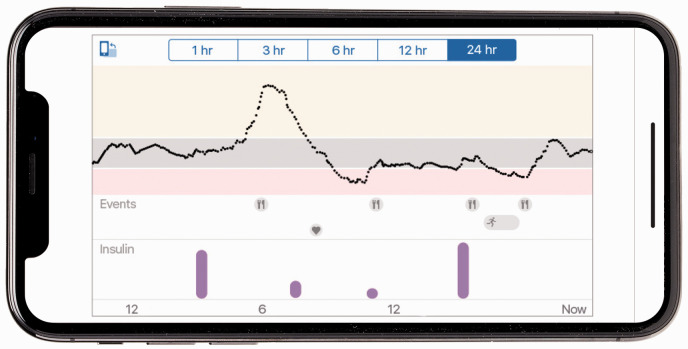
There are two subcategories of CGM: flash glucose monitoring, sometimes referred to as intermittently scanned CGM and real time monitoring (RT-CGM). Flash glucose monitoring systems, of which Freestyle Libre is the only certified system for use in pregnancy, require a scanning device to read glucose levels, sending readings to either smartphone app using near field communication (NFC) technology or a hand-held reader. RT-CGM systems measure glucose readings at high frequency (e.g. every five minutes) and display the data without the need to scan. Data is displayed on a connected phone app or hand-held device. This data can be accessed instantaneously by the user, a relative/friend or a healthcare professional. Both systems have options to link with web-based software, allowing data to be analysed remotely by specialist teams (Figure 2).
Figure 2.
Example of data retrieved from a CGM device.
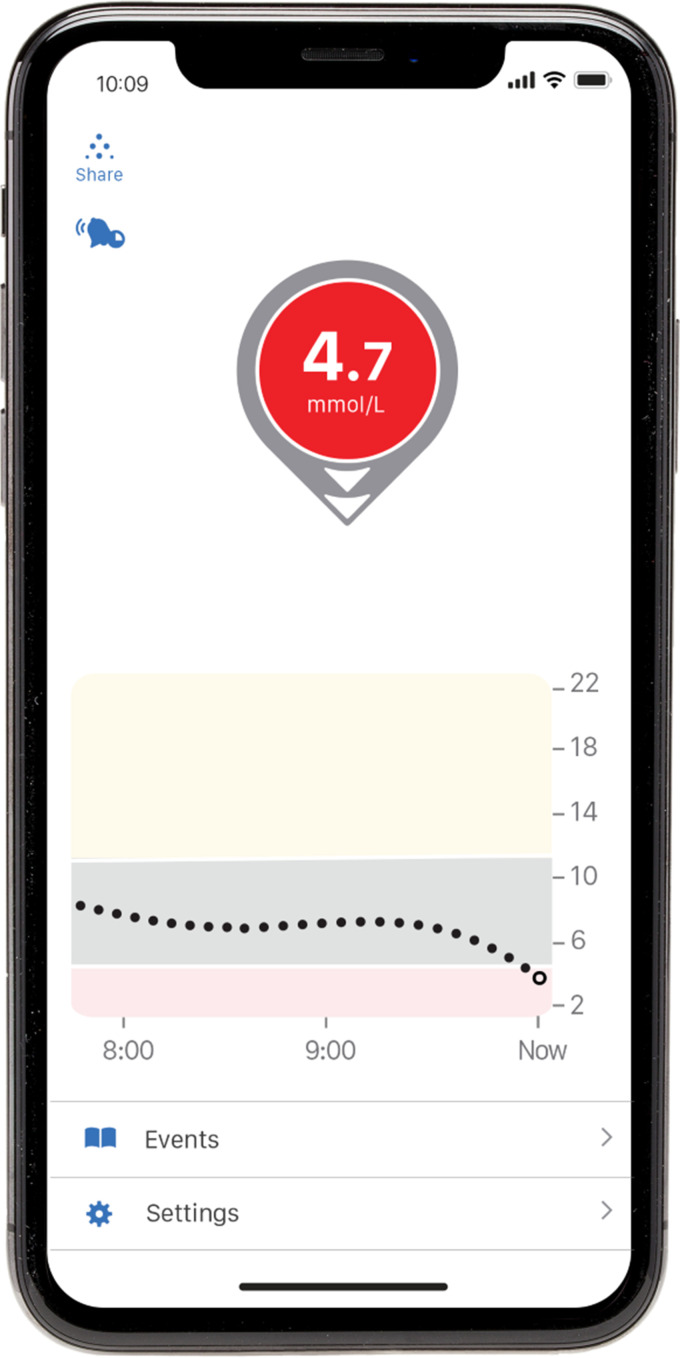
Not only are readings displayed numerically, they are often accompanied by a directional trend (Figure 3), which provides an important advantage; this extra data can alert the user to changing glucose levels and avoid hypo or hyperglycaemia. RT-CGM systems also have the added function of alarming at adjustable high and low glucose threshold targets to warn the user of an impending hypoglycaemic event or the possible need for a correction dose of insulin.
Figure 3.
An example of a single glucose reading from a CGM device.
In order to maintain accuracy in glucose readings, RT-CGM devices may require calibration. Two commonly used systems in practice are Medtronic and Dexcom.
Pitfalls with using CGM include allergic reactions to adhesives and reduced accuracy when glucose readings fall within the hypoglycaemia range; thus, low CGM glucose readings should be double-checked with a standard CBG. 9 Cross-checking should also be performed during and after exercise, when utilising sick day rules or if the sensor reading seems inappropriate. 9 Overnight, trends may also be less accurate if a user happens to roll over and lie on top of the sensor.
The use of CGM reduces the need for and discomfort from standard CBG monitoring; thus, the associated surge of interest within the population of people with diabetes is understandable.
CGM in pregnancy: The evidence
A concerning feature of the National Pregnancy in Diabetes (NPID) audit in 2018 was that the risks of complications in pregnant women with diabetes remains unchanged in over five years. 10 This begs the question: could CGM help bring about the ‘step-changes’ suggested in order to improve outcomes in this cohort of people?
Early studies dating back to 2004 suggested that CGM sensor readings are safe, accurate in their glucose measurements and well tolerated during pregnancy.11,12 More recent studies confirmed the safety and accuracy of flash glucose monitoring and CGM in pregnancy.13,14
Additional studies demonstrated that CGM identified nocturnal hypoglycaemia 15 and post-prandial hyperglycaemia more accurately than conventional CBG monitoring. 16 This can lead to intensification of treatment; Mclachlan et al. demonstrated that the use of CGM altered clinical management because of this extra information. 16
Later studies investigated the effect of CGM on pregnancy outcome. Murphy et al. randomised 71 women with diabetes (46 Type 1, 25 Type 2) to CGM or standard care. The CGM was offered intermittently, every four to six weeks for seven days between weeks 8 and 32 of pregnancy and was blinded. Women using CGM demonstrated improved glycaemic control in the third trimester and reduced risk of macrosomia, with lower mean HbA1c values between 32 and 36 weeks’ gestation. 17
Other studies, however, did not replicate such outcome benefits: Petrovski et al. compared the use of constant CGM against the use of intermittent CGM and found no difference in HbA1c or maternal and fetal outcomes, which included neonatal hypoglycaemia, macrosomia and need for caesarean section, although the power of the study was limited by a sample size of 25 women, all who were pump users for over a year and thus had good baseline management, reflected by mean HbA1c below 7%. 18
Two further studies, one conducted by Secher et al. and another by Voormolen et al. also demonstrated no significant improvements in glycaemic control or pregnancy outcome with larger sample sizes (n = 154 and n = 300, respectively).19,20 Whilst the former studied women with pre-existing type 1 diabetes (T1DM), the cohort in the study by Voormolen et al. included women with GDM and type 2 diabetes (T2DM) as well as T1DM. Outcomes included presence of macrosomia and HbA1c. However, the CGM groups in both studies utilised CGM on an intermittent basis.19,20 Additionally, the non-CGM group in the study by Secher et al. was defined as measuring blood glucose seven times daily, which may not be entirely representative of the compliance of monitoring in the general population. 19
The Continuous Glucose Monitoring in Pregnant Women with Type 1 diabetes (CONCEPTT) trial (2017) has been one of the key providers of evidence supporting the use of CGM in the antenatal period to date. 19 The study randomly assigned 325 women (215 pregnant women and 110 women planning pregnancy) to CGM in addition to standard CBG monitoring or standard CBG monitoring alone. The findings demonstrated significant improvements in neonatal health outcomes. There were fewer cases of large-for-gestational-age (LGA), neonatal intensive care unit (NICU) admissions exceeding 24 h and neonatal hypoglycaemic events, with a number needed to treat (NNT) to prevent NICU admission and large-for-dates at six, and an NNT to prevent neonatal hypoglycaemia, eight. CGM was also associated with shorter hospital stay. This benefit like arose from a reduced exposure to maternal hyperglycaemia, with longer duration in target glucose range at 34 weeks’ gestation (68% vs. 61% in favour of CGM users) and shorter time above target (27% vs. 32% again in favour of CGM users) reflected by a statistically significant improved mean absolute difference in HbA1c of 0.2%. The main adverse effects associated with the use of CGM were skin reactions to the sensor adhesives. 19
There are possible explanations for why the CONCEPTT trial yielded positive outcomes in comparison to those described prior. Firstly, the users of CGM in the CONCEPTT trial had continuous use of RT-CGM. The study also focused on women with T, who will likely have been exposed to more experience and education on diabetes than their GDM counterparts and a cohort where CGM has been shown to be beneficial, even outside of pregnancy. Lastly, it is worth noting that the CONCEPTT study had significantly more power (90%) to show a difference given its larger sample size than other studies. 19
There are some recognised limitations to the evidence supporting the use of CGM in pregnancy. Many studies utilise HbA1c as an endpoint but the use of HbA1c in the assessment of glycaemic control during pregnancy is questionable; NICE (2015) advise monitoring HbA1c in each trimester but make clear that this is to aid the assessment of risk of complications. 2
Most of the studies have researched the role of CGM in pregnant women with T1DM. Although the NPID audit (2018) found a higher prevalence of pregnancy in women with T2DM and increased rate of stillbirth when compared to pregnant women with T1DM, 10 there is yet to be a study directed at exploring the role of CGM in pregnant women with T2DM.
The risks of complications in pregnant women extend to women with GDM, and there is some evidence of benefits in this cohort of women. A systematic review by Yu et al. (2019) revealed increased user satisfaction with CGM compared to self-monitoring of blood glucose, greater detection of hypo- and hyperglycaemia, a greater number of treatment adjustments, lower gestational weight gain but inconclusive effects on maternal and fetal outcomes. 21
Jovanovic (2004) looked at 10 women with GDM and a mean HbA1c of 5.2% and found that CGM unveiled over 5 h per day of previously unrecognised hyperglycaemia with many of these readings occurring shortly after an in-range CBG measurement. 22 This is important to current work studying the mechanisms behind LGA and how CGM may reduce LGA, as there is evidence that women with only modest maternal blood glucose readings above target, have still delivered large neonates. 23 Recent additional analysis of the data from CONCEPTT has also revealed significant positive differences between the glucose profiles of women using RT-CGM compared to women not using CGM with increased time spent in range and lower glucose readings of up to 0.8 mmol/L for 7 h per day – in particular between 8:00 to 12:00 and 16:00 to 19:00. 24 This could prove a significant counter to the threat of LGA described by Law et al. (2019). Their study showed that women who delivered an LGA infant had mean glucoses just 0.4 mmol/L higher than those without, with the principal driver for this being higher glucoses for 6 h per day. 25
In contrast, little benefit has been shown with the use of CGM during the pre-conception period. 26 There is also sparse literature on the use of CGM in the perinatal period, with Cordua et al. (2013) reporting no significant difference in the prevalence of neonatal or maternal hypoglycaemia, although their study had a small sample size (n = 60). 27
The availability of CGM in pregnancy
CGM is recognised to be more expensive than conventional CBG and this has limited its use. Farrar & Campbell (2018) raise the point that in the CONCEPTT study, women randomised to the CGM arm were subject to more frequent healthcare provider visits, which would add to the cost of CGM devices and their associated consumables. 28 This is acknowledged by the authors of CONCEPTT, who suggest that this potential extra cost may be offset by the improved outcomes. 26
In 2019, the CONCEPTT group proceeded to examine the cost-effectiveness of the use of CGM in pregnancy using their study cohort. 29 Although the cost of using RT-CGM was £1232 dearer than standard CBG monitoring, when the total annual costs of managing the pregnancies were analysed, they discovered yearly cost savings of over £9 million. These savings occurred from reduced NICU admissions, need for hypoglycaemia treatment and length-of stay in hospital. 29 In the same year, NHS England (NHSE) outlined criteria for the funding of flash glucose monitoring in people with T1DM, with pregnancy being one such criteria. 30 Following the publication of CONCEPTT, the importance of diabetes in pregnancy has led to the offering of CGM to all pregnant women with T1DM as part of the NHSE’s 10-year plan (NHSE 2019), which would fall in line with some countries which already do so. 31
In December 2020, the National Institute for Health and Care Excellence (NICE) issued updated guidance stating that RT-CGM should be considered for all pregnant women with T1DM. Flash glucose monitoring should be offered either if there is an inability to use continuous glucose monitoring or if an explicit preference for such has been indicated. 2 Additionally, CGM should be offered to pregnant women using insulin who do not have T1DM if they have problematic severe hypoglycaemia or highly variable blood glucose levels.
With the availability of CGM in pregnancy increasing in the near future, a better understanding of these devices is essential for all healthcare professionals assessing women with diabetes in pregnancy.
Managing CGM in pregnancy
Blood glucose targets for CGM users differ to NICE targets for standard CBG monitoring, 2 as the focus shifts towards a time-in-range (Table 1), 32 which has been set as a useful parameter in evaluating blood glucose control in CGM-users. 33 The time-in-range of 70% was based on findings from the CONCEPTT study where the CGM group achieved 68% mean time in a target of 3.5–7.8 mmol/L.
Table 1.
Current recommended glucose targets during pregnancy.
| CBG targets (NICE 2015) (mmol/L) | CGM targets forsensor glucose (mmol/L) |
|---|---|
| Fasting <5.3 | 3.5–7.8 more than 70%of the time |
| 1 h post prandial <7.8 | >7.8 less than 25% of the time |
| 2 h post prandial <6.4 | <3.5 less than 4% of the time |
| >4 | <3 less than 1% of the time |
CBG: capillary blood glucose; CGM: continuous glucose monitoring.
As implied by Table 1, routine post-prandial CBG monitoring is not absolutely required in CGM users. However, they should be advised to continue utilising standard CBG monitoring in any situation when correction is required – for hypoglycaemia or hyperglycaemia. 9
The abundance of data accessible via CGM devices can confuse or overwhelm. 34 The Association of British Clinical Diabetologists (ABCD) (2020) have set out recommendations to counter this, by advising pregnant women not to correct post-prandial hyperglycaemia unless it persists for more than 3 h after the meal, nor should they correct hyperglycaemia that occurs secondary to the correction of a hypoglycaemic episode unless it persists for over 2 h and the sensor demonstrates an upward trend. 9 Pursuing the CGM trend after treating a hypoglycaemia, whereby further correction is given if readings remain below target should be avoided, as the lag between CBG and CGM readings often lead to overtreatment of hypoglycaemia. 9
One key benefit of using CGM is the enhanced ability to deliver care remotely using a virtual environment. This is likely to shape our approach to managing pregnant women with diabetes and has already proved useful in extreme circumstances such as the recent COVID-19 pandemic, which forced a shift to virtual consultations.
Conclusions
Without doubt, CGM has been revolutionary in the management of diabetes and evidence supports its use in pregnant women. Its role has demonstrated positive neonatal outcomes with very few adverse effects. The increased detection of maternal hyperglycaemia and hypoglycaemia facilitates optimisation of insulin dosing and dietary adjustment. CGM will also help to empower women to manage their diabetes during pregnancy, improving the experience of pregnancy for them and their support networks.
However, an observational study of 186 pregnancies in women with T1DM utilising CGM showed that despite CGM, glucose control remained sub-optimal on a day-to-day basis and the incidence of LGA remained above 50%. 35 Along with the data from the NPID audit (2018), there is evidence that suggests more changes will be necessary to truly further optimise outcomes of pregnant women with diabetes and adequately train clinicians with their management of such devices. 10 The NHSE 10-year plan and recently updated NICE guidelines are a promising start to tackling these changes.
This paper has demonstrated how technology has already helped achieve significant positive outcomes in pregnancy for people with diabetes, but technology in the world of diabetes continues to evolve rapidly with insulin pumps and closed loop systems delivering fine-tuned, individualised approaches to glycaemic control. Studies are beginning to delve deeper into the benefits of these systems and how they may impact on pregnancy outcomes. With technology comes excitement; the hope that these devices will lead to improved pregnancy outcomes and experience for all women with diabetes.
Acknowledgements
Many thanks to Dexcom for supplying the photographs required for the educational purposes of this article
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: Not required.
Informed consent: Not required.
Guarantor: AB.
Contributorship: Both Authors were directly involved in the writing of this article.
ORCID iD: Adrian Li https://orcid.org/0000-0003-4514-0376
References
- 1.Ali S, Dornhorst A. Diabetes in pregnancy: health risks and management. Postgrad Med J 2011; 87: 417–427. [DOI] [PubMed] [Google Scholar]
- 2.National Institute for Health and Care Excellence (NICE). Diabetes in pregnancy: management from preconception to the postnatal period. London: NICE, 2015, www.nice.org.uk/guidance/ng3 (accessed 17 December 2020). [PubMed] [Google Scholar]
- 3.Parsons J, Sparrow K, Ismail K, et al. Experiences of gestational diabetes and gestational diabetes care: a focus group and interview study. BMC Pregnancy Childbirth 2018; 18: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. The Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med 2008; 359: 1464–1476. [DOI] [PubMed] [Google Scholar]
- 5.Battelino T, Phillip M, Bratina N, et al. Effect of continuous glucose monitoring on hypoglycaemia in type 1 diabetes. Diabetes Care 2011; 34: 795–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polonsky W, Hessler D. What are the quality of life-related benefits and losses associated with real-time continuous glucose monitoring? A survey of current users. Diabetes Technol Ther 2013; 15: 295–301. [DOI] [PubMed] [Google Scholar]
- 7.National Institute of Diabetes and Digestive and Kidney Diseases. Continuous glucose monitoring, www.niddk.nih.gov/health-information/diabetes/overview/managing-diabetes/continuous-glucose-monitoring (2017. accessed 21 November 2020).
- 8.Rebrin K, Sheppard N, Steil G. Use of subcutaneous interstitial fluid glucose to estimate blood glucose: revisiting delay and sensor offset. J Diabetes Sci Technol 2010; 4: 1087–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Association of British Clinical Diabetologists (ABCD). Using diabetes technology in pregnancy. Best practice guideline. West Midlands: ABCD, 2020. [Google Scholar]
- 10.NHS Digital. National Pregnancy in Diabetes (NPID) Audit 2018. Report, England Wales and the Isle of Man 2019.
- 11.Kerssen A, Valk H, Visser G. The continuous glucose monitoring system during pregnancy of women with type 1 diabetes mellitus: accuracy assessment. Diabetes Technol Ther 2004; 6: 645–651. [DOI] [PubMed] [Google Scholar]
- 12.Bühling KJ, Kurzidim B, Wolf C, et al. Introductory experience with the continuous glucose monitoring system (CGMS; Medtronic Minimed) in detecting hyperglycemia by comparing the self-monitoring of blood glucose (SMBG) in non-pregnant women and in pregnant women with impaired glucose tolerance and gestational diabetes. Exp Clin Endocrinol Diabetes 2004; 112: 556–560. [DOI] [PubMed] [Google Scholar]
- 13.Scott E, Bilous R. and Kautzky-Willer. Accuracy, user acceptability, and safety evaluation for the freestyle Libre flash glucose monitoring system when used by pregnant women with diabetes. Diabetes Technol Ther 2018; 20: 180–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castorino K, Polsy S, O’Malley G, et al. Performance of the dexcom G6 continuous glucose monitoring system in pregnant women with diabetes. Diabetes Technol Ther 2020; 22: 943–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yogev Y, Ben-Haroush A, Chen R, et al. Continuous glucose monitoring for treatment adjustment in diabetic pregnancies – a pilot study. Diabetic Med 2003; 20: 558–562. [DOI] [PubMed] [Google Scholar]
- 16.Mclachlan K, Jenkins A, O’Neal D. The role of continuous glucose monitoring in clinical decision-making in diabetes in pregnancy. Obstet Gynaecol 2007; 47: 186–190. [DOI] [PubMed] [Google Scholar]
- 17.Murphy H, Rayman G, Lewis K, et al. Effectiveness of continuous glucose monitoring in pregnant women with diabetes: randomised clinical trial. Bmj 2008; 337: a1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petrovski G, Dimitrovski C, Bogoev M, et al. Is there a difference in pregnancy and glycemic outcome in patients with type 1 diabetes on insulin pump with constant or intermittent glucose monitoring? A pilot study. Diabetes Technol Ther 2011; 13: 1109–1113. [DOI] [PubMed] [Google Scholar]
- 19.Secher A, Ringholm L, Andersen HU, et al. The effect of real-time continuous glucose monitoring in pregnant women with diabetes. A randomised controlled trial. Diabetes Care 2013; 36: 1877–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Voormolen D, DeVries JH, Sanson R, et al. Continuous glucose monitoring during diabetic pregnancy (GlucoMOMS): a multicentre randomized controlled trial. Diabetes Obes Metab 2018; 20: 1894–1902. [DOI] [PubMed] [Google Scholar]
- 21.Yu Q, Aris I, Tan KH, et al. Application and utility of continuous glucose monitoring in pregnancy: a systematic review. Front Endocrinol (Lausanne) 2019; 10: 697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jovanovic L. The role of continuous glucose monitoring in gestational diabetes mellitus. Diabetes Technol Ther 2004; 2: 67–71. [DOI] [PubMed] [Google Scholar]
- 23.Persson B, Hanson U, Marcus C. Gestational diabetes mellitus and paradoxical fetal macrosomia – a case report. Early Hum Dev 1995; 41: 203–213. [DOI] [PubMed] [Google Scholar]
- 24.Scott E, Feig D, Murphy H, et al. Continuous glucose monitoring in pregnancy: importance of analyzing temporal profiles to understand clinical outcomes. Diabetes Care 2020; 43: 1178–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Law G, Alnaji A, Alrefaii L, et al. Suboptimal nocturnal glucose control is associated with large for gestational age in treated gestational diabetes mellitus. Diabetes Care 2019; 42: 810–815. [DOI] [PubMed] [Google Scholar]
- 26.Feig D, Donovan L, Corcoy R, et al. Continuous glucose monitoring in pregnant women with type 1 diabetes (CONCEPTT): a multicentre international randomised controlled trial. Lancet 2017; 390: 2347–2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cordua S, Secher A, Ringholm L, et al. Real-time continuous glucose monitoring during labour and delivery in women with Type 1 diabetes – observations from a randomized controlled trial. Diabet Med 2013; 30: 1374–1381. [DOI] [PubMed] [Google Scholar]
- 28.Farrar D, Campbell M. Does continuous glucose monitoring during pregnancy improve glycaemic and health outcomes in women with type 1 diabetes? – What the CONCEPTT trial adds. Ann Transl Med 2018; 6: 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murphy H, Feig D, Sanchez JJ, et al. Modelling potential cost savings from use of real-time continuous glucose monitoring in pregnant women with type 1 diabetes. Diabet Med 2019; 36: 1652–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.NHS Engl. Flash glucose monitoring: national arrangements for funding of relevant diabetes patients 2019. NHS England https://www.england.nhs.uk/diabetes/digital-innovations-to-support-diabetes-outcomes/flash-glucose-monitoring/
- 31.National Health Service England (NHSE). The NHS long term plan, www.longtermplan.nhs.uk/wp-content/uploads/2019/08/nhs-long-term-plan-version-1.2.pdf (2019, accessed 12 December 2020).
- 32.Battelino T, Danne T, Bergenstal R, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care 2019; 42: 1593–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gabbay M, Rodacki M, Calliari LE, et al. Time in range: a new parameter to evaluate blood glucose control in patients with diabetes. Diabetol Metab Syndr 2020; 12: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Diabetes UK. Continuous glucose monitoring, www.diabetes.org.uk/guide-to-diabetes/managing-your-diabetes/testing/continuous-glucose-monitoring-cgm (accessed 12 December 2020).
- 35.Kristensen K, Ögge LE, Sengpiel V, et al. Continuous glucose monitoring in pregnant women with type 1 diabetes: an observational cohort study of 186 pregnancies. Diabetologica 2019; 62: 1143–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]