Abstract
We investigated the presence and severity of coronary artery disease (CAD) in orthotopic liver transplantation (OLT) candidates using coronary artery calcium score (CACS) and coronary computed tomography angiography (CCTA) as compared with the prevalence of normal and abnormal single-photon emission computed tomography (SPECT) myocardial perfusion imaging (MPI). A total of 140 prospective OLT candidates without known CAD underwent coronary artery calcium (CAC) scans with (n = 77) or without CCTA and coronary computed tomography angiography–derived fractional flow reserve (FFRCT; n = 57) using a dual-source computed tomography (CT) and were followed for 2.6 ± 1.4 years. Coronary plaque was quantified using the segment-involvement score (SIS) and segment stenosis score (SSS). The mean age was 59 ± 6 years, and 65.0% of patients were male. Mean Agatston CACS was 367 ± 653, and 15.0% of patients had CACSs of 0; 83.6% received a SPECT MPI, of which 95.7% were interpreted as normal/probably normal. By CCTA, 9.1% had obstructive CAD (≥70% stenosis), 67.5% had nonobstructive CAD, and 23.4% had no CAD. Nonobstructive CAD was diffuse with mean SIS 3.0 ± 2.9 and SSS 4.5 ± 5.4. Only 14 patients had high risk-findings (severe 3v CAD, n = 4, CACS >1000 n = 10) that prompted X-ray angiography in 3 patients who had undergone CCTA, resulting in revascularization of a high-risk obstruction in 1 patient who had a normal SPECT study. Patients with end-stage liver disease have a high prevalence of nonobstructive CAD by CCTA, which is undiagnosed by SPECT MPI, potentially underestimating cardiovascular risk. Deferring X-ray angiography unless high-risk CCTA findings are present is a potential strategy for avoiding unnecessary X-ray angiography.
It was previously hypothesized that liver disease might provide a protective effect against CAD.(1) This hypothesis was initially supported by the high rate of normal nuclear perfusion studies seen in patients with end-stage liver disease (ESLD) undergoing orthotopic liver transplantation (OLT) evaluation.(2) However, in the early 2000s, it was recognized that there was a significantly higher rate of longterm cardiovascular events due to coronary artery disease (CAD) in patients with OLT.(3) In fact, cardiovascular complications are the leading cause of nongraft-related posttransplant death.(3,4) Furthermore, in Asia where the most prominent etiology of ESLD is hepatitis B virus (HBV) instead of alcohol, the prevalence of occult obstructive CAD by coronary computed tomography angiography (CCTA) was similar between patients with and without cirrhosis with a high prevalence of nonobstructive CAD occurring among patients with cirrhosis.(5)
Traditional stress testing has limitations for detecting CAD prior to OLT. The majority of patients with ESLD have limited functional capacity(6) and often require pharmacological stress testing.(2) Practice guidelines recommend dobutamine stress echocardiography as the initial screening test for cardiac risk stratification in OLT candidates with cardiovascular risk factors.(7) However, patients with ESLD often cannot reach target heart rates, resulting in nondiagnostic rates as high as >50% and a reported pooled sensitivity for CAD of only 33%.(8) Similarly, adenosine single-photon emission computed tomography (SPECT) myocardial perfusion imaging (MPI) has low sensitivity (~37%) for the detection of CAD, which is likely due to chronic vasodilatation associated with ESLD that results in insufficient augmentation in coronary blood flow with adenosine.(8,9) Anatomical coronary assessment with CCTA is not limited by these hemodynamic abnormalities, and computed tomography (CT) provides the additional advantage of simultaneously obtaining a coronary artery calcium score (CACS), which in itself adds prognostic information.(10,11) CCTA recently demonstrated superior sensitivity for the evaluation of obstructive CAD compared with other noninvasive modalities before kidney transplantation.(12) Coronary computed tomography angiography–derived fractional flow reserve (FFRCT) has been shown to correlate well with invasive fractional flow reserve (FFR)(13) and can safely reduce downstream invasive angiography compared with usual care,(14) which would be of great interest in cohorts of greater bleeding risk such as those with ESLD. In the present study, we hypothesized that CACS and CCTA with or without FFRCT would demonstrate a significant burden of CAD in OLT candidates despite a high prevalence of normal perfusion on SPECT MPI.
Patients and Methods
STUDY DESIGN
We conducted a prospective, observational, single-center study enrolling patients with ESLD referred for cardiac evaluation during pre-OLT evaluation. The local institutional review board approved the protocol, and all patients gave written informed consent. Patients were recruited consecutively from April 2014 through January 2018. Inclusion criteria were ESLD patients ≥18 years of age under consideration for OLT with CAD risk factors or a family history of CAD who met the clinical criteria for preoperative stress testing. Exclusion criteria for CCTA and CACS were patients with pregnancy, atrial fibrillation, and body mass index (BMI) >40 kg/m2. Patients with renal impairment (estimated glomerular filtration rate [eGFR] <50 mL/minute/1.73 m2), contraindication to iodinated contrast agents, and hypotension or intolerance to sublingual nitroglycerin were excluded from undergoing CCTA but underwent CACS.
Patients underwent cardiac risk stratification following our institutional protocols. At our institution, stress testing for OLT evaluation is most frequently performed using SPECT MPI and is performed for all patients >50 years old and for patients with a personal history or strong family history of CAD. In patients under age 50 years, male sex, a history of diabetes, chronic kidney disease (CKD) or known vascular disease were also considered risk factors that would prompt cardiac risk stratification. All patients meeting criteria for cardiac risk stratification were eligible for coronary CT evaluation. Patients enrolled in this study underwent CACS ± CCTA based on inclusion/exclusion criteria as described with a goal of characterizing the presence and extent of atherosclerosis. In patients who could not receive contrast, coronary artery calcium (CAC) was used to assess for the presence and extent of atherosclerotic plaque. Providers were blinded to the CT results unless high-risk findings were identified, which were defined as a left main stenosis >50%, 3-vessel disease (≥70% luminal stenosis in all 3 coronary territories in arterial segments with a diameter ≥2 mm), or CACS >1000. The intent of blinding the clinicians to the CCTA result was to prevent CCTA from altering the planned cardiac risk stratification strategy and to prevent patients from inadvertently being denied OLT based on a research study. The severity of liver disease was calculated from the Model for End-Stage Liver Disease as previously described.(15) The 10-year atherosclerotic cardiovascular disease (ASCVD) risk scores were calculated to estimate cardiac risk.(16) All patients were followed up by phone call and chart review every 6 months for 2 years to assess for any further cardiac testing or adverse cardiovascular outcomes in the preoperative, perioperative, and postoperative time frames. In a post hoc exploratory pilot analysis, all CCTA studies were submitted for an FFRCT analysis to determine the feasibility of performing FFRCT in this cohort of patients.
CCTA, FFRCT, AND CACS ACQUISITION AND INTERPRETATION
CACS and CCTA scans for all patients were performed on a 2 × 128-slice dual-source CT scanner (SOMATOM Definition Flash, Siemens Healthineers, Erlangen, Germany) following Society of Cardiovascular Computed Tomography guidelines for the performance and acquisition of CCTA.(17) CACS was performed during a high-pitch spiral acquisition mode. CACS was quantified using the Agatston score.(18) The Multi-Ethnic Study of Atherosclerosis (MESA) distribution of CAC percentiles by race, sex, and age were calculated for each patient.(19) CCTA was acquired with either prospective or retrospective electrocardiogram gating. If the heart rate was regular and under 60 bpm with or without beta-blocker administration, a prospective acquisition was used. Otherwise, a retrospective electrocardiogram-gating with tube current modulation was applied. Patients undergoing CCTA received an injection of 60 mL of iohexol (Omnipaque 350 mg/mL, GE Healthcare, Chicago, IL) contrast at a flow rate of 5 mL/second followed by a 60-mL saline bolus. All CCTA patients received 0.4 mg of sublingual nitroglycerin for coronary vasodilation, unless the patient was relatively hypotensive at baseline or had a contraindication. Patients with a heart rate greater than the target heart rate of <60 bpm at the time of CCTA received metoprolol (5 mg intravenously every 5 minutes for a maximum of 3 doses) for acute heart rate control as tolerated.
All coronary segments were visually analyzed and graded per current guidelines(20) with the use of dedicated software (Aquarius iNtuition Ver4.4.11, Terarecon, San Mateo, CA). The CCTA readers were blinded to available coronary angiography or other stress-testing data. All stenoses graded ≥50% were confirmed by a second reader. In case of disagreement between the 2 readers, a consensus decision was obtained. The senior reader had level 3 training with >10 years of experience in interpreting CCTA studies. Obstructive CAD was defined as a coronary segment with a diameter ≥2 mm and a ≥50% reduction in luminal stenosis. Atherosclerotic plaque burden was quantified by obtaining the segment-involvement score (SIS) and segment stenosis score (SSS) for each patient undergoing CCTA.(21) No patients or segments were excluded from the analysis.
After enrollment was completed for a pilot study, all acquired CCTA data sets were transmitted for central analysis (HeartFlow Inc., Redwood City, CA) of FFRCT as previously described.(22) The lowest FFRCT value, 2-cm downstream of any focal narrowing, involving a major coronary artery ≥2 mm in diameter was registered. Patients were classified as having ischemia if ≥1 vessel had an FFRCT ≤ 0.80.
STATISTICAL ANALYSIS
Analyses were performed using SAS, version 9.4 (SAS Institute, Inc. Cary, NC). Continuous variables were expressed as mean ± standard deviation (SD). Categorical variables were reported as percentages.
Results
PATIENTS
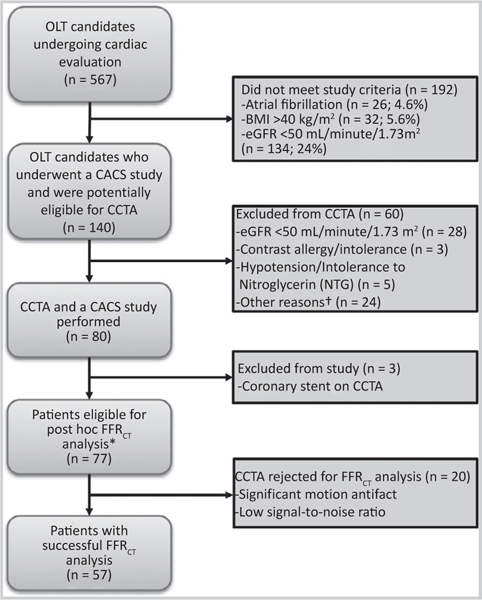
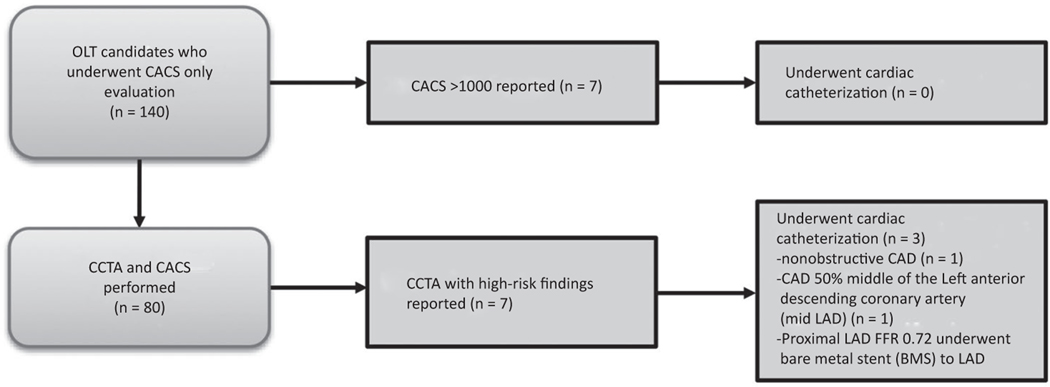
A total of 140 patients (mean age 59 ± 6 years, 65.0% male) were included in this study (Fig. 1; Table 1): 77 patients that underwent CCTA were included in the analysis (3 excluded), and all 140 had a CACS analysis. Over the time of the study, there were 567 patients undergoing OLT evaluation who were potentially eligible. Of these patients, 26 (4.6%) were excluded for atrial fibrillation; 32 (5.6%) were excluded for a BMI >40 kg/m2; and 134 (23.6%) were excluded for GFR < 50 mL/minute/1.73 m2, as specified by our institutional review board protocol (Fig. 1). Three patients enrolled in the study were excluded due the presence of coronary stents on their CCTA. Baseline characteristics are summarized in Table 1. Etiology of ESLD was alcohol and/or hepatitis C virus (HCV) in 55.7% of the patients.
FIG. 1.
Flow diagram of patients in the study who underwent CACS ± CCTA and post hoc FFRCT analysis. Significant coronary findings on CCTA prompting liver transplantation team notification (n = 8). †There were 15 patients who were on metformin, which would have needed to be held prior to the study; 5 patients did not want to have contrast for research; and 4 procedures could not be performed for logistical reasons.
TABLE 1.
Baseline Characteristics
| Demographics | Value (n = 140) |
|---|---|
| Age, years | 59 ± 6 |
| Sex, male | 91 (65.0) |
| Caucasian | 130 (92.9) |
| BMI, kg/m2 | 30.2 ± 4.9 |
| Etiology of liver disease | |
| HCV | 41 (29.3) |
| NASH | 38 (27.1) |
| Alcohol | 23 (16.4) |
| Alcohol and HCV | 14 (10.0) |
| Other | 24 (17.1) |
| Presence of HCC | 21 (15.0) |
| Cardiovascular risk factors | |
| Diabetes | 55 (39.3) |
| Hypertension | 70 (50.0) |
| Dyslipidemia | 50 (35.7) |
| Smoking history | 78 (55.7) |
| 10-year ASCVD risk, % | 16.4 ± 11.5 |
| Medications | |
| Aspirin | 8 (5.7) |
| Beta-blocker | 58 (41.4) |
| Statin | 10 (7.1) |
NOTE: Data are given as mean ±SD or n (%).
CACS ANALYSIS
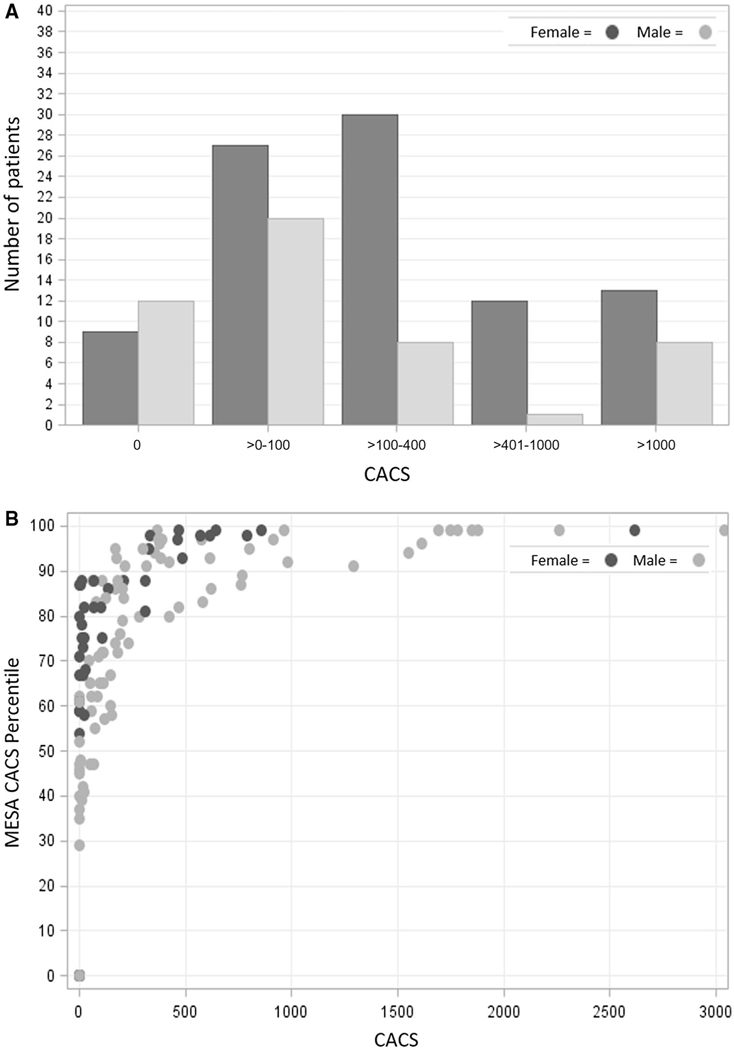
Table 2 shows the breakdown of CACS studies. The mean Agatston CACS was 367.2 ± 653.3. Only 21 (15.0%) had a calcium score of 0, of whom 57.1% were female, and 34 (24.3%) had a CACS >400 as shown in Fig. 2A. Mean MESA CACS percentile was 66 ± 33% (Fig. 2B). Seven patients met reporting criteria based on a CAC-only study with a CACS >1000. None of these patients underwent coronary angiography as part of their OLT workup.
TABLE 2.
Imaging Study Characteristics
| Values | |
|---|---|
|
| |
| CACS (n = 140) | |
| 0 | 21 (15.0) |
| >0–100 | 47 (33.6) |
| >100–400 | 38 (27.1) |
| >401–1000 | 21 (15.0) |
| >1000 | 13 (9.3) |
| CCTA (n = 77)* | |
| Grade 0: normal | 18 (23.4) |
| Grade 1: minimal (<25% luminal stenosis) | 27 (35.1) |
| Grade 2: mild (25%–49% luminal stenosis) | 14 (18.2) |
| Grade 3: moderate (50%–69% luminal stenosis) | 11 (14.3) |
| Grade 4: severe (70%–99% luminal stenosis) | 7 (9.1) |
| Grade 5: occluded | 0 (0) |
| FFRCT (n = 57) | |
| >0.80 | 49 (86.0) |
| ≤0.80 | 8 (14.0) |
| SPECT MPI (n = 117)† | |
| Normal | 101 (86.3) |
| Probably normal | 11 (9.4) |
| Probably abnormal | 2 (1.7) |
| Abnormal | 3 (2.6) |
| Invasive coronary angiography (n = 16) | |
| Angiographically normal coronary arteries | 6 (37.5) |
| Nonobstructive CAD | 8 (50) |
| Obstructive CAD (≥70% luminal stenosis) | 2 (12.5) |
NOTE: Data are given as mean ± SD or n (%).
Stenosis grade.
Myocardial perfusion.
FIG. 2.
(A) CACS distribution by sex. (B) MESA CAC percentile versus measured CACS.
CCTA STUDIES
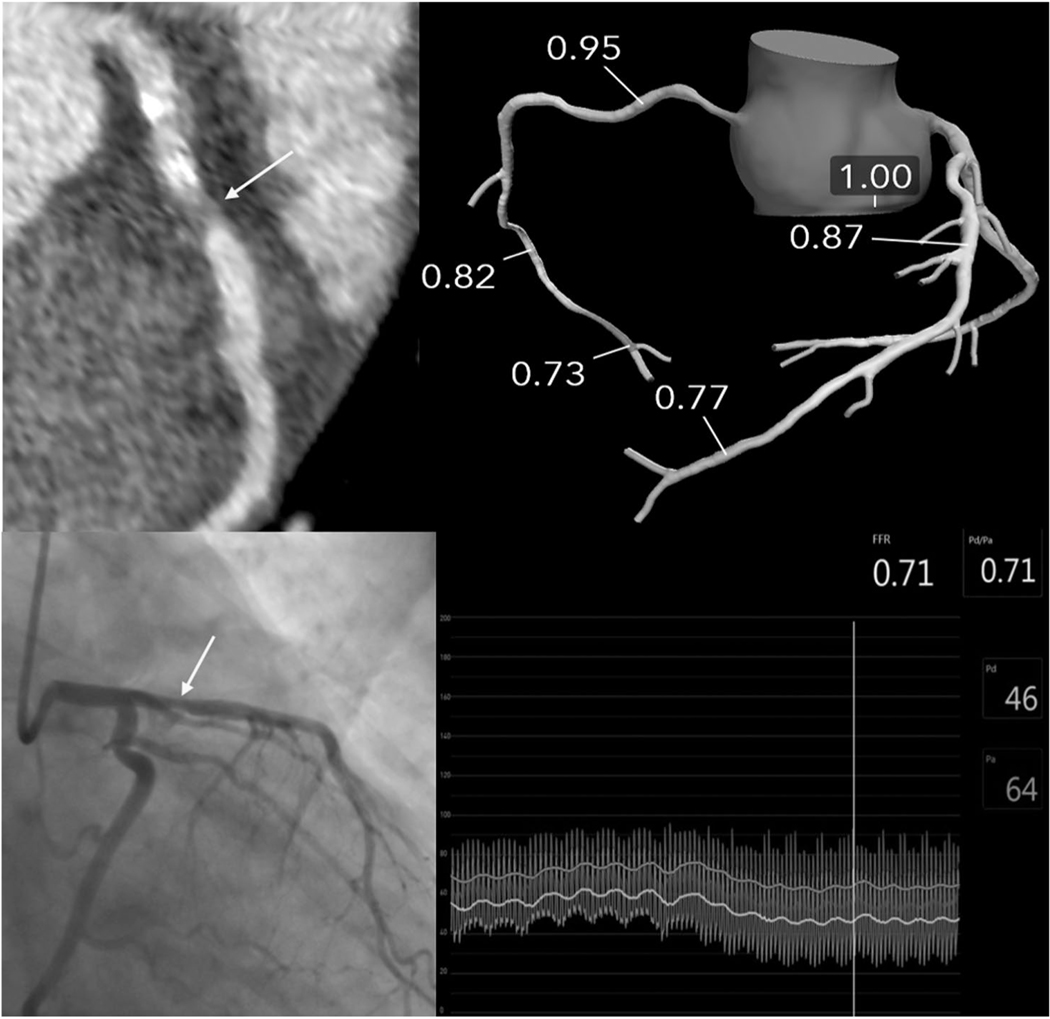
There was a high prevalence of diffuse CAD with mean SIS 3.0 ± 2.9 and mean SSS 4.5 ± 5.4 as defined by CCTA. As shown in Table 2, 67.6% of patients had nonobstructive disease, whereas 9.1% had obstructive CAD. Only 4 patients met criteria for high risk obstructive CAD, and of these, 3 underwent coronary angiography. An additional 3 subjects that underwent both CACS and coronary CTA met high risk criteria by having a CACS >1000. The other patient showed a severe proximal left anterior descending coronary artery (LAD) stenosis (mixed calcified and noncalcified plaque; Fig. 4). Subsequent coronary angiography showed a significant proximal LAD stenosis (FFR, 0.72), and a bare metal stent was placed. The mean dose-length product for the CCTA and CAC was 6.3 ± 4.2 miliSievert (mSv) when using a k value of 0.14 mSv per miliGray (mGy-cm).(23) There were 36 patients enrolled in the study who could have potentially undergone CCTA but who only underwent CAC as detailed in Fig. 1.
FIG. 4.
Images for a patient with ESLD with normal SPECT MPI but a severe proximal LAD lesion on CCTA. The liver transplant team was notified and obtained a coronary angiogram with invasive FFR showing hemodynamic significant (0.71) proximal LAD lesion. FFRCT did not show hemodynamic significance; however, the patient did not receive sublingual nitroglycerin prior to the CCTA.
FFRCT ANALYSIS
An FFRCT analysis was performed successfully in 57/77 (74.0%) patients. As FFRCT was done as a post hoc feasibility pilot study, protocols were not completely optimized, which led to a relatively high rejection rate. Motion or noise artifacts were the main reasons for rejected analysis. Overall, 6/57 (10.5%) patients had a minimum of 1 vessel ≥2 mm in diameter with coronary stenosis associated with FFRCT ≤0.80. Only 1 patient had 2 vessels (LAD and left circumflex coronary artery [LCx]) with FFRCT ≤0.80. Of the 16 patients with a CCTA showing stenosis of grade 3 (50%−69% luminal stenosis) or grade 4 (70%−99% luminal stenosis), 5 patients had potentially hemodynamically significant lesions based on an FFRCT ≤0.80. Only 3/6 patients with significant disease by FFRCT had coronary angiograms within 1 year of their CCTA. All 3 were interpreted as nonobstructive disease; however, none of these patients had invasive FFR assessment done. Only 1 patient who had known obstructive CAD confirmed by invasive FFR, as discussed earlier, did not have significant disease by FFRCT (0.86). However, this patient did not receive sublingual nitroglycerin, which has been shown to improve the accuracy of FFRCT, and had severe stenosis graded on visual assessment of the CCTA (Fig. 4).
SPECT SCANS
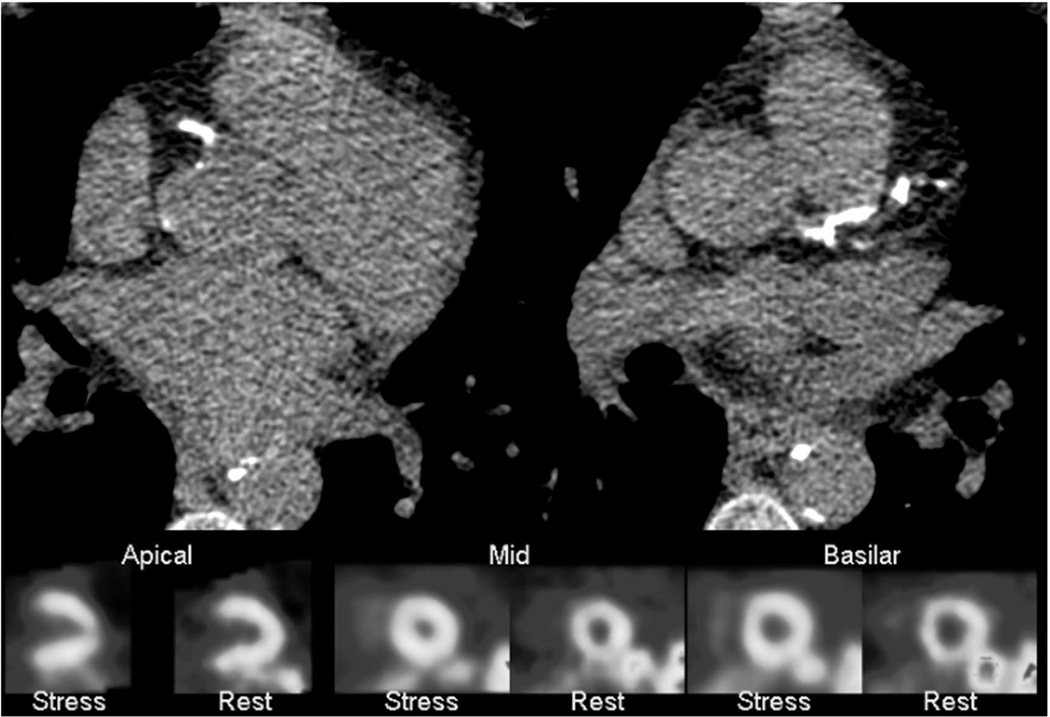
Of the patients in the cohort, 117 (83.6%) had a SPECT MPI performed as part of their liver transplant evaluation, either before or after enrollment in the study. Left ventricle (LV) perfusion was interpreted as normal or probably normal in 112 (95.7%). Of the patients with concurrent CCTA (n = 64), 44 (68.8%) had nonobstructive CAD, and 3 (4.7%) had obstructive CAD. A total of 101 (86.3%) SPECT studies were interpreted as normal. In this group with normal SPECT MPI studies, the mean CAC was 346 ± 635. Figure 5 illustrates how a patient with normal LV perfusion can be falsely reassured of having a low risk when their corresponding CACS shows diffuse coronary artery calcifications.
FIG. 5.
Images for a patient with ESLD and a CACS of 859 with normal LV perfusion on SPECT MPI prior to OLT. The patient developed new systolic heart failure and was found to have 3-vessel CAD 1.3 years after OLT.
INVASIVE CORONARY ANGIOGRAPHY
As part of their transplant evaluation, 16 patients underwent coronary angiography. Five of the patients had had a coronary angiogram within the year prior to enrollment, and 11 received coronary angiography after study enrollment as part of their OLT evaluation. As shown in Table 2, only 2 of these patients had obstructive CAD and 2 of these had a coronary angiogram prior to OLT. One of these patients underwent CACS only, which was 17.8, and had a SPECT MPI read as having probably normal LV perfusion. The other patient underwent CCTA and FFRCT, as discussed earlier (Fig. 4).
FOLLOW-UP FINDINGS
At a mean follow-up of 2.6 ± 1.4 years, 55 (39.3%) patients underwent transplantation and 40 (28.6%) died. After OLT, 6 (10.9%) died of noncardiac causes, 3 (5.5%) had type 2 nonST-elevation myocardial infarctions (NSTEMIs), and 3 (5.5%) had cardiac events occurred. One patient had a type 1 NSTEMI 1.1 years after OLT, was found to have 3-vessel CAD, and underwent 3 drug-eluting stents. This patient had a CACS only, with a score of 422 and SPECT MPI prior to OLT interpreted as small area of inferior ischemia versus diaphragmatic attenuation artifact. A second patient had an acute coronary syndrome (ACS) event (unstable angina) 3.1 years after OLT, was found to have 3-vessel CAD, and underwent coronary artery bypass graft (CABG). He had CACS (194) only and a SPECT MPI interpreted as normal LV perfusion. Another patient developed new systolic heart failure and was found to have 3-vessel CAD 1.3 years after OLT and was treated medically. This patent had a CACS (859) only and a SPECT MPI prior to OLT interpreted as normal as shown in Fig. 4. For those who underwent OLT and experienced cardiac events, mean CACS was 491.6 ± 337.9. Despite a high rate of normal studies, 10.8% of the cohort underwent repeat SPECT MPI testing within 27.2 ± 17.9 months, but only 1 patient was ultimately found to have obstructive CAD on a coronary angiogram.
Discussion
The present study demonstrates that CACS and CCTA can be used for risk stratification in ESLD patients undergoing cardiac evaluation prior to OLT. ESLD patients in our study were found to have a low prevalence of obstructive CAD but a high prevalence of diffuse nonobstructive CAD. This high prevalence of CAD in this cohort was underdiagnosed by traditional stress tests such as SPECT MPI, with none being interpreted as demonstrating definite reversible ischemia. Our results demonstrate that deferring coronary angiography in patients without a high-risk CCTA (left main disease, 3 vessel disease, or CACS >1000) appears to be safe.
Although guidelines suggest that dobutamine stress echo is the recommended initial screening test in OLT candidates with cardiac risk factors,(7) clinical practice varies significantly by institutional preferences. At our institution, SPECT has typically been performed. We report that 95.7% of the SPECT MPI studies were interpreted as normal or probably normal in our cohort, reflecting the low yield of this modality as a screening study in this population. This high rate of normal perfusion studies is similar to a recent large cohort (n = 2500) of SPECT MPI studies performed for OLT cardiac risk evaluation.(24) The concern with this modality for assessing cardiovascular risk in ESLD is 2-fold:
Underdiagnosis of obstructive CAD in patients with ESLD, likely related to decreased arterial resistance in ESLD due to an increased production/activity of vasodilator factors and decreased vascular response to vasoconstrictors.(9)
Functional testing does not provide any assessment of nonobstructive CAD.
The identification of atherosclerotic plaque by CACS and CCTA may provide improved risk stratification by identifying patients who are at higher risk of adverse cardiovascular outcomes and identifying patients who may benefit from primary prevention medications, such as statins, following liver transplantation. High-risk plaque characteristics (positive remodeling or low-attenuation plaque) on CCTA predict an increased risk for nonfatal myocardial infarction or coronary heart disease death even in the absence of significant luminal narrowing in patients presenting with angina chest pain.(25) CACS provides a marker of the overall amount of plaque and consistently demonstrates a strong association with adverse events in primary and secondary prevention studies.(10,11) A retrospective study in OLT candidates found that a CACS >400 was associated with increased cardiovascular complications within 1 month after OLT.(26) In our study, 85.0% of patients had evidence of coronary calcification on CACS with over half of the patients having a CACS >100 and over a quarter of patients with a CACS >400. Despite this high burden of atherosclerosis, only 7.1% of patients were on a statin. A recent large observational study of patients without known CAD showed that patients with severe coronary artery calcification (CACS >400) had significant cardiovascular risk reduction on statin therapy, with a number needed to treat of 12 to prevent a first major adverse cardiovascular event over 10 years.(11) The mean low-density lipoprotein–cholesterol (LDL-C) in our study was <100 mg/dL. This is consistent with the known effects of ESLD resulting in impaired hepatic production.(7) This lower LDL-C may further falsely reassure providers into not prescribing statins. However, there is evidence for the safety of statins in ESLD, and data suggest that they are beneficial in reducing liver decompensation and death.(27) Future studies are need to understand whether statin therapy could lower cardiovascular risk after OLT when a significant CAD burden is revealed by CT.
We demonstrated that the prevalence of obstructive CAD (≥70% luminal stenosis) on CCTA was low as has been shown previously in patients evaluated for OLT by CCTA.(5) Given the low prevalence of obstructive CAD among patients referred for cardiac evaluation prior to OLT, the impact of CCTA on identifying actionable high-risk lesions may be limited. Less than one-fifth of our cohort underwent coronary angiography as part of their evaluation before liver transplant, and of those, only 12.5% had obstructive CAD. Our results demonstrate that deferring coronary angiography in patients without a high-risk CCTA (left main disease, 3 vessel disease, or CACS >1000) appears to be safe. CCTA has not been prospectively studied to evaluate cardiac risk factors in patients undergoing OLT assessment. As such, our study adds to this literature because it was prospective; performed in the United States and not Asia, where the most prominent etiology of ESLD is HBV instead of alcohol; included CACS and FFRCT; and included correlation with available SPECT MPI and invasive coronary angiography.
To the best of our knowledge, FFRCT has not been studied in patients with ESLD. We report higher eligibility for the FFRCT analysis in our cohort than what was seen from FFRCT analysis applied to the Prospective Multicenter Imaging Study for Evaluation of Chest Pain (PROMISE) cohort (74.0% versus 66.8%), suggesting that acceptable CT image quality can be obtained in this cohort with unique hemodynamic profiles.(28) Prospective CCTA studies that take into account the requirements for FFR-CT have higher acceptance rates (85–90%).(29)
As the prevalence of nonalcoholic steatohepatitis (NASH) and the average age of patients undergoing OLT continues to rise, cardiovascular etiologies have as expected become a leading cause of morbidity and morbidity after liver transplantation.(3) In 1 study, cardiovascular disease was responsible for 21% of deaths for those surviving 3 years after OLT.(8) Our study, which was not powered for outcomes, showed a lack of immediate cardiac events after OLT and had few up to 3.1 years after. An older study from the 1990s showed that patients with obstructive CAD who underwent liver transplant had very high mortality (50%) immediately after or up to 3 years after liver transplantation.(30) Thus, it is important to effectively screen for CAD for 2 major purposes:
Identify those patients with obstructive CAD because they appear to be the patients of highest risk of mortality in the perioperative setting.
Identify those patients with nonobstructive CAD because they may benefit from cardiovascular risk reduction with medical therapy and lifestyle modification to improve their longterm outcomes after OLT.
CCTA with CACS with or without FFRCT can effectively screen for both of these patients with ESLD.
Outcomes from studies comparing CCTA versus standard-of-care testing in patients with chest pain suggest that increased detection of nonobstructive CAD by CCTA may have an impact on patient management and longer-term cardiovascular outcomes. The PROMISE trial showed that the risk of death or nonfatal myocardial infarction was lower in the CCTA group versus the group that underwent stress testing (hazard ratio [HR], 0.66; P < 0.05).(28) Similarly, the 5-year follow-up from the Scottish Computed Tomography (SCOT) heart study demonstrated a lower risk of cardiac death or nonfatal myocardial infarction was lower in the CCTA group (HR, 0.59; P = 0.004) likely due to the higher initiation of preventive therapies in the CCTA group (odds ratio, 1.40).(31) This study also demonstrated that CACS was the strongest predictor of poor outcomes in this cohort. Although these studies did not specifically look at liver transplant candidates, medical management of CAD may be important in this cohort given its potential to impact longterm cardiovascular events and not just acute cardiovascular events in the perioperative period.
Our study, although prospective, was not randomized, so only associative observations could be made between the performance of SPECT MPI and CCTA. However, given the low prevalence of obstructive CAD and the low number of adverse events, a definitive comparison between techniques would require a large randomized multicenter clinical trial to have adequate power to detect a difference on outcomes between standard of care versus CT-guided care. Our pilot study demonstrates that such a study would be feasible in this patient population. Not all patients had SPECT MPI for comparison and coronary angiography was only performed if deemed necessary by the treating physicians, the incidence of which our study did not significantly increase. Notably, there was an extremely low rate of abnormal SPECT MPI despite a very high prevalence of diffuse nonobstructive CAD.
Although in our research study a glomerular filtration rate (GFR) <50 mL/minute/1.73 m2 was considered as a contraindication to receiving contrast, current American College of Radiology recommendations suggest that there are limited data that patients with eGFR ≥30 mL/minute/1.73 m2 have a significant risk of acute kidney injury from iodinated contrast agents,(32) and an eGFR >30 mL/minute/1.73 m2 is often used clinically as a threshold for administering contrast in patients with CKD. Among the patients enrolled in the study, 85.7% of patients had a GFR >40 mL/minute/1.73 m2, and 92.8% had a GFR >30 mL/minute/1.73 m2. Thus, more than 90% of patients undergoing cardiac evaluation would have been able to receive a CCTA if it was being done for clinical reasons.
In conclusion, CCTA ± FFRCT is a safe, feasible, and reliable study for ruling out obstructive CAD and diagnosing nonobstructive CAD in OLT candidates. SPECT MPI appears to provide a very low yield in this population with high normal rates even in those with obstructive CAD likely due to poor response of vasodilator agents in patients with ESLD. LDL-C levels likely underestimate cardiovascular risk in this population, and statins are underused. Future studies are needed to understand whether statin therapy can improve longterm cardiovascular outcomes when significant CAD burden is revealed by CCTA.
FIG. 3.
Flow diagram of patients meeting the criteria requiring reporting to the liver transplant team for high-risk findings.
Acknowledgments:
The authors thank our study coordinators Caroline Flournoy, Ph.D., and Sara Prince, R.N.
This work was supported by the University of Virginia Health System Clinical Research Support Grant. Michael Salerno is supported in part by National Institutes of Health grant R01-HL-131919. Adrián I. Löffler, Jorge A. Gonzalez, and Roshin C. Mathew were supported by National Institutes of Health grant 5T32-EB-003841.
Jorge A. Gonzalez is a speaker for HeartFlow Inc. and Boston Scientific. Klaus D. Hagspiel receives research support from Siemens Healthineers.Christopher M. Kramer is on the advisory board for a clinical trial funded by HeartFlow. Campbell Rogers is a full-time employee of HeartFlow and receives salary and stock options from HeartFlow. Neeral L. Shah is on the advisory board for Dova Pharmaceuticals. Michael Salerno receives grant support from AstraZeneca and research support from Siemens.
Abbreviations:
- ACS
acute coronary syndrome
- ASCVD
atherosclerotic cardiovascular disease
- BMI
body mass index
- CAC
coronary artery calcium
- CABG
coronary artery bypass graft
- CACS
coronary artery calcium score
- CAD
coronary artery disease
- CCTA
coronary computed tomography angiography
- CKD
chronic kidney disease
- CT
computed tomography
- eGFR
estimated glomerular filtration rate
- ESLD
end-stage liver disease
- FFR
fractional flow reserve
- FFRCT
coronary computed tomography angiography–derived fractional flow reserve
- GFR
glomerular filtration rate
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- HR
hazard ratio
- LAD
left anterior descending coronary artery
- LCx
left circumflex coronary artery
- LDL-C
low-density lipoprotein–cholesterol
- LV
left ventricle
- MESA
Multi-Ethnic Study of Atherosclerosis
- mGy-cm
miliGray
- MPI
myocardial perfusion imaging
- mSv
miliSievert
- NASH
nonalcoholic steatohepatitis
- NSTEMI
non-ST-elevation myocardial infarction
- OLT
orthotopic liver transplantation
- Pa
arterial pressure
- Pd
distal pressure
- SD
standard deviation
- SIS
segment-involvement score
- SPECT
single-photon emission computed tomography
- SSS
segment stenosis score
REFERENCES
- 1).Howell WL, Manion WC. The low incidence of myocardial infarction in patients with portal cirrhosis of the liver: a review of 639 cases of cirrhosis of the liver from 17,731 autopsies. Am Heart J 1960;60:341–344. [DOI] [PubMed] [Google Scholar]
- 2).Kryzhanovski VA, Beller GA. Usefulness of preoperative noninvasive radionuclide testing for detecting coronary artery disease in candidates for liver transplantation. Am J Cardiol 1997;79:986–988. [DOI] [PubMed] [Google Scholar]
- 3).Therapondos G, Flapan AD, Plevris JN, Hayes PC. Cardiac morbidity and mortality related to orthotopic liver transplantation. Liver Transpl 2004;10:1441–1453. [DOI] [PubMed] [Google Scholar]
- 4).Fouad TR, Abdel-Razek WM, Burak KW, Bain VG, Lee SS. Prediction of cardiac complications after liver transplantation. Transplantation 2009;87:763–770. [DOI] [PubMed] [Google Scholar]
- 5).An J, Shim JH, Kim SO, Lee D, Kim KM, Lim YS, et al. Prevalence and prediction of coronary artery disease in patients with liver cirrhosis: a registry-based matched case-control study. Circulation 2014;130:1353–1362. [DOI] [PubMed] [Google Scholar]
- 6).Dunn MA, Josbeno DA, Schmotzer AR, Tevar AD, DiMartini AF, Landsittel DP, Delitto A. The gap between clinically assessed physical performance and objective physical activity in liver transplant candidates. Liver Transpl 2016;22:1324–1332. [DOI] [PubMed] [Google Scholar]
- 7).Martin P, DiMartini A, Feng S, Brown R, Fallon M. Evaluation for liver transplantation in adults: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Hepatology 2014;59:1144–1165. [DOI] [PubMed] [Google Scholar]
- 8).Ehtisham J, Altieri M, Salamé E, Saloux E, Ollivier I, Hamon M. Coronary artery disease in orthotopic liver transplantation: pretransplant assessment and management. Liver Transpl 2010;16:550–557. [DOI] [PubMed] [Google Scholar]
- 9).Davidson CJ, Gheorghiade M, Flaherty JD, Elliot MD, Reddy SP, Wang NC, et al. Predictive value of stress myocardial perfusion imaging in liver transplant candidates. Am J Cardiol 2002;89:359–360. [DOI] [PubMed] [Google Scholar]
- 10).Hou ZH, Lu B, Gao Y, Jiang SL, Wang Y, Li W, Budoff MJ. Prognostic value of coronary CT angiography and calcium score for major adverse cardiac events in outpatients. JACC Cardiovasc Imaging 2012;5:990–999. [DOI] [PubMed] [Google Scholar]
- 11).Mitchell JD, Fergestrom N, Gage BF, Paisley R, Moon P, Novak E, et al. Impact of statins on cardiovascular outcomes following coronary artery calcium scoring. J Am Coll Cardiol 2018;72:3233–3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Winther S, Svensson M, Jørgensen HS, Bouchelouche K, Gormsen LC, Pedersen BB, et al. Diagnostic performance of coronary CT angiography and myocardial perfusion imaging in kidney transplantation candidates. JACC Cardiovasc Imaging 2015;8:553–562. [DOI] [PubMed] [Google Scholar]
- 13).Koo BK, Erglis A, Doh JH, Daniels DV, Jegere S, Kim HS, et al. Diagnosis of ischemia-causing coronary stenoses by noninvasive fractional flow reserve computed from coronary computed tomographic angiograms. Results from the prospective multicenter DISCOVER-FLOW (Diagnosis of Ischemia-Causing Stenoses Obtained Via Noninvasive Fractional Flow Reserve) study. J Am Coll Cardiol 2011;58:1989–1997. [DOI] [PubMed] [Google Scholar]
- 14).Douglas PS, Pontone G, Hlatky MA, Patel MR, Norgaard BL, Byrne RA, et al. ; for PLATFORM Investigators. Clinical outcomes of fractional flow reserve by computed tomographic angiography-guided diagnostic strategies vs. usual care in patients with suspected coronary artery disease: the prospective longitudinal trial of FFR(CT): outcome and resource impacts study. Eur Heart J 2015;36:3359–3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, et al. A model to predict survival in patients with end-stage liver disease. Hepatology 2001;33:464–470. [DOI] [PubMed] [Google Scholar]
- 16).Goff DC Jr., Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129:S49–S73. [DOI] [PubMed] [Google Scholar]
- 17).Abbara S, Blanke P, Maroules CD, Cheezum M, Choi AD, Han BK, et al. SCCT guidelines for the performance and acquisition of coronary computed tomographic angiography: a report of the society of Cardiovascular Computed Tomography Guidelines Committee: Endorsed by the North American Society for Cardiovascular Imaging (NASCI). J Cardiovasc Comput Tomogr 2016;10:435–449. [DOI] [PubMed] [Google Scholar]
- 18).Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 1990;15:827–832. [DOI] [PubMed] [Google Scholar]
- 19).McClelland RL, Chung H, Detrano R, Post W, Kronmal RA. Distribution of coronary artery calcium by race, gender, and age: results from the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation 2006;113:30–37. [DOI] [PubMed] [Google Scholar]
- 20).Leipsic J, Abbara S, Achenbach S, Cury R, Earls JP, Mancini GJ, et al. SCCT guidelines for the interpretation and reporting of coronary CT angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr 2014;8:342–358. [DOI] [PubMed] [Google Scholar]
- 21).Min JK, Shaw LJ, Devereux RB, Okin PM, Weinsaft JW, Russo DJ, et al. Prognostic value of multidetector coronary computed tomographic angiography for prediction of all-cause mortality. J Am Coll Cardiol 2007;50:1161–1170. [DOI] [PubMed] [Google Scholar]
- 22).Nørgaard BL, Leipsic J, Gaur S, Seneviratne S, Ko BS, Ito H, et al. ; for NXT Trial Study Group. Diagnostic performance of noninvasive fractional flow reserve derived from coronary computed tomography angiography in suspected coronary artery disease: the NXT trial (Analysis of coronary blood flow using CT angiography: next steps). J Am Coll Cardiol 2014;63:1145–1155. [DOI] [PubMed] [Google Scholar]
- 23).Halliburton SS, Abbara S, Chen MY, Gentry R, Mahesh M, Raff GL, et al. Society of cardiovascular computed tomography. SCCT guidelines on radiation dose and dose-optimization strategies in cardiovascular CT. J Cardiovasc Comput Tomogr 2011;5:198–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Duvall WL, Singhvi A, Tripathi N, Henzlova MJ. SPECT myocardial perfusion imaging in liver transplantation candidates. J Nucl Cardiol 2020;27:254–265. [DOI] [PubMed] [Google Scholar]
- 25).Williams MC, Moss AJ, Dweck M, Adamson PD, Alam S, Hunter A, et al. Coronary artery plaque characteristics associated with adverse outcomes in the SCOT-HEART study. J Am Coll Cardiol 2019;73:291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26).Kong YG, Kang JW, Kim YK, Seo H, Lim TH, Hwang S, et al. Preoperative coronary calcium score is predictive of early postoperative cardiovascular complications in liver transplant recipients. Br J Anaesth 2015;114:437–443. [DOI] [PubMed] [Google Scholar]
- 27).Kim RG, Loomba R, Prokop LJ, Singh S. Statin use and risk of cirrhosis and related complications in patients with chronic liver diseases: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2017;15:1521–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28).Lu MT, Ferencik M, Roberts RS, Lee KL, Ivanov A, Adami E, et al. Noninvasive FFR derived from coronary CT angiography: management and outcomes in the PROMISE trial. JACC Cardiovasc Imaging 2017;10:1350–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).Gonzalez JA, Lipinski MJ, Flors L, Shaw PW, Kramer CM, Salerno M. Meta-analysis of diagnostic performance of coronary computed tomography angiography, computed tomography perfusion, and computed tomography-fractional flow reserve in functional myocardial ischemia assessment versus invasive fractional flow reserve. Am J Cardiol 2015;116:1469–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30).Plotkin JS, Scott VL, Pinna A, Dobsch BP, De Wolf AM, Kang Y. Morbidity and mortality in patients with coronary artery disease undergoing orthotopic liver transplantation. Liver Transpl Surg 1996;2:426–430. [DOI] [PubMed] [Google Scholar]
- 31).Newby DE, Adamson PD, Berry C, Boon NA, Dweck MR, et al. ; for SCOT-HEART Investigators. Coronary CT angiography and 5-year risk of myocardial infarction. N Engl J Med 2018;379:924–933. [DOI] [PubMed] [Google Scholar]
- 32).American College of Radiology Committee on Drugs and Contrast Media. ACR Manual on Contrast Media. https://www.acr.org/-/media/ACR/files/clinical-resources/contrast_media.pdf. Accessed June 20, 2020.