Abstract
Introduction
COVID-19 has a wide range of clinical manifestations. Neurological manifestations in COVID-19 patients were demonstrated during the pandemic, including cognitive impairment. This study aimed to determine any relationship between COVID-19 and cognitive complaints, such as dementia, mild cognitive impairment (MCI), or subjective cognitive decline (SCD).
Methods
We performed a systematic review of MEDLINE via Ebsco, Cochrane EMBASE, SCOPUS, and LILACS electronic databases of observational studies with COVID-19 patients confirmed by serology or PCR who developed new cognitive impairment or deteriorated from previous cognitive impairment after infection. This review protocol was recorded on PROSPERO with registration number CRD 42021241590.
Results
A total of 3.520 articles were retrieved and read. Twenty-two studies were selected for our review. A wide range of cognitive assessment tools (n = 25) was used. The most described affected domains in these studies were executive functions, attention, and episodic memory. Thirteen studies showed a pattern of cognitive impairment in processing speed, inattention, or executive dysfunction assessed through working memory.
Conclusion
This review highlights the high frequency of cognitive impairment after COVID-19 infection. However, we were unable to differentiate whether the cognitive impairment found corresponded to mild cognitive impairment or dementia through data from selected studies, and this issue serves as one objective of future studies to be addressed on this topic.
Keywords: COVID-19, Cognitive impairment, SARS-CoV-2 infection, Dementia, Risk factor
Abbreviations: AD, Alzheimer's disease; MoCA, Montreal cognitive assessment; MCI, Mild cognitive impairment
1. Introduction
In November 2019, an unknown cause outbreak of pneumonia in Wuhan, Hubei province, China, began to attract Chinese health authorities’ attention. COVID-19 was declared a pandemic by the World Health Organization on March 11, 2020, with high levels of contamination and mortality in China, Italy, and Spain, and later in other countries (WHO, 2021). COVID-19 has a wide range of clinical manifestations (Guan et al., 2019). Of note, neurological manifestations in COVID-19 patients were demonstrated during the pandemic (Wu et al., 2020). In a study conducted in Wuhan, Hubei province, 36.4% of the patients presented some neurological manifestation, with central involvement being more common (dizziness, headache, altered level of consciousness, stroke, ataxia, and epilepsy). Also, patients with severe disease were more likely to develop neurological disorders, especially disorders of consciousness, acute cerebrovascular disease, and musculoskeletal disease (Mao et al., 2020).
Aside from general neurological manifestations, cognitive impairment was evaluated in COVID-19 patients. A Chinese study evaluated the cognition of 29 COVID-19 patients using digital questionnaires, relating cognitive complaints to high C-reactive protein levels during the disease's acute phase (Zhou et al., 2020). Another study evaluated cognitive impairment in outpatients, using Mini-Mental State Evaluation (MMSE), Montreal Cognitive Assessment (MoCA), Hamilton Rating Scale for Depression, and Functional Independence Measure (FIM), finding 80% of cognitive impairment (Alemanno et al., 2021). Moreover, different cognitive presentations have been described, such as encephalopathy associated with severe conditions (Delorme et al., 2020) and akinetic mutism associated with frontal hypometabolism (Cani et al., 2021).
Regarding cognitive manifestations pathophysiology, a more severe neurological manifestations in patients with APOE 4 allele of Apolipoprotein E has been described (Lumsden et al., 2020). This association is significant since the same allele confers a higher risk of sporadic Alzheimer's disease (AD) (Poirier et al., 1993).
The growing population aging over the past few decades has been associated with increased cognitive disorders. Data from Alzheimer's Disease International (ADI) report 46.8 million people living with dementia worldwide in 2015. This number is estimated to be around 74.5 million in 2030 and 131.5 million in 2050. Alzheimer's disease is the most common form of dementia and accounts for 50–70% of dementia cases (Prince et al., 2015). The prevalence of mild cognitive impairment (MCI) is 12–18% among adults over 65 years of age, and the annual progression rates from MCI to AD are 10–15% (Ding et al., 2015). Neuropathological alterations of amnestic MCI are intermediate between normal individuals and those with Alzheimer's disease, involving tau protein neurofibrillary tangles, beta-amyloid deposits, and neurodegeneration. Also, a minor injury burden emerges when MCI represents a stage before AD, which is theoretically susceptible to medicating action (Petersen et al., 2006). Besides these conditions, subjective cognitive decline is a condition before MCI and a possible dementia precursor. It represents individuals with cognitive complaints without objective evidence of cognitive impairment (Jessen et al., 2014). Therefore, it is crucial to identify whether COVID-19 possible neurologic lesions are associated with more significant cognitive impairment.
This study aimed to determine any relationship between COVID-19 and cognitive complaints, such as dementia, mild cognitive impairment (MCI), or subjective cognitive decline (SCD).
2. Methods
We performed a systematic review of observational studies based on the recommendation from the Cochrane Collaboration (Higgins & Green, 2011). This review protocol was registered on PROSPERO – International Prospective Register of Systematic Reviews— under registration number CRD 42021241590.
2.1. Search strategy
The search strategy will be performed to enhance methodological transparency and improve the reproducibility of the findings, following the PRISMA checklist (Moher et al., 2015). This systematic review study included six stages: 1 – formulating the central research question (theme identification); Step 2 – defining the inclusion and exclusion criteria and literature search; Step 3 – categorizing primary studies (defining data to be extracted from the selected studies); Step 4 – assessing the studies included; Step 5 – interpreting results; 6 – performing knowledge synthesis of the results obtained from the studies assessed (Jackson, 1980; Mendes et al., 2008; Whittemore & Knafl, 2005).
Additionally, we used the PICo method (Population; Interest; Context) acronym (Miller, 2001), where “P” is the study population (patients with confirmed COVID-19); “I” is the interest evaluated (cognitive impairment); and “Co” is the context (risk of developing cognitive impairment). Then, we elaborated the guiding question of this review to ensure scientific literature systematic search: “Do COVID-19 patients develop greater cognitive impairment?”.
Studies were retrieved from five electronic bibliographic databases: MEDLINE via Ebsco, Cochrane Central Register of Controlled Trials (CENTRAL), Excerpta Medica database (EMBASE), SCOPUS, and Latin American and Caribbean Health Sciences Literature (LILACS), from inception until March 30, 2021. No restriction regarding the publication date was considered in this systematic review. No filters were used in order to find as many results as possible, leaving this filtering for a later decision. The reference section of the included studies was considered for additional relevant studies. The search strategy consisted only of crucial terms according to a pre-established PICo acronym. Two researchers (JWLTJ and ACCS) carried out a search strategy in all databases independently. Also, bibliographic software EndNote (https://www.myendnoteweb.com/) was used to store, organize, and manage all the references and ensure a systematic and comprehensive search.
First of all, we identified the existence of specific subject headings index in each database (such as MeSH terms, Emtree terms, and DeCS-Health Science Descriptors) and their synonyms (keywords). The natural language was used with controlled language (descriptors) to expand search results (Araújo, 2020; Siddaway et al., 2019). The search terms were combined using the Boolean operators ‘AND’ and ‘OR’ (Nunes et al., 2018).
2.2. Study selection
We included studies with COVID-19 patients confirmed by serology or PCR who developed new cognitive impairment or worsened from previous cognitive impairment after infection. There was no restriction regarding age, previous disease, gender, or ethnicity. Inclusion criteria were articles in English, and COVID-19 confirmed by serology or PCR with new or deteriorated cognitive impairment with detailed cognitive evaluation. Exclusion criteria were studies focusing on psychiatric manifestations, guidelines, and institutional protocols. Regarding the study design, we included prospective or retrospective and cross-sectional studies. This systematic review had no restrictions concerning the settings of the target population.
2.3. Screening and data extraction
Initially, two researchers independently conducted a database search and sent it to the Mendeley reference manager to exclude duplicate documents. After duplicates exclusion, two independent investigators (JWLTJ and ACCS) screened the studies from their titles and abstracts information. Any disagreement ensued a new evaluation of the article and, if disagreement persisted, a third reviewer (PBN) would make a final decision. The resulting studies were evaluated according to eligibility criteria. Then, articles were read in their entirety using the same previous procedure. Study selection results were reported using PRISMA Flowchart. A standardized form was used for data extraction once achieving consensus on selected studies. Information extracted included country, author, year, objectives, study design, the prevalence of cognitive impairment in selected studies, primary results, median age, sample size and type, time of evaluation from COVID-19 onset, and the cognitive and functional assessment instruments used for diagnosis.
2.4. Qualitative synthesis
For the qualitative synthesis of the systematic review, the analyzes will follow three steps: 1) preliminary synthesis of the included studies, from the identification of their characteristics, their clinical contexts, the tools used to measure cognitive decline and the cognitive results found; 2) exploration of the relationships between study data, identifying those that work with similar instruments and outcomes or even different instruments dealing with similar outcomes; 3) and evaluation of the robustness of the synthesis, which includes criticism about the methodological quality of the included studies and how this quality influences the results of the primary studies (Popay et al., 2006).
In addition, we dicotomized studies between those that included acute and subacute phase patients (<12 weeks from COVID-19 onset) from those that evaluated cognitive impairment after this phase (>12 weeks from COVID-19 onset), in order to differentiate COVID-19 acute and subacute from chronic effects on cognition (Nalbandian et al., 2021).
At the end of the synthesis process, the analysis of the relationships within and between the studies described led to an overall assessment of the strength of the available evidence to draw conclusions based on a narrative synthesis (Popay et al., 2006). Display data matrices were developed in the form of tables to present the results of the systematic review. Heterogeneity was qualitatively explored in the organization of the analysis process, considering methodological similarities and differences between the included studies.
2.5. Risk of bias in individual studies
Cochrane Collaboration Risk of Bias Tool was employed to classify the included studies’ risk of bias, considering the following domains: inadequate patient selection and inclusion; controlling for confounding factors; blinding of investigators and outcome evaluators; incomplete outcome data. Risk of bias was thus classified as low, high, or unknown » http://handbook-5-1.cochrane.org/.
3. Results
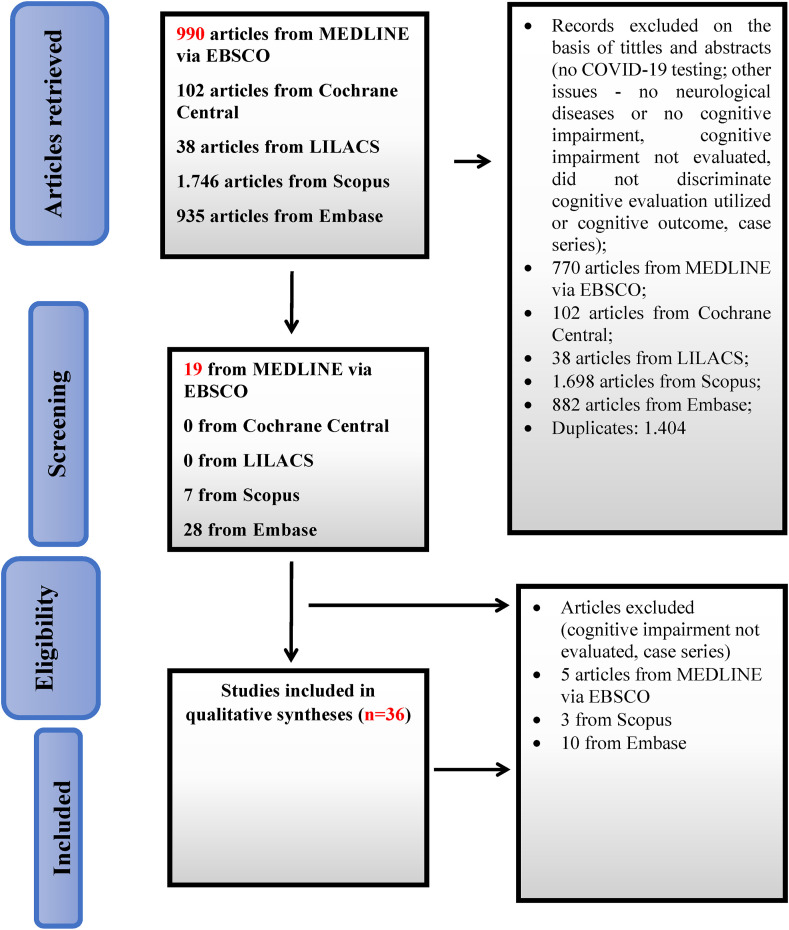
Fig. 1 shows the flowchart of the study selection process according to the PRISMA guidelines. A total of 3,520 articles were retrieved and read. Twenty-two studies were selected for our review. Sixteen studies included patients in COVID-19 acute and subacute phases and 6 involved only patients after 12 weeks of onset.
Fig. 1.
Flowchart of the study selection process.
Table 1 describes the studies assessed. Sample sizes ranged from 14 to 195 participants. The studies were conducted in 10 countries, and most of them were from Italy (27.7%) and Germany (18.8%). Median age ranged from 36.2 years (SD = 11.7) (Amalakanti et al., 2021) to 67.23 years (SD = 12.89) (Alemanno et al., 2021). Regarding the type of studies found, 63.6% were cohort, 31.9% were cross-sectional, and 4.5% were case–control.
Table 1.
Articles with cognitive assessment before or at 12 weeks of COVID-19 infection.
| Authors, Year | Country | Study Design | Primary Study Objective | Sample type and evaluation date | Sample Size | Participant Age | Cognitive Assessment Tool used | Main Results |
|---|---|---|---|---|---|---|---|---|
| Jaywant et al. (2021) | United States | Cross-sectional | To assess frequency, severity and profile of cognitive dysfunction | Hospitalized patients; 43.2 (SD = 19.2) days after initial admission. | 57 patients | Mean age (SD) 64.5 years (13.9) | Brief Memory and Executive Test (BMET) | 81% had cognitive impairment, ranging from mild to severe. Deficits in working memory (55%), set-shifting (47%), divided attention (46%), and processing speed (40%). |
| Puchner et al. (2021) | Austria | Observational cohort study | To explore the dysfunctions and outcome of COVID-19 survivors after early post-acute rehabilitation | Discharged critical or severe COVID-19 individuals (mean in-hospital stay of 30 days) | 14 patients | 57 (SD ± 10) years∗ | Logical Memory I & II of Wechsler Memory Scale-IV (WMSIV), subtest of the verbal and visual memory test (VVM) and test of attentional performance (TAP) | 29% with cognitive deficits of concentration, memory and/or executive functions |
| Darley et al. (2021) | Australia | Prospective cohort study | To determine the prevalence of persistent symptoms, lung function, quality of life, neurocognitive and olfactory abnormalities during the recovery period | 69 days after diagnosis; IQR, 64–83 days. Discharged patients | 78 patients | 47 years (standard deviation, 16 years) | CogState Cognitive Test Battery | Cognitive impairment no dementia in 8 patients (10.25%) |
| Alemanno et al. (2021) | Italy | Prospective cohort study | To investigate COVID-19 impact on cognitive functions in disease sub-acute phase | Five to twenty days after symptoms onset discharged | 87 patients | Mean age 67.23 ± 12.89 years | MoCA, MMSE | 80% with cognitive impairment |
| Blazhenets et al. (2021) | Germany | Prospective cohort study | To assess 18 F-FDG PET and MoCA performance in eight selected patients presenting for a follow-up in the chronic stage | 37 ± 19 days after COVID-19 symptom onset; discharged | 31 patients | 66.00 (14.23) [39–89] | MoCA | 8 patients with cognitive impairment and FDG-PET alteration detected and included |
| Monti et al. (2021) | Italy | Prospective cohort study | To assess the quality of life of invasively ventilated COVID-19 ARDS survivors. |
By phone by a trained investigator after a median of 61 (51–71) days from ICU discharge. | 39 patients | 56 ± 10.5 years | MMSE telephone version | 1 (2.6%) with cognitive impairment |
| Zhou et al. (2020) | China | Cross-sectional | To evaluate the impacts of COVID-19 on cognitive functions in recovered patients and its relationship with inflammatory profiles | Did not mention evaluation time after Covid; recovered patients | 29 patients and 29 controls | Patients (47.00 ± 10.54 years) and controls (42.48 ± 6.94 years) | iPad-based online neuropsychological tests, including the Trail Making Test (TMT), Sign Coding Test (SCT), Continuous Performance Test (CPT), and Digital Span Test (DST) |
CPT - COVID-19 patients had a lower correct number CPT 2 and CPT 3 compared with the controls (9.83 ± 1.93 vs 8.21 ± 1.90). |
| Hellmuth et al. (2020) | United States | Prospective cohort study | To present early findings on reported cognitive symptoms in an observational cohort of SARS-CoV-2 in recovery. | At least 14 days from symptom onset, Recovered patients; Outpatient and previous hospitalized patients | 100 patients | 41 years (IQR range: 36–55) ∗∗ | Questionnaire about deterioration or new concentration, memory, or thinking complaint | 20 reported cognitive complaints |
| Hosp et al. (2021) | Germany | Prospective cohort study | To comprehensively characterize the neurological sequelae of COVID-19 in the subsample of patients affected severely enough to require inpatient treatment. | <30 days symptom onset; Hospitalized patients | 29 patients, with 26 completed MoCA and 15 extensive neuropsychological testing | 65.2 (14.4) ∗ | MoCA (26); Extensive neuropsychological testing (Hopkins verbal learning revised (HVLT-R), Trail Making Test, Stroop test, Digit span and Fluency) (15) |
MoCA test, 14 (54%) were mild to moderately impaired (MoCA 18–25) and four (15%) were severely impaired (MoCA 10–17). |
| Almeria et al. (2020) | Spain | Prospective cohort study | To evaluate the impact of COVID-19 on neurocognitive performance. | 10 and 35 days from hospital discharge | 39 patients | 47.6 (8.9) | Test de Aprendizaje Verbal España-Complutense (TAVEC) with three lists for the Learning, Interference and Recognition to assess verbal memory; Visual Reproduction of the Wechsler Memory Scale –IV (WMSIV), Digits forward and Backward, Letter and Numbers, Trail Making Test A and B (TMT), Symbol Digit Modalities Test (SDMT), Stroop, Phonemic and Semantic fluency and Boston Naming | Pathological scores (PT _ 30) were seen in TAVEC-1 (2 [5.7%]), TAVEC-5 (2 [5.7%]), TAVECTotal (1 [2.9%]), TAVEC-B (2 [5.7%]), TAVEC-IMR (1 [2.9%]), TAVEC-IMRSC (2 [5.7%]), TAVEC-DFR (2[5.7%]), TAVEC-DFRSC (3 [8.6%]), TAVEC-REC (2 [5.7%]), Inverse Digits (3 [8.6%]), TMT-A (1 [2.9%]), TMT-B (3 [8.6%]), SDMT (2[5.7%]), Stroop Color (1 [2.9%]), Stroop Interference (1 [2.9%]), Semantic Fluency (2 [5.7%]), Phonemic Fluency (4 [11.4%]), FCRO copy (1 [2.9%]), BNT (1 [2.9%]). |
| Versace et al. (2021) | Italy | Cross-sectional | To explore, with TMS, the activity of the main inhibitory intracortical circuits within the primary motor cortex (M1) in a sample of patients complaining of fatigue and presenting executive dysfunction after resolution of COVID-19 | Discharged patients, 9–13 weeks from disease onset | 12 patients; 10 controls. | 67 ± 9.6 patients | FAB | Diminished executive functions, as documented by abnormal scores corrected for age and education on the FAB (12.2 ± .7) |
| Ortelli et al. (2021) | Italy | Cross-sectional | To provide a comprehensive clinical, neurophysiological, and neuropsychological profile of fatigued patients suffering from neurological manifestations related to SARSCoV-2, who recovered from the acute phase of COVID-19. | Discharged patients, 9–13 weeks from disease onset | 12 patients, 12 controls | 67 ± 9.6 patients 64.3 ± 10.5 controls |
MoCA, FAB, computerized attentive tasks: Vigilance Task (VT), Stroop Interference Task (SIT), Navon Task (NT) | Patients X Controls; Montreal Cognitive Assessment (MoCA) 17.8 (5.3) X 26.8 (3.1); Frontal Assessment Battery (FAB) 12.3 (2.3) X 16.7 (1.2); SD in brackets |
| Woo et al. (2020) | Germany | Cross-sectional | To detect cognitive deficits in 18 young patients without diagnosed cognitive pre-conditions after recovery from COVID-19 and discovered widespread sub-clinical deficits. | 11 discharged non-ICU patients (61%), 6 outpatients (33%) and 1 patient did not seek medical care (6%); 20–105 days from disease onset | 18 patients; 10 controls | Patients - mean, 42.2 years; SD, 14.3 years; Controls - mean, 38.4 years; SD, 14.4 years. |
Modified Telephone Interview for Cognitive Status (TICS-M) | Post-COVID-19 patients scored significantly lower results in the TICS-M (mean, 38.83; range, 31–46) compared to healthy controls (mean, 45.8; range, 43–50) (Fig. 1A), especially regarding short-term memory, attention and concentration/language tasks |
| Méndez et al. (2021) | Spain | Cross-sectional | To evaluate neurocognitive function, psychiatric symptoms and QoL in COVID-19 survivors shortly after hospital discharge. | Discharged patients by telephone 1–3 months from onset | 179 patients | 57 [49; 67] (Median [1st, 3rd quartile]) | Verbal learning – immediate, and delayed memory subtests from the Subtest Screen for Cognitive Impairment in Psychiatry (SCIP), animal naming test (ANT) from the Controlled Oral Word Association Test (COWAT) for semantic verbal fluency and the subtest Digit Span backward from the Wechsler Adult Intelligence Scale, Third Edition (WAIS-III) for working memory | Immediate verbal memory - 38% moderate and 11.2% severe Impairment; Delayed memory - 11.8% moderate and 2.8% severe; Semantic verbal fluency - 34.6% moderate and 8.4% severe; Working memory - 6.1% moderate and 1.1% severe; Overall, 58.7% moderate and 18.4% severe neurocognitive impairment. |
| Ermis et al. (2021) | Germany | Prospective cohort | To investigate the spectrum of symptoms | Hospitalized in-house patients. | 53 total patients; 13 patients with cognitive evaluation | Median age 63 years IQR 54–73 years) ∗ | MoCA | cognitive impairment (61.5%); deficits primarily in executive function, attention, language and delayed recall |
| De Lorenzo et al. (2020) | Italy | Retrospective and prospective cohort | To investigate whether COVID-19 leaves behind residual dysfunction, and identify patients who might benefit from post-discharge monitoring. | 31.9% Discharged from ED 68.1% had been hospitalized Patients were assessed after a median IQR] time from hospital discharge of 23 [20–29] days | 185 patients | 57 (48; 67) | MoCA | 47 (25.4%) cognitive impairment |
| Soldati et al. (2021) | Brazil | Prospective cohort study | To evaluate TICS’ utility to screen cognitive dysfunction in severe COVID patients. | Discharged patients; 43–136 days after from discharge. | 23 patients | Mean age 53.6 ± 11.7 years. | TICS | MCI was detected in 13% patients. |
| Leth et al. (2021) | Denmark | Prospective cohort study | To inform the duration of symptoms after the initial phase of COVID-19, including hospitalized and nonhospitalized patients. | Discharged patients <12 weeks after discharge. | 49 patients | Median age (IQR) 58 years (48–73) | Orientation, memory, and concentration (OMC) test | Impaired OMC test at 6 weeks 8/38 (21%); at 12 weeks 4/38 (11%) |
| Mazza et al. (2021) | Italy | Prospective cohort study | To study psychopathological and neurocognitive impact of COVID-19 in survivors three-month after clinical recovery. | Discharged patietns in an ambulatory evaluation 3 months after discharge. | 130 patients were cognitive evaluated. | mean age 58.5 ± 12.8, age range from 26 to 87 years∗ total sample | Brief Assessment of Cognition in Schizophrenia (BACS) | 78% of the sample showed poor performances in at least one cognitive domain, with executive functions and psychomotor coordination being impaired in 50% and 57% of the sample. |
| van den Borst et al. (2021) | Netherlands | Prospective cohort study | To comprehensively assess health domains in patients from acute COVID-19. | Discharged and non hospitalized patients 3 months after recovery. | 124 patients (97 discharged patients). | Age, mean (SD), years 59 (14) |
Telephone Interview of Cognitive Status (TICS), Cognitive Failure Questionnaire (CFQ) | Problems in mental and/or cognitive function were found in 36% of patients. |
Animal naming test (ANT) from the Controlled Oral Word Association Test (COWAT), Brief Memory and Executive Test (BMET), Brief Repeatable Battery of Neuropsychological Tests (BRB-NT), Cognitive Failures Questionnaire (CFQ), Continuous Performance Test (CPT), Digital Span Test (DST), Frontal Assessment Battery (FAB), Hopkins verbal learning revised (HVLT-R), Logical Memory I & II of Wechsler Memory Scale-IV (WMSIV), Mini-Mental State Examination (MMSE), Modified Telephone Interview for Cognitive Status (TICS-M), Montreal Cognitive Assessment (MoCA), Navon Task (NT), Screen for Cognitive Impairment in Psychiatry Danish Version (SCIP-D), Sign Coding Test (SCT), Stroop Interference Task (SIT), Subtest of the verbal and visual memory test (VVM), Subtest Screen for Cognitive Impairment in Psychiatry (SCIP), Symbol Digit Modalities Test (SDMT), Test de Aprendizaje Verbal España-Complutense (TAVEC), Test of attentional performance (TAP), Trail Making Test (TMT), Vigilance Task (VT), Visual Reproduction of the Wechsler Memory Scale–IV (WMSIV), Wechsler Adult Intelligence Scale Third Edition (WAIS-III), Orientation, memory, and concentration (OMC) test, Brief Assessment of Cognition in Schizophrenia (BACS).
∗ Mean age of the total sample (does not discriminate from those who only performed the cognitive assessment).
∗∗ Only refers to the mean age of those who reported cognitive symptoms.
Different types of samples were found. Most involved hospitalized or discharged patients (81.8%), while the others evaluated only outpatients never hospitalized due to COVID-19 (18.1%).
Not all studies cited the frequency of cognitive impairment. In the studies cited in Table 1, cognitive impairment varied from 2.6% (Monti et al., 2021) to 81% (Jaywant et al., 2021). In studies after 12 weeks, cognitive impairment varied from 21% (Del Brutto et al., 2021) to 65% (Ferrucci et al., 2021; Miskowiak et al., 2021).
A wide range of cognitive assessment tools (n = 25) were used. Of note, the Montreal Cognitive Assessment (MoCA) was the most frequently used (50.0%). In addition, the frontal assessment battery and the mini mental state examination (MMSE) were used in only 2 studies each. In the case of the MMSE, in one of the studies a telephone version was used. Another battery used by telephone in a study was the Modified Telephone Interview for Cognitive Status (TICS-M). Studies that detailed education showed participants with high education, with only one including patients with less than eight study years (Woo et al., 2020). Importantly, detailed information about patients’ education was not available in more 40.9% of studies.
Thirteen of the 22 selected studies used tests that listed the most affected cognitive domains and described them. The affected domains most described in these studies were executive functions, attention, and episodic memory, described respectively in 9, 7, and 7 studies. All 13 studies showed a pattern of cognitive impairment in processing speed, inattention, or executive dysfunction assessed through working memory (Table 3 ). However, data from selected studies could not differentiate whether the cognitive impairment found corresponded to mild cognitive impairment or dementia. The studies have not evaluated the loss in daily life activities. Table 4 shows the studies that cite the average scores found for the MMSE, MoCA and FAB.
Table 3.
Articles with detailed impaired cognitive domains.
| Authors, Year | Cognitive Assessment Tool used | Impaired cognitive domains | Sample type |
|---|---|---|---|
| Jaywant et al. (2021) | Brief Memory and Executive Test (BMET) | Working memory [55%], set-shifting (21/44 [47%]), divided attention (18/39 [46%]) | Inpatients |
| Puchner et al. (2021) | Logical Memory I & II of Wechsler Memory Scale-IV (WMSIV), subtest of the verbal and visual memory test (VVM), and test of attentional performance (TAP) | Processing speed: psychomotor speed was the most frequent impairment | Discharged patients |
| Darley et al. (2021) | CogState Cognitive Test Battery | Cognitive of concentration, memory, or executive function deficits were found. | 64–83 days after discharge |
| Graham et al. (2021) | NIH Toolbox | Attention (median Tscore 41.5 [37, 48.25]; p < .001 vs US median of 50) and working memory | Ambulatory 4 months after SARS-CoV2 + |
| Zhou et al. (2020) | iPad-based online neuropsychological tests, including the Trail Making Test (TMT), Sign Coding Test (SCT), Continuous Performance Test (CPT), and Digital Span Test (DST) | Attention function changes | – |
| Hosp et al. (2021) | MoCA (26); Extensive neuropsychological testing (Hopkins' verbal learning revised (HVLT-R), Trail Making Test, Stroop test, Digit span and Fluency) (15) | The Word list learning on the Hopkins Verbal Learning Test–Revised (representing the cognitive domain memory) was affected most frequently (7/14) as were executive functions [digit span reverse (6/15); categorical fluency (6/13)]. Tests for attention were less frequently impaired | Inpatients |
| Miskowiak et al. (2021) | Screen for Cognitive Impairment in Psychiatry Danish Version (SCIP-D), Trail Making Test- Part B (TMT-B), Cognitive Failures Questionnaire (CFQ) | Comparing patients with the matched HC group; Patients displayed moderate impairments in verbal learning and working memory Patients' delayed memory performance was unimpaired, whereas there was only a non-significant trend toward verbal fluency and psychomotor speed impairments in patients compared with HC (VFT: p = .08; PMT: p = .09). | 3–4 months after discharge |
| Almeria et al. (2020) | Test de Aprendizaje Verbal España-Complutense (TAVEC) with three lists for the Learning, Interference and Recognition to assess verbal memory; Visual Reproduction of the Wechsler Memory Scale –IV (WMSIV), Digits forward and Backward, Letter and Numbers, Trail Making Test A and B (TMT), Symbol Digit Modalities Test (SDMT), Stroop, Phonemic and Semantic fluency and Boston Naming | Attention, memory and executive function domains; T score lower than 30 was observed in memory domains, attention and semantic fluency (2 [5.7%]) in working memory and mental flexibility (3 [8.6%]) and in phonetic fluency (4 [11.4%]). | 10–35 days after discharge |
| Versace et al. (2021) | FAB | Executive functions | 2–3 months after discharge |
| Ortelli et al. (2021) | MoCA, FAB, computerized attentive tasks: Vigilance Task (VT), Stroop Interference Task (SIT), Navon Task (NT) | Executive dysfunction | 2–3 months after discharge |
| Woo et al. (2020) | Modified Telephone Interview for Cognitive Status (TICS-M) | Short-term memory, attention and concentration/language tasks | 20–100 days from Covid-19 |
| Méndez et al. (2021) | Verbal learning – immediate, and delayed memory subtests from the Subtest Screen for Cognitive Impairment in Psychiatry (SCIP), animal naming test (ANT) from the Controlled Oral Word Association Test (COWAT) for semantic verbal fluency and the subtest Digit Span backward from the Wechsler Adult Intelligence Scale, Third Edition (WAIS-III) for working memory | Amongst survivors, the prevalence of moderately impaired immediate verbal memory and learning was 38%, delayed verbal memory (11.8%), verbal fluency (34.6%) and working memory (executive function) (6.1%), respectively. | Discharged patients by telephone 1–3 months from onset |
| Ferrucci et al. (2021) | Brief Repeatable Battery of Neuropsychological Tests (BRB-NT) | Of all patients, 42.1% had processing speed deficits, while 26.3% showed delayed verbal recall deficits | Neuropsychological assessment between 4 and 5 months (mean _ SD = 4.43 _ 1.22 months) after hospital discharge. |
| Crivelli et al. (2022) | MoCA, Trail Making A, Digit Span Forwards, Digit-Symbol Coding, Craft Story, Rey Auditory Verbal Learning Test, Delayed Recall from the Benson Figure Test, Trail Making B, Wisconsin Card Sorting Test, Stroop Test, phonological fluency. Benson Figure and Clock Drawing Test, Multilingual Naming Test and semantic fluency | Memory (p = .016, Cohen's d = .73), attention (p < .001, Cohen's d = 1.2), executive functions (p < .001, Cohen's d = 1.4), and language (p = .002, Cohen's d = .87). | Outpatients 142 days from disease onset |
| Becker et al. (2021) | Number Span forward and backward, Trail Making Test Parts A and B, phonemic and category fluency and the Hopkins Verbal Learning Test–Revised | Hospitalized patients more impairments in attention (odds ratio [OR]: 2.8; 95%CI: 1.3–5.9), executive functioning (OR: 1.8; 95%CI: 1.0–3.4), category fluency (OR: 3.0; 95%CI: 1.7–5.2), memory encoding (OR: 2.3; 95%CI: 1.3–4.1) and memory recall (OR: 2.2; 95%CI: 1.3–3.8) than outpatient group. ED Patients more impaired category fluency (OR: 1.8; 95%CI: 1.1–3.1) and memory encoding (OR: 1.7; 95% CI: 1.0–3.0) than outpatients. | Ambulatory or discharged patients 7.6 months from disease onset |
| Albu et al. (2021) | Barcelona Test which is based on the Benton Temporal Orientation Test, Digit Span forward, Rey Auditory Verbal Learning Test (RAVLT), Digit Span backward, PMR task (a Spanish version of the FAS letter fluency task) |
Low scores on orientation [X2 (1) = .97, p = .33], attention [X2 (1) = 1.01, p = .32], verbal learning [X2 (1) = 1.77, p = .18], long-term verbal memory [X2 (1) = .28, p = .60], verbal recognition [X2 (1) = 1.44, p = .23], working memory [X2 (1) = 1.45, p = .50] or executive control [X2 (1) = .18, p = .89]. | Discharged patients 89–124 days from onset. |
| García-Sánchez et al. (2022) | MoCA, CPT-II, RAVLT, ROCFT, Digit Span Forward and Backward, BNT, Block Design, Coding, Symbol Search, TMT, Stroop, verbal fluency tasks, and the 15-Objects Test | Attention deficits were the most frequent types of deficits in patients with single-domain impairment (19.0%), significantly exceeding deficits in EF (p = .01), ST/WM (p = .001), and Language (p < .001). Furthermore, attention was the cognitive domain that was most frequently impaired in conjunction with other domains in patients with multiple-domain impairment, especially with Learning and Long-Term Memory and Executive Functioning. | Discharged and outpatients, 187 days after diagnosis. |
| Vannorsdall et al. (2022) | Rey Auditory Verbal Learning Test (RAVLT) Oral Trail Making Test parts A and B, digit span forward and backward, Phonemic and semantic verbal fluency, |
Non-ICU patients -> Mild/moderate impairment was particularly common on Oral Trail Making Test part A, category-cued verbal fluency, RAVLT acquisition, and RAVLT delayed recall. Post-ICU patients -> elevated rates of impairment were observed across all domains with the exception of number span forward. More than onethird of post-ICU patients performed in the mild/moderately impaired range on the Oral Trail Making Test part A, letter- and category-cued verbal fluency, RAVLT acquisition, and RAVLT delayed recall. |
Discharged patients four months after an initial diagnosis of COVID-19 by telephone interview. |
Animal naming test (ANT) from the Controlled Oral Word Association Test (COWAT), Brief Memory and Executive Test (BMET), Brief Repeatable Battery of Neuropsychological Tests (BRB-NT), Cognitive Failures Questionnaire (CFQ), Continuous Performance Test (CPT), Digital Span Test (DST), Frontal Assessment Battery (FAB), Hopkins verbal learning revised (HVLT-R), Logical Memory I & II of Wechsler Memory Scale-IV (WMSIV), Modified Telephone Interview for Cognitive Status (TICS-M), Montreal Cognitive Assessment (MoCA), Navon Task (NT), Screen for Cognitive Impairment in Psychiatry Danish Version (SCIP-D), Sign Coding Test (SCT), Stroop Interference Task (SIT), Subtest of the verbal and visual memory test (VVM), Subtest Screen for Cognitive Impairment in Psychiatry (SCIP), Symbol Digit Modalities Test (SDMT), Test de Aprendizaje Verbal España-Complutense (TAVEC), Test of attentional performance (TAP), Trail Making Test (TMT), Vigilance Task (VT), Visual Reproduction of the Wechsler Memory Scale–IV (WMSIV), Wechsler Adult Intelligence Scale Third Edition (WAIS-III), Continuous Performance Test (CPT-II), Rey Auditory Verbal Learning Test (RAVLT), Rey–Osterrieth Complex Figure Test (ROCFT), Boston Naming Test (BNT), Trail Making Test (TMT).
Table 4.
MMSE/MoCA and FAB mean scores.
| Authors, Year | Cognitive Assessment Tool used | MMSE/MoCA values | FAB values |
|---|---|---|---|
| Alemanno et al. (2021) | MoCA, MMSE | MoCA: group 1 (21.65 ± 5.23), group 2 (16.83 ± 7.11), group 3 (15.90 ± 6.97), group 4 (19.11 ± 6.83); MMSE: group 1 (26.77 ± 2.77), group 2 (22.78 ± 5.80), group 3 (22.24 ± 6.23), group 4 (22.89 ± 6.97) | |
| Blazhenets et al. (2021) | MoCA | MoCA (± standard deviation) global score of 19.1 ± 4.5 at the subacute stage; 23.4 ± 3.6 at the post acute stage | |
| Monti et al. (2021) | MMSE telephone version | Italian telephone Mini Mental State Examination (I-tel MMSE), median (IQR) 22 (21–22) | |
| Hosp et al. (2021) | MoCA; Extensive neuropsychological testing (Hopkins verbal learning revised (HVLT-R), Trail Making Test, Stroop test, Digit span and Fluency), | MoCA global score <26 = 18 patients (69%) mean score (SD) 19.11 (4.14), MoCA global score 18–25 = 14 patients (54%) mean score (SD) 20.93 (2.05), MoCA global score 10–17 = 4 patients (15%) mean score (SD) 12.75 (2.49), MoCA global score ≥ 26 = 8 patients (31%) mean score (SD) 27.75 (1.16) | |
| Versace et al. (2021) | FAB | FAB (12.2 ± .7) | |
| Ortelli et al. (2021) | MoCA, FAB, computerized attentive tasks: Vigilance Task (VT), Stroop Interference Task (SIT), Navon Task (NT) | Mean MoCA scores -> patients - 17.8 (5.3); Controls - 26.8 (3.1). |
Mean FAB scores -> patients - 12.3 (2.3); controls - 16.7 (1.2). |
| Ermis et al. (2021) | MoCA | Mean MoCA scores (SD) 23 (5.02) | |
| Del Brutto et al. (2021) | MoCA | Mean (±SD) score in the MoCA performed 6 months after the start of the SARS-CoV-2 pandemic in the village was 20.2 ± 4.2 points. | |
| Amalakanti et al. (2021) | MoCA | Age 18–29 -> Mean MoCA Score in cases 25.9 ± 2.1 × Mean MoCA score in controls 27 ± 1.7; Age 30–49 -> Mean MoCA Score in cases 25.9 ± 2.3 × Mean MoCA score in controls 25.6 ± 4.3; Age 50 and above -> Mean MoCA Score in cases 24 ± 3.5 × Mean MoCA score in controls 24.5 ± 3.5 |
|
| Rass et al. (2021) | MoCA | All patients MoCA <26–29 patients, Mean scores (SD) MoCA 28 (26–29); Severe disease requiring ICU MoCA <26 - 8 patients, Mean scores (SD) MoCA 28 (25–28); Moderate severity, hospitalization, non-ICU MoCA <26–20 patients, Mean scores (SD) 28 (25–29); Mild severity, outpatient, MoCA <26–1 patient, Mean scores (SD) 29 (28–30) |
|
| Crivelli et al. (2022) | MoCA, Trail Making A, Digit Span Forwards, Digit-Symbol Coding, Craft Story, Rey Auditory Verbal Learning Test, Delayed Recall from the Benson Figure Test, Trail Making B, Wisconsin Card Sorting Test, Stroop Test, phonological fluency. Benson Figure and Clock Drawing Test, Multilingual Naming Test and semantic fluency | Mean MoCA scores -> Controls - 27.22 (1.99); Patients - 26.49 (2.90). |
|
| Frontera et al. (2021) | Telephone MoCA | 101 cases – median MoCA 17 (13–19) 114 controls - median MoCA 18 (15–19) |
|
| García-Sánchez et al. (2022) | MoCA, CPT-II, RAVLT, ROCFT, Digit Span Forward and Backward, BNT, Block Design, Coding, Symbol Search, TMT, Stroop, verbal fluency tasks, and the 15-Objects Test | Hospitalized patients had lower MoCA scores (M = 15.8; SD = 3.8) than non-hospitalized ones (M = 17.8; SD = 2.5). | |
| Walle-Hansen et al. (2021) | MoCA | MoCA total score g < 75 years 25.3 (3.8), ≥ 75 years 21.7 (5.8) |
Animal naming test (ANT) from the Controlled Oral Word Association Test (COWAT), Brief Memory and Executive Test (BMET), Brief Repeatable Battery of Neuropsychological Tests (BRB-NT), Cognitive Failures Questionnaire (CFQ), Continuous Performance Test (CPT), Digital Span Test (DST), Frontal Assessment Battery (FAB), Hopkins verbal learning revised (HVLT-R), Logical Memory I & II of Wechsler Memory Scale-IV (WMSIV), Modified Telephone Interview for Cognitive Status (TICS-M), Montreal Cognitive Assessment (MoCA), Navon Task (NT), Screen for Cognitive Impairment in Psychiatry Danish Version (SCIP-D), Sign Coding Test (SCT), Stroop Interference Task (SIT), Subtest of the verbal and visual memory test (VVM), Subtest Screen for Cognitive Impairment in Psychiatry (SCIP), Symbol Digit Modalities Test (SDMT), Test de Aprendizaje Verbal España-Complutense (TAVEC), Test of attentional performance (TAP), Trail Making Test (TMT), Vigilance Task (VT), Visual Reproduction of the Wechsler Memory Scale–IV (WMSIV), Wechsler Adult Intelligence Scale Third Edition (WAIS-III).
Regarding the studies that evaluated patients in the acute/subacute phases (Table 1), all of them evaluated patients who were still or previously hospitalized. In comparison, in the studies that evaluated patients only after 12 weeks (Table 2 ), 50% included only outpatients subjects. Also, regarding sample differences, 75% of the studies in Table 1 included patients who had been in the ICU at some point, whereas only 16.6% of the studies in Table 2 had patients admitted to the ICU at some time. Furthermore, regarding the main objectives, 50% of the studies in Table 1 had as the main objective to assess cognitive impairment due to the disease, while 66.6% of the studies in Table 2 aimed to assess cognitive impairment after 12 weeks of illness.
Table 2.
Articles with cognitive assessment after 12 weeks of COVID-19 infection.
| Authors, Year | Country | Study Design | Primary Study Objective | Sample type and evaluation date | Sample Size | Participant Age | Cognitive Assessment Tool used | Main Results |
|---|---|---|---|---|---|---|---|---|
| Del Brutto et al. (2021) | Ecuador | Prospective cohort study | To assess cognitive decline 6 months after mild Covid-19. | Outpatients 6 months from disease onset | 52 patients; 41 controls. | Mean age of participants was 62.6 ± 11 years | MoCA (compare pre-pandemic with post -pandemic MoCA decay (≥4 points) | Cognitive decline in 21% patients and in 2% controls |
| Graham et al. (2021) | United States | Prospective cohort study | To characterize the spectrum of neurologic manifestations in non-hospitalized Covid-19 “long haulers”. | On average at 4.72 months after symptom onset in the SARS-CoV-2+ group compared to 5.82 months in the SARS-CoV-2 group | 100 ambulatory patients (50 + e 50-); 36 with cognitive evaluation | 43.2 (SD-11.3) years∗ | NIH Toolbox | SARS-CoV-2 patients had significantly worse attention (median Tscore 41.5) and working memory (median T-score 43); |
| Miskowiak et al. (2021) | Denmark | Prospective cohort study | To investigate frequency, pattern and severity of cognitive impairments 3–4 months after COVID-19 hospital discharge, their relation to subjective cognitive complaints, quality of life and illness Variables |
Discharged patients 3–4 months after discharge | 29 patients | 56.2 (10.6) | Screen for Cognitive Impairment in Psychiatry Danish Version (SCIP-D), Trail Making Test- Part B (TMT-B), Cognitive Failures Questionnaire (CFQ) | Using SCIP total scores ≥.5 SD as cut-off, a total of n = 19 (65%) of patients was classified as cognitively impaired. |
| Amalakanti et al. (2021) | India | Case control study | To detect MCI in asymptomatic COVID-19 subjects with MoCA | Outpatients; | 93 asymptomatic patients; 102 controls. | Patients was 36.2 ± 11.7 and that of the controls was 35.6 ± 9.8 | MoCA | COVID-19 patients secured lower scores than controls in the domains of visuoperception, naming and fluency Fluency .9 ± .6 × 1.6 ± .7 Visuoperception 2.4 ± .7 × 2.8 ± .7 Naming 3.6 ± .5 × 3.9 ± 0.2 |
| Ferrucci et al. (2021) | Italy | Cross-sectional | To study the occurrence of cognitive abnormalities in the months following hospital discharge. | Neuropsychological assessment between 4 and 5 months (Mean _ SD = 4.43 _ 1.22 months) after hospital discharge. | 38 patients | 53.45 (12.64) | Brief Repeatable Battery of Neuropsychological Tests (BRB-NT) |
Cognitive impairment in 60.5% (had obtained scores below cutoffs in at least one task of the BRB-NT) 42.1% had processing speed deficits, while 26.3% showed delayed verbal recall deficits. 10.5% showed deficits in immediate verbal recall |
| Rass et al. (2021) | Austria | Prospective cohort | To assess neurological manifestations and health-related Quality of life (QoL) 3 months after COVID-19. | Discharged patients 102 (interquartile range [IQR], 91–110) days after disease onset. | 135 patients | Median age was 56 (IQR, 48–68) | MoCA | Cognitive impairments (MoCA) were found in 23% of patients (in severe COVID-19 patients 29%, moderate 30%, mild 3%). |
| Crivelli et al. (2022) | Argentina | Prospective cohort study | To describe the cognitive profile of a cohort of COVID-19 survivors that attended a neurological clinic | Outpatients 142 days from disease onset | 45 patients; 45 controls | Mean age of participants was 50 (43–63) years | MoCA, Trail Making A, Digit Span Forwards, Digit-Symbol Coding, Craft Story, Rey Auditory Verbal Learning Test, Delayed Recall from the Benson Figure Test, Trail Making B, Wisconsin Card Sorting Test, Stroop Test, phonological fluency. Benson Figure and Clock Drawing Test, Multilingual Naming Test and semantic fluency | No significant differences were found in the screening measures (MoCA p = .15). Significant differences between groups were found in cognitive composites of memory (p = .016, Cohen's d = .73), attention (p < .001, Cohen's d = 1.2), executive functions (p < .001, Cohen's d = 1.4), and language (p = .002, Cohen's d = .87). |
| Becker et al. (2021) | United States | Cross-sectional | To investigate rates of cognitive impairment in survivors of COVID-19 who were treated in outpatient, emergency department (ED), or inpatient hospital settings. | Ambulatory or discharged patients 7.6 months from disease onset | Total = 740; Outpatients = 379, Emergency department = 165, Hospital = 196. | Mean age 49.0 (14.2) years | Number Span forward and backward, Trail Making Test Parts A and B, phonemic and category fluency and the Hopkins Verbal Learning Test–Revised | Hospitalized patients more impairments in attention (odds ratio [OR]: 2.8; 95%CI: 1.3–5.9), executive functioning (OR: 1.8; 95%CI: 1.0–3.4), category fluency (OR: 3.0; 95%CI: 1.7–5.2), memory encoding (OR: 2.3; 95%CI: 1.3–4.1) and memory recall (OR: 2.2; 95%CI: 1.3–3.8) than outpatient group. ED Patients more impaired category fluency (OR: 1.8; 95%CI: 1.1–3.1) and memory encoding (OR: 1.7; 95% CI: 1.0–3.0) than outpatients. |
| Pilotto et al. (2021) | Italy | Prospective cohort study | To evaluate general and neurological manifestations after 6 months of follow-up and their potential relationship with premorbid conditions and severity of respiratory infection. | Discharged patients 6 months from discharge. | 105 were evaluated using a standard neurological examination and cognitive screening. | 64.8 ± 12.6 years | MoCA | Cognitive deficits in 17.5%. |
| Albu et al. (2021) | Spain | Cross-sectional | To characterize persistent symptoms, physical, neurological and respiratory sequelae and their impact on daily life activities and quality of life in post COVID-19 patients. | Discharged patients 89–124 days from onset. | 30 patients total; 16 post ICU patients e 14 non-ICU patients. | 54 (43.8–64.75) years | Barcelona Test which is based on the Benton Temporal Orientation Test, Digit Span forward, Rey Auditory Verbal Learning Test (RAVLT), Digit Span backward, PMR task (a Spanish version of the FAS letter fluency task) |
Cognitive impairment was found in 63.3% of patients, with a similar profile in both sub-groups. |
| Gautam et al. (2022) | United Kingdom | Retrospectivecase series | To assess the medium-term effects of coronavirus disease 2019 (COVID-19) on survivors of severe disease. | Discharged patients 4–7 months after disease onset. | 200 patients | Mean age (SD) 56.5 years (13.2) | MoCA | In 12.5% of patients, some cognitive impairment was noted, mainly in concentration and short-term recall. |
| Frontera et al. (2021) | United States | Prospective cohort study | To compare global functional outcomes between COVID-19 hospital survivors with and without neurological complications using an ordinal analysis of the modified Rankins Scale (mRS). | Discharged patients 6-month from infection. Assessments were conducted by telephone interview among case and control hospital survivors. | 196 cases and 186 controls | Median Age (IQR)-years Cases 68 (55–77); Controls 69 (57–78). | Telephone MoCA | (50%) had impaired cognition (telephone MOCA<18) |
| García-Sánchez et al. (2022) | Spain | Prospective cohort study | To analyze the frequency of deficits for specific cognitive domains and to discern the frequency of single and multiple-domain impairments and to understand which combinations of deficits were a specific feature of post-COVID-19 cognitive impairment. | Discharged and outpatients, 187 days after diagnosis. | 63 patients (33 previous hospitalized). | Mean age of 51.1 years (SD = 12.5; range: 22–78) | MoCA, CPT-II, RAVLT, ROCFT, Digit Span Forward and Backward, BNT, Block Design, Coding, Symbol Search, TMT, Stroop, verbal fluency tasks, and the 15-Objects Test |
Multiple-domain impairment (60.3%) was more frequent than impairment in only one domain (39.7%) (p = .02). Attention deficits were the most frequent types of deficits in patients with single-domain impairment (19.0%), significantly exceeding deficits in EF (p = .01), ST/WM (p = .001), and Language (p < .001). Furthermore, attention was the cognitive domain that was most frequently impaired in conjunction with other domains in patients with multiple-domain impairment, especially with Learning and Long-Term Memory and Executive Functioning. |
| Valdes et al. (2022) | United States | Prospective cohort study | To investigated the relationship between demographics, social determinants of health and cognitive outcomes 6-months after hospitalization for COVID-19. | Discharged patients 6-month from infection. Assessments were conducted by telephone interview. | 215 patients. | Median Age years (IQR)- normal Moca patients: 62 years (51–69); abnormal Moca patients: 68 (57–77). | Telephone MoCA | 106/215 [49%] abnormal t-MoCA results). Significant univariate predictors of abnormal t-MoCA included older age, ≤12 years of education, unemployment pre-COVID, Black race, and a pre-COVID history of cognitive impairment (all p < .05). |
| Walle-Hansen et al. (2021) | Norway | Prospective cohort study | To study age related change in functional status and mortality among patients aged 60 years and older after hospitalisation due to COVID-19. | Discharged patients 6-month from infection in an ambulatory evaluation. | 106 patients. | Mean age was 74.3 years (range 60–96) | MoCA | Forty-six of the participants (43%) experienced a negative change in cognitive function 6 months after the COVID-19 hospitalisation, with a higher proporton reporting cognitive decline among persons 75 years and older, compared to younger persons (59% vs 37%, p <.05). |
| Vannorsdall et al. (2022) | United States | Prospective cohort study | To characterize post-acute neuropsychiatric functioning. | Discharged patients four months after an initial diagnosis of COVID-19 by telephone interview. | 82 patients | Age, mean (sd); range, years 54.5 (14.6); 26–85 | Rey Auditory Verbal Learning Test (RAVLT), Oral Trail Making Test parts A and B, digit span forward and backward, Phonemic and semantic verbal fluency, |
67% demonstrated at least 1 abnormally low cognitive score. |
Brief Repeatable Battery of Neuropsychological Tests (BRB-NT), Cognitive Failures Questionnaire (CFQ), Montreal Cognitive Assessment (MoCA), Navon Task (NT), Screen for Cognitive Impairment in Psychiatry Danish Version (SCIP-D), Trail Making Test (TMT), Rey Auditory Verbal Learning Test (RAVLT), Rey–Osterrieth Complex Figure Test (ROCFT), Boston Naming Test (BNT), Continuous Performance Test (CPT-II).
∗ Mean age of the total sample (does not discriminate from those who only performed the cognitive assessment).
The studies did not find specific alterations in structural neuroimaging exams (brain computed tomography or magnetic resonance imaging). Two of the selected studies evaluated patients in the acute phase with a cranial positron emission tomography scan, finding frontoparietal hypometabolism in patients with encephalopathy (Blazhenets et al., 2021; Hosp et al., 2021).
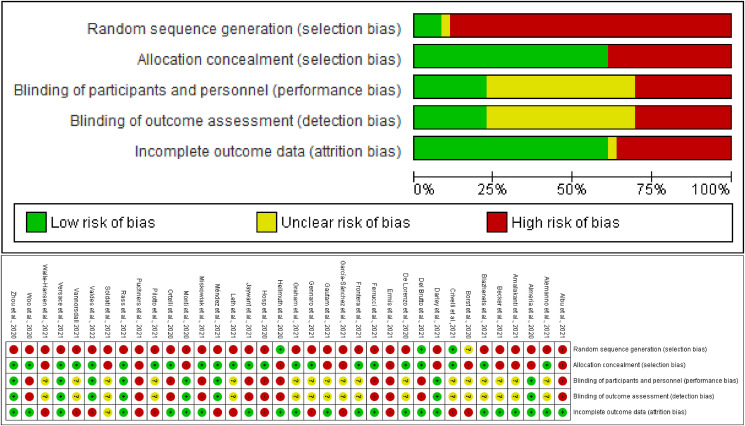
All articles were submitted to bias risk assessment (Fig. 2 ). The overall risk of selection bias was high in 20 studies, while the risk for controlling for confounding factors and attrition was low in 16 and 18 studies, respectively. In turn, the risk of performance bias and detection bias was high in 10 studies each.
Fig. 2.
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies (above). Risk of bias summary: review authors' judgements about each risk of bias item for each included study (below).
4. Discussion
We carried out a critical review of cognitive impairment in confirmed COVID-19 patients. Our results revealed a possibility of cognitive impairment, even in mildly symptomatic subjects six months after disease onset (Del Brutto et al., 2021). The importance of this systematic review showed a possible relationship between SARS-Cov2 infection and the development or deterioration of complaints and cognitive impairment. Moreover, cognitive impairment does not occur only in severe cases and in the acute disease phase.
Neurologic symptoms may persist in the postacute phase and constitute a “long Covid” syndrome (Graham et al., 2021). Because of this, and to differentiate cognitive impairment during acute/subacute phase from chronic phase, we separated the studies according to disease phase of patients’ evaluation. Cognitive impairment still occurred after 12 weeks of disease onset (Table 2). However, we still need more data to determine whether this cognitive impairment, when it occurs, is lasting or improves after a certain period of time.
Cognitive impairment was found after other infections, including coronavirus infections (Rogers et al., 2020). Furthermore, as COVID-19 can cause critical illnesses in some patients, ARDS and delirium may occur, both previously related to cognitive impairment (Honarmand et al., 2020; Pfoh et al., 2015). Thus, Rass et al (Rass et al., 2021) showed cognitive impairment in 23% of COVID-19 patients (in severe COVID-19 patients 29%, moderate 30%, and mild 3%). Additionally, the disease leading to hypoxemia with repercussions on memory is expected since the hippocampus is sensitive to low oxygen concentrations (Sartori et al., 2012).
Most selected studies evaluated previously hospitalized patients or even hospitalized patients. We cannot rule out that the cognitive impairment was caused by injuries related to hospitalization, delirium, or even the remaining acute phase of the disease or hypoxemia. Furthermore, McLoughlin et al (Mcloughlin et al., 2020) evaluated a group of patients with COVID-19 with delirium and compared it to individuals without delirium in the acute phase. All patients in this study were evaluated four weeks later with a TICS scale over the telephone. No significant differences were found regarding the instrument applied between the groups. Another possible cause that has not yet found support in the medical literature was a direct virus action. Such a direct role of the virus does not provide robust evidence in studies of general neurological manifestations evaluating cerebrospinal fluid or necropsy studies (Matschke et al., 2020; Neumann et al., 2020).
Conversely, Del Brutto and coworkers evaluated only outpatients without previous hospitalization and with mild disease (Del Brutto et al., 2021). In this study, patients had mild COVID-19, not requiring hospitalization. They were followed up on an outpatient basis before the pandemic and had a regular cognitive assessment. The authors evaluated patients six months after symptom onset with a control group and identified a drop ≥4 points in the MoCA applied to patients before the pandemic.
Other studies have pointed out possible causes of COVID-19 related cognitive impairment. Zhou et al. evaluated discharged patients through scales applied via digital devices, finding compromised attention of patients, also relating such findings to high inflammatory markers (Zhou et al., 2020). This last finding is interesting, as previous evidence shows a possible causal role of microglial inflammation and the later emergence of Alzheimer's disease (Mandrekar-Colucci & Landreth, 2010). Another possible cause of cognitive impairment associated with COVID-19 is ischemic changes associated with COVID-19 since cerebrovascular changes denote the risk of cognitive impairment and dementia (Pendlebury et al., 2019; Solomon et al., 2020). Also, endothelial lesions described in COVID-19 can impair brain metabolites clearance, including beta-amyloid peptides, which are involved in Alzheimer's disease (Varga et al., 2020; Weller et al., 2008).
Regarding patient assessment methods, as would be expected, some studies used tele-assistance services during the critical phase of the pandemic in their respective countries (Mcloughlin et al., 2020; Zhou et al., 2020). They are essential alternatives and can therefore play a triage role (Iqbal, 2020). However, it is essential to mention the lack of studies with extensive neuropsychological assessment.
This review study has some important limitations. First, some studies do not mention cognitive impairment prevalence or incidence. Furthermore, almost all studies did not have patients’ previous cognitive assessments. Furthermore, no information was available about cognitive impairment subtype (MCI, AD, other neurodegenerative diseases, e.g.) in almost all studies. Another caveat is that most studies did not have control groups, precluding direct comparison. In addition, it was not possible to compare the most affected cognitive domains between studies before and after 12 weeks of Covid-19 infection. Another potential limitation of this systematic review was the wide variety of cognitive assessment instruments employed, influencing external validity. Lastly, no study included in this review used a complete cognitive neuropsychological evaluation, the gold standard assessment method. These limitations made it impossible to perform a meta-analysis.
On the other hand, a strength of this systematic review is its reproducible and transparent procedure for literature systematic review. When publishing the research, the protocol is essential to display strategy clarity and reduce bias risk (Silagy et al., 2002). These results provide evidence to inform, support, and customize shared decision-making from the healthcare providers, stakeholders, and governments. In this sense, this systematic review delivers relevant evidence about the impact of the COVID-19 on patients’ cognitive performance to address the gap in the literature and guide essential strategies, detect cognitively impaired patients (SCD, MCI, and dementia) with the establishment of proper treatment, cognitive rehabilitation, and psychoeducation. The possibility of cognitive impairment after infection further emphasizes the importance of preventive measures for COVID-19.
In terms of future directions, it is essential to implement clinical research using control groups, using extensive neuropsychological assessment to determine the most affected cognitive domains, employing imaging and cerebrospinal fluid (CSF) biomarkers for neurogenerative diseases such as Alzheimer's disease, in order to elucidate specific pathophysiological changes by COVID-19. Ideally, individuals with prior neurocognitive follow-up, including biomarkers and prior cognitive assessment, should be included in future studies. Likewise, the inclusion of patients with previous cognitive impairment is essential to evaluate these patients' evolution. Another important group to be assessed is the low education patients, bringing psychometric data from this population and assessing whether low education, via low cognitive reserve, adversely affects cognition after the COVID-19 condition.
This review highlights the high frequency of cognitive impairment after COVID-19 infection. However, we could not differentiate whether the cognitive impairment identified corresponded to mild cognitive impairment or dementia through data from selected studies, which is one objective of future studies to be addressed on this topic.
Author contributions
Conception and design of work: JT-J, AS, JB and PB-N. Acquisition, analysis or interpretation of data and work: JT-J, AS, JB and PB-N. Drafting the work: JT-J, AS, JB, MS-N, DO, JS-N and PB-N. All authors were involved in critical revision of the manuscript for important intellectual content.
Declaration of competing interest
One of the authors (Pedro Braga Neto) received funding from the Brazilian National Council for Scientific and Technological Development (CNPq) as research grant funding and from the Brazilian Funding Grant Number 88881.505364/2020-01 from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES).
José Wagner Leonel Tavares Júnior, Ana Célia Caetano de Souza, José Wicto Pereira Borges, Danilo Nunes Oliveira, José Ibiapina Siqueira Neto, Manoel Alves Sobreira Neto declare that they have no conflict of interest.
Acknowledgments
One of the authors (Pedro Braga Neto) received funding from the Brazilian National Council for Scientific and Technological Development (CNPq) as research grant funding. We also acknowledge the Brazilian Funding Grant Number 88881.505364/2020-01 from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES).
Reviewed 7 February 2022
Action editor Brad Dickerson
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cortex.2022.04.006.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Albu S., Zozaya N.R., Murillo N., García-Molina A., Chacón C.A.F., Kumru H. What’s going on following acute covid-19? Clinical characteristics of patients in an out-patient rehabilitation program. NeuroRehabilitation. 2021;48(4) doi: 10.3233/NRE-210025. [DOI] [PubMed] [Google Scholar]
- Alemanno F., Houdayer E., Parma A., et al. Covid-19 cognitive deficits after respiratory assistance in the subacute phase: A covid-rehabilitation unit experience. Plos One. 2021;16(2) doi: 10.1371/journal.pone.0246590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeria M., Cejudo J.C., Sotoca J., Deus J., Krupinski J. Cognitive profile following COVID-19 infection: Clinical predictors leading to neuropsychological impairment. Brain, Behavior, & Immunity – Health. 2020;9:100163. doi: 10.1016/j.bbih.2020.100163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amalakanti S., Arepalli K.V.R., Jillella J.P. Cognitive assessment in asymptomatic COVID-19 subjects. VirusDis. 2021;32(1):146–149. doi: 10.1007/s13337-021-00663-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araújo W.C.O. Recuperação da informação em saúde: Construção, modelos e estratégias. Convergências em Ciência da Informação. 2020;3(2):100–134. doi: 10.33467/conci.v3i2.13447. [DOI] [Google Scholar]
- Becker J.H., Lin J.L., Doernberg M., Stone K., Navis A., Festa J.R., et al. Assessment of cognitive function in patients after COVID-19 infection. JAMA Network Open. 2021;4(10) doi: 10.1001/jamanetworkopen.2021.30645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazhenets G., Schröter N., Bormann T., et al. Slow but evident recovery from neocortical dysfunction and cognitive impairment in a series of chronic COVID-19 patients. Journal of Nuclear Medicine. 2021 doi: 10.2967/jnumed.121.262128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cani I., Barone V., D'Angelo R., et al. Frontal encephalopathy related to hyperinflammation in COVID-19. Journal of Neurology. 2021;268(1):16–19. doi: 10.1007/s00415-020-10057-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crivelli L., Calandri I., Corvalán N., Carello M.A., Keller G., Martínez C., et al. Cognitive consequences of COVID-19: results of a cohort study from South America. Arquivos de Neuro-psiquiatria. 2022;80(3) doi: 10.1590/0004-282X-ANP-2021-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darley D.R., Dore J.G., Cysique L., et al. Persistent symptoms up to four months after community and hospital-managed SARS-CoV-2 infection. The Medical Journal of Australia. 2021 doi: 10.5694/mja2.50963. (AMPCo Pty Ltd.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lorenzo R., Conte C., Lanzani C., et al. Residual clinical damage after COVID-19: A retrospective and prospective observational cohort study. Plos One. 2020;15(10) doi: 10.1371/journal.pone.0239570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Brutto O.H., Wu S., Mera R.M., Costa A.F., Recalde B.Y., Issa N.P. Cognitive decline among individuals with history of mild symptomatic SARS-CoV-2 infection: A longitudinal prospective study nested to a population cohort. European Journal of Neurology: the Official Journal of the European Federation of Neurological Societies. 2021:1–9. doi: 10.1111/ene.14775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme C., Paccoud O., Kas A., et al. Covid-19-related encephalopathy: A case series with brain FDG-PET/CT findings. European Journal of Neurology. 2020;27(12):2651–2657. doi: 10.1111/ene.14478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding D., Zhao Q., Guo Q., et al. Prevalence of mild cognitive impairment in an urban community in China: A cross-sectional analysis of the Shanghai aging study. Alzheimer's & Dementia: the Journal of the Alzheimer's Association. 2015;11(3):300–309. doi: 10.1016/j.jalz.2013.11.002. Available from: [DOI] [PubMed] [Google Scholar]
- Ermis U., Rust M.I., Bungenberg J., et al. Neurological symptoms in COVID-19: A cross-sectional monocentric study of hospitalized patients. Neurological Research and Practice. 2021;3(1):1–12. doi: 10.1186/s42466-021-00116-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrucci R., Dini M., Groppo E., et al. Long-lasting cognitive abnormalities after COVID-19. Brain Sciences. 2021;11(2):235. doi: 10.3390/brainsci11020235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frontera J.A., Yang D., Lewis A., Patel P., Medicherla C., Arena V., et al. A prospective study of long-term outcomes among hospitalized COVID-19 patients with and without neurological complications. Journal of the Neurological Sciences. 2021 doi: 10.1016/j.jns.2021.117486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Sánchez C., Calabria M., Grunden N., Pons C., Arroya J.A., Gómez-Anson B., et al. Neuropsychological deficits in patients with cognitive complaints after COVID-19. Brain and Behavior. 2022;12(3) doi: 10.1002/brb3.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam N., Madathil S., Tahani N., Bolton S., Parekh D., Stockley J., et al. Medium-Term Outcomes in Severely to Critically Ill Patients With Severe Acute Respiratory Syndrome Coronavirus 2 Infection. Clinical Infectious Diseases. 2022;74(2) doi: 10.1093/cid/ciab341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham E.L., Clark J.R., Orban Z.S. Persistent neurologic symptoms and cognitive dysfunction in non-hospitalized Covid-19 “long haulers”. Annals of Clinical and Translational Neurology. 2021;8(5):1073–1085. doi: 10.1002/acn3.51350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W.J., Ni Z.Y., Hu Y., et al. Clinical characteristics of coronavirus disease 2019 in China. The New England Journal of Medicine. 2019:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellmuth J., Barnett T.A., Asken B.M., et al. Persistent COVID-19-associated neurocognitive symptoms in non-hospitalized patients. Journal of NeuroVirology. 2021;27(1):191–195. doi: 10.1007/s13365-021-00954-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J.P.T., Green S., The Cochrane Collaboration . 2011. Cochrane handbook for systematic reviews of interventions.http://handbook.cochrane.org Version 5.1.0 [updated March 2011]. [Online]. Available: [Google Scholar]
- Honarmand K., Lalli R.S., Priestap F., et al. Natural history of cognitive impairment in critical illness survivors a systematic review. American Journal of Respiratory and Critical Care Medicine. 2020;202(2):193–201. doi: 10.1164/rccm.201904-0816CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosp J.A., Dressing A., Blazhenets G., et al. Cognitive impairment and altered cerebral glucose metabolism in the subacute stage of COVID-19. Brain. 2021;144(4):1263–1276. doi: 10.1093/brain/awab009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal M.H. Telemedicine: An innovative twist to primary health care in rural Bangladesh. Journal of Primary Care & Community Health. 2020;11:1–10. doi: 10.1177/2150132720950519. Article reuse guidelines: sagepub.com/journals-permissions (journals.sagepub.com/home/jpc) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson G.B. Methods for integrative reviews. Review of Educational Research. 1980;50(3):438–460. doi: 10.3102/00346543050003438. [DOI] [Google Scholar]
- Jaywant A., Vanderlind W.M., Alexopoulos G.S., Fridman C.B., Perlis R.H., Gunning F.M. Gunning. Frequency and profile of objective cognitive deficits in hospitalized patients recovering from COVID-19. Neuropsychopharmacology. 2021:1–6. doi: 10.1038/s41386-021-00978-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen F., Amariglio R.E., Van Boxtel M., et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer's disease. Alzheimer’s & Dementia. 2014;10(6):844–852. doi: 10.1016/j.jalz.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leth S., Gunst J.D., Dahl V.N., Hansen K.S., Søgaard O., Østergaard L., et al. Persistent symptoms in patients recovering from COVID-19 in Denmark. Open Forum Infectious Diseases. 2021;8(4) doi: 10.1093/ofid/ofab042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumsden A.L., Mulugeta A., Zhou A., Hyppönen E. Apolipoprotein E (APOE) genotype-associated disease risks: A phenome-wide, registry-based, case-control study utilizing the UK Biobank. EBioMedicine. 2020;59:102954. doi: 10.1016/j.ebiom.2020.102954. http://creativecommons.org/licenses/by-nc-nd/4.0/ The Authors. Published by Elsevier B.V. This is an open access article under the CC BY-NC-ND license. Available at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrekar-Colucci S., Landreth G.E. Microglia and inflammation in Alzheimer's disease. CNS & Neurological Disorders Drug Targets. 2010;9(2):156–167. doi: 10.2174/187152710791012071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L., Jin H., Wang M., et al. Neurological manifestations of hospitalized patients with COVID-19 in Wuhan, China: A retrospective case series study (February 24, 2020) Lancet Neurology. 2020 Manuscript Draft. [Google Scholar]
- Matschke J., Lütgehetmann M., Hagel C., et al. Neuropathology of patients with COVID-19 in Germany: A post-mortem case series. Lancet Neurology. 2020;19(11):919–929. doi: 10.1016/S1474-4422(20)30308-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazza M.G., Palladini M., Lorenzo R., Magnaghi C., Polleti S., Furlan S., et al. Persistent psychopathology and neurocognitive impairment in COVID-19 survivors: Effect of inflammatory biomarkers at three-month follow-up. Brain, Behavior and Immunity. 2021 doi: 10.1016/j.bbi.2021.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcloughlin B.C., Miles A., Webb T.E., et al. Functional and cognitive outcomes after COVID-19 delirium. Eur Geriatr Med. 2020;11(5):857–862. doi: 10.1007/s41999-020-00353-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes K.D.S., Silveira R.C.C.P., Galvão C.M. Revisão integrativa: Método de pesquisa para a incorporação de evidências na saúde e na enfermagem. Texto Contexto – enferm. 2008;17(4):758–764. doi: 10.1590/S0104-07072008000400018. [DOI] [Google Scholar]
- Mendez R., Balanzá-Martínez V., Luperdi S.C. Short-term neuropsychiatric outcomes and quality of life in COVID-19 survivors. Journal of Internal Medicine. 2021 doi: 10.1111/joim.13262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S.A. US National Center for Dental Hygiene Research. University of Southern California; Los Angeles: 2001. PICO worksheet and search strategy. [Google Scholar]
- Miskowiak K.W., Johnsen S., Sattler S.M., et al. Cognitive impairments four months after COVID-19 hospital discharge: Pattern, severity and association with illness variables. European Neuropsychopharmacology. 2021;46:39–48. doi: 10.1016/j.euroneuro.2021.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D., Shamseer L., Clarke M., et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. [Systematic Reviews Electronic Resource] 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti G., Leggieri C., Fominskiy E., et al. Two-months quality of life of COVID-19 invasively ventilated survivors; an Italian single-center study. Acta Anaesthesiologica Scandinavica. 2021:1–9. doi: 10.1111/aas.13812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalbandian A., Sehgal K., Gupta A., et al. Post-acute COVID-19 syndrome. Nature Medicine. 2021;27:601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann B., Schmidbauer M.L., Dimitriadis K., et al. Cerebrospinal fluid findings in COVID-19 patients with neurological symptoms. Journal of the Neurological Sciences. 2020;418:117090. doi: 10.1016/j.jns.2020.117090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes M.D.R., Bomfim E., Olson K., et al. Interventions minimizing fatigue in children/adolescents with cancer: An integrative review. J Child Health Care. 2018;22(2):186–204. doi: 10.1177/1367493517752498. [DOI] [PubMed] [Google Scholar]
- Ortelli P., Ferrazzoli D., Sebastianelli L. Neuropsychological and neurophysiological correlates of fatigue in post-acute patients with neurological manifestations of COVID-19: Insights into a challenging symptom. Journal of the Neurological Sciences. 2021;420:117271. doi: 10.1016/j.jns.2020.117271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendlebury S.T., Rothwell P.M., Oxford Vascular S. Incidence and prevalence of dementia associated with transient ischaemic attack and stroke: Analysis of the population-based Oxford Vascular study. Lancet Neurology. 2019;18(3):248–258. doi: 10.1016/S1474-4422(18)30442-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen R.C., Parisi J.E., Dickson D.W., et al. Neuropathologic features of amnestic mild cognitive impairment. Archives of Neurology. 2006;63(5):665–672. doi: 10.1001/archneur.63.5.665. [DOI] [PubMed] [Google Scholar]
- Pfoh E.R., Chan K.S., Dinglas V.D., et al. Cognitive screening among acute respiratory failure survivors: A cross-sectional evaluation of the mini-mental state examination. Critical Care: the Official Journal of the Critical Care Forum. 2015;19(1):1–11. doi: 10.1186/s13054-015-0934-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilotto A., Cristillo V., Cotti Piccinelli S., Zoppi N., Bomzi G., Sattin D., et al. Long-term neurological manifestations of COVID-19: prevalence and predictive factors. Neurological Sciences. 2021;42(12) doi: 10.1007/s10072-021-05586-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier J., Davignon J., Bouthillier D., et al. Apolipoprotein E polymorphism and Alzheimer's disease. The Lancet. 1993;342(8873):697–699. doi: 10.1016/0140-6736(93)91705-q. [DOI] [PubMed] [Google Scholar]
- Popay J., Roberts H., Sowden A., Petticrew M., Arai L., Rodgers M., Britten N., Roen K., Duffy S. University of Lancaster; UK: 2006. Guidance on the conduct of narrative synthesis in systematic reviews. Results of an ESRC funded research project. Unpublished report. [Google Scholar]
- Prince M.J., Wimo A., Guerchet M.M., Ali G.C., Wu Y.T., Prina M. 2015. World Alzheimer report 2015-the global impact of dementia: An analysis of prevalence, incidence, cost and trends. [Google Scholar]
- Puchner B., Kirchmair R., Pizzini A., et al. Beneficial effects of multi-disciplinary rehabilitation in European. Journal of Pediatric Rehabilitation Medicine. 2021:189–198. doi: 10.23736/S1973-9087.21.06549-7. [DOI] [PubMed] [Google Scholar]
- Rass V., Beer R., Schiefecker A.J., et al. Neurological outcome and quality of life 3 months after COVID-19: A prospective observational cohort study. European Journal of Neurology: the Official Journal of the European Federation of Neurological Societies. 2021:1–12. doi: 10.1111/ene.14803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J.P., Chesney E., Oliver D., et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: A systematic review and meta-analysis with comparison to the COVID-19 pandemic. The Lancet Psychiatry. 2020;7(7):611–627. doi: 10.1016/S2215-0366(20)30203-0. www.thelancet.com/psychiatry. Acessed July 7, 2020 Available at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartori A.C., Vance D.E., Slater L.Z., Crowe M. The impact of inflammation on cognitive function in older adults: Implications for health care practice and research. The Journal of Neuroscience Nursing: Journal of the American Association of Neuroscience Nurses. 2012;44(4):206. doi: 10.1097/JNN.0b013e3182527690. http://doi:10.1097/JNN.0b013e3182527690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddaway A.P., Wood A.M., Hedges L.V. How to do a systematic review: A best practice guide for conducting and reporting narrative reviews, meta-analyses, and meta-Syntheses. Annual Review of Psychology. 2019;70(1):747–770. doi: 10.1146/annurev-psych-010418-102803. [DOI] [PubMed] [Google Scholar]
- Silagy C.A., Middleton P., Hopewell S. Publishing protocols of systematic reviews: Comparing what was done to what was planned. JAMA. 2002;287(21):2831–2834. doi: 10.1001/jama.287.21.2831. [DOI] [PubMed] [Google Scholar]
- Soldati A.B., Almeida C., Lima M., Araujo A., Araújo-Leite M.A., Silva M.T.T., et al. Telephone Screening of Cognitive Status (TICS) in severe COVID-19 patients: Utility in the era of social isolation. eNeurologicalSci. 2021 doi: 10.1016/j.ensci.2021.100322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon I.H., Normandin E., Bhattacharyya S., et al. Neuropathological features of Covid-19. The New England Journal of Medicine. 2020;383(10):989–992. doi: 10.1056/NEJMc2019373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdes E., Fuchs B., Morrison C., Charvet L., Lewis A., Thawani S., et al. Demographic and social determinants of cognitive dysfunction following hospitalization for COVID-19. Journal of the Neurological Sciences. 2022 doi: 10.1016/j.jns.2022.120146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Borst B., Peters J.B., Brink M., Schoon Y., Bleeker-Rovers C.P., Schers S., et al. Comprehensive health assessment 3 months after recovery from acute coronavirus disease 2019 (COVID-19) Clinical Infectious Diseases. 2021 doi: 10.1093/cid/ciaa1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannorsdall T.D., Brigham E., Fawzy A., Raju S., Gorgone A., Pletnikova A., et al. Cognitive Dysfunction, Psychiatric Distress, and Functional Decline After COVID-19. Journal of the Academy of Consultation-liaison Psychiatry. 2022 doi: 10.1016/j.jaclp.2021.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga Z., Flammer A.J., Steiger P., et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versace V., Sebastianelli L., Ferrazzoli D., et al. Intracortical GABAergic dysfunction in patients with fatigue and dysexecutive syndrome after COVID-19. Clinical Neurophysiology. 2021;132(5):1138–1143. doi: 10.1016/j.clinph.2021.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walle-Hansen M.M., Ranhoff A.H., Mellingsæter M., Wang-Hansen M.S., Myrstad M. Health-related quality of life, functional decline, and long-term mortality in older patients following hospitalisation due to COVID-19. BMC Geriatrics. 2021 doi: 10.1186/s12877-021-02140-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller R.O., Subash M., Preston S.D., Mazanti I., Carare R.O. Perivascular drainage of amyloid-β peptides from the brain and its failure in cerebral amyloid angiopathy and Alzheimer's disease. Brain Pathology. 2008;18(2):253–266. doi: 10.1111/j.1750-3639.2008.00133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittemore R., Knafl K. The integrative review: Updated methodology. Journal of Advanced Nursing. 2005;52(5):546–553. doi: 10.1111/j.1365-2648.2005.03621.x. [DOI] [PubMed] [Google Scholar]
- WHO 2021. https://www.who.int/emergencies/diseases/novel-coronavirus-2019
- Woo M.S., Malsy J., Pöttgen J., et al. Frequent neurocognitive deficits after recovery from mild COVID-19. Brain Communications. 2020;2(2) doi: 10.1093/braincomms/fcaa205. fcaa205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Xu X., Chen Z., et al. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain, Behavior, and Immunity. 2020 doi: 10.1016/j.bbi.2020.03.031. 2020 Jul. Available at elsevier BV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H., Lu S., Chen J., et al. The landscape of cognitive function in recovered COVID-19 patients. Journal of Psychiatric Research. 2020;129:98–102. doi: 10.1016/j.jpsychires.2020.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.