Abstract
Purpose
We report 2 cases of ischemic retinal events occurring soon after administration of the Moderna and Johnson & Johnson/Janssen COVID-19 vaccines. To our knowledge, these are the first reports of isolated ischemic retinal events occurring after COVID-19 vaccination.
Observations
A 57-year-old female had new onset floaters of the left eye within days of her second Moderna COVID-19 vaccination, which progressively worsened prompting her to present for evaluation. She was diagnosed with a branch retinal vein occlusion in the left eye. A 20-year-old female presented with persistent central scotomata in both eyes, which she first noticed two days after her Johnson & Johnson/Jannsen COVID-19 vaccination. She was diagnosed with acute macular neuroretinopathy of both eyes.
Conclusions and Importance
The potential side effects of COVID-19 vaccines are still being established; however, there has been concern over pro-thrombotic events with these vaccines, with most concerns directed toward the Johnson & Johnson vaccine. We observed likely transient pro-thrombotic retinal milieu in patients who received these vaccines though it remains unclear whether there is a shared mechanism between systemic response to the COVID-19 spike protein and the highly pro-thrombotic state seen in COVID-19 infections. In the case of our patients, we postulate their immunologic responses to the vaccines – and possibly a resultant pro-thrombotic state – may have precipitated their ischemic retinal events. We thus recommend that patients with ocular symptoms after COVID-19 vaccination undergo comprehensive ophthalmologic evaluation.
Keywords: COVID-19 vaccine, Moderna Pfizer, J&J, Johnson & Johnson Janssen, SARS-CoV-2, Vaccination
1. Introduction
Vaccination against the SARS-CoV-2 (COVID-19) virus represents one of the largest public health vaccination efforts in global history, with vaccines from 3 manufacturers—Pfizer-BioNTech (Mainz, Germany), Moderna (Cambridge, MA, USA), and Johnson & Johnson's Janssen (New Brunswick, New Jersey, USA)—currently approved in the United States under Emergency Use Authorization by the Food and Drug Administration (FDA).1 These vaccines have demonstrated dramatic reduction of morbidity and mortality from COVID-19 and are an invaluable tool in returning to pre-pandemic life. Despite excellent phase III safety data, the full real-world, phase IV, safety profile of these vaccines is not yet known. For example, from April 13 – April 25, 2021, the Centers for Disease Control (CDC) suspended use of the Johnson & Johnson's vaccine due to reports for rare thrombosis and thrombocytopenia syndromes, nearly all occurring in women under age 50.2 Despite reports of systemic ischemic events after COVID-19 vaccination, there is limited data on ocular sequelae due to the same.
2. Case report 1
A 57-year-old female with a history of hypertension and dry eye syndrome presented with flashes and floaters in her left eye for three weeks duration. Her medications included fluticasone, hydrochlorothiazide, fexofenadine, and artificial tears. She had no known allergies. On April 12, 2021, she received the second dose of the Moderna vaccine and had a fever followed by the onset of floaters, which progressively worsened prompting evaluation three weeks later. Her visual acuity was 20/20 in each eye; intraocular pressures were unremarkable; there was no relative afferent pupillary defect; and her visual fields were full to confrontation. Dilated examination of the right eye was unremarkable; dilated examination of the left eye revealed a supero-temporal branch retinal vein occlusion (BRVO) with superficial intraretinal hemorrhages scattered along the superior arcade without macular edema (Fig. 1). Her blood pressure at her most recent primary care visit was 124/77 mmHg. Observation was recommended given absence of macular edema and preserved visual acuity. Macular edema developed and visual acuity declined to 20/30 at two months after initial presentation for which she was treated with intravitreal aflibercept monthly for 6 months. Visual acuity at most recent follow-up was 20/25, and OCT demonstrated persistent foveal-sparing macular edema; she did not receive a booster vaccine.
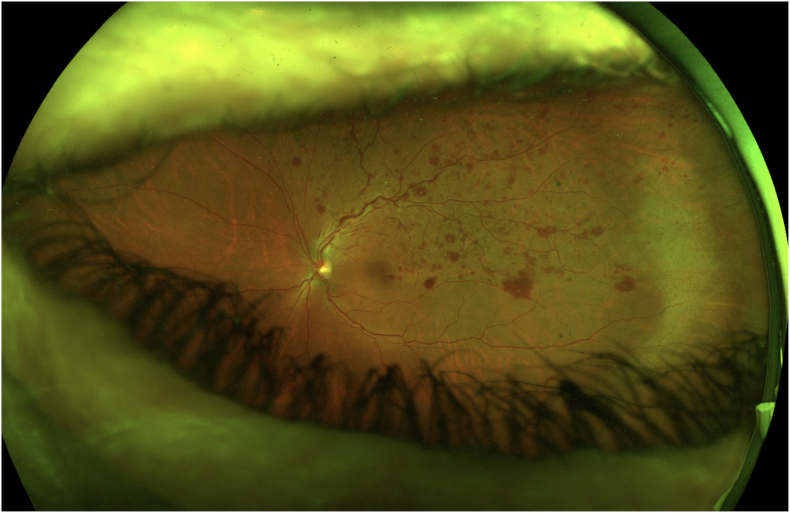
Fig. 1.
Widefield pseudocolor fundus photograph of the left eye demonstrating supero-temporal scattered intraretinal hemorrhages, arteriovenous nicking, and marked venous tortuosity, indicative of branch retinal vein occlusion.
3. Case report 2
A 20-year-old Caucasian female with a history of allergic rhinitis and myopia presented with 1 week of new, persistent central scotomata in both eyes (Fig. 2 – Amsler Grid). Her medications included fluticasone, loratadine, and norgestimate-ethinyl estradiol. She had no known allergies. The patient reported receiving a Johnson & Johnson/Janssen (J&J) vaccine on April 12, 2021, 8 days prior to presentation and 1 day prior to the CDC announcement temporarily halting use of the J&J vaccination. One day after vaccination, she developed subjective fever, chills, myalgias, and headaches; symptoms responded well to oral ibuprofen. The following day, she noted new central blind spots in both eyes. She discontinued her birth control medication due to her concern for thrombosis. An MRI brain without contrast was ordered by her primary care physician and was without acute abnormalities.
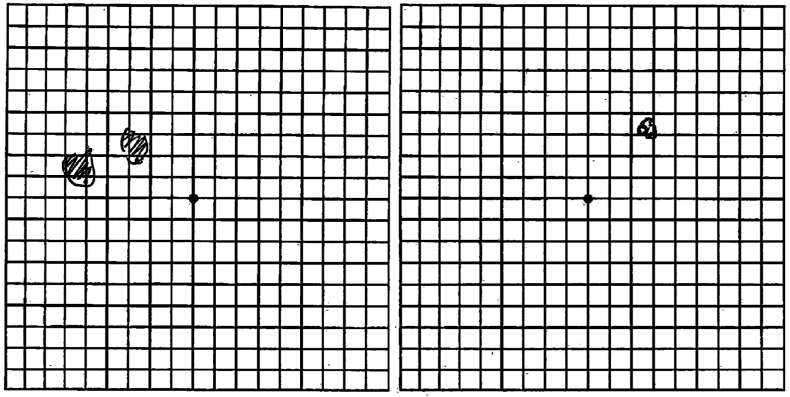
Fig. 2.
Amsler grid drawings by patient illustrating her perceived visual field deficits in the left (left image) and right (right image) eyes.
At presentation, her visual acuity with correction was 20/20 in each eye. Her intraocular pressures were normal; her pupils reacted briskly to light, and there was no relative afferent pupillary defect. Her peripheral visual fields were full to confrontation; however central visual field testing with Amsler grid revealed central defects in both eyes (Fig. 2). Her extraocular movements of both eyes were intact. Her anterior segment examination was normal, with no evidence of inflammation. Dilated fundus examination was normal; there were no clinically observable ocular findings to explain her visual symptoms (Fig. 3).
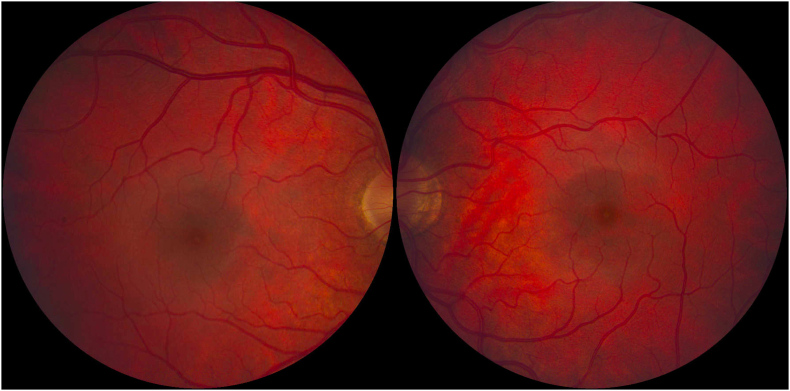
Fig. 3.
Normal appearing 60-degree color fundus photos of the right (left image) and left (right image) eyes. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
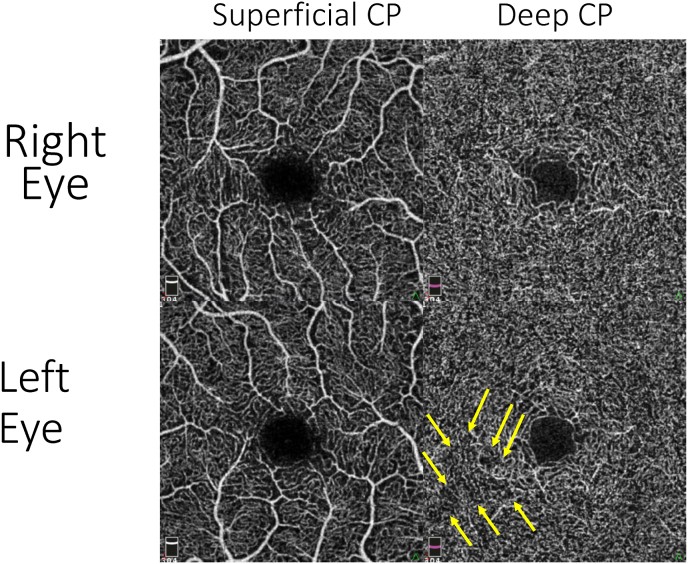
Multimodal imaging was obtained. Color fundus, autofluorescence, and red-free photos were unremarkable in both eyes. Near-infrared fundus photos revealed hyporeflective, well-defined, teardrop-shaped lesions, with spectral domain optical coherence tomography (SD-OCT) demonstrating corresponding ellipsoid zone disruption (Fig. 4). These lesions perfectly corresponded to her Amsler grid findings (i.e., supero-temporal scotoma in the right eye corresponding to an inferonasal macular lesion in the right eye and two supero-temporal scotomata corresponding to two inferonasal macular lesions in the left eye). Optical coherence tomography angiography (OCT-A) demonstrated a trace perfusion defect in the deep capillary plexus of the left eye and slight alteration of the foveal avascular zone in both eyes (Fig. 5).
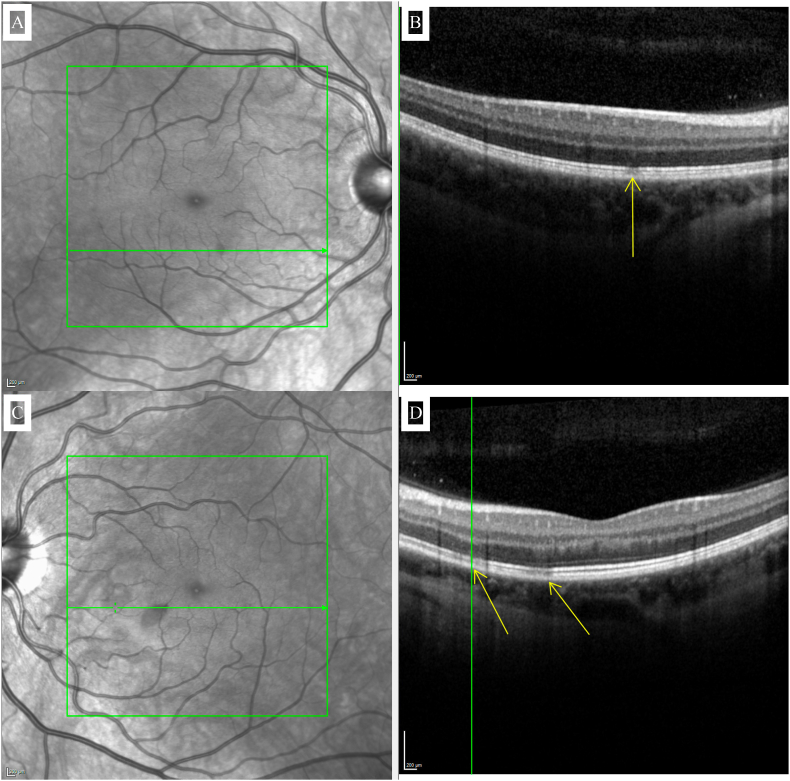
Fig. 4.
Near-infrared reflectance images (insets A and C) demonstrating well-defined, hyporeflective, teardrop-shaped lesions of the right and left eyes respectively with corresponding ellipsoid zone disruptions evident on spectral domain-OCT highlighted by the arrows (insets B and D) at corresponding locations of the right and left eyes respectively.
Fig. 5.
Optical coherence tomography-angiography demonstrating trace perfusion defect in deep capillary plexus of the left eye in the inferonasal macula corresponding to the AMN lesion (arrows) and alteration of the foveal avascular zone of both eyes.
The patient was diagnosed with acute macular neuroretinopathy (AMN) of both eyes, and further history was obtained to identify other potential risk factors for the development of AMN. The patient reported sparse caffeine use (1 cup of coffee every 2–3 days) but had self-discontinued caffeine after researching her symptoms. She denied any recent infections, trauma, and confirmed that she was not on any sympathomimetic agents. There was no family history of autoimmunity or coagulopathy.
The patient was advised to abstain from coffee and oral birth control for the time-being. At one and three months after initial presentation, visual acuity remained 20/20 and her scotomata were progressively less subjectively noticeable though not completely resolved. She did not receive further COVID-19 vaccination.
4. Discussion
While COVID-19 infection is known to cause ocular ischemic events as consequence of systemic vascular derangements, isolated ischemic retinal events attributed to the COVID-19 vaccination have not been previously described.
A broad range of potential ocular side effects of all three currently approved COVID-19 vaccines have been reported in the Centers for Disease Control and Prevention's Vaccine Adverse Event Reporting System (VAERS). The VAERS database contains unverified reports submitted voluntarily by healthcare providers, vaccine manufacturers, and the public. Inclusion of a symptom in the VAERS database linked to a vaccine is not documentation that the symptom was caused by the vaccine but is simply a report that the event occurred in temporal proximity to vaccine administration. As of the moment of this writing, there are 7,537 reports of blurred vision and 4,372 reports of visual impairment potentially related to the three currently approved COVID-19 vaccines in the United States, which account for 0.9% and 0.5% of the currently filed 799,732 events.3 We take these reports as a suggestion that COVID-19 vaccines may have ocular side effects that are currently not well understood.
Retinal vein occlusions are relatively common with the 5-year incidence of branch retinal vein occlusions estimated at 0.6% based on the Beaver Dam Eye Study.4 Risk factors for BRVO include increasing age, hypertension, hyperlipidemia, hypercoagulable disorders, glaucoma, and use of various medications including oral contraceptives and COX-2 inhibitors.5 Presentation is variable ranging from asymptomatic to central or peripheral visual deficits with funduscopy findings including flame hemorrhages, dot and blot hemorrhages, cotton wool spots, hard exudates, retinal edema, and/or dilated tortuous veins.5 The pathogenesis of BRVO is thought to involve a combination of venous compression at arteriovenous crossings, degenerative venular changes, and hypercoagulability.
Acute macular neuroretinopathy is a rare condition thought to arise from ischemia of the deep retinal capillary plexus6; between 1975 and 2014, 101 cases were reported in the literature.7 Patients present with acute, symptomatic paracentral scotomas with preserved visual acuity; 54% of cases are bilateral. Typically, patients are young, Caucasian, and female with 76% of cases being in patients ages 11 through 40 (mean 29.5 years) and 84% of cases being in females. Classically, the diagnosis manifests with teardrop or wedge-shaped reddish-brown macular lesions with their apices toward the fovea though these are only observed in 24% of cases.7
Outer retinal capillary nonperfusion, decreased vessel density, and alteration of the foveal avascular zone morphology have all been identified in eyes with AMN.8,9 Reduction in flow of the deep capillary plexus in the retina's outer plexiform layer has also been shown.10, 11, 12 Several environmental triggers have been identified: infection or febrile illness (48%), oral contraceptives (36%), use of epinephrine or ephedrine (8%), severe bodily trauma (6%), systemic shock (5%), and intravenous contrast (2%). AMN has also been reported after the influenza vaccination.13
The potential side effects of COVID-19 vaccines are still being established as real-world phase IV safety data is collected. However, there has been concern over pro-thrombotic events with these vaccines, with most concerns directed toward the J&J vaccine.14, 15, 16 Numerous cases of AMN and retinal vein occlusion possibly related to recent COVID-19 vaccination have been reported since our initial observations, though the majority of these reports pertain to the Pfizer and AstraZeneca vaccines – see Table 1, Table 2.
Table 1.
Summary of reported cases of acute macular neuroretinopathy occurring after COVID-19 vaccination.
| Publication Date | Diagnosis | Age | Sex | Hormonal Birth Control Use? | Vaccine | Onset of Symptoms After Vaccine |
|---|---|---|---|---|---|---|
| September 1, 202117 | AMN (bilateral) | 20 | Female | Vaginal hormonal contraception | Pfizer | 2 days |
| September 23, 202118 | AMN (bilateral) | 23 | Female | Oral contraception | AstraZeneca | 1 day |
| November 17, 202119 | AMN (bilateral) | 21 | Female | No | AstraZeneca | 3 days |
| January 1, 202220 | AMN (unilateral) | 22 | Female | No | AstraZeneca | 5 days |
| January 1, 202221 | AMN (bilateral) | 26 | Female | Oral contraception | Johnson & Johnson | 2 days |
| January 20, 202222 | AMN (bilateral) | 25 | Female | Oral contraception | AstraZeneca | 1 day |
| January 20, 202223 | AMN (bilateral) | 31 | Female | Oral contraceptive | AstraZeneca | 2 days |
| January 20, 202223 | AMN (bilateral) | 19 | Female | No | AstraZeneca | 1 day |
| June 22, 202124 | AMN (unilateral) | 27 | Female | Oral contraception | AstraZeneca | 2 days |
| June 30, 202125 | AMN (unilateral) | 22 | Female | Oral contraception | AstraZeneca | 2 days |
| June 30, 202125 | AMN (unilateral) | 28 | Female | Oral contraception | AstraZeneca | 2 days |
| July 21, 202126 | AMN (bilateral) | 21 | Female | Oral contraception | AstraZeneca | 3 days |
Table 2.
Summary of reported cases of retinal vein occlusion occurring after COVID-19 vaccination.
| Publication Date | Diagnosis | Age | Sex | Risk factor | Vaccine | Onset of Symptoms After Vaccine |
|---|---|---|---|---|---|---|
| August 23, 202127 | CRVO with CME | 50 | Male | None | Pfizer | 15 minutes |
| September 25, 202130 | Non-ischemic CRVO | 52 | Male | None | Pfizer | 14 days |
| November 17, 202119 | Combined BRAO and BRVO | 81 | Female | Hypertension | Pfizer | 12 days |
| December 5, 202128 | RVO | 68 | Female | None | AstraZeneca | 1 day |
| December 5, 202128 | RVO | 76 | Male | Hypertension | Pfizer | 3 days |
| December 5, 202128 | RVO | 85 | Female | Diabetes mellitus, hypertension | Pfizer | 1 day |
| December 5, 202128 | RVO | 59 | Male | Diabetes mellitus, hypertension | AstraZeneca | 2 days |
| December 5, 202128 | RVO | 61 | Male | None | AstraZeneca | 2 days |
| December 5, 202128 | RVO | 79 | Male | Diabetes mellitus | Pfizer | 2 days |
| December 5, 202128 | RVO | 77 | Female | Hypertension | Pfizer | 16 days |
| December 5, 202128 | RVO | 63 | Male | Diabetes mellitus | Pfizer | 13 days |
| December 5, 202128 | RVO | 51 | Female | Hypertension | AstraZeneca | 21 days |
| December 5, 202128 | RVO | 81 | Female | Hypertension | Pfizer | 4 days |
| December 5, 202128 | RVO | 61 | Male | Hypertension | AstraZeneca | 3 days |
| December 9, 202129 | HRVO with CME | 74 | Female | None | Moderna | 2 days |
| January 9, 202231 | Combined CRAO and CRVO | 54 | Female | None | Moderna | 2 days |
| January 1, 202232 | CRVO with CME | 50 | Male | Diabetes mellitus | AstraZeneca | 4 days |
| January 1, 202232 | CRVO without CME | 43 | Female | None | AstraZeneca | 3 days |
| February 3, 202233 | BRVO with CME | 71 | Male | None | AstraZeneca | 2 days |
| February 3, 202233 | HRVO with CME | 58 | Male | None | AstraZeneca | 3 days |
| February 3, 202233 | BRVO with CME | 73 | Female | Hypertension | AstraZeneca | 3 days |
| February 3, 202233 | BRVO with CME | 47 | Female | None (Hyperthyroidism on treatment) | Pfizer | 5 days |
| February 3, 202233 | Non-ischemic CRVO with CME | 36 | Male | None | Pfizer | 1–3 days |
We observed likely transient pro-thrombotic retinal mileu in patients who received both the Moderna and J&J vaccine. It remains unclear whether there is a shared mechanism between systemic response to the COVID-19 spike protein and the highly pro-thrombotic state seen in COVID-19 infections. In the case of our patients, we postulate their immunologic responses to the vaccines --and possibly a resultant pro-thrombotic state --may have precipitated their ischemic retinal events. Regardless, at this time we strongly believe the benefits of COVID vaccination overwhemingly outweigh its risks.
5. Conclusions
We report on two cases of ischemic retinal events (BRVO and AMN) occurring after COVID-19 vaccination, which may or may not be related. The potential ocular side effects of these vaccines remain unknown and thus we recommend that patients with ocular symptoms after COVID-19 vaccination undergo comprehensive ophthalmologic evaluation with multimodal imaging to assess for potential complications.
Conflicts of interest
Potential conflict of interest exists:
Bottom of Form.
We wish to draw the attention of the Editor to the following facts, which may be considered as potential conflicts of interest, and to significant financial contributions to this work:
The nature of potential conflict of interest is described below:
No conflict of interest exists.
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Funding
Funding was received for this work.
All of the sources of funding for the work described in this publication are acknowledged below:
[List funding sources and their role in study design, data analysis, and result interpretation]
No funding was received for this work.
Intellectual property
We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing we confirm that we have followed the regulations of our institutions concerning intellectual property.
Research ethics
We further confirm that any aspect of the work covered in this manuscript that has involved human patients has been conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged within the manuscript.
IRB approval was obtained (required for studies and series of 3 or more cases)
Written consent to publish potentially identifying information, such as details or the case and photographs, was obtained from the patient(s) or their legal guardian(s).
Authorship
The International Committee of Medical Journal Editors (ICMJE) recommends that authorship be based on the following four criteria:
-
1.
Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; AND
-
2.
Drafting the work or revising it critically for important intellectual content; AND
-
3.
Final approval of the version to be published; AND
-
4.
Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Contact with the editorial office
The Corresponding Author declared on the title page of the manuscript is:
Patient consent
Consent to publish the case report was not obtained. This report does not contain any personal information that could lead to the identification of the patient.
Acknowledgments and Disclosures
No funding or grant support. The following authors have no financial disclosures: AP, JFA, RP. All authors attest that they meet the current ICMJE criteria for Authorship.
References
- 1.Prevention CfDCa. Different COVID-19 Vaccines. Accessed April 7, 2022.
- 2.Prevention CfDCa CDC recommends use of Johnson & Johnson's Janssen COVID-19 vaccine resume. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/JJUpdate.html
- 3.Prevention CfDCa . VAERS); 2021. Data from: The Vaccine Adverse Event Reporting System. [Google Scholar]
- 4.Klein R., Klein B.E., Moss S.E., Meuer S.M. The epidemiology of retinal vein occlusion: the Beaver Dam Eye Study. Trans Am Ophthalmol Soc. 2000;98:133–141. ; discussion 141-3. [PMC free article] [PubMed] [Google Scholar]
- 5.Jaulim A., Ahmed B., Khanam T., Chatziralli I.P. Branch retinal vein occlusion: epidemiology, pathogenesis, risk factors, clinical features, diagnosis, and complications. An update of the literature. Retina. May 2013;33(5):901–910. doi: 10.1097/IAE.0b013e3182870c15. [DOI] [PubMed] [Google Scholar]
- 6.Rahimy E., Sarraf D. Paracentral acute middle maculopathy spectral-domain optical coherence tomography feature of deep capillary ischemia. Curr Opin Ophthalmol. May. 2014;25(3):207–212. doi: 10.1097/ICU.0000000000000045. [DOI] [PubMed] [Google Scholar]
- 7.Bhavsar K.V., Lin S., Rahimy E., et al. Acute macular neuroretinopathy: a comprehensive review of the literature. Surv Ophthalmol. 2016 Sep-Oct 2016;61(5):538–565. doi: 10.1016/j.survophthal.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Pecen P.E., Smith A.G., Ehlers J.P. Optical coherence tomography angiography of acute macular neuroretinopathy and paracentral acute middle maculopathy. JAMA Ophthalmol. Dec. 2015;133(12):1478–1480. doi: 10.1001/jamaophthalmol.2015.4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kulikov A.N., Maltsev D.S., Leongardt T.A. Retinal microvasculature alteration IN paracentral acute middle maculopathy and acute macular neuroretinopathy: a quantitative optical coherence tomography angiography study. Retin Cases Brief Rep. 2020;14(4):343–351. doi: 10.1097/ICB.0000000000000709. [DOI] [PubMed] [Google Scholar]
- 10.Chu S., Nesper P.L., Soetikno B.T., Bakri S.J., Fawzi A.A. Projection-resolved OCT angiography of microvascular changes in paracentral acute middle maculopathy and acute macular neuroretinopathy. Invest Ophthalmol Vis Sci. 06. 01 2018;59(7):2913–2922. doi: 10.1167/iovs.18-24112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Y.C., Chen S.N. Microvascular change in acute macular neuroretinopathy by using optical coherence tomography angiography. Taiwan J Ophthalmol. 2019 Apr-Jun 2019;9(2):118–121. doi: 10.4103/tjo.tjo_83_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nemiroff J., Kuehlewein L., Rahimy E., et al. Assessing deep retinal capillary ischemia in paracentral acute middle maculopathy by optical coherence tomography angiography. Am J Ophthalmol. Feb. 2016;162:121–132.e1. doi: 10.1016/j.ajo.2015.10.026. [DOI] [PubMed] [Google Scholar]
- 13.Shah P., Zaveri J.S., Haddock L.J. Acute macular neuroretinopathy following the administration of an influenza vaccination. Ophthalmic Surg Lasers Imaging Retina. 10 01 2018;49(10):e165–e168. doi: 10.3928/23258160-20181002-23. [DOI] [PubMed] [Google Scholar]
- 14.MacNeil J.R., Su J.R., Broder K.R., et al. Updated recommendations from the advisory committee on immunization practices for use of the Janssen (Johnson & Johnson) COVID-19 vaccine after reports of thrombosis with thrombocytopenia syndrome among vaccine recipients - United States, April 2021. MMWR Morb Mortal Wkly Rep. Apr 30 2021;70(17):651–656. doi: 10.15585/mmwr.mm7017e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li X., Ostropolets A., Makadia R., et al. Characterizing the incidence of adverse events of special interest for COVID-19 vaccines across eight countries: a multinational network cohort study. medRxiv. Apr 17 2021 doi: 10.1101/2021.03.25.21254315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.See I., Su J.R., Lale A., et al. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, march 2 to April 21, 2021. JAMA. 06 22 2021;325(24):2448–2456. doi: 10.1001/jama.2021.7517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valenzuela D.A., Groth S., Taubenslag K.J., Gangaputra S. Acute macular neuroretinopathy following Pfizer-BioNTech COVID-19 vaccination. Am J Ophthalmol Case Rep. Dec 2021;24:101200. doi: 10.1016/j.ajoc.2021.101200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drüke D., Pleyer U., Hoerauf H., Feltgen N., Bemme S. Acute macular neuroretinopathy (AMN) following COVID-19 vaccination. Am J Ophthalmol Case Rep. Dec 2021;24:101207. doi: 10.1016/j.ajoc.2021.101207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Girbardt C., Busch C., Al-Sheikh M., et al. Retinal vascular events after mRNA and Adenoviral-Vectored COVID-19 vaccines-A case series. Vaccines (Basel) Nov 17 2021;9(11) doi: 10.3390/vaccines9111349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zaheer N., Renju M.P., Chavan R. Acute macular neuroretinopathy after COVID-19 vaccination. Retin Cases Brief Rep. Jan 01 2022;16(1):9–11. doi: 10.1097/ICB.0000000000001196. [DOI] [PubMed] [Google Scholar]
- 21.Patel S.N., Yonekawa Y. Acute macular neuroretinopathy after SARS-COV-2 vaccination. Retin Cases Brief Rep. Jan 01 2022;16(1):5–8. doi: 10.1097/ICB.0000000000001195. [DOI] [PubMed] [Google Scholar]
- 22.Gabrielle P.H., Baudin F., Ben Ghezala I., et al. Bilateral acute macular neuroretinopathy in a young woman after the first dose of Oxford-AstraZeneca COVID-19 vaccine. Am J Ophthalmol Case Rep. Mar. 2022;25 doi: 10.1016/j.ajoc.2022.101281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franchi A., Rauchegger T., Palme C., et al. Two cases of acute macular neuroretinopathy associated with the Adenovirus-based COVID-19 vaccine Vaxzevria (Astrazeneca) Ocul Immunol Inflamm. Jan 20 2022:1–6. doi: 10.1080/09273948.2022.2027463. [DOI] [PubMed] [Google Scholar]
- 24.Bøhler A.D., Strøm M.E., Sandvig K.U., Moe M.C., Jørstad Ø. Acute macular neuroretinopathy following COVID-19 vaccination. Eye (Lond) Jun 22 2021 doi: 10.1038/s41433-021-01610-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mambretti M., Huemer J., Torregrossa G., Ullrich M., Findl O., Casalino G. Acute macular neuroretinopathy following Coronavirus Disease 2019 vaccination. Ocul Immunol Inflamm. May 19 2021;29(4):730–733. doi: 10.1080/09273948.2021.1946567. [DOI] [PubMed] [Google Scholar]
- 26.Book B.A.J., Schmidt B., Foerster A.M.H. Bilateral acute macular neuroretinopathy after vaccination against SARS-CoV-2. JAMA Ophthalmol. Jul 01 2021;139(7) doi: 10.1001/jamaophthalmol.2021.2471. [DOI] [PubMed] [Google Scholar]
- 27.Bialasiewicz A.A., Farah-Diab M.S., Mebarki H.T. Central retinal vein occlusion occurring immediately after 2nd dose of mRNA SARS-CoV-2 vaccine. Int Ophthalmol. Dec 2021;41(12):3889–3892. doi: 10.1007/s10792-021-01971-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park H.S., Byun Y., Byeon S.H., Kim S.S., Kim Y.J., Lee C.S. Retinal hemorrhage after SARS-CoV-2 vaccination. J Clin Med. Dec 05 2021;(23):10. doi: 10.3390/jcm10235705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sacconi R., Simona F., Forte P., Querques G. Retinal vein occlusion following two doses of mRNA-1237 (Moderna) immunization for SARS-Cov-2: a case report. Ophthalmol Ther. Feb. 2022;11(1):453–458. doi: 10.1007/s40123-021-00441-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Endo B., Bahamon S., Martínez-Pulgarín D.F. Central retinal vein occlusion after mRNA SARS-CoV-2 vaccination: a case report. Indian J Ophthalmol. 10. 2021;69(10):2865–2866. doi: 10.4103/ijo.IJO_1477_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ikegami Y., Numaga J., Okano N., Fukuda S., Yamamoto H., Terada Y. Combined central retinal artery and vein occlusion shortly after mRNA-SARS-CoV-2 vaccination. QJM. 01 09 2022;114(12):884–885. doi: 10.1093/qjmed/hcab287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sonawane N.J., Yadav D., Kota A.R., Singh H.V. Central retinal vein occlusion post-COVID-19 vaccination. Indian J Ophthalmol. Jan. 2022;70(1):308–309. doi: 10.4103/ijo.IJO_1757_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peters M.C., Cheng S.S.H., Sharma A., Moloney T.P. Retinal vein occlusion following COVID-19 vaccination. Clin Exp Ophthalmol. Feb 03 2022 doi: 10.1111/ceo.14056. [DOI] [PubMed] [Google Scholar]