Abstract
Background
Occult breast cancer (OBC) is a special type of breast cancer. Because of its rarity, clinicopathological information is still insufficient, causing a controversial condition about its treatment recommendation. Thus, we aimed to clarify major clinicopathological information, treatment strategies and prognosis of OBC based on a large population.
Methods
We retrospectively collected adult female OBC population from Surveillance, Epidemiology, and End Results database. We divided the whole cohort into two groups based on surgical treatment in-breast. Descriptive analysis of 18 clinicopathological variables was conducted. Survival analysis was performed based on different clinicopathological factors. Univariate and multivariate Cox regression analysis was performed to identify potential independent predictor for prognosis of OBC.
Results
1189 OBC patients were in final analysis and most of them were diagnosed as an early-stage carcinoma. Patients received breast-conserving treatment (BCT) was nearly two times of ones received mastectomy. Patients receiving radiotherapy in BCT group were significantly more than patients receiving radiotherapy in mastectomy group (61.76 vs. 50.9%, P < 0.001). After a median follow-up period of 62 months, 5-year and 10-year overall survival (OS) of all subjects was 81.6% and 68.8%, respectively. No significant difference in OS and breast-cancer specific survival (BCSS) was found between mastectomy and local breast-conserving surgery. Older age and larger number of positive lymph nodes causes a worse prognosis whereas radiotherapy brought a better clinical outcome for OBC patients.
Conclusions
OBC has a generally good prognosis. Less-intensive surgery does not negatively impact clinical outcomes of OBC while additional radiotherapy is totally beneficial to prolong OS and BCSS.
Keywords: Occult breast cancer, Surgical treatment, Systemic therapy, Radiotherapy, Survival
Background
Occult breast cancer (OBC) is a special type of breast cancer. It is first reported and described by Halsted as “cancerous axillary glands with non-demonstrable cancer of the mamma” in 1907 [1]. The present definition of OBC is a kind of primary breast cancer diagnosed histologically by biopsy of axillary lymph nodes but without clinical or radiographic finding (mammography and ultrasonography) in the breast itself [2, 3]. The incidence of OBC among all types of breast cancer is about 0.1 to 1% [4].
Because of its rarity and lack of primary focus within the breast, the clinicopathological traits of OBC, including lymph node metastasis, immunohistochemistry [hormone status/human epidermal factor receptor-2 (HER-2) status, etc.], is still unclear [5–8]. Moreover, prognosis of OBC is still debatable. 10-year overall survival of OBC in different reports varied from 45% to nearly 70% [9].
The National Comprehensive Cancer Network (NCCN) guidelines of breast disease recommended that patients diagnosed with OBC by magnetic resonance imaging (MRI) be treated in two ways according to nodal condition: (1) for T0N1M0 disease, Breast conserving therapy (BCT) [axillary lymph node dissection (ALND) plus whole breast radiotherapy with or without nodal radiotherapy] or ALND plus mastectomy (modified radical mastectomy, MRM) with or without radiotherapy; (2) for T0N2-3M0 disease, neoadjuvant systemic therapy (chemotherapy, endocrine therapy and targeting therapy) followed by MRM [9, 10]. Up till now, research of OBC is largely derived from case reports as well as insufficient number of retrospective clinical studies with small sample size [11–13]. Shortage of comprehensive clinical research and insufficient number of subjects in various retrospective studies hurdled determination of a more general and individualized therapeutic options [11, 12].
In our study, we collected the latest edition of population-based data from the Surveillance, Epidemiology, and End Results (SEER) database. The main goal of our study is to further investigate the main clinicopathological features of OBC and acquire the most updated information of association between different types of treatment and prognosis.
Methods
Ethical statement
No patient informed consent is necessary because SEER database is publicly available and all data have been de-identified. We have submitted our application and obtained the approval of usage for the updated data of SEER database.
Data source and study cohort
We conducted a retrospective population-based cohort study using SEER 18 registry research data (Nov 2020 submission). The database included data from 18 population-based cancer registries (2000–2018) and covered approximately 27.8% of US population. Data-retrieving software SEER*STAT software ver. 8.3.9.1 was used to collect finally wanted data. The inclusion criteria include “Female”; age of diagnosis from 18 to 85; year of diagnosis from 2004 through 2018; diagnosis is primary breast cancer; American Joint Committee on Cancer breast staging T is T0 and M is M0; A known number of examined lymph nodes; positive lymph node not less than one (N+). Diagnosis not by histology, male patients, unknown laterality; breast cancer staging not T0, N staging with N0 or Nx, breast cancer with distal metastasis and unknown staging were all excluded.
Clinicopathological variables include age, year of diagnosis, race, marital status, grade, number of examined lymph nodes, number of positive lymph nodes, N staging, surgery types, radiation, chemotherapy, types of systemic therapy, subtypes of breast cancer, estrogen receptor (ER), progesterone receptor (PR), and HER-2. For the reason that HER-2 status was not recorded until 2010, it is not available in patients whose diagnosis were between 2004 and 2009. Subtypes of breast cancer includes Luminal A (ER-positive and/or PR-positive, HER-2 negative), Luminal B (ER-positive and/or PR-positive, HER-2 positive), HER-2 positive (ER and PR-negative, HER-2 positive) and triple negative (ER negative, PR negative and HER-2 negative).
Study endpoints and definition
In our study, OS served as primary outcome. It was defined as from the date of diagnosis to the date of death, no matter if the patient was dead from breast cancer or other reasons. Breast cancer-specific survival (BCSS) was secondary outcome. It was calculated from the day of diagnosis to the date of death caused by breast cancer.
Statistical analysis
For continuous variables, Shapiro tests will be conducted to identify whether they meet the criteria of normal distribution. If so, t-test will be used. If not, Wilcoxon rank sum and signed rank tests will be conducted. For categorical variables, Chi-square test or Fisher’s exact test will be utilized to describe the difference of rate for every clinicopathological variables between two groups. Survival curves were illustrated by the Kaplan–Meier method and the difference in OS and BCSS rate in different groups was identified using log-rank test. Hazard ratio (HR) with 95% confidence intervals (CI) was calculated by Cox proportional hazard regression model to determine the factors significantly related with outcome. Clinicopathological factors with P value < 0.1 in univariate analysis would be selected to be further analyzed in multivariate analysis. Significant association between selected factors and major outcomes was defined as P value of 0.05 or less. All the statistical analyses were two-sided and all the P-value less than 0.05 was set as a significance level except univariate analysis.
The above statistical analysis was conducted by R software version of 4.0.4. Main packages necessary for R program include "survival" (version 3.2-7), “survminer” (version 0.4.8), ”ggplot2” (version 3.3.3) and “ggthemes” (version 4.2.4).
Results
Baseline clinicopathological description of OBC cohort
A total of 1189 patients diagnosed as OBC were included in ultimate analysis from 2004 to 2018. Mean age of complete OBC cohort was 59.88 years and the range of age is from 26 through 85. White patients predominated the whole population with a percentage of 79.20% while Asian or Pacific islander accounted for nearly 10%. Majority of OBC patients were married women (n = 699, 58.80%). Ductal carcinoma was the main histological types of OBC. For N staging and the general staging, patients with stage-N1/stage-IIA OBC took nearly two thirds of the whole population (n = 721, 60.60%). Breast-conserving surgery (BCS) were carried out in 120 patients (10.10%) whereas mastectomy was carried out in 422 patients (35.50%). More than half of all patients received radiotherapy and nearly 80% the whole cohort took chemotherapy. For subtypes of OBC, Luminal A subtype was the commonest one (n = 326, 27.40%) while HER-2 positive OBC merely made up of about 6.20% (n = 74) (Table 1).
Table 1.
Demographic and tumor characteristics
| Characteristic | Number of patients | Percentage (%) |
|---|---|---|
| N | 1189 | 100 |
| Age (year) | ||
| < 40 | 54 | 4.54 |
| 40–49 | 176 | 14.80 |
| 50–59 | 348 | 29.27 |
| 60–69 | 343 | 28.85 |
| ≥ 70 | 268 | 22.54 |
| Mean of age (year) | 59.88 | |
| Median of age (year, range) | 60.00 (26–85) | |
| Race | ||
| White | 942 | 79.23 |
| Black | 139 | 11.69 |
| Asian or Pacific Islander | 95 | 7.99 |
| American Indian/Alaska Native | 9 | 0.76 |
| Unknown/other | 4 | 0.34 |
| Marital status | ||
| Not married | 449 | 37.76 |
| Married | 699 | 58.79 |
| Unknown | 41 | 3.45 |
| Grade | ||
| I/II | 65 | 5.47 |
| III | 223 | 18.76 |
| IV | 10 | 0.84 |
| Unknown | 891 | 74.94 |
| Laterality | ||
| Right | 565 | 47.52 |
| Left | 624 | 52.48 |
| Histology | ||
| Ductal carcinoma | 476 | 40.03 |
| Lobular carcinoma | 54 | 4.54 |
| Mixed type | 25 | 2.10 |
| Other | 634 | 53.32 |
| N | ||
| N1mi | 31 | 2.61 |
| N1 | 721 | 60.64 |
| N2 | 228 | 19.18 |
| N3 | 209 | 17.58 |
| No. of examined LN | ||
| 1–3 | 182 | 15.31 |
| 4–9 | 177 | 14.89 |
| ≥ 10 | 675 | 56.77 |
| Other/unknown | 155 | 13.04 |
| No. of positive LN | ||
| 1–3 | 615 | 51.72 |
| 4–9 | 212 | 17.83 |
| ≥ 10 | 148 | 12.45 |
| Other/unknown | 214 | 18.00 |
| Stage | ||
| IB | 31 | 2.61 |
| IIA | 722 | 60.72 |
| IIIA | 228 | 19.18 |
| IIIC | 208 | 17.49 |
| Surgery | ||
| BCS | 120 | 10.09 |
| Mastectomy | 422 | 35.49 |
| None/unknown | 647 | 54.42 |
| Radiation | ||
| Yes | 688 | 57.86 |
| None/unknown | 501 | 42.13 |
| Chemotherapy | ||
| Yes | 929 | 78.13 |
| No/unknown | 260 | 21.87 |
| Type of systemic therapy | ||
| Neoadjuvant | 118 | 9.92 |
| Adjuvant | 583 | 49.03 |
| Neoadjuvant + adjuvant | 104 | 8.75 |
| Only systemic therapy, no surgery | 28 | 2.35 |
| Other | 190 | 15.98 |
| No/unknown | 166 | 13.96 |
| Subtypes | ||
| Luminal A | 326 | 27.42 |
| Luminal B | 111 | 9.34 |
| HER2 positive | 74 | 6.22 |
| Triple negative | 148 | 12.45 |
| Unknown | 530 | 44.58 |
| ER | ||
| Negative | 414 | 34.82 |
| Borderline/unknown | 100 | 8.41 |
| Positive | 675 | 56.77 |
| PR | ||
| Negative | 598 | 50.29 |
| Borderline/unknown | 126 | 10.60 |
| Positive | 465 | 39.11 |
| HER2 | ||
| Negative | 474 | 39.87 |
| Positive | 187 | 15.73 |
| Unknown | 528 | 44.41 |
| Vital status | ||
| Alive | 917 | 77.12 |
| Dead | 272 | 22.88 |
BCS breast-conserving surgery, MRM modified radical mastectomy, RM radical mastectomy
Comparison of clinicopathological features in different treatment
We extracted OBC patients received either BCT/non-mastectomy [BCS of the breast (n = 120) or no surgical treatment of the breast (n = 628)] or mastectomy into further analysis (Table 2). Totally, 748 women had BCT therapy and 422 patients received mastectomy procedure. No significant difference was in demographic characteristics such as racial composition (P = 0.14) and marital status (P = 0.64). Average age of patients in BCT groups was significantly higher than that in mastectomy group (61.16±11.35 vs. 57.65±11.72, P < 0.001). Speaking of features of OBC, no significant difference was found for tumor grade, laterality between BCT and mastectomy group (P = 0.30 and P = 0.15, respectively). For tumor histology, it was marginally associated with surgical types (P = 0.04). For lymph node stage, majority of patients in BCT group had a relatively early N1 stage (64.30%) whereas proportion of patients in mastectomy group with stages of N2 and N3 were higher than that of patients in BCT group (22.51% vs. 17.51% for N2; 19.43% vs. 16.58% for N3, P < 0.001). A larger proportion of patients in mastectomy group were examined more than 10 lymph nodes (65.40% vs. 52.14%, P < 0.001) while a smaller part of patients in mastectomy group were examined 1-3 lymph nodes (11.61 vs. 17.51, P < 0.001). For number of positive lymph nodes, about half of the patients in both groups had 1–3 positive nodes but a larger proportion of patients in mastectomy group got 4–9 positive nodes (22.04% vs. 15.64%, P < 0.001). More than 60% of the patients in BCT group were diagnosed as IIA (64.30% vs. 53.55%) OBC while a higher proportion of population in mastectomy group were diagnosed as higher stages of OBC (IIIA: 22.75% vs. 17.51%; IIIC 19.19% vs. 16.58%, P < 0.001). Rate of Luminal A OBC topped in both BCS groups and mastectomy groups, with 28.61% and 25.12% respectively. HER-2 positive OBC has the lowest rate in both groups (less than 10%). When discussing three immunohistochemistry results apart (ER/PR/HER-2), however, no significant association was found between each of them with surgical procedure (P>0.05). Regarding non-surgical management, higher proportion of patients in BCT received radiation compared with that of patients in mastectomy group (61.76% vs. 50.95%, P < 0.001). Different from radiotherapy, a greater percentage of patients in mastectomy group received chemotherapy than that of patients in BCT group (83.18% vs. 75.67%, P = 0.003). For patterns of systemic therapy, sequential neoadjuvant and adjuvant therapy were more popular in population from mastectomy group compared with that from BCT group (12.80% vs. 6.55%, P < 0.001).
Table 2.
Basic data of variables in OBC patients with breast-conserving therapy (BCT) and mastectomy
| Variables | Surgery | P-value | |||
|---|---|---|---|---|---|
| BCT | Mastectomy | ||||
| N | Percentage (%) | N | Percentage (%) | ||
| N | 748 | 100 | 422 | 100 | |
| Age | < 0.001 | ||||
| < 40 | 24 | 3.21 | 29 | 6.87 | |
| 40–49 | 91 | 12.17 | 83 | 19.67 | |
| 50–59 | 216 | 28.88 | 126 | 29.86 | |
| 60–69 | 223 | 29.81 | 114 | 27.01 | |
| ≥ 70 | 194 | 25.94 | 70 | 16.59 | |
| Mean (± SD) | 61.16 ± 11.35 | 57.65 ± 11.72 | < 0.001* | ||
| Race | 0.14** | ||||
| White | 587 | 78.48 | 340 | 80.57 | |
| Black | 92 | 12.30 | 45 | 10.66 | |
| Asian or Pacific Islander | 57 | 7.62 | 36 | 8.53 | |
| American Indian/Alaska Native | 9 | 1.20 | 0 | 0 | |
| Unknown/other | 3 | 0.40 | 1 | 0.24 | |
| Marital status | 0.64 | ||||
| Not married | 289 | 34.17 | 153 | 36.3 | |
| Married | 433 | 61.67 | 256 | 60.7 | |
| Unknown | 26 | 4.17 | 13 | 3.1 | |
| Grade | 0.30 | ||||
| I/II | 35 | 4.68 | 28 | 6.64 | |
| III | 135 | 18.05 | 85 | 20.14 | |
| IV | 7 | 0.94 | 2 | 0.47 | |
| Unknown | 571 | 76.34 | 307 | 72.75 | |
| Laterality | 0.15 | ||||
| Right | 342 | 45.72 | 212 | 50.24 | |
| Left | 406 | 54.28 | 210 | 49.76 | |
| Histology | 0.04 | ||||
| Ductal carcinoma | 277 | 37.03 | 192 | 45.50 | |
| Lobular carcinoma | 37 | 4.95 | 16 | 3.79 | |
| Mixed type | 16 | 2.14 | 9 | 2.13 | |
| Other | 418 | 55.88 | 205 | 48.58 | |
| N | < 0.001 | ||||
| N1mi | 12 | 1.60 | 19 | 4.50 | |
| N1 | 481 | 64.30 | 226 | 53.55 | |
| N2 | 131 | 17.51 | 95 | 22.51 | |
| N3 | 124 | 16.58 | 82 | 19.43 | |
| No. of examined LN | < 0.001 | ||||
| 1–3 | 131 | 17.51 | 49 | 11.61 | |
| 4–9 | 100 | 13.37 | 72 | 17.06 | |
| ≥ 10 | 390 | 52.14 | 276 | 65.40 | |
| Other/Unknown | 127 | 16.98 | 25 | 5.92 | |
| No. of positive LN | < 0.001 | ||||
| 1–3 | 376 | 50.27 | 227 | 53.79 | |
| 4–9 | 117 | 15.64 | 93 | 22.04 | |
| ≥ 10 | 86 | 11.50 | 60 | 14.22 | |
| Other/Unknown | 169 | 22.59 | 42 | 9.95 | |
| Stage | < 0.001 | ||||
| IB | 12 | 1.60 | 19 | 4.50 | |
| IIA | 481 | 64.30 | 226 | 53.55 | |
| IIIA | 131 | 17.51 | 96 | 22.75 | |
| IIIC | 124 | 16.58 | 81 | 19.19 | |
| Radiation | < 0.001 | ||||
| Yes | 462 | 61.76 | 215 | 50.95 | |
| None/Unknown | 286 | 38.24 | 207 | 49.05 | |
| Chemotherapy | 0.003 | ||||
| Yes | 566 | 75.67 | 351 | 83.18 | |
| No/Unknown | 182 | 24.33 | 71 | 16.82 | |
| Type of systemic therapy | < 0.001 | ||||
| Neoadjuvant | 61 | 8.16 | 55 | 13.03 | |
| Adjuvant | 380 | 50.80 | 196 | 46.45 | |
| Neoadjuvant + adjuvant | 49 | 6.55 | 54 | 12.80 | |
| Only systemic therapy, no surgery | 28 | 3.74 | 0 | 0 | |
| Other | 113 | 15.11 | 74 | 17.54 | |
| No/Unknown | 117 | 15.64 | 43 | 10.19 | |
| Subtypes | 0.48 | ||||
| Luminal A | 214 | 28.61 | 106 | 25.12 | |
| Luminal B | 65 | 8.69 | 44 | 10.43 | |
| HER2 + | 51 | 6.82 | 23 | 5.45 | |
| Triple negative | 93 | 12.43 | 52 | 12.32 | |
| Unknown | 325 | 43.45 | 197 | 46.68 | |
| ER | 0.89 | ||||
| Negative | 259 | 34.63 | 149 | 35.31 | |
| Borderline/Unknown | 61 | 8.16 | 37 | 8.77 | |
| Positive | 428 | 57.22 | 236 | 55.92 | |
| PR | 0.76 | ||||
| Negative | 372 | 49.73 | 215 | 50.95 | |
| Borderline/Unknown | 77 | 10.29 | 47 | 11.14 | |
| Positive | 299 | 39.97 | 160 | 37.91 | |
| HER2 | 0.44 | ||||
| Negative | 307 | 41.04 | 158 | 37.44 | |
| Positive | 118 | 15.78 | 67 | 15.88 | |
| Unknown | 323 | 43.18 | 197 | 46.68 | |
BCT breast-conserving therapy, ER estrogen, PR progesterone receptor, HER-2 human epidermal growth factor receptor-2, SD standard deviation
*Wilcoxon Rank Sum and Signed Rank Tests
**Fisher's Exact Test
Survival analysis in OBC cohort
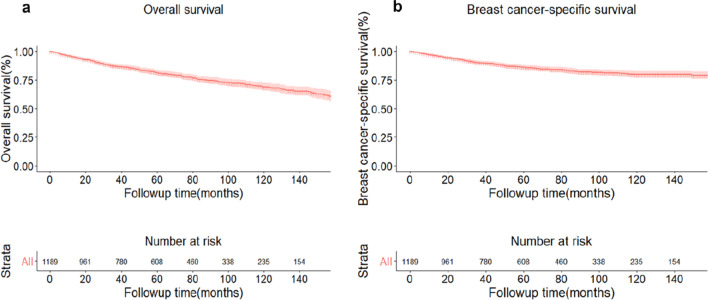
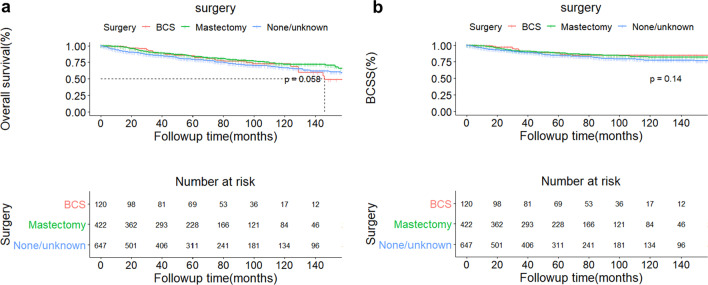
In the whole OBC cohort, the median follow-up duration was 62 months. Totally, 272 people died from all reasons. 168 died because of disease of breast cancer, among which 14 patients received BCS and 53 female received mastectomy. The 5-year and 10-year overall survival of the whole population was 81.60% (95% CI:79.20–84.10%) and 68.8% (95%CI: 65.30–72.40%), respectively (Fig. 1a). The 5-year and 10-year OBC-specific survival of the whole population was 86.10% (95% CI:83.90–88.30%) and 79.7% (95%CI: 76.80–82.80%), respectively (Fig. 1b). No matter OS and BCSS for the whole cohort, both of them failed to reach median survival timepoints. And both patients in BCS group and mastectomy group were followed up for a median time of 62 months. For population in BCS group, the 5-year and 10-year OS was 84.50% (95% CI 77.60–92.10%) and 72.60% (95% CI: 63.30–83.30%) (Fig. 2a); the 5-year and 10-year BCSS was 88.50% (95% CI 82.30–95.20%) and 84.40% (95% CI: 71.10–92.40%) (Fig. 2b). For mastectomy cohort, the 5-year and 10-year OS was 83.50% (95% CI 79.60–87.60%) and 71.90% (95% CI: 66.20–78.00%) (Fig. 2a); the 5-year and 10-year BCSS was 87.80% (95% CI 84.40–91.30%) and 82.00% (95% CI: 77.30–87.00%) (Fig. 2b). The median survival time of OS for BCS group was 12.17 months while median survival time for mastectomy group and median survival time of BCSS for BCS group were not reached (Fig. 2a, b).
Fig. 1.
Curves of overall survival (a) and breast cancer-specific survival (b) of the whole cohort of occult breast cancer patients
Fig. 2.
Curves of overall survival (a) and breast cancer-specific survival (b) of 3 cohorts of patients receiving BCS (pink), mastectomy (green) and no surgery (blue). The dotted line in (a) marked the median overall survival timepoint of patients receiving BCS. BCS breast-conserving surgery
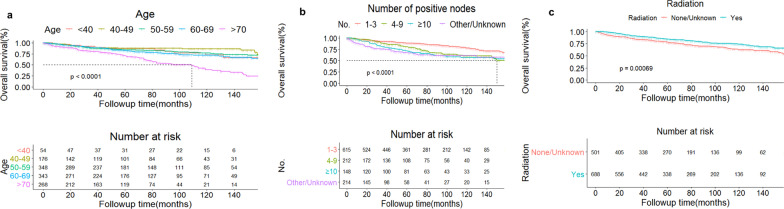
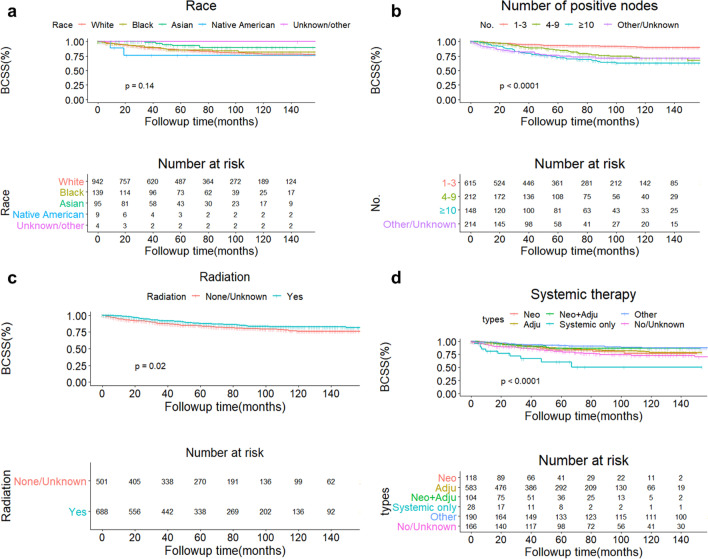
Univariate and multivariate COX regression analysis for OS and BCSS were conducted (Tables 3 and 4, respectively). Age, marital status, grade, N staging, number of positive lymph node, stage, radiation, chemotherapy, type of systemic therapy, tumor subtypes, ER and PR were factors significantly associated with OS according to univariate analysis (P < 0.10) (Table 3). By multivariate analysis, it was found that age, number of positive lymph nodes and radiation were independent predictive factors for OS (P < 0.05) (Table 4; Fig. 3a–c). For BCSS, age, race, grade, N staging, number of positive lymph node, stage, radiation, chemotherapy, type of systemic therapy, tumor subtypes, ER and PR were selected to enter multivariate analysis (P < 0.10) (Table 3). In multivariate analysis, independent predictive factors of BCSS included race, number of positive lymph node, radiation and types of systemic therapy (P < 0.05) (Table 4; Fig.4a–d). Patients older than 70 has a significant gloomy overall survival than that with age less than 40 (hazard ratio, HR = 2.15, 95% CI = 1.13–4.08). Compared with white OBC patients, Asian/Pacific-island patients tended to get a better BCSS (HR = 0.39, 95%CI = 0.16–0.97, P = 0.04) but native Americans had a relatively poor survival (HR = 4.25, 95% CI = 1.01–17.99, P = 0.04). More positive lymph node is associated with both poor OS and BCSS (P < 0.001). Patients receiving radiation tended to get a better prognosis no matter statistical analysis of OS or BCSS (Table 4).
Table 3.
Univariable analysis of OS and BCSS in OBC patients
| Variables | OS | BCSS | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Age (year) | 1.05 | 1.03–1.06 | < 0.0001 | 1.03 | 1.02–1.04 | < 0.001 |
| < 40 | Reference | Reference | ||||
| 40–49 | 0.63 | 0.31–1.31 | 0.22 | 0.82 | 0.34–1.97 | 0.66 |
| 50–59 | 0.93 | 0.49–1.77 | 0.83 | 0.99 | 0.44–2.20 | 0.98 |
| 60–69 | 1.14 | 0.60–2.16 | 0.68 | 1.10 | 0.50–2.45 | 0.81 |
| ≥ 70 | 2.69 | 1.44–5.01 | 0.002 | 2.03 | 0.93–4.46 | 0.08 |
| Race | ||||||
| White | Reference | Reference | ||||
| Black | 1.30 | 0.93–1.81 | 0.12 | 0.88 | 0.55–1.42 | 0.61 |
| Asian or Pacific Islander | 0.75 | 0.44–1.26 | 0.28 | 0.37 | 0.15–0.89 | 0.03 |
| American Indian/Alaska Native | 1.11 | 0.28–4.49 | 0.88 | 1.78 | 0.44–7.17 | 0.42 |
| Unknown/other | 8.34 × 10–7 | 0-INF | 0.99 | 7.65 × 10−7 | 0-INF | 0.99 |
| Marital status | ||||||
| Not married | Reference | Reference | ||||
| Married | 0.71 | 0.56–0.91 | 0.007 | 0.77 | 0.56–1.06 | 0.11 |
| Unknown | 1.25 | 0.69–2.26 | 0.47 | 1.31 | 0.63–2.72 | 0.47 |
| Grade | ||||||
| I/II | Reference | Reference | ||||
| III | 2.59 | 1.03–6.54 | 0.04 | 2.59 | 1.03–6.54 | 0.04 |
| IV | 0.99 | 0.12–8.52 | 1.00 | 0.99 | 0.12–8.52 | 1.00 |
| Unknown | 1.81 | 0.74–4.42 | 0.20 | 1.81 | 0.74–4.42 | 0.20 |
| Laterality | ||||||
| Right | Reference | Reference | ||||
| Left | 1.03 | 0.81–1.31 | 0.79 | 1.11 | 0.82–1.51 | 0.50 |
| Histology | ||||||
| Ductal carcinoma | Reference | Reference | ||||
| Lobular carcinoma | 0.85 | 0.44–1.62 | 0.61 | 1.24 | 0.59–2.60 | 0.57 |
| Mixed type | 0.69 | 0.28–1.70 | 0.43 | 0.77 | 0.24–2.47 | 0.67 |
| Other | 0.85 | 0.66–1.08 | 0.18 | 1.03 | 0.75–1.43 | 0.84 |
| N | ||||||
| N1mi | Reference | Reference | ||||
| N1 | 2.15 | 0.53–8.70 | 0.28 | 1.01 | 0.32–16.46 | 0.41 |
| N2 | 2.84 | 0.69–11.67 | 0.15 | 1.01 | 0.64–33.65 | 0.13 |
| N3 | 4.00 | 0.98–16.32 | 0.05 | 1.01 | 1.04–54.08 | 0.05 |
| No. of positive LN | ||||||
| 1–3 | Reference | Reference | ||||
| 4–9 | 1.82 | 1.32–2.52 | < 0.001 | 2.77 | 1.79–4.28 | < 0.001 |
| ≥ 10 | 2.22 | 1.59–3.10 | < 0.001 | 4.42 | 2.91–6.71 | < 0.001 |
| Other/Unknown | 1.01 | 1.99–3.77 | < 0.001 | 4.09 | 2.67–6.25 | < 0.001 |
| Stage | ||||||
| IB | Reference | Reference | ||||
| IIA | 2.15 | 0.53–8.70 | 0.28 | 1.01 | 0.32–16.46 | 0.4123 |
| IIIA | 2.84 | 0.69–11.63 | 0.15 | 1.01 | 0.63–33.51 | 0.1312 |
| IIIC | 4.02 | 0.98–16.40 | 0.05 | 1.01 | 1.04–54.37 | 0.0453 |
| Surgery | ||||||
| BCS | Reference | Reference | ||||
| Mastectomy | 0.82 | 0.53–1.26 | 0.36 | 1.08 | 0.60–1.94 | 0.80 |
| None/Unknown | 1.13 | 0.76–1.69 | 0.55 | 1.44 | 0.82–2.52 | 0.20 |
| Radiation | ||||||
| None/Unknown | Reference | Reference | ||||
| Yes | 0.66 | 0.52–0.84 | 0.0007 | 0.70 | 0.52–0.95 | 0.02 |
| Chemotherapy | ||||||
| Yes | Reference | Reference | ||||
| No/Unknown | 0.55 | 0.43–0.71 | < 0.001 | 0.73 | 0.52–1.02 | 0.07 |
| Type of systemic therapy | ||||||
| Neoadjuvant | Reference | Reference | ||||
| Adjuvant | 0.97 | 0.61–1.55 | 0.91 | 0.92 | 0.53–1.60 | 0.76 |
| Neoadjuvant + adjuvant | 0.53 | 0.24–1.15 | 0.11 | 0.73 | 0.32–1.66 | 0.45 |
| Only systemic therapy, no surgery | 3.42 | 1.71–6.83 | 0.0005 | 3.58 | 1.61–7.97 | 0.002 |
| Other | 0.27 | 0.37–1.08 | 0.09 | 0.52 | 0.27–1.04 | 0.06 |
| No/Unknown | 0.25 | 1.01–2.70 | 0.04 | 1.39 | 0.76–2.53 | 0.28 |
| Subtypes | ||||||
| Luminal A | Reference | Reference | ||||
| Luminal B | 0.62 | 0.30–1.27 | 0.19 | 0.69 | 0.28–1.66 | 0.40 |
| HER2 positive | 1.31 | 0.70–2.43 | 0.40 | 1.80 | 0.89–3.64 | 0.10 |
| Triple negative | 1.63 | 1.03–2.57 | 0.04 | 2.03 | 1.17–3.54 | 0.01 |
| Unknown | 1.28 | 0.91–1.81 | 0.16 | 1.52 | 0.98–2.35 | 0.06 |
| ER | ||||||
| Negative | Reference | Reference | ||||
| Borderline/Unknown | 1.08 | 0.73–1.61 | 0.70 | 1.18 | 0.73–1.90 | 0.50 |
| Positive | 0.71 | 0.55–0.92 | 0.009 | 0.59 | 0.43–0.81 | 0.001 |
| PR | ||||||
| Negative | Reference | Reference | ||||
| Borderline/Unknown | 1.08 | 0.76 -1.56 | 0.66 | 1.06 | 0.68–1.66 | 0.80 |
| Positive | 0.78 | 0.60–1.01 | 0.06 | 0.54 | 0.38–0.77 | 0.0007 |
| HER2 | ||||||
| Negative | Reference | Reference | ||||
| Positive | 0.74 | 0.46–1.19 | 0.21 | 0.85 | 0.49–1.47 | 0.56 |
| Unknown | 1.08 | 0.81–1.43 | 0.62 | 1.15 | 0.81–1.63 | 0.43 |
CI confidence interval, ER estrogen receptor, HER-2 human epidermal growth factor receptor-2, N N staging of OBC, PR progesterone receptor
Table 4.
Multivariable analysis of OS and BCSS in OBC patients
| Variables | OS | BCSS | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Age (year) | ||||||
| < 40 | Reference | |||||
| 40–49 | 0.57 | 0.27–1.19 | 0.13 | 0.64 | 0.26–1.56 | 0.32 |
| 50–59 | 0.82 | 0.43–1.58 | 0.56 | 0.82 | 0.36–1.85 | 0.64 |
| 60–69 | 1.04 | 0.55–1.99 | 0.90 | 0.97 | 0.43–2.18 | 0.93 |
| ≥ 70 | 2.15 | 1.13–4.08 | 0.02 | 1.52 | 0.67–3.41 | 0.31 |
| Race | ||||||
| White | Reference | Reference | ||||
| Black | – | – | – | 0.90 | 0.56–1.47 | 0.68 |
| Asian or Pacific Islander | – | – | – | 0.39 | 0.16–0.97 | 0.04 |
| American Indian/Alaska Native | – | – | – | 4.25 | 1.01–17.99 | 0.05 |
| Unknown/other | – | – | – | 1.23 × 10–6 | 0-INF | 0.99 |
| Marital status | ||||||
| Not married | Reference | – | – | – | ||
| Married | 0.80 | 0.62–1.02 | 0.07 | – | – | – |
| Unknown | 1.25 | 0.68–2.31 | 0.48 | – | – | – |
| Grade | ||||||
| I/II | Reference | Reference | ||||
| III | 0.90 | 0.51–1.59 | 0.71 | 1.71 | 0.65–4.47 | 0.27 |
| IV | 0.66 | 0.22–2.04 | 0.47 | 0.65 | 0.07–5.73 | 0.70 |
| Unknown | 0.70 | 0.41–1.19 | 0.18 | 1.26 | 0.50–3.16 | 0.63 |
| N | ||||||
| N1mi | Reference | Reference | ||||
| N1 | 1.34 × 1011 | 0-INF | 0.99 | 1.17 × 1013 | 0-INF | 1.00 |
| N2 | 1.72 × 106 | 0-INF | 0.99 | 4.04 × 107 | 0-INF | 1.00 |
| N3 | 3.44 | 0.79–14.92 | 0.10 | 4.47 | 0.58–34.38 | 0.15 |
| No. of positive LN | ||||||
| 1–3 | Reference | Reference | ||||
| 4–9 | 2.09 | 1.17–3.73 | 0.01 | 1.96 | 0.95–4.02 | 0.07 |
| ≥ 10 | 1.53 | 0.86–2.73 | 0.15 | 2.28 | 1.16–4.46 | 0.02 |
| Other/Unknown | 2.47 | 1.76–3.47 | < 0.001 | 3.26 | 2.07–5.13 | < 0.001 |
| Stage | ||||||
| IB | Reference | Reference | ||||
| IIA | 1.39 × 10–11 | 0-INF | 0.99 | 1.45 × 10–13 | 0-INF | 1.00 |
| IIIA | 9.80 × 10–7 | 0-INF | 0.99 | 6.46 × 10–8 | 0-INF | 1.00 |
| IIIC | NA | NA | NA | NA | NA | NA |
| Radiation | ||||||
| None/Unknown | Reference | Reference | ||||
| Yes | 0.68 | 0.52–0.88 | 0.003 | 0.63 | 0.45–0.88 | 0.007 |
| Chemotherapy | ||||||
| Yes | Reference | Reference | ||||
| No/Unknown | 0.87 | 0.52–1.44 | 0.59 | 1.08 | 0.51–2.28 | 0.84 |
| Type of systemic therapy | ||||||
| Neoadjuvant | Reference | Reference | ||||
| Adjuvant | 0.93 | 0.58–1.50 | 0.77 | 1.00 | 0.57–1.75 | 0.99 |
| Neoadjuvant + adjuvant | 0.50 | 0.23–1.10 | 0.08 | 0.69 | 0.30–1.59 | 0.38 |
| Only systemic therapy, no surgery | 1.88 | 0.90–3.90 | 0.09 | 2.40 | 1.03–5.60 | 0.04 |
| Other | 0.55 | 0.32–0.96 | 0.04 | 0.46 | 0.23–0.93 | 0.03 |
| No/Unknown | 1.02 | 0.51–2.06 | 0.95 | 1.23 | 0.48–3.17 | 0.67 |
| Subtypes | ||||||
| Luminal A | Reference | Reference | ||||
| Luminal B | 0.63 | 0.31–1.31 | 0.22 | 0.65 | 0.26–1.60 | 0.35 |
| HER2 positive | 1.10 | 0.54–2.22 | 0.79 | 1.31 | 0.56–3.02 | 0.53 |
| Triple negative | 1.42 | 0.81–2.50 | 0.22 | 1.66 | 0.81–3.40 | 0.17 |
| Unknown | 1.53 | 1.02–2.27 | 0.04 | 1.64 | 0.97–2.76 | 0.06 |
| ER | ||||||
| Negative | Reference | Reference | ||||
| Borderline/Unknown | 1.13 | 0.51–2.54 | 0.76 | 1.63 | 0.63- 4.18 | 0.31 |
| Positive | 0.78 | 0.53–1.14 | 0.20 | 0.87 | 0.52–1.43 | 0.57 |
| PR | ||||||
| Negative | Reference | Reference | ||||
| Borderline/Unknown | 0.85 | 0.41–1.79 | 0.68 | 0.72 | 0.29–1.74 | 0.46 |
| Positive | 0.99 | 0.69–1.43 | 0.98 | 0.78 | 0.49–1.24 | 0.30 |
BCSS breast cancer-specific survival, CI confidence interval, ER estrogen receptor, HR hazard ratio, INF infinite, N N staging of OBC, NA not available, OS overall survival, PR progesterone receptor
Fig. 3.
Curves of overall survival according to 5 groups based on different ages (a), 4 groups based on different number of positive nodes (b) and 2 groups based on different radiation status (c). The dotted line in Fig. 3A marked the median overall survival timepoint of patients older than 70 (a) and patients with 4–9 positive nodes (b)
Fig. 4.
Curves of BCSS according to 5 groups based on different races (a), 4 groups based on different number of positive nodes (b), 2 groups based on different radiation status (c) and 6 groups based on different patterns of systemic therapy (d). Adju adjuvant systemic therapy, BCSS breast cancer-specific survival, Neo neoadjuvant systemic therapy
Discussion
In our study, we collected nearly 1200 OBC patients for 15 years and the data is the most up-to-date one. We found that OBC was predominated by elderly population. Majority of OBC patients were diagnosed at relative early stage (Stage IIA) and Luminal A type of OBC took up nearly 30% of all subjects. Radiotherapy and chemotherapy were common and important treatments for OBC patients. In this study, we found that older patients tended to receive less-invasive surgical intervention. Patients in mastectomy group were found more involved lymph nodes thus more patients were diagnosed as advanced stage OBC (stage IIIA and IIIC) compared with subjects in BCT group. Patients treated by radiotherapy had a significant difference between two surgical groups, in which more than 60% subjects in BCT group were accepted this therapy but only half of women took radiotherapy in mastectomy groups (P < 0.001). Speaking of survival analysis, the whole OBC cohort generally had optimistic prognosis. 5-year OS and BCSS exceeding 80% and both of the survival analysis did not reach the timepoint of median survival. When the whole cohort was grouped according to types of surgery, both of the 5 and 10-year BCSS in BCS and mastectomy group were higher than 5 and 10-year OS in the same group. However, no significant difference was found no matter in OS or BCSS between BCS and mastectomy group (P = 0.058 for OS and P = 0.14 for BCSS). For Cox regression analysis, we found that age was an independent predictor for OS and higher age meant a poorer survival. Race served as an independent predictor for BCSS and Asian/Pacific natives has a better clinical outcome while native Americans had a relatively poor survival. Radiotherapy was clinically beneficial to OBC patients, no matter according to OS or BCSS. But no significant benefit was found between survival and systemic therapy. This finding was also supported by the result derived from multivariate analysis of tumor subtypes immunohistochemistry: ER, PR, HER-2 and tumor subtypes were not independent predictors for neither OS or BCSS.
Although NCCN guidelines stated that mastectomy was recommended for OBC patients, the optimal surgical therapy for the ipsilateral breast in OBC has been controversial [14]. According to a meta-analysis collecting 7 qualified studies, no significant difference in overall survival between ALND plus mastectomy and ALND combined with breast radiation [7]. Likewise, in a clinical trial carried on by Rueth’s team, no significant survival disparity was found between BCT and mastectomy (P = 0.7). No local and distal recurrence occurred within 5 years, either [15]. A clinical study based on National Cancer Database collected 190 patients diagnosed from 2004 to 2014 [16]. Treatment strategies included mastectomy alone, radiation alone and mastectomy combined with radiation. No significant difference in OS was found between each two of these three strategies (mastectomy vs. radiation, P = 0.650; mastectomy + radiation vs. mastectomy, P = 0.393; mastectomy vs. radiation, P = 0.872). These findings are also supported by two retrospective study from China and Korean respectively, suggesting that mastectomy was not more clinically beneficial than BCT [8, 17]. Even some studies found that a less-intensive approach would be beneficial for OBC patient. In another research also based on National Cancer Database, 1231 OBC patients were grouped into MRM± radiotherapy (N = 592), radiotherapy + ALND (N = 342), ALND alone (N = 106) and no breast surgery (N = 191). They found that patients treated by ALND and radiotherapy have significantly better OS compared with patients received MRM with/without radiotherapy (HR = 0.475, 95% CI 0.306-0.736, P = 0.001). Multivariate analysis proved that ALND with radiation was an independent protective predictor of OS (HR 0.509, 95% CI 0.321-0.808, P = 0.004). However, a retrospective study performed by Wang et al. drew an opposite conclusion. Their study included 51 OBC cases from a single center, in which 38 had ALND plus Mastectomy and the other 13 patients had ALND only [18]. 28 of 38 patients having mastectomy had been found the primary tumor in the breast by pathology. Recurrence rate in mastectomy group was 26%, much lower than that in ALND-only group (77%). Patients with mastectomy had a significantly promising disease-free survival and overall survival compared with patients merely received ALND (P < 0.001). Hence, this study drew a conclusion that mastectomy based on ALND is necessary for OBC patients.
For radiotherapy, we found that radiotherapy is clinically beneficial for OBC. This finding is in line with majority of previous clinical research. A study from the UK collected 29 OBC patients across more than 25 years. Median follow-up time was 44 months. Amongst, 16 patients got local radiotherapy and 13 patients did not receive local radiation. Local recurrence rate was 12.50% in radiation group while 69% of patients in non-radiotherapy group got local recurrence (P = 0.02). Moreover, radiotherapy could significantly improve relapse-free survival (HR = 0.31, P = 0.04) and local relapse-free survival (HR = 0.09, P = 0.004). Overall survival, unfortunately, was not positively impacted by radiation therapy [19]. In 2010, another research conducted by team from UK led by Masinghe reported 53 OBC patients diagnosed from 1974 to 2003. Similar to the above studies, this study was also a mixture of clinical and pathological OBC. All the patients received axillary surgery but only 25 of them (47%) had ALND. 5-year recurrence rate of ipsilateral breast was 16.40% (95% CI 4.30–28.50%) in population receiving radiotherapy, lower than that in non-radiation population (35.80%, 95% confidence interval 7.60–64.10%). Similar results were presented in 10-year ipsilateral relapse rate, where radiotherapy group was 23.20% (95% CI 8.90–37.60%) and non-radiotherapy group was 51.90% (95% CI 17.40–86.40%). Furthermore, patients receiving radiotherapy had a significantly higher 5-year and 10-year BCSS rate compared with patients without radiation [P = 0.0073; 5-year: 72.80% (95% CI 59.10–86.50%) vs. 58.30% (95% CI 30.40–86.20%); 10-year: 66.20% (95% CI 51.00–81.50%) vs. 14.6% (0.00–43.30%), respectively). Radiotherapy was the only significant predictor of survival [20]. In a Korean study, 3 of 66 OBC patients did not receive breast radiation. In 15 patients received BCS therapy (blind local excision of the breast), no positive finding of tumor was identified in all these patients. The local recurrence rate was significantly lower in patients without breast radiation than that in patients with breast radiation (6.30% vs. 66.70%; P = 0.02). Breast radiation could significantly improve 8-year disease-free survival in patients with breast radiation compared with patients without ipsilateral breast radiation (89.50% vs. 50.00%, P = 0.02). The shortcoming of this study was that number of patients without breast radiation was much lower than that of radiation group so that the conclusion should be further examined [21]. Different from studies above, research team from Memorial Sloan Kettering Cancer Center gave an opposite answer for this topic. In this study, all the 38 OBC patients were evaluated by MRI before recruited, stricter examination than the above studies. Among the subjects, 25 patients received ALND + whole breast radiotherapy while 13 patients got MRM (6 patients got chest-wall radiation) [22]. This totally met the criteria of NCCN guideline mentioned before. To make the population in different groups more homogenous, all the patients got chemotherapy. Surprisingly, no recurrence occurred in lymph node during a median follow-up of 7 years. Even relapse of two breast occurred in patients receiving ALND combined with whole breast radiotherapy. No local recurrence occurred in patients with MRM. Similar rates of distal failure were between the two groups (7.70% in MRM cohort vs. 8.00% in ALND + radiotherapy group). Nevertheless, the conclusion of this study should be further discussed because of a small sample size.
For systemic therapy, especially chemotherapy for OBC patients, no specific guidelines, recommendation and consensus have been laid out. Conventionally, neoadjuvant chemotherapy was commonly used for locally advanced/ inoperable/inflammatory breast cancers [23]. This kind of regimen can degrade the tumor stage and serve to evaluate treatment response [24]. Several studies reported results of effectiveness of neoadjuvant chemotherapy in treating of OBC. A case series from China including 5 OBC patients suggested an effective neoadjuvant chemotherapy with pathological complete response (PCR) rate reached 80% (4/5). Among the 4 patients, they were all received mastectomy followed by radiotherapy, two of whom also underwent endocrine therapy. Another study led by Rueth et.al collected 36 OBC patients. Nearly 95% of subjects (34/36) received chemotherapy and the rate of neoadjuvant therapy was up to almost 70% (25/36). In 33 patients with ALND, 15 of them got neoadjuvant therapy. Thus, the PCR rate of neoadjuvant therapy was about 60%, which supported the effectiveness of neoadjuvant therapy in the case series above [15]. In the most recent study with the largest sample size, Cohen et.al collected 684 OBC patients and 214 (31.3%) of them received neoadjuvant chemotherapy. PCR was recognized in 55 of 214 (26%) patients took neoadjuvant therapy. In our study, counterintuitively, we found that patients without chemotherapy tended to have a better prognosis, partly because patients administered with chemotherapy had a relatively higher tumor stage. In our study, 21% cases with stage-IIIA OBC and 19% cases with stage-IIIC cases in chemotherapy group patients, whereas both of the stage-IIIA and stage-IIIC OBC patients accounted for 13% in the other group (P = 0.002, χ2test). As the number of studies focusing on neoadjuvant chemotherapy was small and the PCR rate varied among these studies by virtue of sample size in huge disparity, it is still debatable whether systemic therapy is beneficial for OBC patients.
In our study, several limitations are necessary to be noticed: (1) For systemic therapy, current database is shortage of specific data such as the chemotherapy formula and dosage of each drug. Thus, we could not get more information about chemotherapy and its relationship with survival. Moreover, data of endocrine therapy is not available in current version of SEER database; (2) SEER database predominately describes clinical characteristic of American citizens including white and black citizens. Data of Asian OBC patients were insufficient so that it is unclear whether the results of this analysis were also generalized to Asian population; (3) Because the data of HER-2 status originates from 2010, it is not available in the cases diagnosed before 2010, which impacted the descriptive results and following analysis about HER-2 status, tumor subtypes as well as their associations with OS and BCSS; (4) For radiation part in SEER database, we did not know the exact number of patients unknown information of radiation and we could not separately summarize number of patients with or without radiation. Hence, some sort of deviation of the survival result might be existed. We hope that more clear information can be retrieved in updated edition of this database.
Conclusion
Majority of OBC patients had a relatively optimistic prognosis. A less-intensive surgical therapy is acceptable for OBC treatment without cost on OS or BCSS. Radiotherapy was beneficial for clinical outcomes of OBC patients. More information of systemic therapy is expected to be obtained to refine the treatment recommendation for OBC.
Acknowledgements
We thanked Jin Bian and Junwei Zhang for help and advice of data management. We also thanked support team of SEER database for answering questions about the usage of SEERstat and related knowledge of different datasets.
Abbreviations
- ALND
Axillary lymph node dissection
- BCS
Breast-conserving surgery
- BCT
Breast-conserving therapy
- BCSS
Breast-cancer specific survival
- CI
Confidence intervals
- ER
Estrogen receptor
- HER-2
Human epidermal factor receptor-2
- HR
Hazard ratio
- MRI
Magnetic resonance imaging
- MRM
Modified radical mastectomy
- NCCN
National Comprehensive Cancer Network
- OBC
Occult breast cancer
- OS
Overall survival
- PCR
Pathological complete response
- PR
Progesterone receptor
- SEER
Surveillance, Epidemiology, and End Results
Authors' contributions
ZJZ and XL put forward the idea of this study and ZJZ wrote the manuscript. ZJZ, TZ and YY analyzed and interpreted data of OBC patients. All authors read and approved the final manuscript.
Funding
This work was supported by International Science and Technology Cooperation Projects (2016YFE0107100 and 2015DFA30650), CAMS Clinical and Translational Medicine Research Funds (2019XK320006), CAMS Innovation Fund for Medical Science (CIFMS) (2017-I2M-4-003 and 2018-I2M-3-001), Capital Special Research Project for Health Development (2014-2-4012), Beijing Natural Science Foundation (L172055 and 7192158), the Fundamental Research Funds for the Central Universities (3332018032) and National Ten-thousand Talent Program.
Availability of data and materials
The datasets generated and integrated are available in Surveillance, Epidemiology, and End Results (SEER) database (https://seer.cancer.gov/). We selected the SEER research plus data, 18 Registries, Nov 2020 Sub (2000–2018) to perform further analysis. Software for data retrieving and integration is SEER* Stat 8.3.9.1, which is successfully downloaded from official website of SEER database (https://seer.cancer.gov/seerstat/).
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declared that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zijun Zhao, Email: fallingflower@163.com.
Xin Lu, Email: luxinln@163.com.
References
- 1.Halsted WSI. The results of radical operations for the cure of carcinoma of the breast. Ann Surg. 1907;46(1):1–19. doi: 10.1097/00000658-190707000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vlastos G, Jean ME, Mirza AN, et al. Feasibility of breast preservation in the treatment of occult primary carcinoma presenting with axillary metastases. Ann Surg Oncol. 2001;8(5):425–431. doi: 10.1007/s10434-001-0425-6. [DOI] [PubMed] [Google Scholar]
- 3.Fayanju OM, Jeffe DB, Margenthaler JA. Occult primary breast cancer at a comprehensive cancer center. J Surg Res. 2013;185(2):684–689. doi: 10.1016/j.jss.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao KL, Liu Y, Scherpelz KP, et al. Occult primary breast cancer presenting with brachial plexopathy: a case report. SAGE Open Med Case Rep. 2021;9:2050313x20985646. doi: 10.1177/2050313X20985646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baron PL, Moore MP, Kinne DW, et al. Occult breast cancer presenting with axillary metastases. Updated management. Arch Surg. 1990;125(2):210–214. doi: 10.1001/archsurg.1990.01410140088014. [DOI] [PubMed] [Google Scholar]
- 6.Yang H, Li L, Zhang M, et al. Application of neoadjuvant chemotherapy in occult breast cancer: five case reports. Medicine (Baltimore) 2017;96(40):e8200. doi: 10.1097/MD.0000000000008200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Macedo FI, Eid JJ, Flynn J, et al. Optimal surgical management for occult breast carcinoma: a meta-analysis. Ann Surg Oncol. 2016;23(6):1838–1844. doi: 10.1245/s10434-016-5104-8. [DOI] [PubMed] [Google Scholar]
- 8.Sohn G, Son BH, Lee SJ, et al. Treatment and survival of patients with occult breast cancer with axillary lymph node metastasis: a nationwide retrospective study. J Surg Oncol. 2014;110(3):270–274. doi: 10.1002/jso.23644. [DOI] [PubMed] [Google Scholar]
- 9.Walker GV, Smith GL, Perkins GH, et al. Population-based analysis of occult primary breast cancer with axillary lymph node metastasis. Cancer. 2010;116(17):4000–4006. doi: 10.1002/cncr.25197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology, Breast Cancer, version 2. 2022. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.
- 11.Blanchard DK, Shetty PB, Hilsenbeck SG, et al. Association of surgery with improved survival in stage IV breast cancer patients. Ann Surg. 2008;247(5):732–738. doi: 10.1097/SLA.0b013e3181656d32. [DOI] [PubMed] [Google Scholar]
- 12.Olson JA, Jr, Morris EA, Van Zee KJ, et al. Magnetic resonance imaging facilitates breast conservation for occult breast cancer. Ann Surg Oncol. 2000;7(6):411–415. doi: 10.1007/s10434-000-0411-4. [DOI] [PubMed] [Google Scholar]
- 13.Varadarajan R, Edge SB, Yu J, et al. Prognosis of occult breast carcinoma presenting as isolated axillary nodal metastasis. Oncology. 2006;71(5–6):456–459. doi: 10.1159/000107111. [DOI] [PubMed] [Google Scholar]
- 14.Ofri A, Moore K. Occult breast cancer: where are we at? Breast. 2020;54:211–215. doi: 10.1016/j.breast.2020.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rueth NM, Black DM, Limmer AR, et al. Breast conservation in the setting of contemporary multimodality treatment provides excellent outcomes for patients with occult primary breast cancer. Ann Surg Oncol. 2015;22(1):90–95. doi: 10.1245/s10434-014-3991-0. [DOI] [PubMed] [Google Scholar]
- 16.Tsai C, Zhao B, Chan T, et al. Treatment for occult breast cancer: a propensity score analysis of the National Cancer Database. Am J Surg. 2020;220(1):153–160. doi: 10.1016/j.amjsurg.2019.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He M, Tang LC, Yu KD, et al. Treatment outcomes and unfavorable prognostic factors in patients with occult breast cancer. Eur J Surg Oncol. 2012;38(11):1022–1028. doi: 10.1016/j.ejso.2012.08.022. [DOI] [PubMed] [Google Scholar]
- 18.Wang X, Zhao Y, Cao X. Clinical benefits of mastectomy on treatment of occult breast carcinoma presenting axillary metastases. Breast J. 2010;16(1):32–37. doi: 10.1111/j.1524-4741.2009.00848.x. [DOI] [PubMed] [Google Scholar]
- 19.Shannon C, Walsh G, Sapunar F, et al. Occult primary breast carcinoma presenting as axillary lymphadenopathy. Breast. 2002;11(5):414–418. doi: 10.1054/brst.2002.0455. [DOI] [PubMed] [Google Scholar]
- 20.Masinghe SP, Faluyi OO, Kerr GR, et al. Breast radiotherapy for occult breast cancer with axillary nodal metastases—does it reduce the local recurrence rate and increase overall survival? Clin Oncol (R Coll Radiol) 2011;23(2):95–100. doi: 10.1016/j.clon.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Kim H, Park W, Kim SS, et al. Outcome of breast-conserving treatment for axillary lymph node metastasis from occult breast cancer with negative breast MRI. Breast. 2020;49:63–69. doi: 10.1016/j.breast.2019.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCartan DP, Zabor EC, Morrow M, et al. Oncologic outcomes after treatment for MRI occult breast cancer (pT0N+) Ann Surg Oncol. 2017;24(11):3141–3147. doi: 10.1245/s10434-017-5965-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaufmann M, von Minckwitz G, Bear HD, et al. Recommendations from an international expert panel on the use of neoadjuvant (primary) systemic treatment of operable breast cancer: new perspectives 2006. Ann Oncol. 2007;18(12):1927–1934. doi: 10.1093/annonc/mdm201. [DOI] [PubMed] [Google Scholar]
- 24.Kaufmann M, von Minckwitz G, Mamounas EP, et al. Recommendations from an international consensus conference on the current status and future of neoadjuvant systemic therapy in primary breast cancer. Ann Surg Oncol. 2012;19(5):1508–1516. doi: 10.1245/s10434-011-2108-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and integrated are available in Surveillance, Epidemiology, and End Results (SEER) database (https://seer.cancer.gov/). We selected the SEER research plus data, 18 Registries, Nov 2020 Sub (2000–2018) to perform further analysis. Software for data retrieving and integration is SEER* Stat 8.3.9.1, which is successfully downloaded from official website of SEER database (https://seer.cancer.gov/seerstat/).