Abstract
In acute stroke, neuroinflammation can nowadays be analyzed by local cerebral aspiration of pial-ischemic blood during mechanical thrombectomy. Recently, Shaw et al. reported on differences in leukocyte subpopulations within the occluded cerebrovascular compartment. In their study, a main proportion of granulocytes was lost during isolation. By immediate analysis, we found a reproducible increase in absolute local granulocytes without variations in absolute lymphocyte and monocyte numbers. Flow-cytometric phenotyping confirmed a high proportion of granulocytes and a local shift towards CD4+ T cells. Thus, immediate analysis appears to be critical to observe distinct local responses of leukocytes to acute ischemic stroke.
Keywords: Acute ischemic stroke, flow cytometry, immune cells, leukocytes, T cells
In acute experimental stroke, there is strong evidence that infiltrating immune cells in concert with platelets exert deleterious effects within the ischemic brain. 1 In human acute ischemic stroke, the method of local cerebral aspiration of arterial blood from the occluded ischemic compartment only recently has opened an avenue to investigate cellular and molecular processes of the acute stroke immune response. These unique samples are obtained from within the collateral circulation distal to an embolic occlusion and, importantly, before recanalization through mechanical thrombectomy (MT) is achieved.2,3 Thereby, we observed a robust local infiltration of granulocytes during hyperacute human stroke.2,4 In parallel, the Blood and Clot Thrombectomy Registry and Collaboration (BACTRAC) collected the clot and arterial blood during standard of care thrombectomy for laboratory analysis in which leukocytes are isolated and then cryopreserved.3,5 Recently, Shaw et al. reported on flow-cytometry analysis of 16 stroke patients from this biobank. Their analysis uncovered differences in lymphocyte subsets within the intracranial arterial blood compared to the systemic circulation, but the observed granulocyte fraction was exceedingly low. 5 We here report on a shift in leukocyte subsets sampled directly from the secluded cerebral vasculature before MT. In this cohort of 36 stroke patients, we performed immediate flow-cytometry analysis of these whole blood samples conserving all leukocyte subpopulations.
From July 2020 to May 2021, we prospectively observed large-vessel occlusion (LVO) stroke patients undergoing emergency MT. Local microcatheter aspiration within the cerebral ischemic arterial compartment and the cervical internal carotid artery (systemic control) was performed as previously described.2,4 A cohort of patients meeting all a priori defined inclusion criteria comprised 43 patients. 2 Due to the time-critical flow-cytometry work-up, only 36 patients admitted during regular working hours could be analyzed. In brief, Citrate-Phosphate-Dextrose-Adenine (CPDA) 1-anticoagulated whole blood samples of the different arterial regions were immediately stained and analyzed by flow-cytometry using the Multitest™ 6-color TBNK reagent (#644611, BD Biosciences, Heidelberg, Germany) and BD Trucount Tubes (#340334, BD Biosciences, Heidelberg, Germany) according to the manufacturer’s instructions. We calculated absolute cell numbers using the bead population of the Trucount Tubes. Samples were analyzed using a FACS Lyric (BD Biosciences, Heidelberg, Germany) and evaluated through FACSuite Software V1.4 (BD Biosciences, Heidelberg Germany) and FlowJo V10.8.1 (TreeStar). Ethical approval was obtained by the local ethics committee of the University of Würzburg, Germany (approval #135/17). All patients or their legal representatives provided written informed consent.
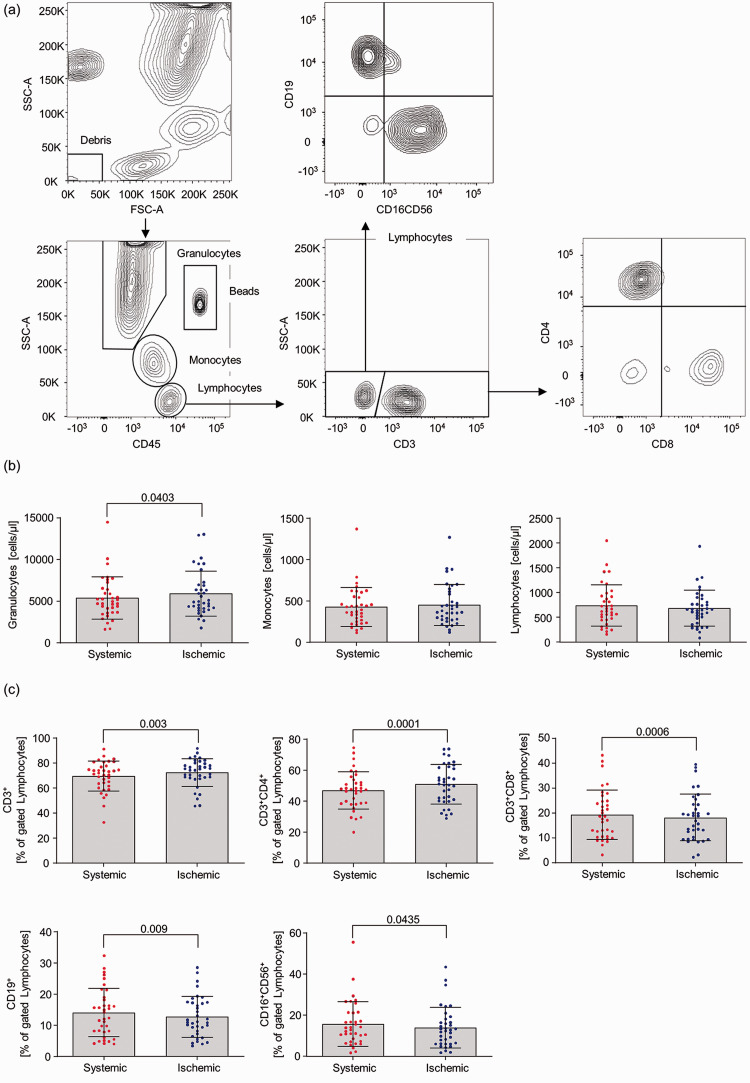
Flow-cytometry based analysis of leukocyte populations (Figure 1(a)) revealed a significant increase in absolute granulocyte numbers in ischemic blood samples when compared to intraindividual systemic controls (systemic 5394 2540 vs ischemic 5901 2706, p = 0.0403). No difference could be observed in absolute monocyte and lymphocyte numbers between the sampling locations (Figure 1(b)). Detailed immune phenotyping of lymphocytes, represented as percent of gated lymphocytes, uncovered an overrepresentation of CD3+ T cells under occlusion condition (systemic 69.56 12.00 vs ischemic 72.45 11.12, p = 0.003). Especially percentages of CD4+ T cells were significantly increased within the ischemic compartment (systemic 46.99 12.03 vs ischemic 50.96 12.81, p = 0.0001), while percentages of CD8+ T, B and NK cells concomitantly decreased in these probes (Figure 1(c)).
Figure 1.
Analysis of leukocyte populations within systemic vs local ischemic blood samples of stroke patients. (a) Gating strategy. (b) Counting of absolute granulocytes, monocytes and lymphocytes numbers. (c) Percentage composition of the lymphocyte population. Statistical analyses were performed using GraphPad Prism 5.0 (GraphPad Software, San Diego, USA). Data are given as mean and SD. Gaussian distribution was tested using the D’Agostino-Pearsons Test followed by a Wilcoxon signed-rank Test or a paired Student’s t Test. P-values <0.05 (two-sided) were considered statistically significant.
To date, the recent study of Shaw and colleagues and our present work are the first studies using flow-cytometry to analyze leukocyte subpopulations in ischemic blood samples locally aspirated during MT. 5 While leukocyte isolation and cryopreservation led to a significant cellular loss within the granulocyte population in the study by Shaw et al., we could show by automatic cell counting that the absolute numbers of granulocytes increased within the secluded ischemic vasculature and made up the predominant leukocyte population, confirming our previous results from two independent prospective cohorts, which were based on manual cell counting.2,4 In line with the data of Shaw and colleagues, we detected a shift within the lymphocyte population towards higher percentages of T cells in the ischemic compartment compared to systemic blood samples. This was due to a decrease in the number of B and NK cells, but an increase of CD4+ helper/inducer T cells at the cost of decreasing cytotoxic/suppressor CD8+ T cells. While the timing and relevance of granulocyte recruitment for infarct formation in human stroke have just been framed by clinical observation under occlusion condition,6,7 there is experimental evidence that T cells, in particular CD4+ T cells, act synergistically with platelets and promote infarct expansion already before recanalization. 8 Accordingly, early clinical trials depleting lymphocytes from the circulation have proven promising as adjunct treatments to MT in acute ischemic stroke. 9 Moreover, systemic alterations in T cell subsets, in particular CD4+ regulatory T cells, have recently been suggested as biomarkers to predict stroke outcome. Thus, it will be important to elucidate how intracerebral T cell responses relate to lymphocyte shifts in the systemic circulation. 10
Taken together, pial blood sampling is a powerful tool to gain insight into ultra-early stroke pathophysiology, with biobanking being a proven tool to access soluble mediators, and immediate cell analysis being critical to define leukocellular responses, which seem to emerge from the vascular compartment to infiltrate the ischemic brain. A major challenge for the future is to disclose intravascular platelet-leukocyte interactions and their mechanistic impact on stroke evolution in human stroke patients.
Acknowledgements
We thank Yanyan Xiong, Julian Kunz and Thomas Günthner-Lengsfeld for their support in data acquisition and patient handling. We also thank the CSF laboratory of the Department of Neurology for excellent technical support.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The project described was supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) [project number 374031971] TR 240 to M.P., G.S. and M.K.S., Gerok position to A.M.K. was supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) [project number 413657723] (Clinician Scientist-Programme UNION CVD).
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iDs
Lena Zimmermann https://orcid.org/0000-0003-4350-3655
Alexander M Kollikowski https://orcid.org/0000-0002-0288-5928
References
- 1.Stoll G, Nieswandt B. Thrombo-inflammation in acute ischaemic stroke – implications for treatment. Nat Rev Neurol 2019; 15: 473–481. [DOI] [PubMed] [Google Scholar]
- 2.Kollikowski AM, Schuhmann MK, Nieswandt B, et al. Local leukocyte invasion during hyperacute human ischemic stroke. Ann Neurol 2020; 87: 466–479. [DOI] [PubMed] [Google Scholar]
- 3.Fraser JF, Collier LA, Gorman AA, et al. The blood and clot thrombectomy registry and collaboration (BACTRAC) protocol: novel method for evaluating human stroke. J Neurointerv Surg 2019; 11: 265–270. [DOI] [PubMed] [Google Scholar]
- 4.Strinitz M, Pham M, Marz AG, et al. Immune cells invade the collateral circulation during human stroke: prospective replication and extension. IJMS 2021; 22: 9161–9109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shaw BC, Maglinger GB, Ujas T, et al. Isolation and identification of leukocyte populations in intracranial blood collected during mechanical thrombectomy. J Cereb Blood Flow Metab 2022; 42: 280–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schabitz WR, Minnerup J. Neutrophils in acute stroke pathophysiology. Stroke 2019; 50: e44–e45. [DOI] [PubMed] [Google Scholar]
- 7.Kollikowski AM, Pham M, Marz AG, et al. Platelet activation and chemokine release are related to local neutrophil-dominant inflammation during hyperacute human stroke. Transl Stroke Res 2021. DOI: 10.1007/s12975-021-00938-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schuhmann MK, Bieber M, Franke M, et al. Platelets and lymphocytes drive progressive penumbral tissue loss during middle cerebral artery occlusion in mice. J Neuroinflammation 2021; 18: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fu Y, Zhang N, Ren L, et al. Impact of an immune modulator fingolimod on acute ischemic stroke. Proc Natl Acad Sci U S A 2014; 111: 18315–18320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li S, Huang Y, Liu Y, et al. Change and predictive ability of circulating immunoregulatory lymphocytes in long-term outcomes of acute ischemic stroke. J Cereb Blood Flow Metab 2021; 41: 2280–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]