Abstract
Candida species other than Candida albicans frequently cause nosocomial infections in immunocompromised patients. Some of these pathogens have either variable susceptibility patterns or intrinsic resistance against common azoles. The availability of a rapid and reproducible susceptibility-testing method is likely to help in the selection of an appropriate regimen for therapy. A flow cytometry (FC) method was used in the present study for susceptibility testing of Candida glabrata, Candida guilliermondii, Candida krusei, Candida lusitaniae, Candida parapsilosis, Candida tropicalis, and Cryptococcus neoformans based on accumulation of the DNA binding dye propidium iodide (PI). The results were compared with MIC results obtained for amphotericin B and fluconazole using the NCCLS broth microdilution method (M27-A). For FC, the yeast inoculum was prepared spectrophotometrically, the drugs were diluted in either RPMI 1640 or yeast nitrogen base containing 1% dextrose, and yeast samples and drug dilutions were incubated with amphotericin B and fluconazole, respectively, for 4 to 6 h. Sodium deoxycholate and PI were added at the end of incubation, and fluorescence was measured with a FACScan flow cytometer (Becton Dickinson). The lowest drug concentration that showed a 50% increase in mean channel fluorescence compared to that of the growth control was designated the MIC. All tests were repeated once. The MICs obtained by FC for all yeast isolates except C. lusitaniae were in very good agreement (within 1 dilution) of the results of the NCCLS broth microdilution method. Paired t test values were not statistically significant (P = 0.377 for amphotericin B; P = 0.383 for fluconazole). Exceptionally, C. lusitaniae isolates showed higher MICs (2 dilutions or more) than in the corresponding NCCLS broth microdilution method for amphotericin B. Overall, FC antifungal susceptibility testing provided rapid, reproducible results that were statistically comparable to those obtained with the NCCLS method.
There has been an apparent shift in infections caused by Candida spp., with non-albicans Candida spp. assuming an ever-increasing role in the pathogenesis of candidemia (1). The newer antifungals have been effective in the treatment of systemic fungal infections and offer a potent alternative to potentially toxic amphotericin B therapy (8). It is well documented, however, that several pathogenic yeasts have either intrinsic or acquired resistance to the azole antifungal drugs (17). It is advisable, therefore, to determine the antifungal susceptibility patterns of patient isolates, which may assist in making appropriate decisions regarding the best therapeutic option (13). The National Committee for Clinical Laboratory Standards (NCCLS) reference broth dilution method (M27-A) is a benchmark currently used in diagnostic laboratories for antifungal susceptibility testing of pathogenic yeasts (10).
A number of investigators have reported flow cytometry (FC) methods to obtain rapid susceptibility results for Candida albicans (4–6, 11, 12, 14–16, 19). These methods measure the effects of the change in membrane potential due to antifungal compounds, the change in metabolic activity due to membrane damage, or the uptake of DNA binding dye in the yeast cell. An improved FC susceptibility-testing method was previously developed in our laboratory (16). The method used sodium deoxycholate for permeability and propidium iodide (PI), a membrane-impermeant DNA-intercalating dye, to detect increased permeability of the cell membrane after antifungal treatment. We have further modified this method for other pathogenic Candida spp. and Cryptococcus neoformans and compared the MIC obtained by the FC method with that obtained by the reference NCCLS broth microdilution method.
MATERIALS AND METHODS
Organisms.
Eighty-two isolates of various yeasts (11 Candida glabrata, 10 Candida guilliermondii, 10 Candida krusei, 11 Candida lusitaniae, 13 Candida parapsilosis, 11 Candida tropicalis, and 16 Cryptococcus neoformans isolates) were tested in parallel by the FC assay and the NCCLS broth microdilution method. The test organisms were either recent clinical isolates or from laboratory culture collections. These cultures were maintained at −20°C on potato dextrose agar. Before the assays, the cultures were passaged twice on Sabouraud dextrose agar at 35°C.
Quality control strains.
Two quality control strains recommended by NCCLS, C. parapsilosis ATCC 22019 and C. krusei ATCC 6258, were included with each series of experiments.
Antifungal agent.
Amphotericin B was purchased from Sigma Chemical Company (St. Louis, Mo.), and fluconazole was a gift from Roerig/Pfizer Pharmaceuticals (New York, N.Y.). Stock solutions of amphotericin B and fluconazole were prepared in dimethyl sulfoxide at concentrations of 1,600 and 6,400 μg/ml, respectively, and stored at −70°C.
Antifungal susceptibility testing using NCCLS broth microdilution test.
The broth microdilution test was performed in accordance with standard M27-A (10). Briefly, serial twofold dilutions of amphotericin B and fluconazole were prepared with RPMI 1640 in microtiter plates. The microtiter plates were stored at −70°C and thawed as required. Inoculum preparation was slightly modified from the NCCLS method by not using a match to 0.5 MacFarland standard; instead, samples of 24- or 48-h-old cultures were suspended in 0.85% saline and the cells were counted in a hemocytometer to yield stock suspensions of 1 × 106 to 5 × 106 cells/ml and diluted to the final concentration of 0.5 × 103 to 2.5 × 103 CFU/ml. The mixture of drugs and inoculum was incubated at 35°C and read after 48 h; the incubation period was up to 72 h for C. neoformans.
FC susceptibility test.
The FC assay was performed essentially as described in an earlier report (16). Briefly, serial twofold dilutions of amphotericin B ranging from 0.03 to 16 μg/ml and of fluconazole ranging from 0.06 to 64 μg/ml were prepared with RPMI 1640 containing l-glutamine without bicarbonate buffered to pH 7.0 with MOPS (morpholinepropanesulfonic acid). The yeast isolates were grown on Sabouraud dextrose agar plates for 18 to 24 h at 35°C. Yeast suspensions were prepared in 0.85% sterile saline. The yeast cell density was adjusted spectrophotometrically to 0.5 MacFarland standard. One-half milliliter of the yeast suspension was added to 0.5 ml of serial drug dilution solution and incubated at 35°C. The growth control tube contained yeast suspension and RPMI 1640 without drugs. For non-albicans species, the mixture of drug and yeast suspension was incubated for 2 h for amphotericin B and 4 h for fluconazole. At the end of incubation, 200 μl of the mixture of yeast and drug were placed in 12- by 75-mm tubes (Falcon; Becton Dickinson, Lincoln Park, N.J.). Two hundred microliters of 25 mM sodium deoxycholate (Sigma Chemical Company) and 5 μl of PI (200 μg/ml) were added to each dilution, and the tubes were gently mixed by flicking them with the fingers. Controls included samples containing viable cells, heat-killed cells, cells with sodium deoxycholate, and cells with PI and sodium deoxycholate. Each tube was analyzed with a FACScan flow cytometer (Becton Dickinson) with Cell Quest software for data acquisition and analysis. The sample volume was 75 μl, and the sample flow rate was 10 μl/min. The instrument settings were as follows: forward scatter, 3.73 linear gain; side scatter, 270 V log; fluorescence (FL2), 457 V log; and threshold value, 52. Each sample was analyzed for 10,000 events or yeast cells. Electronic gates were set up based on live cells used in control experiments. Cell debris and clusters below the gates were not included in sample analyses. The samples were analyzed for forward scatter, side scatter, log of red fluorescence, and mean channel fluorescence (MCF; the intensity of fluorescence of yeasts labeled with PI). The instrument was calibrated and DNA beads were aligned on a daily basis, according to the manufacturer's instructions. The MIC was defined as the lowest concentration of drug that showed an increase of 50% in MCF compared to that of the growth control. If an abrupt increase in MCF occurred for two drug dilutions yielding values much lower or higher than 50%, then the higher drug concentration was taken as the MIC (see Fig. 2). All samples were tested twice.
FIG. 2.
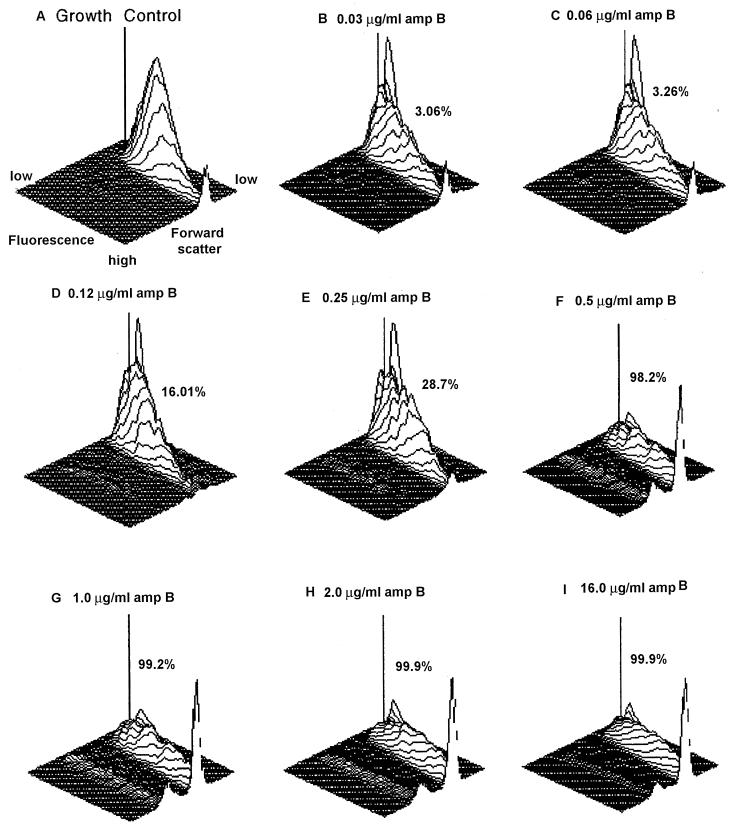
Effect of amphotericin B on C. parapsilosis ATCC 22019. (A) 3-D plot depicting growth control. (B to I) 3-D plots illustrating increasing concentrations of amphotericin B, with dead cells indicated as percent MCF. The dilution at which the percent MCF increased to 50% or more was the MIC of the isolate. In this isolate, an abrupt increase of percent MCF from 28.7% at 0.25 μg to 98.3% at 0.5 μg of amphotericin B/ml was seen; therefore, the MIC is considered to be 0.5 μg/ml.
FC susceptibility testing for Cryptococcus neoformans.
Drug dilutions for amphotericin B and fluconazole were prepared in RPMI 1640 and in yeast nitrogen base with 1% dextrose. The inoculum was prepared from 48-h-old cultures as described in a previous report (16). The inoculum was added to the drug dilutions. The suspensions containing amphotericin B and fluconazole with Cryptococcus neoformans were incubated at 35°C for 4 and 6 h, respectively. The flow cytometer settings and controls for Cryptococcus neoformans were similar to those used for other yeast isolates, and MCF was used to calculate the MICs.
Data analysis.
For comparisons of MICs calculated by the NCCLS method and FC, MIC-0 represented the proportion of isolates with similar MICs by two methods, and MIC-1 represented the proportion of isolates with MICs within 1 dilution by two methods. Additionally, paired t test values (P) were calculated for amphotericin B and fluconazole using Microsoft Excel software (version 5.0). P values of ≤0.001 were considered highly significant.
RESULTS
NCCLS broth microdilution analysis.
The MIC results were read as described in standard M27-A (10). For amphotericin B, the lowest drug concentration that showed complete growth inhibition was considered the MIC for an isolate, while 50% growth inhibition compared to the growth control was considered the fluconazole MIC. The MICs for quality control strains were within the published range with each run of experiments (details not shown). The MICs for various yeasts obtained by NCCLS broth microdilution are summarized in Table 1.
TABLE 1.
MICs for various yeasts obtained by NCCLS broth microdilution and FC methods
| Yeast (no.) | Amphotericin B (μg/ml)
|
Fluconazole (μg/ml)
|
||||||
|---|---|---|---|---|---|---|---|---|
| NCCLS
|
FC
|
NCCLS
|
FC
|
|||||
| Range | MIC | Range | MIC | Range | MIC | Range | MIC | |
| C. glabrata (11) | 0.06–1.0 | 0.5 | 0.12–2.0 | 1.0 | 2–>64 | 16.0 | 2–>64 | 32.0 |
| C. guilliermondii (10) | 0.06–1.0 | 0.06 | 0.12–2.0 | 0.12 | 0.25–16.0 | 4.0 | 0.5–32.0 | 4.0 |
| C. krusei (10) | 0.03–2.0 | 1.0 | 0.06–1.0 | 0.5 | 0.12–>64 | 64.0 | 0.25–>64 | 64.0 |
| C. lusitaniae (11) | 0.06–1.0 | 0.12 | 0.06–2.0 | 1.0 | 0.5–2.0 | 1.0 | 0.25–4.0 | 1.0 |
| C. parapsilosis (13) | 0.12–1.0 | 0.5 | 0.12–0.5 | 0.5 | 0.25–2.0 | 0.25 | 0.12–2.0 | 0.5 |
| C. tropicalis (11) | 0.03–0.5 | 0.25 | 0.03–1.0 | 0.5 | 0.06–32 | 0.5 | 0.06–16.0 | 0.5 |
| Cryptococcus neoformans (16) | 0.03–0.5 | 0.03 | 0.03–1.0 | 0.06 | 0.06–4.0 | 0.5 | 0.12–4.0 | 1.0 |
FC analysis of yeast cells.
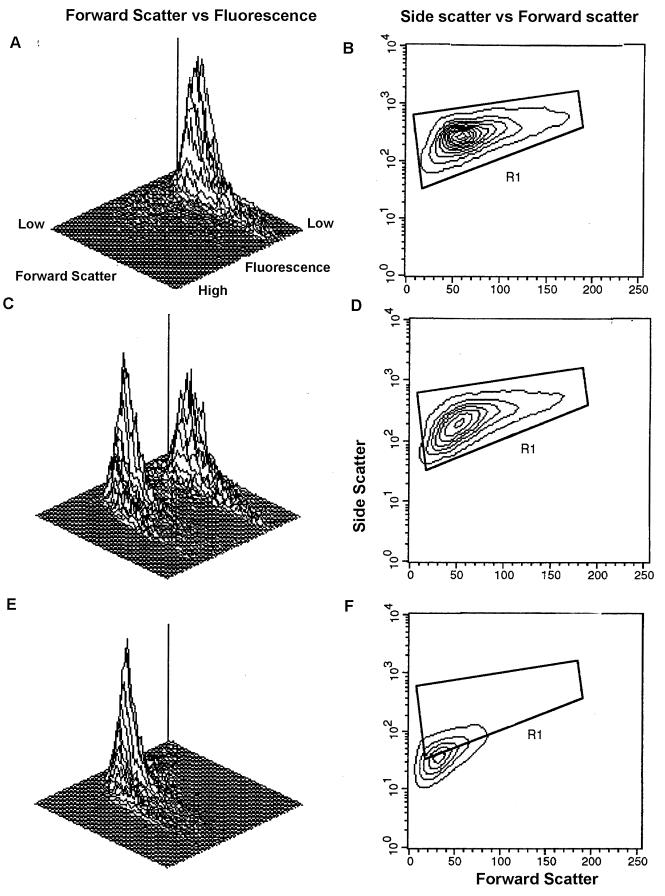
The instrument parameters described above were set up based on FC analyses of live and dead cells in control experiments. Initially, control tubes containing viable and heat-killed cells of all isolates were stained with PI to measure the MCF from log 1 to log 3. Control tubes containing viable cells stained with PI fluoresced in log 1 scale, while heat-killed cells fluoresced in log 3 scale (Fig. 1A and E). The combination of 50% live and 50% dead cells with intermediate PI staining is depicted in Fig. 1C. Based on control live cells, an electronic gate (R1) was set up excluding clusters and cell debris (Fig. 1B). A marked shift of dead cells from R1 is shown in Fig. 1F. The experiments were repeated twice with identical results (data not shown).
FIG. 1.
FC analyses of control cells. C. parapsilosis ATCC 22019 was used to establish analytical parameters. (A) 3-D plot illustrating forward scatter versus fluorescence for live cells. Fluorescence was seen in log 1 scale. (B) Two-dimensional contour plot depicting the electronic gate (R1) set up for live cells. The results of 50% of the events are shown. (C) 3-D plot illustrating two fluorescence peaks for a mixture of 50% live and 50% dead cells. (D) Two-dimensional contour plot depicting 50% live and dead cells in the R1 gate. (E) 3-D plot illustrating heat-killed cells. Fluorescence was seen in log 3 scale. (F) Two-dimensional contour plot depicting dead cells with complete shift in forward scatter and fluorescence compared to panel A. These cells were small and shifted out of the gate R1.
FC susceptibility testing.
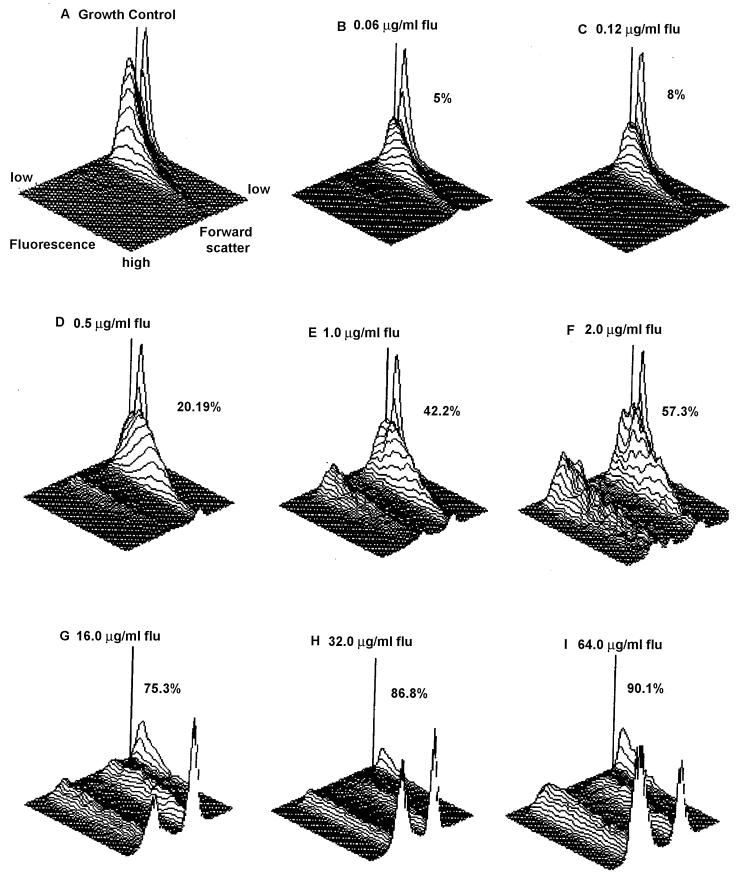
The mean PI fluorescence intensity of untreated control cells was analyzed first. An incubation time of less than 2 h did not yield adequate fluorescence (data not shown). When cells treated with increasing concentrations of antifungals were analyzed, cell membrane damage was noticeable by increased cellular fluorescence intensity that resulted from increased uptake of PI. The gradual increase in MCF obtained with increasing concentrations of amphotericin B is illustrated in Fig. 2 by means of three-dimensional (3-D) plots. The amphotericin B concentration contributing to a 50% or more MCF increase compared to the growth control was considered the MIC, which is represented by C. parapsilosis ATCC 22019 (Fig. 2). The gradual increase in MCF with increasing concentrations of fluconazole is depicted in Fig. 3. The fluconazole concentration (2 μg/ml) at which an MCF of 57.3% was obtained compared to the growth control was considered the MIC. A summary of MICs obtained for all yeast isolates is presented in Table 1.
FIG. 3.
Effect of fluconazole on C. parapsilosis ATCC 22019. (A) 3-D plot depicting growth control. (B to I) 3-D plots illustrating increasing concentrations of fluconazole, with dead cells indicated as percent MCF. The MIC of the isolate was the drug concentration at which the MCF was equal to or more than 50%. In this isolate, an increase of percent MCF from 42.2% at 1.0 μg to 57.3% at 2.0 μg of fluconazole/ml was seen; therefore, 2.0 μg/ml was considered the MIC.
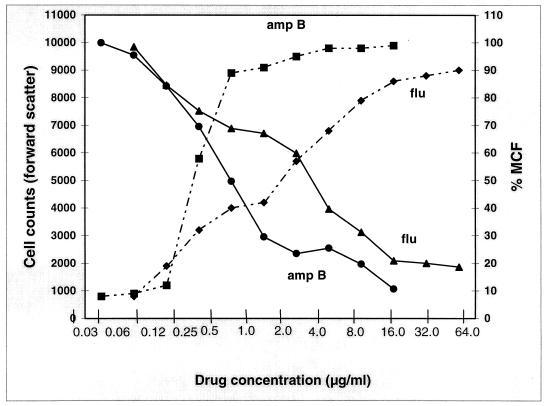
The correlation of antifungal concentration with cell counts in forward scatter and the percentage of MCF is depicted in Fig. 4. At the lowest concentration of both drugs (0.03 μg/ml for amphotericin B and 0.06 μg/ml for fluconazole), the cell counts were highest, with a negligible MCF. There was a gradual increase in the MCF with a concomitant decline in cell counts at increasing drug concentrations. As expected, the highest MCFs were observed with 16 μg of amphotericin B and 64 μg of fluconazole. Additionally, there were few detectable cells with the highest amphotericin B treatment, while 15% of the cells were still detectable after incubation with 64 μg of fluconazole.
FIG. 4.
Correlation of amphotericin B (amp B) and fluconazole (flu) concentrations with both cell counts in forward scatter (solid lines) and percentage MCF (dashed lines) obtained for C. parapsilosis ATCC 22019. At the lowest drug concentration, i.e., 0.03 μg/ml and 0.06 μg/ml for amphotericin B and fluconazole, respectively, the MCF was negligible while cell counts were highest. As the drug concentrations increased, there was a proportional MCF increase and cell count decrease. Note fewer detectable cells with amphotericin B (16 μg/ml) versus a residual population seen at the highest concentration of fluconazole (64 μg/ml).
FC susceptibility testing of Cryptococcus neoformans did not yield consistent results due to the poor growth of test strains in RPMI 1640 (details not shown). Subsequently, RPMI 1640 was replaced with yeast nitrogen broth–1% dextrose and the incubation period was raised to 4 and 6 h for amphotericin B and fluconazole, respectively. These changes yielded consistent MICs that were comparable to the results obtained with the broth microdilution method.
Comparison of NCCLS and FC methods.
A comparison of the data obtained by the FC and NCCLS methods is presented in Table 2. FC MICs were within one drug dilution of those of the reference method for most yeast isolates. A good percentage of agreement (see Materials and Methods) is evident between the values obtained by the NCCLS and FC methods (Table 2). Amphotericin B MIC-0 values for all isolates showed 95 to 99% agreement between the NCCLS and FC methods, while fluconazole MIC-0 values were 96 to 99% in agreement. Paired t test results also showed no significant difference in the results obtained by the two methods (P = 0.377 and 0.383 for amphotericin B and fluconazole, respectively). The only exception to good agreement between the NCCLS and FC methods was seen with C. lusitaniae, for which the MICs obtained by the two methods were quite variable.
TABLE 2.
Agreement among MICs obtained by NCCLS (M27-A) broth microdilution and FC methods
| Organism (no.) | % Agreement
|
|||
|---|---|---|---|---|
| Amphotericin B
|
Fluconazole
|
|||
| MIC-0a | MIC-1b | MIC-0a | MIC-1b | |
| C. glabrata (11) | 98.3 | 93.6 | 99 | 96 |
| C. guilliermondii (10) | 94.5 | 91.6 | 99 | 97.3 |
| C. krusei (10) | 99 | 91 | 98.6 | 93.6 |
| C. lusitaniae (11) | 64.3 | 70.2 | 98.3 | 97 |
| C. parapsilosis (13) | 98.6 | 93.2 | 98.8 | 96 |
| C. tropicalis (11) | 96 | 94.3 | 94 | 91.3 |
| C. neoformans (16) | 98 | 93.4 | 95 | 92 |
Proportion of isolates with same MIC by NCCLS and FC methods.
Proportion of isolates with MIC ± 1 dilution difference by NCCLS and FC methods.
DISCUSSION
The results of this study suggested that Candida spp. and Cryptococcus neoformans could be tested for amphotericin B and fluconazole by FC within 2 to 6 h of incubation, respectively. Amphotericin B, a fungicidal drug, caused shrinkage of yeast cells, as was evident from a decrease in the ratio of forward scatter and side scatter (16). Fluconazole, which is known to inhibit cell growth by disruption of sterol biosynthesis, led to an increase in cell size, apparently due to the accumulation of culture medium. This resulted in an increase in the ratio of forward scatter and side scatter. Subsequently, these cells also disintegrated, perhaps due to increased turgor pressure. Since fluconazole acts by blocking ergosterol formation, an incubation period that is longer than a single generation time seemed an essential prerequisite in order to detect this metabolic effect. Our culture conditions, dilution scheme, and incubation conditions were comparable to those recommended by NCCLS. The results reinforced our previous report that the MCF was a reliable indicator of the MIC for all strains, including strains for which the MICs were high. Interestingly, we did not notice any “trailing effect,” characterized by the lack of a definite reading endpoint, as observed in the NCCLS tests of susceptibility to azoles. Perhaps a shorter incubation and cumulative analyses of individual cells minimized the trailing artifact. It has also been suggested recently that adjustment of the medium pH could eliminate this artifact in the NCCLS test (9).
The present study utilized sodium deoxycholate to enhance the diffusion of PI across the cell wall, enhancing its penetration into the damaged yeast cell membranes. The growth controls did not show dye uptake in the presence of deoxycholate (7). However, the technique as used is only applicable to the antifungal agents that directly or indirectly affect fungal membrane integrity. Previously, Green et al. (4) used PI without sodium deoxycholate and obtained results (MICs) in 6 h. In the present study, the combination of PI with sodium deoxycholate gave faster results, perhaps because deoxycholate enhanced PI penetration.
In previous FC studies, MICs obtained by FC susceptibility testing were compared to those obtained with the standard NCCLS broth macrodilution method (4–6, 12, 16, 19). The investigators mainly used C. albicans and Saccharomyces cerevisiae. In the present study, we have used non-albicans Candida species and C. neoformans, as the incidence of serious yeast infections caused by these organisms is increasing (1). Kirk et al. (5) used acridine orange as a florophore for FC testing. These investigators obtained MICs in 8 h and compared them to results obtained with the NCCLS broth macrodilution method. However, acridine orange binds to DNA, RNA, and lysosomes, and therefore, the results are likely to be affected by the growth phase of the fungal cells (2). Ordonez and Wehman (11) and Peyron et al. (12) have used 3,3′-dipentyloxacarbocyanine iodide for FC susceptibility testing of Candida species against amphotericin B. Their assay was completed in 30 min, and the results obtained were comparable with those of NCCLS broth dilution (12). This is a very promising series of reports on rapid susceptibility testing, which ought to be applied to the testing of azole antifungals.
The high MICs of amphotericin B obtained for C. lusitaniae by the FC method were in sharp contrast to the susceptible range obtained with the NCCLS broth microdilution method. It has been suggested that the NCCLS methodology has a limited ability to detect resistance to amphotericin B (18). Instead of RPMI 1640, antibiotic medium 3 was used to discriminate between resistant and susceptible isolates for amphotericin B (18). Antibiotic medium 3 could not be used for FC susceptibility testing because it interfered with PI fluorescence. More investigations are needed to evaluate whether FC could provide a better screen for amphotericin B resistance.
The FC assay for Cryptococcus neoformans did not yield comparable results when RPMI 1640 was used. Other investigators have reported that yeast nitrogen broth with 1% dextrose provided better susceptibility results for Cryptococcus neoformans (3). Our results supported the better efficacy of yeast nitrogen broth for susceptibility testing of Cryptococcus neoformans. The longer incubations needed for Cryptococcus neoformans testing could be due to a longer generation time as well as the presence of a capsule, which may impede the penetration of drugs and fluorophores.
In conclusion, FC antifungal susceptibility testing provided rapid, reproducible results that were comparable to those obtained by the NCCLS method. The time required to obtain MICs by FC susceptibility testing varied from 2 to 6 h compared to the 24 to 72 h required in the NCCLS broth microdilution method. The FC procedure is simple and can be useful in research and clinical practice by providing precise MIC cutoff points. One obvious drawback of this approach is the need for specialized equipment, which limits its use in routine laboratories. Further evaluations are necessary to assess the usefulness of FC as a technique for antifungal susceptibility testing.
ACKNOWLEDGMENTS
We thank Andrea Doney of the Mycology Laboratory for doing NCCLS susceptibility testing of yeast isolates and Robert Dilwith of the Immunology Core, Wadsworth Center, for his skillful operation of the flow cytometer. We also thank an anonymous reviewer for constructive suggestions.
REFERENCES
- 1.Coleman D C, Rinaldi M G, Haynes K A, Rex J H, Summerbell R C, Anaissie E J, Li A, Sullivan D J. Importance of Candida species other than Candida albicans as opportunistic pathogens. Med Mycol. 1998;36(Suppl. 1):156–165. [PubMed] [Google Scholar]
- 2.Darzynkiewicz Z, Kapuscinski J. Acridine orange: a versatile probe of nucleic acids and other cell constituents. In: Melamed M A, Lindmo T, Mendelsohn M L, editors. Flow cytometry and sorting. 2nd ed. New York, N.Y: Wiley-Liss, Inc.; 1990. pp. 291–314. [Google Scholar]
- 3.Ghannoum M A, Ibrahim A S, Fu Y, Shafiq M C, Edwards J E, Criddle R S. Susceptibility testing of Cryptococcus neoformans: a microdilution technique. J Clin Microbiol. 1992;30:2881–2886. doi: 10.1128/jcm.30.11.2881-2886.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Green L, Petersen B, Steimel L, Haeber P, Current W. Rapid determination of antifungal activity by flow cytometry. J Clin Microbiol. 1994;32:1088–1091. doi: 10.1128/jcm.32.4.1088-1091.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kirk S M, Callister S M, Lim L C L, Schell R F. Rapid susceptibility testing of Candida albicans by flow cytometry. J Clin Microbiol. 1997;35:358–363. doi: 10.1128/jcm.35.2.358-363.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee W, Kwak Y. Antifungal susceptibility testing of Candida species by flow cytometry. J Korean Med Sci. 1999;14:21–26. doi: 10.3346/jkms.1999.14.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lehrer R I, Cline M J. Interventions of Candida albicans with human leucocytes and serum. J Bacteriol. 1969;98:996–1004. doi: 10.1128/jb.98.3.996-1004.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewis R E, Klepser M E. The changing face of nosocomial candidemia: epidemiology, resistance, and drug therapy. Am J Health Syst Pharm. 1999;56:525–533. [PubMed] [Google Scholar]
- 9.Marr K A, Rustad T R, Rex J H, White T C. The trailing end point phenotype in antifungal susceptibility testing is pH dependent. Antimicrob Agents Chemother. 1999;43:1383–1386. doi: 10.1128/aac.43.6.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts. M27-A. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1998. [Google Scholar]
- 11.Ordonez J V, Wehman N M. Amphotericin B susceptibility of Candida species assessed by rapid flow cytometric membrane potential assay. Cytometry. 1995;22:154–157. doi: 10.1002/cyto.990220213. [DOI] [PubMed] [Google Scholar]
- 12.Peyron F A, Favel H, Guiraud-Dauriac M, El Mzibri M, Chastin C, Dumenil G, Regli P. Evaluation of a flow cytofluorometric method for rapid determination of amphotericin B susceptibility of yeast isolates. Antimicrob Agents Chemother. 1997;41:1537–1540. doi: 10.1128/aac.41.7.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pfaller M A, Rex J H, Rinaldi M G. Antifungal susceptibility testing: technical advances and potential clinical applications. Clin Infect Dis. 1997;24:776–784. doi: 10.1093/clinids/24.5.776. [DOI] [PubMed] [Google Scholar]
- 14.Pore R S. Antibiotic susceptibility testing by flow cytometry. J Antimicrob Chemother. 1994;34:613–627. doi: 10.1093/jac/34.5.613. [DOI] [PubMed] [Google Scholar]
- 15.Pore R S. Antibiotic susceptibility testing of Candida albicans by flow cytometry. Curr Microbiol. 1990;20:323–328. [Google Scholar]
- 16.Ramani R, Ramani A, Wong S J. Rapid flow cytometric susceptibility testing of Candida albicans. J Clin Microbiol. 1997;35:2320–2324. doi: 10.1128/jcm.35.9.2320-2324.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rex J H, Rinaldi M G, Pfaller M A. Resistance of Candida species to fluconazole. Antimicrob Agents Chemother. 1995;39:1–8. doi: 10.1128/aac.39.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rex J H, Cooper C H, Jr, Merz W G, Galgiani J N, Anaissie E J. Detection of amphotericin B-resistant Candida isolates in a broth-based system. Antimicrob Agents Chemother. 1995;39:906–909. doi: 10.1128/aac.39.4.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wenich C, Linnau K F, Parschalk B, Zedtwitz-Liebenstein K, Georgopoulos A. Rapid susceptibility testing of fungi by flow cytometry using vital staining. J Clin Microbiol. 1997;35:5–10. doi: 10.1128/jcm.35.1.5-10.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]